Antioxidant and Antibacterial Effect of Fruit Peel Powders in Chicken Patties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fruit Peel Powders
2.2. Ingredients Preparation
2.3. Product Formulation
2.4. Product Processing and Storage
2.5. Examinations
2.5.1. Total Phenolic Content Analysis in Fruit Peel Powders
2.5.2. Total Flavonoid Content Analysis in Fruit Peel Powders
2.5.3. Antioxidant Activity Measurement of Fruit Peel Powders Using DPPH Assay
2.5.4. Proximate Compositional Analysis of Chicken Patties
2.5.5. Bacteriological Examination of Chicken Patties
2.5.6. Deterioration Criteria Measurement of the Chicken Patties
2.5.7. Color Evaluation of Chicken Patties
2.5.8. Sensory Analysis of Chicken Patties
2.6. Statistical Analysis
3. Results and Discussion
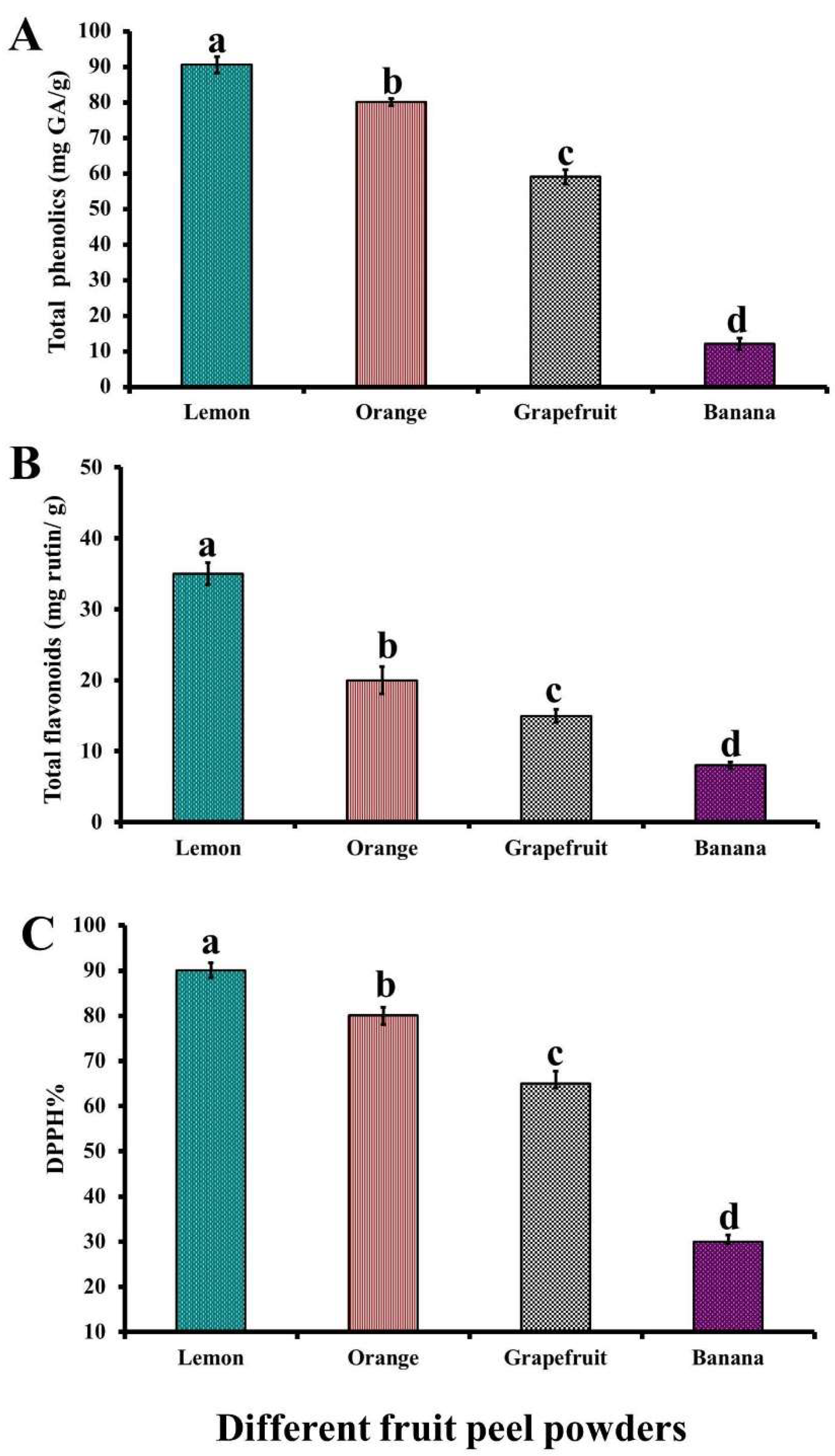
3.1. Total Phenolics, Total Flavonoids, and DPPH% of Fruit Peel Powders
3.2. Proximate Composition Analysis of the Different Fruit Peel Powder-Treated Chicken Patties
3.3. Bacteriological Examination of the Different Fruit Peel Powder-Treated Chicken Patties
3.4. Deterioration Criteria of the Different Fruit Peel Powder-Treated Chicken Patties
3.5. Color Evaluation of the Different Fruit Peel Powder-Treated Chicken Patties
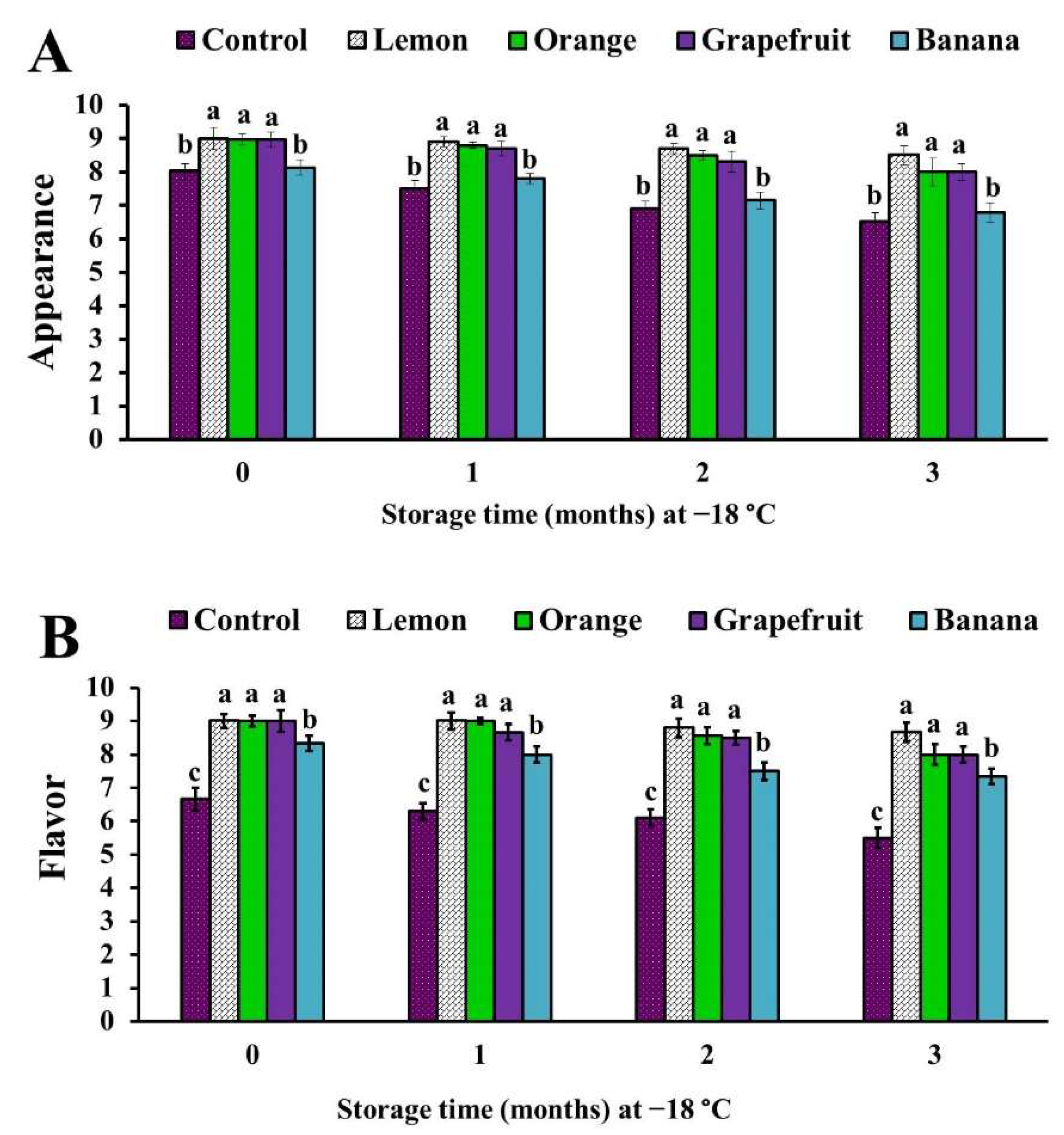
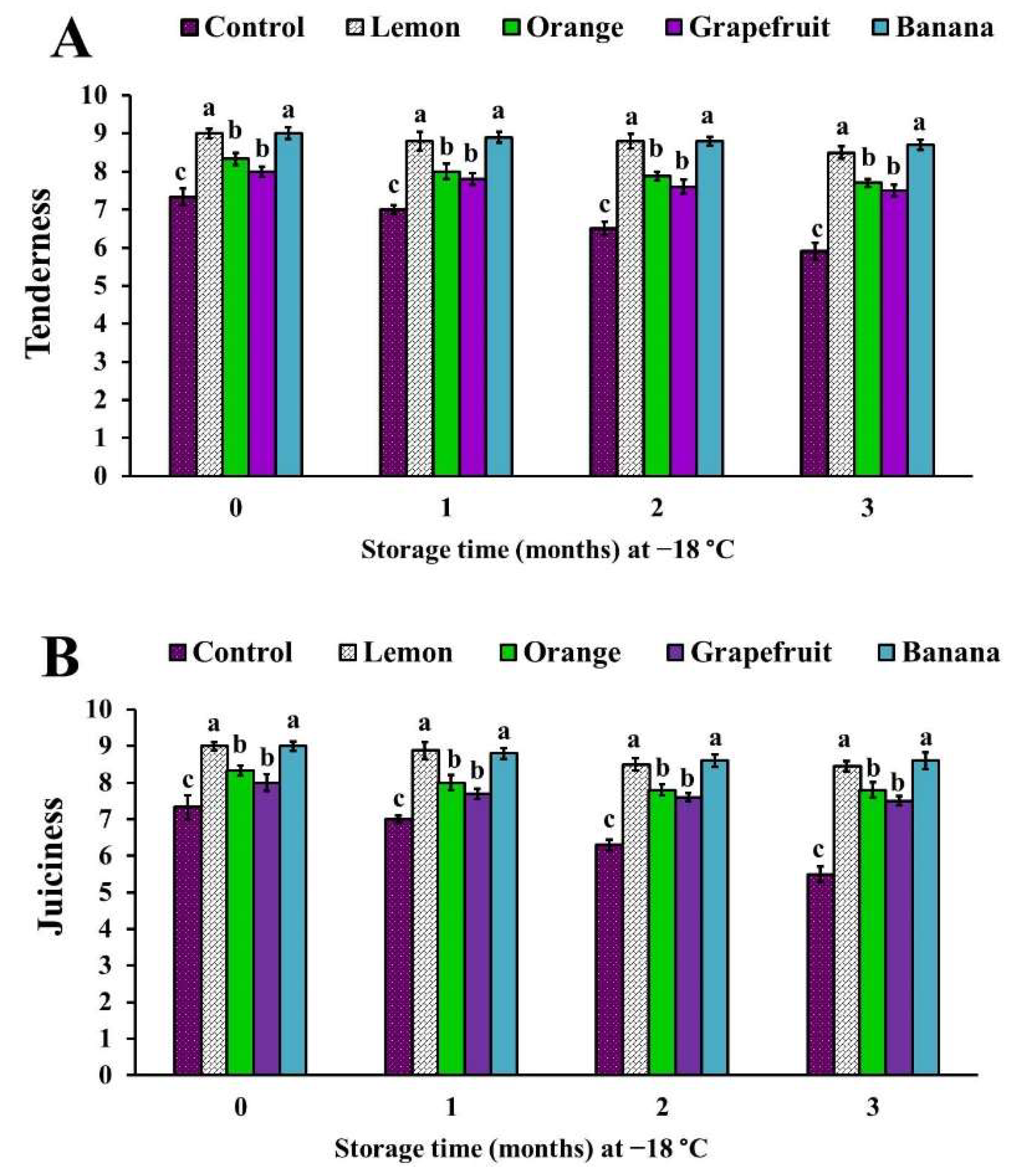
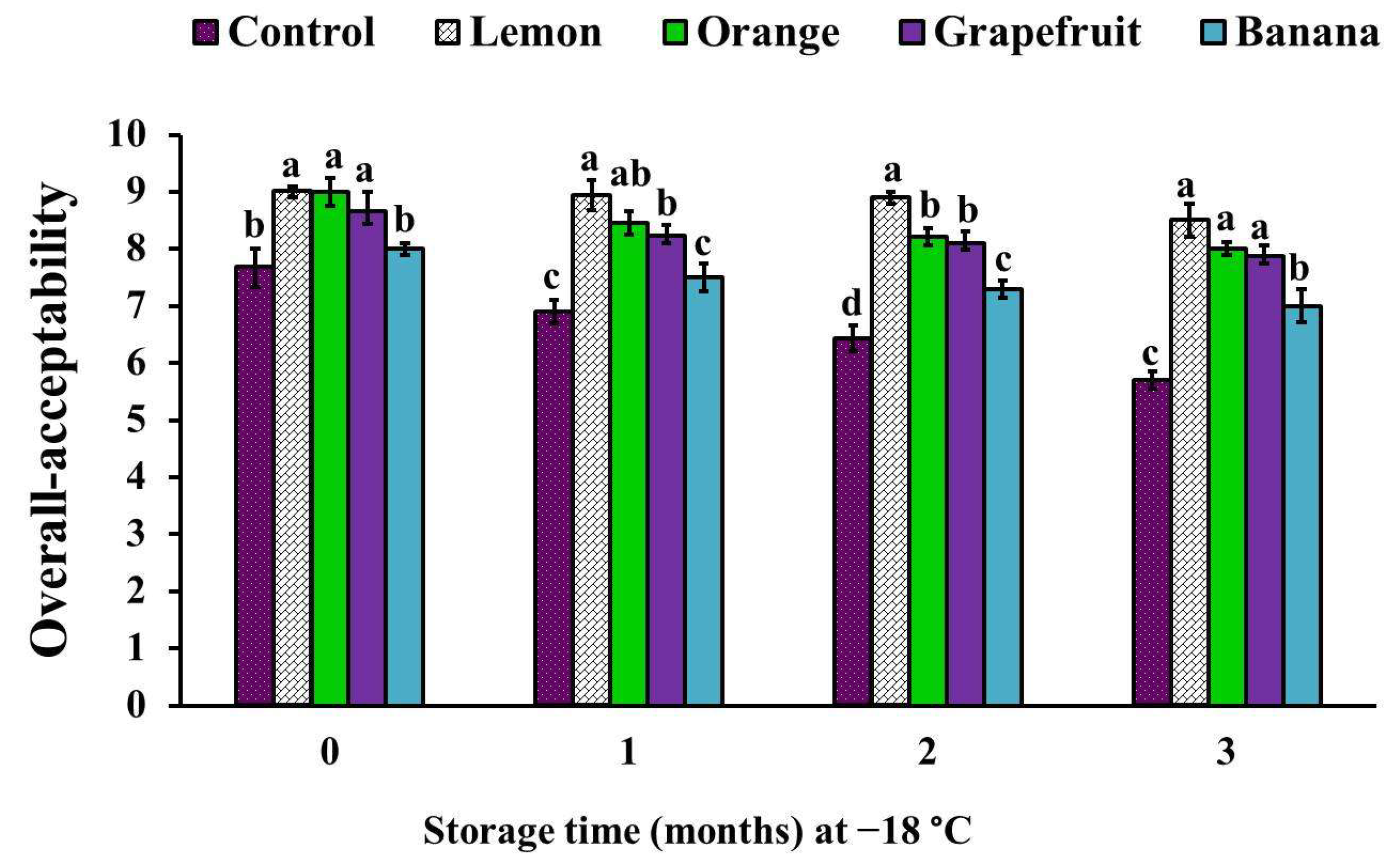
3.6. Sensory Analysis of the Different Fruit Peel Powder-Treated Chicken Patties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kralik, G.; Kralik, Z.; Grčević, M.; Hanžek, D. Quality of chicken meat. In Animal Husbandry and Nutrition; Yucel, B., Turgay, T., Eds.; IntechOpen: London, UK, 2018; Chapter 4; pp. 63–94. [Google Scholar] [CrossRef]
- Bhaisare, D.B.; Thyagarajan, D.; Churchil, R.R.; Punniamurthy, N. Bacterial pathogens in chicken meat: Review. Int. J. Life Sci. Res. 2014, 2, 1–7. [Google Scholar] [CrossRef][Green Version]
- Sasaki, Y.F.; Kawaguchi, S.; Kamaya, A.; Ohshita, M.; Kabasawa, K.; Iwama, K.; Taniguchi, K.; Tsuda, S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res. 2002, 519, 103–119. [Google Scholar] [CrossRef]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-based phenolic molecules as natural preservatives in comminuted meats: A review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Sallam, K.I.; Abd-Elghany, S.M.; Imre, K.; Morar, A.; Herman, V.; Hussein, M.A.; Mahros, M.A. Ensuring safety and improving keeping quality of meatballs by addition of sesame oil and sesamol as natural antimicrobial and antioxidant agents. Food Microbiol. 2021, 99, 103834. [Google Scholar] [CrossRef]
- Czech, A.; Malik, A.; Sosnowska, B.; Domaradzki, P. Bioactive substances, heavy metals, and antioxidant activity in whole fruit, peel, and pulp of citrus fruits. Int. J. Food Sci. 2021. [Google Scholar] [CrossRef]
- Gowe, C. Review on potential use of fruit and vegetables by-products as a valuable source of natural food additives. J. Food Qual. 2015, 45, 47–61. [Google Scholar]
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros, G.; Viuda-Martos, M. Valorization of citrus co-products: Recovery of bioactive compounds and application in meat and meat products. Plants 2021, 10, 1069. [Google Scholar] [CrossRef]
- Mathur, A.; Vema, S.; Gupta, V. Evaluation of in vitro antimicrobial and antioxidants of peel and pulp of some citrus fruits. Int. J. Biotech. Biother. 2011, 2, 12–17. [Google Scholar]
- Aboul-Enein, A.M.; Salama, Z.A.; Gaafar, A.A.; Aly, H.F.; Abou-Elella, F.; Ahmed, H.A. Identification of phenolic compounds from banana peel (Musa paradaisica L.) as antioxidant and antimicrobial agents. J. Chem. Pharm. 2016, 8, 46–55. [Google Scholar]
- Silva, L.B.F.; Miranda, C.N.; Dos Santos, M.; Pereira, P.A.P.; Da Cunha, L.R.; Vieira, S.M.; Gandra, C.M.B. Orange albedo flour as a fat replacer in beef burgers: Adding value to citrus industry by-products. Res. Soc. Dev. 2020, 9, e1599108298. [Google Scholar] [CrossRef]
- Nishad, J.; Koley, T.K.; Varghese, E.; Kaur, C. Synergistic effects of nutmeg and citrus peel extracts in imparting oxidative stability in meat balls. Food Res. Int. 2018, 106, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Foline–Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Abdel-Naeem, H.H.S.; Sallam, K.I.; Zaki, H.M.B.A. Effect of different cooking methods of rabbit meat on topographical changes, physicochemical characteristics, fatty acids profile, microbial quality and sensory attributes. Meat Sci. 2021, 181, 108612. [Google Scholar] [CrossRef] [PubMed]
- Kearsley, M.W.; EL-Khatib, L.; Gunu, C.O.K.A. Rapid determination of total volatile nitrogen in fish and meat. J. Assoc. Public Anal. 1983, 21, 123–128. [Google Scholar]
- Du, M.; Ahn, D.U. Effect of antioxidants on the quality of irradiated sausages prepared with turkey thigh meat. Poult. Sci. 2002, 81, 1251–1256. [Google Scholar] [CrossRef]
- Shin, H.; Choi, Y.; Kim, H.; Ryu, Y.; Lee, S.; Kim, B. Tenderization and fragmentation of myofibrillar proteins in bovine Longissimus dorsi muscle using proteolytic extract from Sarcodon aspratus. Food Sci. Technol. 2008, 41, 1389–1395. [Google Scholar] [CrossRef]
- American Meat Science Association (AMSA). Research Guidelines for Cookery, Sensory Evaluation and Instrumental Tenderness Measurements of Fresh Meat; American Meat Science Association: Chicago, IL, USA, 1995. [Google Scholar]
- Gorinstein, S.; Martin-Belloso, O.; Park, Y.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparision of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Abd El-Khalek, H.H.; Zahran, D.A. Utilization of fruit by-product in ground meat preservation. FSQM 2013, 11, 49–60. [Google Scholar]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Dhanavade, M.J.; Jalkute, C.B.; Ghosh, J.S.; Sonawane, K.D. Study antimicrobial activity of lemon (Citrus lemon L.) peel extract. Br. J. Pharmacol. Toxicol. 2011, 2, 119–122. [Google Scholar]
- Mahmoud, M.H.; Abou-Arab, A.A.; Abu-Salem, F.M. Quality characteristics of beef burger as influenced by different levels of orange peel powder. Am. J. Food Technol. 2017, 12, 262–270. [Google Scholar] [CrossRef]
- Chappalwar, A.M.; Pathak, V.; Goswami, M.; Verma, A.K.; Rajkumar, V. Efficacy of lemon albedo as fat replacer for development of ultra-low-fat chicken patties. J. Food Process. Preserv. 2021, 45, e15587. [Google Scholar] [CrossRef]
- Haque, F.; Rahman, M.H.; Habib, M.; Alam, M.S.; Monir, M.M.; Hossain, M.M. Effect of different levels of orange peel extract on the quality and shelf life of beef muscle during frozen storage. IOSR J. Agric. Vet. Sci. 2020, 13, 43–56. [Google Scholar]
- Wachirasiri, P.; Julakarangka, S.; Wanlapa, S. The effects of banana peel preparations on the properties of banana peel dietary fibre concentrate. Songklanakarin J. Sci. Technol. 2009, 31, 605–611. [Google Scholar]
- Romelle, F.D.; Ashwini, R.P.; Manohar, R.S. Chemical composition of some selected fruit peels. Eur. Food Res. Technol. 2016, 4, 12–21. [Google Scholar]
- Ibrahim, H.M.; Hassan, I.M.; Hamed, A.A.M. Application of lemon and orange peels in meat products: Quality and safety. Int. J. Curr. Microbiol. 2018, 7, 2703–2723. [Google Scholar] [CrossRef]
- Mokbel, M.S.; Hashinaga, F. Antibacterial and antioxidant activities of Banana (Musa, AAA cv. Cavendish) Fruit peel. Am. J. Biochem. Biotechnol. 2005, 1, 125–131. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Hafez, T.A.; Eissawy, M.M. Effect of banana peel extract on sensory and bacteriological quality of marinated beef. Arch. Public Health 2018, 1, 1–11. [Google Scholar]
- Chabuck, G.Z.; Al-Charrakh, H.A.; Hindi, K.N.; Hindi, K.S. Antimicrobial effect of aqueous banana peel extract, Iraq. Pharm. Sci. 2013, 1, 73–75. [Google Scholar]
- Sayari, N.; Sila, A.; Balti, R.; Abid, E.; Hajlaoui, K.; Nasri, M.; Bougatef, A. Antioxidant and antibacterial properties of Citrus paradisi barks extracts during turkey sausage formulation and storage. Biocatal. Agric. Biotechnol. 2015, 4, 616–623. [Google Scholar] [CrossRef]
- Borah, A.; Mahanta, C.L.; Devatkal, S.K.; Narsaiah, K. Utilization of fruit by-product for the shelf life extension of chicken meat ball. JFRT 2014, 2, 153–157. [Google Scholar]
- Clements, R.L. Organic Acids in Citrus Fruits. I. Varietal Differences. J. Food Sci. 2006, 29, 276–280. [Google Scholar] [CrossRef]
- Fernández-López, J.; Zhi, N.; Aleson-Carbonell, L.; Pérez-Alvarez, J.A.; Kuri, V. Antioxidant and antibacterial activities of natural extracts: Application on cooked meat balls. Meat Sci. 2005, 69, 371–380. [Google Scholar] [CrossRef]
- Hasegawa, S.; Berhow, M.A.; Fong, C.H. Analysis of bitter principles in citrus. In Modern Methods of Plant Analysis, Fruit Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 18, pp. 59–80. [Google Scholar] [CrossRef]
- Kealey, K.S.; Kinsella, J.E. Orange juice quality with an emphasis on flavor components. Crit. Rev. Food Sci. Nutr. 1978, 11, 1–40. [Google Scholar] [CrossRef]
| Proximate Composition | Treatments | ||||
|---|---|---|---|---|---|
| Control | Lemon Peel Powder | Orange Peel Powder | Grapefruit Peel Powder | Banana Peel Powder | |
| Moisture (g/100 g) | 63.62 a ± 1.01 | 64.04 a ± 0.93 | 64.37 a ± 0.27 | 64.33 a ± 0.08 | 64.95 a ± 0.52 |
| Protein (g/100 g) | 18.71 b ± 0.71 | 19.17 b ± 1.01 | 19.89 b ± 1.02 | 19.80 b ± 0.95 | 22.18 a ± 0.38 |
| Fat (g/100 g) | 15.55 a ± 0.25 | 14.53 b ± 0.26 | 13.45 b ± 0.60 | 13.57 b ± 0.28 | 10.52 c ± 0.21 |
| Ash (g/100 g) | 2.09 a ± 0.15 | 2.24 a ± 0.09 | 2.28 a ± 0.12 | 2.29 a ± 0.10 | 2.34 a ± 0.11 |
| Microbial Counts | Treatments | Storage Period (Months) | |||
|---|---|---|---|---|---|
| Time Zero | First Month | Second Month | Third Month | ||
| APC | |||||
| Control | 4.51 a,w ± 0.01 | 4.79 a,w ± 0.02 | 5.60 a,x ± 0.03 | 6.60 a,y ± 0.17 | |
| Lemon peel powder | 2.21 d,w ± 0.05 | 2.48 d,w ± 0.07 | 2.70 d,w ± 0.05 | 2.80 d,w ± 0.02 | |
| Orange peel powder | 2.34 d,w ± 0.01 | 2.52 d,w ± 0.23 | 2.98 c,d,w,x ± 0.01 | 3.18 c,d,x ± 0.05 | |
| Grapefruit peel powder | 2.96 c,w ± 0.07 | 3.11 c,w ± 0.00 | 3.38 c,w± 0.04 | 3.57 c,w ± 0.03 | |
| Banana peel powder | 3.45 b,w ± 0.01 | 3.98 b,x ± 0.01 | 4.34 b,x ± 0.25 | 4.90 b,y ± 0.06 | |
| Psychrotrophs | |||||
| Control | 3.13 a,w ± 0.02 | 3.60 a,w ± 0.20 | 4.10 a,x ± 0.20 | 4.97 a,y ± 0.21 | |
| Lemon peel powder | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | |
| Orange peel powder | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | |
| Grapefruit peel powder | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | |
| Banana peel powder | <2.17 b,w ± 0.04 | <2.39 b,w ± 0.11 | 2.46 b,w ± 0.00 | 2.60 b,w ± 0.13 | |
| S. aureus | |||||
| Control | 2.22 a,w ± 0.07 | 2.70 a,w,x ± 0.05 | 3.30 a,x,y ± 0.10 | 3.82 a,y ± 0.04 | |
| Lemon peel powder | <2.00 b,w ± 0.00 | <2.00 b,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | |
| Orange peel powder | <2.00 b,w ± 0.00 | <2.00 b,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | |
| Grapefruit peel powder | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 b,w ± 0.00 | <2.00 b,w ± 0.00 | |
| Banana peel powder | <2.00 b,w ± 0.00 | <2.00 b,w ± 0.00 | 2.72 b,x ± 0.02 | 2.90 b,x ± 0.10 | |
| Enterobacteriaceae | |||||
| Control | 2.15 a,w ± 0.32 | 3.24 a,x± 0.20 | 3.50 a,x± 0.18 | 4.53 a,y ± 0.41 | |
| Lemon peel powder | <2.00 b,w ± 0.00 | <2.00 b,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | |
| Orange peel powder | <2.00 b,w ± 0.00 | <2.00 b,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | |
| Grapefruit peel powder | <2.00 b,w ± 0.00 | <2.00 b,w ± 0.00 | <2.00 c,w ± 0.00 | <2.00 c,w ± 0.00 | |
| Banana peel powder | <2.00 b,w ± 0.00 | <2.00 b,w,x ± 0.00 | 2.00 b,x ± 0.02 | 2.50 b,x ± 0.3 | |
| Parameters | Treatments | Storage Period (Months) | |||
|---|---|---|---|---|---|
| Time Zero | First Month | Second Month | Third Month | ||
| pH | |||||
| Control | 6.28 a,w ± 0.01 | 6.30 a,w,x ± 0.00 | 6.33 a,x ± 0.00 | 6.38 a,y ± 0.00 | |
| Lemon peel powder | 5.99 c,w ± 0.08 | 6.00 c,w ± 0.11 | 6.05 c,w ± 0.15 | 6.13 c,x ± 0.05 | |
| Orange peel powder | 6.15 b,w ± 0.00 | 6.16 b,w ± 0.00 | 6.19 b,w ± 0.01 | 6.24 b,x ± 0.01 | |
| Grapefruit peel powder | 6.15 b,w ± 0.01 | 6.18 b,w ± 0.03 | 6.24 b,x ± 0.01 | 6.26 b,x ± 0.01 | |
| Banana peel powder | 6.28 a,w ± 0.11 | 6.29 a,w ± 0.01 | 6.31 a,w ± 0.00 | 6.33 a,w ± 0.15 | |
| TBA (mg/kg) | |||||
| Control | 0.24 a,w ± 0.05 | 0.58 a,x ± 0.06 | 0.81 a,y ± 0.04 | 0.99 a,z ± 0.06 | |
| Lemon peel powder | 0.05 b,w ± 0.01 | 0.06 c,w ± 0.00 | 0.17 d,x ± 0.01 | 0.30 d,y ± 0.02 | |
| Orange peel powder | 0.05 b,w ± 0.01 | 0.09 c,w ± 0.00 | 0.22 d,x ± 0.01 | 0.37 c,d,y ± 0.01 | |
| Grapefruit peel powder | 0.08 b,w ± 0.02 | 0.11 c,w ± 0.04 | 0.30 c,x ± 0.02 | 0.42 c,y ± 0.03 | |
| Banana peel powder | 0.09 b,w ± 0.00 | 0.24 b,x ± 0.11 | 0.41 b,x ± 0.17 | 0.66 b,y ± 0.02 | |
| TVBN (mg/100 g) | |||||
| Control | 10.59 a,w ± 0.04 | 10.91 a,w ± 0.07 | 11.79 a,x ± 0.05 | 12.85 a,y ± 0.01 | |
| Lemon peel powder | 9.25 c,w ± 0.01 | 9.29 c,w ± 0.03 | 10.44 c,x ± 0.02 | 10.53 c,x ± 0.09 | |
| Orange peel powder | 9.41 c,w ± 0.04 | 9.45 c,w ± 0.01 | 10.55 c,x ± 0.05 | 10.58 c,x ± 0.05 | |
| Grapefruit peel powder | 9.53 c,w ± 0.02 | 9.56 c,w ± 0.02 | 10.62 c,x ± 0.06 | 10.67 c,x ± 0.00 | |
| Banana peel powder | 10.00 b,w ± 0.01 | 10.13 b,w ± 0.07 | 11.17 b,x ± 0.02 | 11.21 b,x ± 0.08 | |
| Parameters | Treatments | Storage Period (Months) | |||
|---|---|---|---|---|---|
| Time Zero | First Month | Second Month | Third Month | ||
| L* | |||||
| Control | 57.71 a,w ± 0.23 | 60.00 a,x ± 0.10 | 62.07 a,y ± 0.14 | 65.23 a,z ± 0.50 | |
| Lemon peel powder | 53.12 b,w ± 0.20 | 54.30 b,w,x ± 0.15 | 56.22 b,x,y ± 0.20 | 59.09 b,y ± 0.19 | |
| Orange peel powder | 53.03 b,w ± 0.14 | 55.00 b,w,x ± 0.14 | 57.89 b,x,y ± 0.17 | 60.30 b,y ± 0.17 | |
| Grapefruit peel powder | 53.14 b.w ± 0.15 | 55.10 b,w,x ± 0.13 | 58.10 b,x,y ± 0.15 | 60.68 b,y ± 0.11 | |
| Banana peel powder | 47.07 c,w ± 0.27 | 49.25 c,w,x ± 0.11 | 49.18 c,w,x ± 0.32 | 50.10 c,x ± 0.22 | |
| a* | |||||
| Control | 11.31 b,w ± 0.15 | 10.00 b,x ± 0.10 | 7.89 b,y ± 0.63 | 6.00 b,z ± 0.32 | |
| Lemon peel powder | 12.76 a,w ± 0.16 | 11.40 a,x ± 0.15 | 9.37 a,y ± 0.11 | 9.20 a,y ± 0.16 | |
| Orange peel powder | 12.33 a,w ± 0.04 | 11.30 a,x ± 0.15 | 9.24 a,y ± 0.11 | 9.05 a,y ± 0.12 | |
| Grapefruit peel powder | 12.25 a,w ± 0.11 | 11.10 a,x ± 0.15 | 9.03 a,y ± 0.17 | 9.00 a,y ± 0.10 | |
| Banana peel powder | 11.40 b,w ± 0.22 | 10.50 b,w ± 0.15 | 8.00 b,x ± 0.26 | 7.11 b,x ± 0.10 | |
| b* | |||||
| Control | 21.54 c,w ± 0.09 | 21.10 c,w ± 0.13 | 20.73 c,w ± 0.11 | 18.75 c,x ± 0.08 | |
| Lemon peel powder | 23.40 b,w ± 0.24 | 23.20 b,w ± 0.17 | 23.05 b,w ± 0.11 | 23.26 b,w ± 0.11 | |
| Orange peel powder | 30.17 a,w ± 0.46 | 29.00 a,w ± 0.14 | 29.63 a,w ± 0.15 | 28.70 a,w ± 0.13 | |
| Grapefruit peel powder | 23.92 b,w ± 0.03 | 23.68 b,w ± 0.11 | 23.95 b,w ± 0.14 | 22.65 b,w ± 0.42 | |
| Banana peel powder | 14.17 d,w ± 0.10 | 13.50 d,w ± 0.13 | 13.56 d,w ± 0.15 | 13.35 d,w ± 0.11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Naeem, H.H.S.; Elshebrawy, H.A.; Imre, K.; Morar, A.; Herman, V.; Pașcalău, R.; Sallam, K.I. Antioxidant and Antibacterial Effect of Fruit Peel Powders in Chicken Patties. Foods 2022, 11, 301. https://doi.org/10.3390/foods11030301
Abdel-Naeem HHS, Elshebrawy HA, Imre K, Morar A, Herman V, Pașcalău R, Sallam KI. Antioxidant and Antibacterial Effect of Fruit Peel Powders in Chicken Patties. Foods. 2022; 11(3):301. https://doi.org/10.3390/foods11030301
Chicago/Turabian StyleAbdel-Naeem, Heba H.S., Hend Ali Elshebrawy, Kálmán Imre, Adriana Morar, Viorel Herman, Raul Pașcalău, and Khalid Ibrahim Sallam. 2022. "Antioxidant and Antibacterial Effect of Fruit Peel Powders in Chicken Patties" Foods 11, no. 3: 301. https://doi.org/10.3390/foods11030301
APA StyleAbdel-Naeem, H. H. S., Elshebrawy, H. A., Imre, K., Morar, A., Herman, V., Pașcalău, R., & Sallam, K. I. (2022). Antioxidant and Antibacterial Effect of Fruit Peel Powders in Chicken Patties. Foods, 11(3), 301. https://doi.org/10.3390/foods11030301











