Use of Bacteriocins and Bacteriocinogenic Beneficial Organisms in Food Products: Benefits, Challenges, Concerns
Abstract
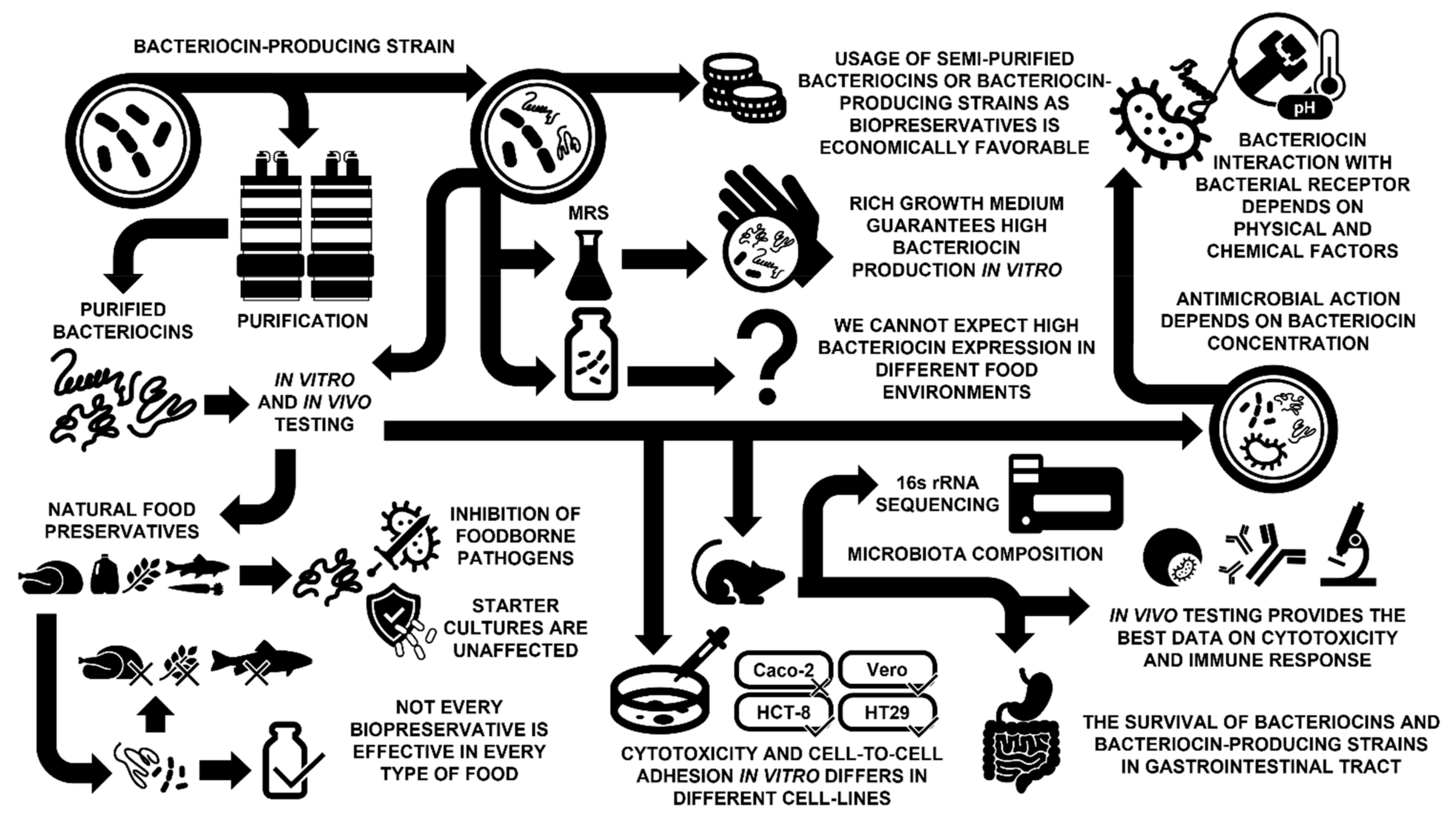
1. Introduction
2. Bacteriocins: What Good Do They Do for Us in Food Products?
3. Effect of Environmental Factors on the Bacteriocin Effectiveness
4. Bacteriocins: Safety Is the Priority
5. Interactions between Bacteriocins and Environmental Factors
6. Application of Bacteriocins
7. How Effective Can the Application of Partially Purified Bacteriocins Be?
8. Limitations of Use of Bacteriocin Producers
9. Spectrum of Activity: Kill Only Bad and Not Good
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; et al. Scientific Opinion on the safety of nisin (E 234) as a food additive in the light of new toxicological data and the proposed extension of use. EFSA J. 2017, 15, 5063. [Google Scholar] [CrossRef]
- Todorov, S.D.; Franco, B.D.G.M.; Tagg, J.R. Bacteriocins of Gram-positive bacteria having activity spectra extending beyond closely-related species. Beneficial Microbs. 2019, 10, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Cornforth, D.M.; Foster, K.R. Competition sensing: The social side of bacterial stress responses. Nat. Rev. Microbiol. 2013, 11, 285–293. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Heilbronner, S.; Krismer, B.; Brötz-Oesterhelt, H.; Peschel, A. The microbiome-shaping roles of bacteriocins. Nat. Rev. Microbiol. 2021, 19, 726–739. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Montville, T.J.; Chung, H.-J.; Chikindas, M.L.; Chen, Y. Nisin A depletes intracellular ATP and acts in bactericidal manner against Mycobacterium smegmatis. Lett. Appl. Microbiol. 1999, 28, 189–193. [Google Scholar] [CrossRef]
- Chung, H.-J.; Montville, T.J.; Chikindas, M.L. Nisin depletes ATP and proton motive force in mycobacteria. Lett. Appl. Microbiol. 2008, 31, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.I.; Noll, K.S.; Xu, S.; Li, J.; Huang, Q.; Sinko, P.J.; Wachsman, M.B.; Chikindas, M.L. Safety, formulation, and in vitro antiviral activity of the antimicrobial peptide subtilosin against herpes simplex virus type 1. Prob. Antimicrob. Prot. 2013, 5, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Quintana, V.M.; Torres, N.I.; Wachsman, M.B.; Sinko, P.J.; Castilla, V.; Chikindas, M. Antiherpes simplex virus type 2 activity of the antimicrobial peptide subtilosin. J. Appl. Microbiol. 2014, 117, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Dicks, L.M.T.; Popov, I.V.; Karaseva, A.; Ermakov, A.M.; Suvorov, A.; Tagg, J.R.; Weeks, R.; Chikindas, M.L. Probiotics at war against viruses: What is missing from the picture? Front. Microbiol. 2020, 11, 1877. [Google Scholar] [CrossRef]
- Bingol, E.B.; Akkaya, E.; Hampikyan, H.; Cetin, O.; Colak, H. Effect of nisin-EDTA combinations and modified atmosphere packaging on the survival of Salmonella enteritidis in Turkish type meatballs. Cyta J. Food. 2018, 16, 1030–1036. [Google Scholar] [CrossRef]
- Cavera, V.L.; Arthur, T.D.; Kashtanov, D.; Chikindas, M.L. Bacteriocins and their position in the next wave of conventional antibiotics. Int. J. Antimicrob. Agents 2015, 46, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Arthur, T.D.; Cavera, V.L.; Chikindas, M.L. On bacteriocin delivery systems and potential applications. Future Microbiol. 2014, 9, 235–248. [Google Scholar] [CrossRef]
- Delves-Broughton, J. Nisin and its application as a food preservative. Int. J. Dairy Technol. 1990, 43, 73–76. [Google Scholar] [CrossRef]
- Verma, A.; Banerjee, R.; Dwivedi, H.; Juneja, V.K. Bacteriocins: Potential in food preservation. In Encyclopedia of Food Microbiology; Academic Press: Cambridge, MA, USA; Elsivier Science: Amsterdam, The Netherlands, 2014; pp. 180–186. [Google Scholar] [CrossRef]
- Arqués, J.L.; Rodríguez, E.; Langa, S.; Landete, J.M.; Medina, M. Antimicrobial activity of lactic acid bacteria in dairy products and gut: Effect on pathogens. Bio.Med. Res. Int. 2015, 2015, 584183. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; Lucas, R.; Grande Burgos, M.J. Bacteriocins for Bioprotection of Foods; CABI Books; CABI International: Wallingford, UK, 2011. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; Omar, N.B.; Lucas, R. Food applications and regulation. In Prokaryotic Antimicrobial Peptides; Springer: New York, NY, USA, 2011; pp. 353–390. [Google Scholar]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a food preservative: Part 1: Physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Xavier, J.; Gopalan, N.; Ramana, K. Immobilization of lactic acid bacteria and application of bacteriocin for preservation of fruit juices and bacteriocin production. Defence Life Sci. J. 2017, 2, 231–238. [Google Scholar] [CrossRef][Green Version]
- Rendueles, C.; Duarte, A.C.; Escobedo, S.; Fernández, L.; Rodríguez, A.; García, P.; Martínez, B. Combined use of bacteriocins and bacteriophages as food biopreservatives. A review. Int. J. Food Microbiol. 2022, 368, 109611. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, C.; Dridi, L.; Bergeron, M.G.; Ouellette, M.; Fliss, I. Nisin is an effective inhibitor of Clostridium difficeile vegetative cells and spore germination. J. Med. Microbiol. 2016, 65, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hoover, D.G. Bacteriocins and their Food applications. Compr. Rev. Food Sci. Food Safety 2006, 2, 82–100. [Google Scholar] [CrossRef]
- Bucheli, J.E.V.; Fugaban, J.I.I.; Holzapfel, W.H.; Todorov, S.D. Combined action of antibiotics and bacteriocins against vancomycin-resistant enterococci. Microorganisms. 2022, 10, 1423. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Ibarra-Sanchez, L.A.; El-Haddad, N.; Mahmoud, D.; Miller, M.J.; Karam, L. Advances in nisin use for preservation of dairy products. J. Dairy Sci. 2020, 103, 2041–2052. [Google Scholar] [CrossRef]
- Abdulhussain, K.R.; Razavi, S.H. Plantaricin bacteriocins: As safe alternative antimicrobial peptides in food preservation. J. Food Safety 2020, 40, e12735. [Google Scholar] [CrossRef]
- Oros-Florez, Z.S.; Garcia-Almendarez, B.E.; Barboza-Corona, J.E.; Salcedo-Hernandez, R. A fast micromethod for the estimation of nisin activity in a soft cheese. Int. J. Dairy Technol. 2019, 72, 282–286. [Google Scholar] [CrossRef]
- Martinez, R.R.C.; Wachsman, M.; Torres, N.I.; LeBlanc, J.G.; Todorov, S.D.; Franco, B.D.G.M. Biochemical, antimicrobial and molecular characterization of a noncytotoxic bacteriocin produced by Lactobacillus plantarum ST71KS. Food Microbiol. 2013, 34, 376–381. [Google Scholar] [CrossRef]
- Favaro, L.; Penna, A.L.B.; Todorov, S.D. Bacteriocinogenic LAB from cheeses—Application in biopreservation? Trends Food Sci. Technol. 2015, 41, 37–48. [Google Scholar] [CrossRef]
- Todorov, S.D. What bacteriocinogenic lactic acid bacteria do in the milk? In Raw Milk. Balance between Hazards and Benefits; Chapter 8; Nero, L.A., de Carvalho, A.F., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2018; pp. 149–174. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Todorov, S.D.; Ivanova, I.V.; Belguesmia, Y.; Choiset, Y.; Rabesona, H.; Chobert, J.-M.; Haertlé, T.; Franco, B.D.G.M. Characterization of a two-peptide plantaricin produced by Lactobacillus plantarum MBSa4 isolated from Brazilian salami. Food Control 2016, 60, 103–112. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Todorov, S.D.; Belguesmia, Y.; Choiset, Y.; Rabesona, H.; Ivanova, I.V.; Chobert, J.M.; Haertle, T.; Franco, B.D.G.M. Purification and characterization of the bacteriocin produced by Lactobacillus sakei MBSa1 isolated from Brazilian salami. J. Appl. Microbiol. 2014, 116, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.S.; Todorov, S.D.; Jurkiewicz, C.H.; Franco, B.D.G.M. Bacteriocin production by Lactobacillus curvatus MBSa2 entrapped in calcium alginate during ripening of salami for control of Listeria monocytogenes. Food Control 2015, 47, 147–153. [Google Scholar] [CrossRef]
- Todorov, S.D.; Koep, K.S.C.; Van Reenen, C.A.; Hoffman, L.C.; Slinde, E.; Dicks, L.M.T. Production of salami from beef, horse, mutton, blesbok (Damaliscus dorcas phillipsi) and springbok (Antidorcas marsupialis) with bacteriocinogenic strains of Lactobacillus plantarum and Lactobacillus curvatus. Meat Sci. 2017, 77, 405–412. [Google Scholar] [CrossRef]
- Enan, G.; El-Essawy, A.A.; Uyttendaele, M.; Debevere, J. Antibacterial activity of Lactobacillus plantarum UG1 isolated from dry sausage: Characterization, production and bactericidal action of plantaricin UG1. Int. J. Food Microbiol. 1996, 30, 189–215. [Google Scholar] [CrossRef]
- Hugas, M. Bacteriocinogenic lactic acid bacteria for the biopreservation of meat and meat products. Meat Sci. 1998, 49, S139–S150. [Google Scholar] [CrossRef]
- Messi, P.; Bondi, M.; Sabia, C.; Battini, R.; Manicardi, G. Detection and preliminary characterization of a bacteriocin (plantaricin 35d) produced by a Lactobacillus plantarum strain. Int. J. Food Microbiol. 2001, 64, 193–198. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Isolation and preliminary characterization of a bacteriocin produced by Lactobacillus plantarum N014 isolated from Nham, a traditional Thai fermented pork. J. Food Protect. 2006, 69, 1937–1943. [Google Scholar] [CrossRef]
- Todorov, S.D.; Ho, P.; Vaz-Velho, M.; Dicks, L.M.T. Characterization of bacteriocins produced by two strains of Lactobacillus plantarum isolated from beloura and chouriço, traditional pork products from Portugal. Meat Sci. 2010, 84, 334–343. [Google Scholar] [CrossRef]
- Todorov, S.D. Diversity of bacteriocinogenic lactic acid bacteria isolated from boza, a cereal-based fermented beverage from Bulgaria. Food Control 2010, 21, 1011–1021. [Google Scholar] [CrossRef]
- Todorov, S.D.; Franco, B.D.G.M.; Vaz-Velho, M. Bacteriocin producing lactic acid bacteria from and for production of salami-like products. A review. Int. Rev. Food Sci. Technol. 2009, 1, 57–61. [Google Scholar]
- Todorov, S.D.; von Mollendorff, J.W.; Moelich, E.; Muller, N.; Witthuhn, R.C.; Dicks, L.M.T. Evaluation of potential probiotic properties of Enterococcus mundtii, its survival in boza and in situ bacteriocin production. Food Technol. Biotechnol. 2009, 47, 178–191. [Google Scholar]
- Todorov, S.D.; Holzapfel, W.H.; Nero, L.A. Safety evaluation and bacteriocinogenic potential of Pediococcus acidilactici strains isolated from artisanal cheeses. LWT–Food Sci. Technol. 2021, 139, 110550. [Google Scholar] [CrossRef]
- Vaz-Velho, M.; Jacome, S.; Noronha, L.; Todorov, S.; Fonseca, S.; Pinheiro, R.; Morais, A.; Silva, J.; Teixeira, P. Comparison of antilisterial effects of two strains of lactic acid bacteria during processing and storage of a Portuguese salami-like product “Alheira”. Chem. Engin. Transact. 2013, 32, 1807–1812. [Google Scholar] [CrossRef]
- Wachsman, M.B.; Castilla, V.; de Ruiz Holgado, A.P.; de Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antiviral. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef]
- Todorov, S.D.; Franco, B.D.G.M.; Wiid, I.J. In vitro study of beneficial properties and safety of lactic acid bacteria isolated from Portuguese fermented meat products. Beneficial Microbs. 2013, 5, 351–366. [Google Scholar] [CrossRef]
- Cavicchioli, V.Q.; de Carvalho, O.V.; de Paiva, J.C.; Todorov, S.D.; Júnior, A.S.; Nero, L.A. Inhibition of Herpes simplex virus 1 and Poliovirus (PV 1-1) by bacteriocins from Lactococcus lactis subsp. lactis and Enterococcus durans strains isolated from goat milk. Int. J. Antimicrob. Agents. 2018, 51, 33–37. [Google Scholar] [CrossRef]
- Carneiro, B.M.; Braga, A.C.S.; Batista, M.N.; Rahal, P.; Favaro, L.; Penna, A.L.B.; Todorov, S.D. Lactobacillus plantarum ST202Ch and Lactobacillus plantarum ST216Ch—What are the limitations for application? J. Nutr. Health Food Engin. 2014, 1, 00010. [Google Scholar]
- Horvath, S. Cytotoxicity of drugs and diverse chemical agents to cell cultures. Toxicol. 1980, 16, 59–66. [Google Scholar] [CrossRef]
- Niles, A.L.; Moravec, R.A.; Riss, T.L. Update on in vitro cytotoxicity assays for drug development. Expert. Opin. Drug. Discov. 2008, 3, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Myers, M. Direct measurement of cell numbers in microtitre plate cultures using the fluorescent dye SYBR green I. J. Immunol. Methods. 1998, 212, 99–103. [Google Scholar] [CrossRef]
- Cook, J.; Mitchell, J. Viability measurements in mammalian cell systems. Anal. Biochem. 1989, 179, 1–7. [Google Scholar] [CrossRef]
- Borenfruend, E.; Puerner, J. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Gonzalez-Nicolini, V.; Fux, C.; Fussenegger, M. A novel mammalian cell-based approach for the discovery of anticancer drugs with reduced cytotoxicity on non-dividing cells. Invest. New Drugs. 2004, 22, 253–262. [Google Scholar] [CrossRef]
- Cox, C.R.; Coburn, P.S.; Gilmore, M.S. Enterococcal cytolysin: A novel two component peptide system that serves as a bacterial defense against eukaryotic and prokaryotic cells. Curr. Protein Pept. Sci. 2005, 6, 77–84. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. Host defense peptides as new weapons in cancer treatment. Cell. Mol. Life. Sci. 2005, 62, 784–790. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. BBA Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef]
- Frazer, A.; Sharratt, M.; Hickman, J. The biological effects of food additives. I. Nisin. J. Sci. Food Agr. 1962, 13, 32–42. [Google Scholar] [CrossRef]
- Vaucher, R.d.A.; Velho Gewehr, C.d.C.; Correa, A.P.; Sant’Anna, V.; Ferreira, J.; Brandelli, A. Evaluation of the immunogenicity and in vivo toxicity of the antimicrobial peptide P34. Int. J. Pharm. 2011, 421, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.K.; Jena, P.K.; Prajapati, B.; Gehlot, L.; Patel, A.K.; Seshadri, S. In Vivo assessment of immunogenicity and toxicity of the bacteriocin TSU4 in BALB/c mice. Prob. Antimicrob. Prot. 2017, 9, 345–354. [Google Scholar] [CrossRef]
- Marlida, Y.; Arnim, A.; Yuherman, Y.; Rusmarilin, H. Toxicity test pediocin N6 powder produced from isolates Pediococcus Pentosaceus strain N6 on white mice. J. Food Pharm. Sci. 2016, 4, 12–16. [Google Scholar] [CrossRef]
- Dover, S.E.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Safety study of an antimicrobial peptide lactocin 160, produced by the vaginal Lactobacillus rhamnosus. Infect. Dis. Obstet. Gynecol. 2007, 2007, 78248. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, R.; Rodrıguez-Cabezas, M.E.; Martın-Escolano, R.; Rubino, S.; Carrido-Barros, M.; Montalban-Lopez, M.; Rosales, M.J.; Sanchez-Moreno, M.; Valdivia, E.; Martinez-Bueno, M.; et al. Preclinical studies of toxicity and safety of the AS-48 bacteriocin. J. Adv. Res. 2019, 20, 129–139. [Google Scholar] [CrossRef]
- Murinda, S.; Rashid, K.; Roberts, R. In vitro assessment of the cytotoxicity of nisin, pediocin, and selected colicins on simian Virus 40-transfected human colon and Vero monkey kidney cells with trypan blue staining viability assays. J. Food Prot. 2003, 66, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.D.; de Oliveira, M.D.; de Paula, S.O.; Baracat-Pereira, M.C.; Breukink, E.; Mantovani, H.C. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiolology 2012, 158, 2851–2858. [Google Scholar] [CrossRef]
- Favaro, L.; Todorov, S.D. Bacteriocinogenic LAB strains for fermented meat preservation: Perspectives, challenges and limitations. Prob. Antimicrob. Prot. 2017, 9, 444–458. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Bacteriocins from lactic acid bacteria: Production, purification, and food applications. J. Mol. Microb. Biotech. 2007, 13, 194–199. [Google Scholar] [CrossRef]
- Fernandez, B.; Le Lay, C.; Jean, J.; Fliss, I. Growth, acid production and bacteriocin production by probiotic candidates under simulated colonic conditions. J. Appl. Microbiol. 2013, 114, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Kheadr, E.; Zihler, A.; Dabour, N.; Lacroix, C.; Le Blay, G.; Fliss, I. Study of the physicochemical and biological stability of pediocin PA-1 in the upper gastrointestinal tract conditions using a dynamic in vitro model. J. Appl. Microbiol. 2010, 109, 54–64. [Google Scholar] [CrossRef]
- Birri, D.J.; Brede, D.A.; Nes, I.F. Salivaricin D, a novel intrinsically trypsin-resistant lantibiotic from Streptococcus salivarius 5M6c isolated from a healthy infant. Appl. Environ. Microbiol. 2012, 78, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.M.; Jung, D.Y.; Jin, D.Y.; Jayabalan, D.R.; Yang, D.S.H.; Suh, J.W. Bacteriocins as food preservatives: Challenges and emerging horizons. Crit. Rev. Food Sci. Nutr. 2018, 58, 2743–2767. [Google Scholar] [CrossRef]
- O’Shea, E.F.; O’Connor, P.M.; Cotter, P.D.; Ross, P.P.; Hill, C. Synthesis of trypsinresistant variants of the Listeria-active bacteriocin salivaricin P. Appl. Environ. Microbiol. 2010, 76, 5356–5362. [Google Scholar] [CrossRef] [PubMed]
- Rollema, H.S.; Kuipers, O.P.; Both, P.; de Vos, W.M.; Siezen, R.J. Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl. Environ. Microbiol. 1995, 61, 2873–2878. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Blake, T.; Mathur, H.; O’Connor, P.M.; Cotter, P.D.; Ross, P.R.; Hill, C. Bioengineering nisin to overcome the nisin resistance protein. Mol. Microbiol. 2019, 111, 717–731. [Google Scholar] [CrossRef]
- Gomaa, A.I.; Martinent, C.; Hammami, R.; Fliss, I.; Subirade, M. Dual coating of liposomes as encapsulating matrix of antimicrobial peptides: Development and characterization. Front. Chem. 2017, 5, 103. [Google Scholar] [CrossRef]
- Habib, W.; Sakr, A. Development and human in vivo evaluation of a colonic drug delivery system. Pharm. Ind. 1999, 61, 1145–1149. [Google Scholar]
- Gough, R.; Cabrera Rubio, R.; O’Connor, P.M.; Crispie, F.; Brodkorb, A.; Miao, S.; Hill, C.; Ross, R.P.; Cotter, P.D.; Nilaweera, K.N.; et al. Oral delivery of nisin in resistant starch-based matrices alters the gut microbiota in mice. Front. Microbiol. 2018, 9, 1186. [Google Scholar] [CrossRef]
- De Groot, A.S.; Scott, D.W. Immunogenicity of protein therapeutics. Trends Immunol. 2007, 28, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Lohans, C.T.; Vederas, J.C. Development of class IIa bacteriocins as therapeutic agents. Int. J. Microbiol. 2011, 2012, 386410. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K.; Johnson, M.C.; Ray, B.; Belden, E.L. Antigenic properties of pediocin AcH produced by Pediococcus acidilactici H. J. Appl. Microbiol. 1990, 69, 211–215. [Google Scholar] [CrossRef]
- Pablo, M.A.; Gaforio, J.J.; Gallego, A.M.; Ortega, E.; Galvez, A.M.; de Cienfuegos Lopez, G.A. Evaluation of immunomodulatory effects of nisin-containing diets on mice. FEMS Immunol. Med. Micro. 1999, 24, 35–42. [Google Scholar] [CrossRef]
- Scholl, D.; Martin, D.W. Antibacterial efficacy of R-type pyocins towards Pseudomonas aeruginosa in a murine peritonitis model. Antimicrob. Agents Chemother. 2008, 52, 1647–1652. [Google Scholar] [CrossRef]
- McCaughey, L.C.; Ritchie, N.D.; Douce, G.R.; Evans, T.J.; Walker, D. Efficacy of species-specific protein antibiotics in a murine model of acute Pseudomonas aeruginosa lung infection. Sci. Rep. 2016, 6, 30201. [Google Scholar] [CrossRef]
- Belguesmia, Y.; Naghmouchi, K.; Chihib, N.E.; Drider, D. Class IIa bacteriocins: Current knowledge and perspectives. In Prokaryotic Antimicrobial Peptides; Drider, D., Rebuffat, S., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Fujiwara, S.; Hashiba, H.; Hirota, T.; Forstner, J.F. Inhibition of the binding of enterotoxigenic Escherichia coli Pb176 to human intestinal epithelial cell line HCT-8 by an extracellular protein fraction containing BIF of Bifidobacterium longum SBT2928: Suggestive evidence of blocking of the binding receptor gangliotetraosylceramide on the cell surface. Int. J. Food Microbiol. 2001, 67, 97–106. [Google Scholar] [CrossRef]
- Fujiwara, S.; Hashiba, H.; Hirota, T.; Forstner, J.F. Proteinaceous factor(s) in culture supernatant fluids of bifidobacteria which prevents the binding of enterotoxigenic Escherichia coli to gangliotetraosylceramide. Appl. Environ. Microbiol. 1997, 63, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; Calasso, M.; Vincentini, O.; Vernocchi, P.; Ndagijimana, M.; De Vincenzi, M.; Dessì, M.R.; Guerzoni, M.E.; Gobbetti, M. Quorum sensing in sourdough Lactobacillus plantarum DC400: Induction of plantaricin A (PlnA) under co-cultivation with other lactic acid bacteria and effect of PlnA on bacterial and Caco-2 cells. Proteomics 2010, 10, 2175–2190. [Google Scholar] [CrossRef]
- Villarante, K.I.; Elegado, F.B.; Iwatani, S.; Zendo, T.; Sonomoto, K.; de Guzman, E.E. Purification, characterization and in vitro cytotoxicity of the bacteriocin from Pediococcus acidilactici K2a2–3 against human colon adenocarcinoma (HT29) and human cervical carcinoma (HeLa) cells. World J. Microbiol. Biotechnol. 2011, 27, 975–980. [Google Scholar] [CrossRef]
- Olejnik-Schmidt, A.K.; Schmidt, M.T.; Sip, A.; Szablewski, T.; Grajek, W. Expression of bacteriocin divercin AS7 in Escherichia coli and its functional analysis. Ann. Microbiol. 2014, 64, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Aasen, I.M.; Møretrø, T.; Katla, T.; Axelsson, L.; Storrø, I. Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl. Microbiol. Biotechnol. 2000, 53, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bogovic-Matijasic, B.; Rogelj, I. Bacteriocin complex of Lactobacillus acidophilus LF221—Production studies in MRS-media at different pH-values and effect against Lactobacillus helveticus ATCC 15009. Process Biochem. 1988, 33, 345–352. [Google Scholar] [CrossRef]
- Krier, F.; Revol-Junelles, A.M.; Germain, P. Influence of temperature and pH on production of two bacteriocins by Leuconostoc mesenteroides subsp. mesenteroides FR52 during batch fermentation. Appl. Microbiol. Biotechnol. 1998, 50, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Matsusaki, H.; Endo, N.; Sonomoto, K.; Ishizaki, A. Lantibiotic nisin Z fermentative production by Lactococcus lactis IO-1: Relationship between production of the lantibiotic and lactate and cell growth. Appl. Microbiol. Biotechnol. 1996, 45, 36–40. [Google Scholar] [CrossRef]
- Mortvedt-Abildgaa, C.I.; Nissen-Meyer, J.; Jelle, B.; Grenov, B.; Skaugen, M.; Nes, I.F. Production and pH-dependent bactericidal activity of lactocin S, a lantibiotic from Lactobacillus sake L45. Appl. Environ. Microbiol. 1995, 61, 175–179. [Google Scholar] [CrossRef]
- Drider, D.; Fimland, G.; Héchard, Y.; McMullen, L.M.; Prévost, H. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 2006, 70, 564–582. [Google Scholar] [CrossRef]
- Soliman, W.; Bhattacharjee, S.; Kaur, K. Adsorption of an antimicrobial peptide on self-assembled monolayers by molecular dynamics simulation. J. Phys. Chem. B. 2010, 114, 11292–11302. [Google Scholar] [CrossRef]
- Grein, F.; Schneider, T.; Sahl, H.G. Docking on lipid II-A widespread mechanism for potent bactericidal activities of antibiotic peptides. J. Mol. Biol. 2019, 431, 3520–3530. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Q.; Breukink, E. Non-lipid II targeting lantibiotics. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183244. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, J.; Wang, C.; Wang, J. Structural basis of pore formation in the mannose phosphotransferase system by pediocin PA-1. Appl. Environ. Microbiol. 2022, 88, e0199221. [Google Scholar] [CrossRef] [PubMed]
- Gadea, R.; Glibota, N.; Perez Pulido, R.; Galvez, A.; Ortega, E. Effects of exposure to biocides on susceptibility to essential oils and chemicals preservatives in bacteria from organic foods. Food Control 2017, 80, 176–182. [Google Scholar] [CrossRef]
- Fernandez-Fuentes, M.A.; Ortega Morente, E.; Abriouel, H.; Perez Pulido, R.; Galvez, A. Isolation and identification of bactéria from organic foods: Sensitivity to biocides and antibiotics. Food Control 2012, 26, 73–78. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Paiva, A.D.; Cruz, A.M.; Vanetti, M.C.; Ferreira, S.O.; Mantovani, H.C. Bovicin HC5 and nisin reduce cell viability and the thermal resistance of Alicyclobacillus acidoterrestris endospores in fruit juices. J. Sci. Food Agric. 2022, 102, 3994–4002. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, A.A.T.; Montovani, H.C.; Vanetto, M.C.D. Bactericidal effect of bovicin HC5 and nisin against Clostridium tyrobutyricum isolated from spoiled mango pulp. Lett. Appl. Microbiol. 2007, 45, 68–74. [Google Scholar] [CrossRef]
- Aymerich, T.; Jofré, A.; Bover-Cid, S. Enterocin A-based antimicrobial film exerted strong antilisterial activity in sliced dry-cured ham immediately and after 6 months at 8 °C. Food Microbiol. 2022, 105, 104005. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.-Z.; Wu, G.; Zhang, Y.-P.; Yang, L.-Y.; Zhang, Y.-M.; Zhao, Z.-S.; Deng, X.-Y.; Zhang, Q.-L. Inhibitory effect of a new bacteriocin RSQ04 purified from Lactococcus lactis on Listeria monocytogenes and its application on model food system. LWT Food Sci. Technol. 2022, 164, 113626. [Google Scholar] [CrossRef]
- Doshi, M.N.; Nair, K.; Hussan, U.; Jaoua, S. Pyocin QDD1: A highly thermostable bacteriocin produced by Pseudomonas aeruginosa QDD1 for the biocontrol of foodborne pathogens Staphylococcus aureus and Bacillus cereus. Biores. Technol. Rep. 2022, 18, 101106. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Jiang, Y.-H.; Li, H.-W.; Zhang, Q.-L. Purification and characterization of Lactobacillus plantarum—Derivated bacteriocin with activity against Staphylococcus argenteus planktonic cells and biofilm. J. Food Sci. 2022, 87, 2718–2731. [Google Scholar] [CrossRef]
- Valledor, S.J.D.; Dioso, C.M.; Bucheli, J.E.V.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Kim, B.; Holzapfel, W.H.; Todorov, S.D. Characterization and safety evaluation of two beneficial, enterocin-producing Enterococcus faecium strains isolated from kimchi, a Korean fermented cabbage. Food Microbiol. 2022, 102, 103886. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Xin, W.G.; Yang, L.Y.; Ying, J.P.; Zhao, Z.S.; Lin, L.B.; Li, X.Z.; Zhang, Q.L. A novel bacteriocin against Staphylococcus aureus from Lactobacillus paracasei isolated from Yunnan traditional fermented yogurt: Purification, antibacterial characterization, and antibiofilm activity. J. Dairy Sci. 2022, 105, 2094–2107. [Google Scholar] [CrossRef] [PubMed]
- Serra-Castello, C.; Costa, J.C.C.P.; Jofre, A.; Bolivar, A.; Perez-Rodriguez, F.; Bover-Cid, S. A mathematical model to predict the antilisterail bioprotective effect of Latilactobacillus sakei CTC494 in vacuum packed cooked ham. Int. J. Food Microbiol. 2022, 363, 109491. [Google Scholar] [CrossRef]
- Zadeh, R.G.; Asgharzadeh, S.; Darbandi, A.; Aliramezani, A.; Jazi, F.M. Characterization of bacteriocins produced by Lactobacillus species against adhesion and invasion of Listeria monocytogenes isolated from different samples. Microbial. Pathogen. 2022, 162, 105307. [Google Scholar] [CrossRef] [PubMed]
- Fugaban, J.I.I.; Holzapfel, W.H.; Todorov, S.D. Probiotic potential and safety assessment of bacteriocinogenic Enterococcus faecium strains with antibacterial activity against Listeria and vancomycin-resistant enterococci. Curr. Res. Microb. Sci. 2021, 30, 100070. [Google Scholar] [CrossRef]
- Abitayeva, G.K.; Urazova, M.S.; Abilkhadirov, A.S.; Sarmurzina, Z.S.; Shaikhin, S.M. Characterization of a new bacteriocin-like inhibitory peptide produced by Lactobacillus sakei B-RKM 0559. Biotechnol. Lett. 2021, 43, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Meng, X.; Shen, H.; Luo, W.; Yao, S.; Yang, J.; Zhu, Q.; Tian, Y.; Wang, S. Purification, molecular characterization of Lactocin 63 produced by Lactobacillus coryniformis FZU63 and its antimicrobial mode of action against Shewanella putrefaciens. Appl. Microbiol. Biotechnol. 2021, 105, 6921–6930. [Google Scholar] [CrossRef]
- Barbosa, J.; Albano, H.; Silva, B.; Almeida, M.H.; Nogueira, T.; Teixeira, P. Characterization of a Lactiplantibacillus plantarum R23 Isolated from Arugula by whole-genome sequencing and its bacteriocin production ability. Int. J. Environ. Res. Public Health. 2021, 18, 5515. [Google Scholar] [CrossRef]
- Sheoran, P.; Tiwari, S.K. Synergistically-acting enterocin LD3 and plantaricin LD4 against Gram-positive and Gram-negative pathogenic bacteria. Probiot. Antimicro. Prot. 2021, 13, 542–554. [Google Scholar] [CrossRef]
- Yan, H.; Lu, Y.; Li, X.; Yi, Y.; Wang, X.; Shan, Y.; Liu, B.; Zhou, Y.; Lu, X. Action mode of bacteriocin BM1829 against Escherichia coli and Staphylococcus aureus. Food Biosci. 2021, 39, 100794. [Google Scholar] [CrossRef]
- Alang, H.; Kusnadi, J.; Ardyati, T.; Suharjono. Optimisation and characterization of enterocin Enterococcus faecalis K2B1 isolated from Toraja’s Belang buffalo milk, South Sulawesi, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21, 1236–1242. [Google Scholar] [CrossRef]
- Ananou, S.; Lotfi, S.; Azdad, O.; Nzoyikorera, N. Production, recovery and characterization of an enterocin with anti-listerial activity produced by Enterococcus hirae OS1. Appl. Food Biotechnol. 2020, 7, 103–114. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Bucheli, J.E.V.; Kim, B.; Holzapfel, W.H.; Todorov, S.D. Safety and beneficial properties of bacteriocinogenic Pediococcus acidilactici and Pediococcus pentosaceus isolated from silage. Lett. Appl. Microbiol. 2021, 73, 725–734. [Google Scholar] [CrossRef]
- De Souza, B.M.S.; Borgonovi, T.F.; Casaroti, S.N.; Todorov, S.D.; Penna, A.L.B. Lactobacillus casei and Lactobacillus fermentum strains isolated from Mozzarella cheese: Probiotic potential, safety, acidification kinetic parameters and viability under gastrointestinal tract conditions. Prob. Antimicrob. Prot. 2019, 11, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Casarotti, S.N.; Penna, A.L.B. Acidification profile, probiotic in vitro gastrointestinal tolerance and viability in fermented milk with fruit flours. Int. Dairy J. 2015, 41, 1–6. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Bialas, W.; Grajek, W. Integrated approach for obtaining bioactive peptides from whey proteins hydrolysedusing a new proteolytic lactic acid bacteria. Food Chem. 2020, 312, 126035. [Google Scholar] [CrossRef]
- Garcia-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jimenez-Flores, R. Lactic acid bactéria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl. Microbiol. Biotechnol. 2019, 103, 5243–5257. [Google Scholar] [CrossRef]
- Biscola, V.; Choiset, Y.; Rabesona, H.; Chobert, J.-M.; Haertle, T.; Franco, B.D.G.M. Brazilian artisanal ripened cheeses as source of proteolityc lactic acid bacteria capable of reducing cow milk allergy. J. Appl. Microbiol. 2018, 125, 564–574. [Google Scholar] [CrossRef]
- Brown, L.; Pingitore, E.V.; Mozzi, F.; Saavedra, L.; Villegas, J.M.; Hebert, E.M. Lactic acid bacteria as cell factories for the generation of bioactive peptides. Prot. Pept. Lett. 2017, 24, 146–155. [Google Scholar] [CrossRef]
- Herran, A.R.; Perez-Andres, J.; Caminero, A.; Nistal, E.; Vivas, S.; Ruiz de Morales, J.M.; Casqueiro, J. Gluten-degrading bacteria are present in the human small intestine of healthy volunteers and celic patiens. Res. Microbiol. 2017, 168, 673–684. [Google Scholar] [CrossRef]
- Perin, L.M.; Belviso, S.; Dal Bello, B.; Nero, L.A.; Cocolin, L. Technological properties and biogenic amines production by bacteriocinogenic lactococci and enterococci strains isolated from raw goat’s milk. J. Food Protect. 2017, 80, 151–157. [Google Scholar] [CrossRef]
- Perin, L.M.; Miranda, R.P.; Todorov, S.D.; Franco, B.D.G.M.; Nero, L.A. Virulence, antibiotic resistenceand biogenic amines of bacteriocinogenic lactococci and enterococci isolated from goat milk. Int. J. Food Microbiol. 2014, 185, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A. What is wrong with enterococcal probiotics? Prob. Antimicrob. Prot. 2020, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Holzapfel, W.H. Traditional cereal fermented foods as sources of functional microorganisms. In Advances in Fermented Foods and Beverages: Improving Quality, Technologies and Health Benefits; Holzapfel, W.H., Ed.; Woodhead Publishing: London, UK, 2013; pp. 123–153. [Google Scholar]
- Von Mollendorff, J.; Todorov, S.D.; Vaz-Velho, M. Cereal-based fermented foods are rich source of probiotics and bacteriocin producer lactic acid bacteria. In Functional Properties of Traditional Foods; ISEKI Food Series Volume 12; Kristbergsson, K., Ötles, S., Eds.; Springer Publishing Group: New York, NY, USA, 2016; pp. 157–188. [Google Scholar] [CrossRef]
- Öncül, N.; Yıldırım, Z. Inhibitory effect of bacteriocins against Escherichia coli O157:H7. Food Sci. Technol. Int. 2019, 25, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Gök, C.M.; Özden, T.B.; Akpinar, K.D.; Tuncer, Y. Bacteriocinogenic properties and safety evaluation of Enterococcus faecium YT52 isolated from boza, a traditional cereal based fermented beverage. J. Verbrauch. Lebensm. 2019, 14, 41–53. [Google Scholar] [CrossRef]
- Koral, G.; Tuncer, Y. Nisin Z—Producing Lactococcus lactis subsp. lactis GYI32 isolated from boza. J. Food Proc. Preserv. 2014, 38, 1044–1053. [Google Scholar] [CrossRef]
- Botes, A.; Todorov, S.D.; von Mollendorff, J.W.; Botha, A.; Dicks, L.M.T. Identification of Lactic Acid Bacteria and Yeast from Boza. Process Biochem. 2007, 42, 267–270. [Google Scholar] [CrossRef]
- Todorov, S.D.; Botes, M.; Guigas, C.; Schillinger, U.; Wiid, I.; Wachsman, M.B.; Holzapfel, W.H.; Dicks, L.M.T. Boza, a natural source of probiotic lactic acid bacteria. J. Appl. Microbiol. 2008, 104, 465–477. [Google Scholar] [CrossRef]
- Von Mollendorff, J.W.; Todorov, S.D.; Dicks, L.M.T. Factors affecting the adsorption of bacteriocins to Latobacillus sakei and Enterococcus sp. Appl. Biochem. Biotechnol. 2007, 142, 209–220. [Google Scholar] [CrossRef]
- Pingitore, E.V.; Todorov, S.D.; Sesma, F.; Franco, B.D.G.M. Application of bacteriocinogenic Enterococcus mundtii CRL35 and Enterococcus faecium ST88Ch in the control of Listeria monocytogenes in fresh Minas cheese. Food Microbiol. 2012, 32, 38–47. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Staliano, C.D.; Vieira, A.D.S.; Villarreal, M.L.M.; Todorov, S.D.; Saad, S.M.I.; Franco, B.D.G.M. Bacteriocin production and inhibition of Listeria monocytogenes by Lactobacillus sakei subsp. sakei 2a in a potentially synbiotic cheese spread. Food Microbiol. 2015, 48, 143–152. [Google Scholar] [CrossRef]
- Schirru, S.; Favaro, L.; Mangia, N.P.; Basaglia, M.; Casella, S.; Comunian, R.; Fancello, F.; Franco, B.D.G.M.; Oliveira, R.P.S.; Todorov, S.D. Comparison of bacteriocins production from Enterococcus faecium strains in cheese whey and optimised commercial MRS medium. Ann. Microbiol. 2014, 64, 321–331. [Google Scholar] [CrossRef]
- Heng, N.C.K.; Wescombe, P.A.; Burton, J.P.; Jack, R.W.; Tagg, J.R. The diversity of bacteriocins in Gram-positive bacteria. In Bacteriocins; Riley, M.A., Chavan, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Andrade, M.S.; de Arauz, L.J.; Pessoa, A.; Penna, T.C.V. Nisin production utilizing skimmed milk aiming to reduce process cost. Appl. Biochem. Biotechnol. 2007, 137, 515. [Google Scholar] [CrossRef] [PubMed]
- Pongtharangkul, T.; Demirci, A. Evaluation of agar diffusion bioassay for nisin quantification. Appl. Microbiol. Biotechnol. 2004, 65, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Corredoira, J.; Coira, A.; Alonso, M.A.; Varela, J. Association between Streptococcus infantarius (Formerly, S. bovis II/1). J. Clin. Microbiol. 2008, 46, 1570. [Google Scholar] [CrossRef]
- Boleij, A.; Gelder, M.H.J.; Swinnkels, D.W.; Tjalsma, H. Clinical importance of Streptococcus gallolyticus infection among colorectal cancer patients: Systematic review and meta-analysis. Clin. Infect. Dis. 2011, 53, 870–878. [Google Scholar] [CrossRef]
- Jans, C.; Kaindi, D.W.M.; Böck, D.; Njage, P.M.K.; Kouamé-Sina, S.M.; Bonfoh, B.; Lacroix, C.; Meile, L. Prevalence and comparison of Streptococcus infantarius subsp. infantarius and Streptococcus gallolyticus subsp. macedonicus in raw and fermented dairy products from East and West Africa. Int. J. Food Microbiol. 2013, 167, 186–195. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dioso, C.M.; Liong, M.-T.; Vasileva, T.; Moncheva, P.; Ivanova, I.V.; Iliev, I. Lukanka, a semi-dried fermented traditional Bulgarian sausage: Role of the bacterial cultures in its technological, safety and beneficial characteristics. Appl. Food Biotechnol. 2022, 9, 255–265. [Google Scholar] [CrossRef]
- Schillinger, U.; Geisen, R.; Holzapfel, W.H. Potential of antagosnistic microorganisms and bacteriocins for the biological preservation of food. Trends Food Sci. Technol. 1996, 7, 158–164. [Google Scholar] [CrossRef]
- Foulquié-Moreno, M.R.; Sarantinopoulos, P.; Tsakalidou, E.; De Vuyst, L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006, 106, 1–24. [Google Scholar] [CrossRef]
- Landman, D.; Quale, J.M. Management of infections due to resistant enterococci: A review of therapeutic options. J. Antimicrob. Chemother. 1997, 40, 161–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.J.; Willems, R.; Weinstein, R.A. Vancomycin-resistant enterococci: Why are they here, and where do they come from? Lancet Infect. Dis. 2001, 1, 314–325. [Google Scholar] [CrossRef]
- Franz, C.M.; Stiles, M.E.; Schleifer, K.H.; Holzapfel, W.H. Enterococci in foods—A conundrum for food safety. Int. J. Food Microbiol. 2003, 88, 105–122. [Google Scholar] [CrossRef]
- Klare, I.; Konstabel, C.; Badstübner, D.; Werner, G.; Witte, W. Occurrence and spread of antibiotic resistances in Enterococcus faecium. Int. J. Food Microbiol. 2003, 88, 269–290. [Google Scholar] [CrossRef]
- Johnson, A.G.; Nguyen, T.V.; Day, R.O. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann. Intern. Med. 1994, 121, 289–300. [Google Scholar] [CrossRef]
- Dos Santos, K.M.O.; de Matos, C.R.; Salles, H.O.; Franco, B.D.G.M.; Arellano, K.; Holzapfel, W.H.; Todorov, S.D. Exploring beneficial/virulence properties of two dairy related strains of Streptococcus infantarius subsp. infantarius. Probiot. Antimicrob. Prot. 2020, 12, 1524–1541. [Google Scholar] [CrossRef]
- Nascimento, L.C.S.; Casarotti, S.N.; Todorov, S.D.; Penna, A.L.B. Probiotic potential and safety of enterococci strains. Ann. Microbiol. 2018, 69, 241–252. [Google Scholar] [CrossRef]
- Hammes, W.P.; Tichaczek, P.S. The potential of lactic acid bacteria for the production of safe and wholesome food. Z. Lebensm. Unters. Forsch. 1994, 198, 193–201. [Google Scholar] [CrossRef]
- Land, M.H.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005, 115, 178–181. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Khoury, C.C. Sepsis associated with Lactobacillus bacteremia in a patient with ischemic colitis. Indian J. Crit. Care Med. 2014, 18, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Järvinen, A. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin. Infect. Dis. 2004, 38, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Karime, C.; Barrios, M.S.; Wiest, N.E.; Stancampiano, F. Lactobacillus rhamnosus sepsis, endocarditis and septic emboli in a patient with ulcerative colitis taking probiotics. BMJ Case Rep. 2022, 15, e249020. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Collins, M.D. Lactic acid bacteria and human clinical infection. J. Appl. Bacteriol. 1993, 75, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Kalschne, D.L.; Womer, R.; Mattana, A.; Sarmento, C.M.; Colla, L.M.; Colla, E. Characterization of the spoilage lactic acid bacteria in “sliced vacuum-packed cooked ham”. Braz. J. Microbiol. 2015, 46, 173–181. [Google Scholar] [CrossRef]
- Andreevskaya, M.; Jääskeläinen, E.; Johansson, P.; Ylinen, A.; Paulin, L.; Björkroth, J.; Auvinen, P. Food spoilage-associated Leuconostoc, Lactococcus, and Lactobacillus species display different survival strategies in response to competition. Appl. Environ. Microbiol. 2018, 84, e00554-18. [Google Scholar] [CrossRef]
- Umu, Ö.C.; Bäuerl, C.; Oostindjer, M.; Pope, P.B.; Hernández, P.E.; Pérez-Martínez, G.; Diep, D.B. The potential of class II bacteriocins to modify gut microbiota to improve host health. PLoS ONE 2016, 11, e0164036. [Google Scholar] [CrossRef]
- De Koning, H.P. Drug resistance in protozoan parasites. Emerg. Top. Life Sci. 2017, 1, 627–632. [Google Scholar] [CrossRef]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional roles of B-vitamins in the gut and gut microbiome. Mol. Nutr. Food Res. 2020, 64, e2000426. [Google Scholar] [CrossRef]
- Ammor, M.S.; Flórez, A.B.; Mayo, B. Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol. 2007, 24, 559–570. [Google Scholar] [CrossRef]
- Teuber, M.; Meile, L.; Schwarz, F. Acquired antibiotic resistance in lactic acid bacteria from food. In Lactic Acid Bacteria: Genetics, Metabolism and Applications; Konings, W.N., Kuipers, O.P., In’t Veld, J.H.J.H., Eds.; Springer: Dordrecht, Germany, 1919. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10 (Suppl. 12), S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Bucheli, J.E.V.; Todorov, S.D.; Holzapfel, W.H. Role of gastrointestinal microbial populations, a terra incognita of the human body in the management of intestinal bowel disease and metabolic disorders. Benef. Microbes. 2022, 22, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Le Blay, G.; Hammami, R.; Lacroix, C.; Fliss, I. Stability and inhibitory activity of pediocin PA-1 against Listeria sp. in simulated physiological conditions of the human terminal ileum. Prob. Antimicrob. Prot. 2012, 4, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Cotter, P.D.; Hill, C.; Ross, R.P. Spontaneous resistance in Lactococcus lactis IL1403 to the lantibiotic lacticin 3147. FEMS Microbiol. Lett. 2006, 260, 77–83. [Google Scholar] [CrossRef]
- Le Lay, C.; Fernandez, B.; Hammami, R.; Ouellette, M.; Fliss, I. On Lactococcus lactis UL719 competitivity and nisin (Nisaplin®) capacity to inhibit Clostridium difficile in a model of human colon. Front. Microbiol. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Bernbom, N.; Licht, T.R.; Brogren, C.-H.; Jelle, B.; Johansen, A.H.; Badiola, I.; Vogensen, F.K.; Nørrung, B. Effects of Lactococcus lactis on composition of intestinal microbiota: Role of nisin. Appl. Environ. Microbiol. 2006, 72, 239–244. [Google Scholar] [CrossRef]
- Dabour, N.; Zihler, A.; Kheadr, E.; Lacroix, C.; Fliss, I. In Vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int. J. Food Microbiol. 2009, 133, 225–233. [Google Scholar] [CrossRef]
- Rea, M.C.; Dobson, A.; O’Sullivan, O.; Crispie, F.; Fouhy, F.; Cotter, P.D.; Shanahan, F.; Kiely, B.; Hill, C.; Ross, R.P. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4639–4644. [Google Scholar] [CrossRef]
- Blay, G.L.; Lacroix, C.; Zihler, A.; Flies, I. In Vitro inhibition activity of nisin A, nisin Z, pediocin PA-1 and antibiotics against common intestinal bacteria. Lett. Appl. Microbiol. 2007, 45, 252–257. [Google Scholar] [CrossRef]
- Dobson, A.; Crispie, F.; Rea, M.C.; O’Sullivan, O.; Casey, P.G.; Lawlor, P.G.; Cotter, P.D.; Ross, P.; Gardiner, G.E.; Hill, C. Fate and efficacy of lacticin 3147-producing Lactococcus lactis in the mammalian gastrointestinal tract. FEMS Microbiol. Ecol. 2011, 76, 602–614. [Google Scholar] [CrossRef]
- Riboulet-Bisson, E.; Sturme, M.H.; Jeffery, I.B.; O’Donnell, M.M.; Neville, B.A.; Forde, B.M.; Claesson, M.J.; Harris, H.; Gardiner, G.E.; Casey, P.G.; et al. Effect of Lactobacillus salivarius bacteriocin Abp118 on the mouse and pig intestinal microbiota. PLoS ONE 2012, 7, e31113. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.F.; Cotter, P.D.; Hogan, A.; O’Sullivan, O.; Joyce, A.; Fouhy, F.; Clarke, S.F.; Marques, T.M.; O’Toole, P.W.; Stanton, C.; et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 2013, 62, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Ross, R.P.; O’Toole, P.W.; Shanahan, F.; Cotter, P.D. Targeting the microbiota to address diet-induced obesity: A time dependent challenge. PLoS ONE 2013, 8, e65790. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Stanton, C.; Hill, C.; Ross, R.P. New developments and applications of bacteriocins and peptides in foods. Annu. Rev. Food Sci. Technol. 2011, 2, 299–329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xie, Y.; Liu, H.; Jin, J.; Duan, H.; Zhang, H. Effects of two application methods of plantaricin BM-1 on control of Listeria monocytogenes and background spoilage bacteria in sliced vacuum-packaged cooked ham stored at 4 °C. J. Food Prot. 2015, 78, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Chandrakasan, G.; Rodrıguez-Hernandez, A.-I.; del Rocıo Lopez-Cuellar, M.; Palma-Rodriquez, H.-M.; Chavarria-Hernandez, N. Bacteriocin encapsulation for food and pharmaceutical applications: Advances in the past 20 years. Biotechnol. Lett. 2019, 41, 453–469. [Google Scholar] [CrossRef]
- Udompijitkul, P.; Paredes-Sabja, D.; Sarker, M.R. Inhibitory effects of nisin against Clostridium perfringens food poisoning and nonfood-borne isolates. J. Food Sci. 2012, 77, M51–M56. [Google Scholar] [CrossRef]
- Bartoloni, A.; Mantella, A.; Goldstein, B.P.; Dei, R.; Benedetti, M.; Sbaragli, S.; Paradisi, F. In-vitro activity of nisin against clinical isolates of Clostridium difficile. J. Chemother. 2004, 16, 119–121. [Google Scholar] [CrossRef]
| Bacteriocin | Producer | Area of Proposed Application | Reference |
|---|---|---|---|
| Bovicin HC5 and nisin | Streptococcus bovis HC5 | Control of Alicyclobacillus acidoterrestris in fruit juices | [109] |
| Bovicin HC5 | Streptococcus bovis HC5 | Clostridium tyrobutyricum, a pathogen associated with spoiled mango pulp | [110] |
| Enterococin A | Enterococcus faecium MMRA | Control of Listeria monocytogenes in sliced dry-cured ham | [111] |
| Bacteriocin RSQ04 | Lactococcus lactis CGMCC20699 | Evaluation of activity against Listeria monocytogenes in model food system | [112] |
| Pyocin QDD1 | Pseudomonas aeruginosa QDD1 | Biocontrol of foodborne pathogens Staphylococcus aureus and Bacillus cereus | [113] |
| Bacteriocin LSB1 | Lactiplantibacillus plantarum LSB1 | Activity against Staphylococcus argenteus planktonic cells and biofilm | [114] |
| Bacteriocins ST20Kc and ST41Kc | Enterococcus faecium ST20Kc and ST41Kc | Control of Listeria monocytogenes and vancomycin-resistant entorococci | [115] |
| Bacteriocin LSX01 | Lacticaseibacillus paracasei LSX01 | Reduction of planktonic cells of Staphylococcus aureus | [116] |
| Bacteriocin CTC494 | Latilactobacillus sakei CTC494 | Anti-listerial activity in vacuum packaged cooked ham | [117] |
| Six bacteriocins | Lacticaseibacillus casei and Lactiplantibacillus plantarum | Activity against 8 different Listeria monocytogenes strains | [118] |
| Bacteriocins ST651ea, ST7119ea, and ST7319ea | Enterococcus faecium ST651ea, ST7119ea, and ST7319ea | Control of Listeria monocytogenes and vancomycin-resistant enterococci in GIT model system | [119] |
| Bacteriocin Sak-59 | Lactobacillus sakei B-RKM 0559 | Activity against meat spoilage bacteria strains of Listeria monocytogenes, Staphylococcus aureus, and pathogenic strains of Serratia marcescens and Escherichia coli | [120] |
| Lactocin 63 | Loigolactobacillus coryniformis FZU63 | Antimicrobial mode of action against Shewanella putrefaciens | [121] |
| Bacteriocin R23 | Lactiplantibacillus plantarum R23 | Anti-Listeria monocytogenes activity | [122] |
| Enterocin LD3 and Plantaricin LD4 | Enterococcus faecium LD3 and Lactiplantibacillus plantarum LD4 | Synergistic effect against Staphylococcus aureus subsp. aureus ATCC25923, Salmonella enterica subsp. enterica serovar Typhimurium ATCC13311, Proteus mirabilis ATCC43071, Pseudomonas aeruginosa ATCC27853, and Escherichia coli ATCC25922 | [123] |
| Bacteriocins ST1607V, ST2104V and ST3105V | Pediococcus acidilactici ST1607V, ST2104V and ST3105V | Bactericidal mode of action against Listeria monocytogenes ATCC7644 and Enterococcus faecium ATCC19434 | [48] |
| Bacteriocin BM1829 | Companilactobacillus crustorum MN047 | Reduction of Escherichia coli and Staphylococcus aureus | [124] |
| Enterocin K2B1 | Enterococcus faecalis K2B1 | Control of foodborne pathogens in dairy products | [125] |
| Bacteriocin OS1 | Enterococcus hirae OS1 | Anti-Listeria activity | [126] |
| Bacteriocins ST3522BG and ST3633BG | Pediococcus acidilactici ST3522BG and Pedioccocus pentosaceus ST3633BG | Anti-Listeria activity in silage fermentation models system | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorov, S.D.; Popov, I.; Weeks, R.; Chikindas, M.L. Use of Bacteriocins and Bacteriocinogenic Beneficial Organisms in Food Products: Benefits, Challenges, Concerns. Foods 2022, 11, 3145. https://doi.org/10.3390/foods11193145
Todorov SD, Popov I, Weeks R, Chikindas ML. Use of Bacteriocins and Bacteriocinogenic Beneficial Organisms in Food Products: Benefits, Challenges, Concerns. Foods. 2022; 11(19):3145. https://doi.org/10.3390/foods11193145
Chicago/Turabian StyleTodorov, Svetoslav Dimitrov, Igor Popov, Richard Weeks, and Michael Leonidas Chikindas. 2022. "Use of Bacteriocins and Bacteriocinogenic Beneficial Organisms in Food Products: Benefits, Challenges, Concerns" Foods 11, no. 19: 3145. https://doi.org/10.3390/foods11193145
APA StyleTodorov, S. D., Popov, I., Weeks, R., & Chikindas, M. L. (2022). Use of Bacteriocins and Bacteriocinogenic Beneficial Organisms in Food Products: Benefits, Challenges, Concerns. Foods, 11(19), 3145. https://doi.org/10.3390/foods11193145







