Glucosinolates and Polyphenols of Colored Cauliflower as Chemical Discriminants Based on Cooking Procedures
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Samples and Sample Treatment
2.4. Data Analysis
3. Results and Discussion
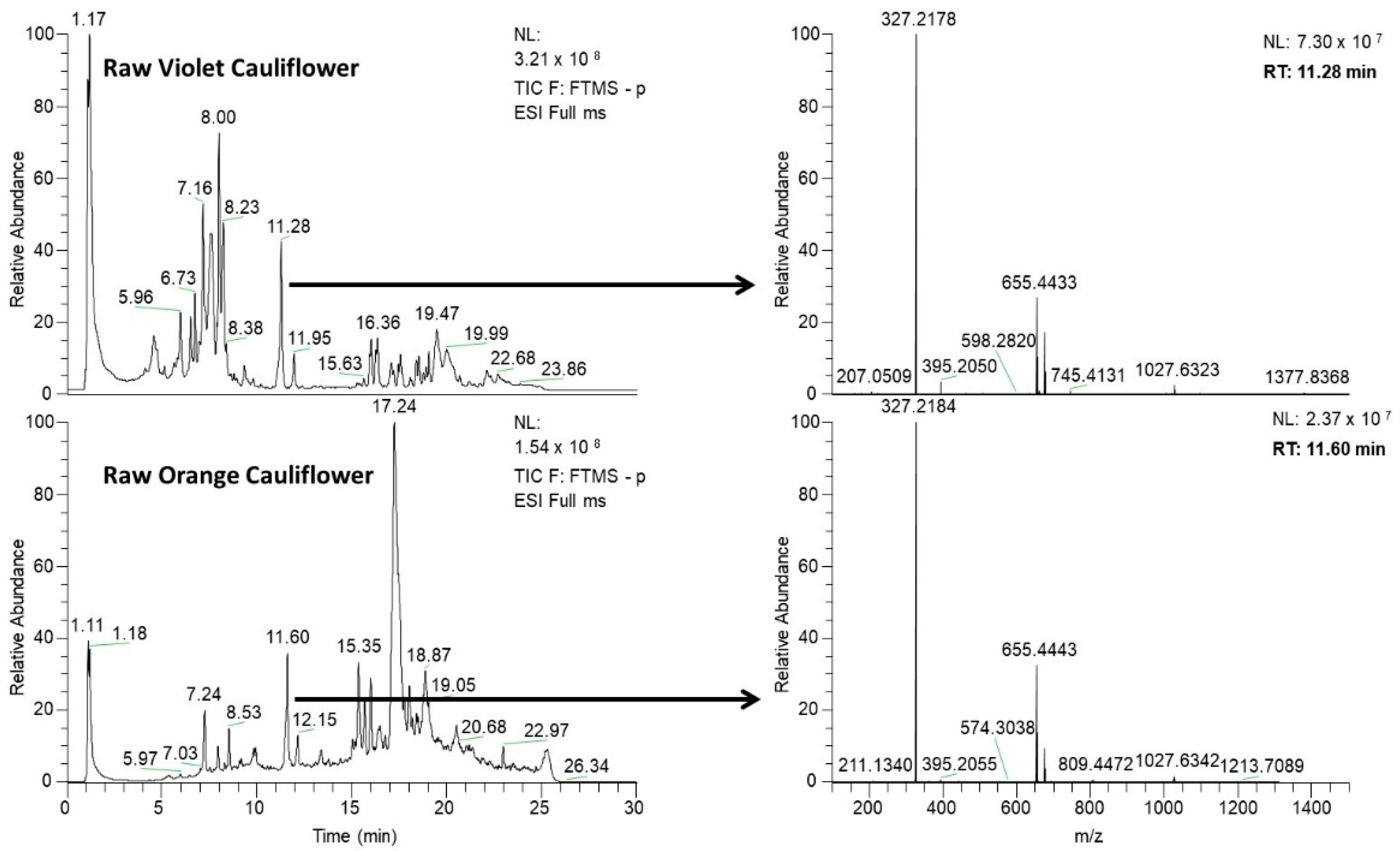
3.1. UHPLC–HRMS Phytochemical Profiling
3.2. Phytochemical Profiles as Chemical Descriptors for Sample Discrimination
3.3. Qualitative Changes in Phytochemical Contents
3.3.1. Violet Cauliflower
3.3.2. Orange Cauliflower
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aǧagündüz, D.; Şahin, T.Ö.; Yilmaz, B.; Ekenci, K.D.; Duyar Özer, Ş.; Capasso, R. Cruciferous Vegetables and Their Bioactive Metabolites: From Prevention to Novel Therapies of Colorectal Cancer. Evid. Based. Complement. Alternat. Med. 2022, 2022, 1534083. [Google Scholar] [CrossRef] [PubMed]
- Mageney, V.; Neugart, S.; Albach, D.C. A Guide to the Variability of Flavonoids in Brassica oleracea. Molecules 2017, 22, 252. [Google Scholar] [CrossRef]
- Aguilera, Y.; Herrera, T.; Benítez, V.; Arribas, S.M.; López De Pablo, A.L.; Esteban, R.M.; Martín-Cabrejas, M.A. Estimation of Scavenging Capacity of Melatonin and Other Antioxidants: Contribution and Evaluation in Germinated Seeds. Food Chem. 2015, 170, 203–211. [Google Scholar] [CrossRef]
- Zietz, M.; Weckmüller, A.; Schmidt, S.; Rohn, S.; Schreiner, M.; Krumbein, A.; Kroh, L.W. Genotypic and Climatic Influence on the Antioxidant Activity of Flavonoids in Kale (Brassica oleracea Var. Sabellica). J. Agric. Food Chem. 2010, 58, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Ellili, A.; Legault, J.; Pichette, A.; Ksouri, R.; Lachaal, M.; Karray-Bouraoui, N. Phenolic Content, Antioxidant and Anti-Inflammatory Activities of Tunisian Diplotaxis Simplex (Brassicaceae). Nat. Prod. Res. 2015, 29, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Farhat, G.; Drummond, S.; Al-Dujaili, E.A.S. Polyphenols and Their Role in Obesity Management: A Systematic Review of Randomized Clinical Trials. Phyther. Res. 2017, 31, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxid. Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxid. Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial Roles of Honey Polyphenols against Some Human Degenerative Diseases: A Review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; D’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.M.; Wessjohann, L.; Gazzino, R.; Costantino, P.; et al. Anti-Inflammatory Activity of a Polyphenolic Extract from Arabidopsis Thaliana in in Vitro and in Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 708. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Ebrahimi, F.; Farzaei, M.H.; Baratpourmoghaddam, A.; Ahmadi, P.; Rostamiasrabadi, P.; Rasouli Amirabadi, A.H.; Rahimi, R. Dietary Polyphenols for Atherosclerosis: A Comprehensive Review and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A Review of the Dietary Flavonoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The Intrinsic Quality of Brassicaceous Vegetables: How Secondary Plant Metabolites Are Affected by Genetic, Environmental, and Agronomic Factors. Sci. Hortic. 2018, 233, 460–478. [Google Scholar] [CrossRef]
- Russo, G.L.; Tedesco, I.; Spagnuolo, C.; Russo, M. Antioxidant Polyphenols in Cancer Treatment: Friend, Foe or Foil? Semin. Cancer Biol. 2017, 46, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea Var. Acephala) as a Superfood: Review of the Scientific Evidence behind the Statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, H.; Childs, H.; Wu, Y.; Yu, L.; Pehrsson, P.R. Glucosinolates in Brassica Vegetables: Characterization and Factors That Influence Distribution, Content, and Intake. Annu. Rev. Food Sci. Technol. 2021, 12, 485–511. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Dwivedi, A.; Plessis, J. Du Sinigrin and Its Therapeutic Benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Razis, A.F.A. Apoptosis as a Mechanism of the Cancer Chemopreventive Activity of Glucosinolates: A Review. Asian Pac. J. Cancer Prev. 2018, 19, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Argentieri, M.P. Brassicaceae: A Rich Source of Health Improving Phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Fujioka, N.; Fritz, V.; Upadhyaya, P.; Kassie, F.; Hecht, S.S. Research on Cruciferous Vegetables, Indole-3-Carbinol, and Cancer Prevention: A Tribute to Lee W. Wattenberg. Mol. Nutr. Food Res. 2016, 60, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Tuli, H.S.; Mittal, S.; Shandilya, J.K.; Tiwari, A.; Sandhu, S.S. Isothiocyanates: A Class of Bioactive Metabolites with Chemopreventive Potential. Tumor Biol. 2015, 36, 4005–4016. [Google Scholar] [CrossRef] [PubMed]
- Herz, C.; Márton, M.R.; Tran, H.T.T.; Gründemann, C.; Schell, J.; Lamy, E. Benzyl Isothiocyanate but Not Benzyl Nitrile from Brassicales Plants Dually Blocks the COX and LOX Pathway in Primary Human Immune Cells. J. Funct. Foods 2016, 23, 135–143. [Google Scholar] [CrossRef]
- Guzmán-Pérez, V.; Bumke-Vogt, C.; Schreiner, M.; Mewis, I.; Borchert, A.; Pfeiffer, A.F.H. BenzylglucosinolateDerived Isothiocyanate from Tropaeolum Majus Reduces Gluconeogenic Gene and Protein Expression in Human Cells. PLoS ONE 2016, 11, e0162397. [Google Scholar] [CrossRef]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-Rich Moringa Oleifera Extract Reduces Weight Gain, Insulin Resistance, and Hepatic Gluconeogenesis in Mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Orlando, P.; Nartea, A.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Dludla, P.V.; Fiorini, R.; Pacetti, D.; Loizzo, M.R.; Lucci, P.; et al. Bioavailability Study of Isothiocyanates and Other Bioactive Compounds of Brassica oleracea L. Var. Italica Boiled or Steamed: Functional Food or Dietary Supplement? Antioxidants 2022, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of Industrial Broccoli Discards (Brassica oleracea Var. Italica) for Their Glucosinolate, Polyphenol and Flavonoid Contents Using UPLC MS/MS and Spectrophotometric Methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; Toxqui-Terán, A.; Espinosa-Solis, V. Physicochemical Properties and Antioxidant Activity of Spray-Dry Broccoli (Brassica oleracea Var. Italica) Stalk and Floret Juice Powders. Molecules 2021, 26, 1973. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients from Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Gródek-Szostak, Z.; Kuboń, M.; Leszczyńska, T.; Borczak, B. Health-Promoting Properties of Fresh and Processed Purple Cauliflower. Sustainability 2019, 11, 4008. [Google Scholar] [CrossRef]
- Nartea, A.; Fanesi, B.; Falcone, P.M.; Pacetti, D.; Frega, N.G.; Lucci, P. Impact of Mild Oven Cooking Treatments on Carotenoids and Tocopherols of Cheddar and Depurple Cauliflower (Brassica oleracea L. Var. Botrytis). Antioxidants 2021, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Nartea, A.; Falcone, P.M.; Torri, L.; Ghanbarzadeh, B.; Frega, N.G.; Pacetti, D. Modeling Softening Kinetics at Cellular Scale and Phytochemicals Extractability in Cauliflower under Different Cooking Treatments. Foods 2021, 10, 1969. [Google Scholar] [CrossRef] [PubMed]
- Florkiewicz, A.; Ciska, E.; Filipiak-Florkiewicz, A.; Topolska, K. Comparison of Sous-Vide Methods and Traditional Hydrothermal Treatment on GLS Content in Brassica Vegetables. Eur. Food Res. Technol. 2017, 243, 1507–1517. [Google Scholar] [CrossRef][Green Version]
- Kapusta-Duch, J.; Kusznierewicz, B.; Leszczyńska, T.; Borczak, B. Effect of Cooking on the Contents of Glucosinolates and Their Degradation Products in Selected Brassica Vegetables. J. Funct. Foods 2016, 23, 412–422. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Dunkel, A.; Szwengiel, A.; Czaczyk, K.; Drożdżyńska, A.; Zawirska-Wojtasiak, R.; Jeleń, H.H. The Relation between Phytochemical Composition and Sensory Traits of Selected Brassica Vegetables. LWT 2022, 156, 113028. [Google Scholar] [CrossRef]
- Martínez, S.; Armesto, J.; Gómez-Limia, L.; Carballo, J. Impact of Processing and Storage on the Nutritional and Sensory Properties and Bioactive Components of Brassica Spp. A Review. Food Chem. 2020, 313, 126065. [Google Scholar] [CrossRef]
- Dos Reis, L.C.R.; de Oliveira, V.R.; Hagen, M.E.K.; Jablonski, A.; Flores, S.H.; de Oliveira Rios, A. Effect of Cooking on the Concentration of Bioactive Compounds in Broccoli (Brassica oleracea Var. Alphina F1) Grown in an Organic System. Food Chem. 2015, 172, 770–777. [Google Scholar] [CrossRef]
- Mazzeo, T.; N’Dri, D.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effect of Two Cooking Procedures on Phytochemical Compounds, Total Antioxidant Capacity and Colour of Selected Frozen Vegetables. Food Chem. 2011, 128, 627–633. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Wong, K.W.; Benzie, I.F.F. The Effect of Cooking on Brassica Vegetables. Food Chem. 2008, 110, 706–710. [Google Scholar] [CrossRef]
- Pellegrini, N.; Chiavaro, E.; Gardana, C.; Mazzeo, T.; Contino, D.; Gallo, M.; Riso, P.; Fogliano, V.; Porrini, M. Effect of Different Cooking Methods on Color, Phytochemical Concentration, and Antioxidant Capacity of Raw and Frozen Brassica Vegetables. J. Agric. Food Chem. 2010, 58, 4310–4321. [Google Scholar] [CrossRef]
- Bongoni, R.; Verkerk, R.; Steenbekkers, B.; Dekker, M.; Stieger, M. Evaluation of Different Cooking Conditions on Broccoli (Brassica oleracea Var. Italica) to Improve the Nutritional Value and Consumer Acceptance. Plant Foods Hum. Nutr. 2014, 69, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.; Pardo-Mates, N.; Hidalgo-Serrano, M.; Saurina, J.; Puignou, L.; Núnez, O. Detection and Quantitation of Frauds in the Authentication of Cranberry-Based Extracts by UHPLC-HRMS (Orbitrap) Polyphenolic Profiling and Multivariate Calibration Methods. J. Agric. Food Chem. 2018, 66, 9353–9365. [Google Scholar] [CrossRef]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Rothwell, J.A.; Medina-Remón, A.; Pérez-Jiménez, J.; Neveu, V.; Knaze, V.; Slimani, N.; Scalbert, A. Effects of Food Processing on Polyphenol Contents: A Systematic Analysis Using Phenol-Explorer Data. Mol. Nutr. Food Res. 2015, 59, 160–170. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Y.; Haytowitz, D.B.; Chen, P.; Pehrsson, P.R. Effects of Domestic Cooking on Flavonoids in Broccoli and Calculation of Retention Factors. Heliyon 2019, 5, 1310. [Google Scholar] [CrossRef]
- Florkiewicz, A.; Socha, R.; Filipiak-Florkiewicz, A.; Topolska, K. Sous-Vide Technique as an Alternative to Traditional Cooking Methods in the Context of Antioxidant Properties of Brassica Vegetables. J. Sci. Food Agric. 2019, 99, 173–182. [Google Scholar] [CrossRef]
- Fechner, J.; Kaufmann, M.; Herz, C.; Eisenschmidt, D.; Lamy, E.; Kroh, L.W.; Hanschen, F.S. The Major Glucosinolate Hydrolysis Product in Rocket (Eruca sativa L.), Sativin, Is 1,3-Thiazepane-2-Thione: Elucidation of Structure, Bioactivity, and Stability Compared to Other Rocket Isothiocyanates. Food Chem. 2018, 261, 57–65. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Płatosz, N.; Bartoszek, A. Phytochemical Composition and Biological Activities of Differently Pigmented Cabbage (Brassica oleracea Var. Capitata) and Cauliflower (Brassica oleracea Var. Botrytis) Varieties. J. Sci. Food Agric. 2019, 99, 5499–5507. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartea, A.; Fanesi, B.; Giardinieri, A.; Campmajó, G.; Lucci, P.; Saurina, J.; Pacetti, D.; Fiorini, D.; Frega, N.G.; Núñez, O. Glucosinolates and Polyphenols of Colored Cauliflower as Chemical Discriminants Based on Cooking Procedures. Foods 2022, 11, 3041. https://doi.org/10.3390/foods11193041
Nartea A, Fanesi B, Giardinieri A, Campmajó G, Lucci P, Saurina J, Pacetti D, Fiorini D, Frega NG, Núñez O. Glucosinolates and Polyphenols of Colored Cauliflower as Chemical Discriminants Based on Cooking Procedures. Foods. 2022; 11(19):3041. https://doi.org/10.3390/foods11193041
Chicago/Turabian StyleNartea, Ancuta, Benedetta Fanesi, Alessandra Giardinieri, Guillem Campmajó, Paolo Lucci, Javier Saurina, Deborah Pacetti, Dennis Fiorini, Natale Giuseppe Frega, and Oscar Núñez. 2022. "Glucosinolates and Polyphenols of Colored Cauliflower as Chemical Discriminants Based on Cooking Procedures" Foods 11, no. 19: 3041. https://doi.org/10.3390/foods11193041
APA StyleNartea, A., Fanesi, B., Giardinieri, A., Campmajó, G., Lucci, P., Saurina, J., Pacetti, D., Fiorini, D., Frega, N. G., & Núñez, O. (2022). Glucosinolates and Polyphenols of Colored Cauliflower as Chemical Discriminants Based on Cooking Procedures. Foods, 11(19), 3041. https://doi.org/10.3390/foods11193041









