Liposomes as Delivery System for Applications in Meat Products
Abstract
1. Introduction
2. General Information of Liposomes
3. Preparation of Liposomes
3.1. Conventional Methods
3.2. Advanced Methods
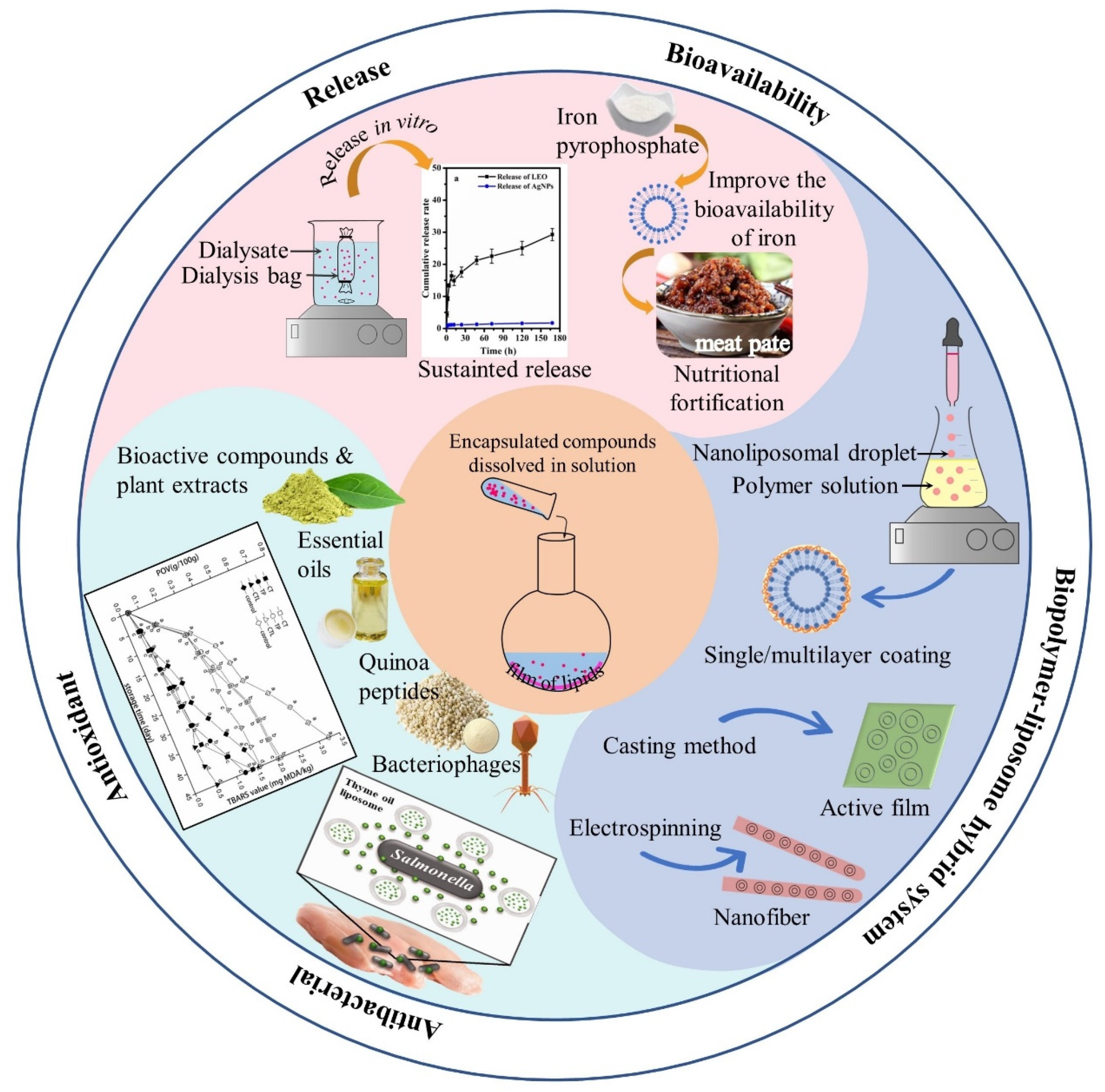
4. Application of Liposomes in Meat Products
4.1. The Release and Bioavailability of Delivered Molecules
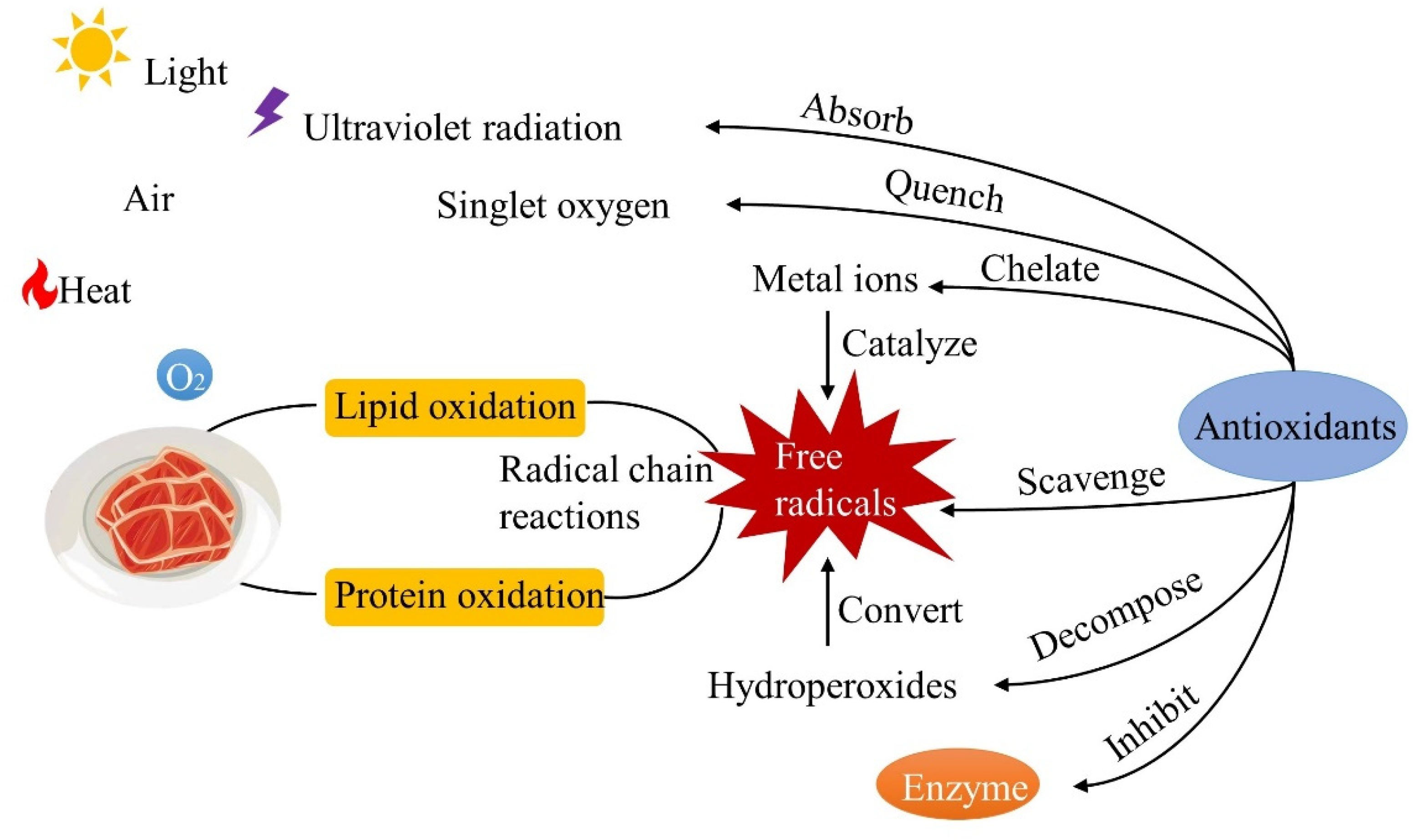
4.2. The Antibacterial and Antioxidant Effects
4.2.1. The Antioxidant and Antibacterial Mechanisms in Food Products
- (1)
- The antioxidant mechanism in food products
- (2)
- The antibacterial mechanism in food products
4.2.2. Applications of Encapsulated Active Compounds in the Meat Industry
- (1)
- Essential oils
- (2)
- Bacteriophages
- (3)
- Bioactive compounds
- (4)
- Peptides
4.3. The Biopolymer-Liposome Hybrid Systems
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef]
- Liu, W.; Hou, Y.; Jin, Y.; Wang, Y.; Xu, X.; Han, J. Research progress on liposomes: Application in food, digestion behavior and absorption mechanism. Trends Food Sci. Technol. 2020, 104, 177–189. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Khanniri, E.; Bagheripoor-Fallah, N.; Sohrabvandi, S.; Mortazavian, A.M.; Khosravi-Darani, K.; Mohammad, R. Application of Liposomes in Some Dairy Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 484–493. [Google Scholar] [CrossRef]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A Review of Manufacturing Techniques and Targeting Strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Hamadou, A.H.; Huang, W.-C.; Xue, C.; Mao, X. Formulation of vitamin C encapsulation in marine phospholipids nanoliposomes: Characterization and stability evaluation during long term storage. LWT-Food Sci. Technol. 2020, 127, 109439. [Google Scholar] [CrossRef]
- Thompson, A.K.; Singh, H. Preparation of Liposomes from Milk Fat Globule Membrane Phospholipids Using a Microfluidizer. J. Dairy Sci. 2006, 89, 410–419. [Google Scholar] [CrossRef]
- Eibl, H.; Kaufmann-Kolle, P. Medical Application of Synthetic Phospholipids as Liposomes and Drugs. J. Liposome Res. 1995, 5, 131–148. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Hou, L.; Sun, X.; Pan, L.; Gu, K. Effects of Phytosterol Butyrate Ester on the Characteristics of Soybean Phosphatidylcholine Liposomes. J. Oleo Sci. 2021, 70, 1295–1306. [Google Scholar] [CrossRef]
- Srihera, N.; Li, Y.; Zhang, T.-T.; Wang, Y.-M.; Yanagita, T.; Waiprib, Y.; Xue, C.-H. Preparation and Characterization of Astaxanthin-loaded Liposomes Stabilized by Sea Cucumber Sulfated Sterols Instead of Cholesterol. J. Oleo Sci. 2022, 71, 401–410. [Google Scholar] [CrossRef]
- Li, J.; Zhai, J.; Dyett, B.; Yang, Y.; Drummond, C.J.; Conn, C.E. Effect of gum arabic or sodium alginate incorporation on the physicochemical and curcumin retention properties of liposomes. LWT-Food Sci. Technol. 2021, 139, 110571. [Google Scholar] [CrossRef]
- Pu, C.; Tang, W.; Li, X.; Li, M.; Sun, Q. Stability enhancement efficiency of surface decoration on curcumin-loaded liposomes: Comparison of guar gum and its cationic counterpart. Food Hydrocoll. 2019, 87, 29–37. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, W.; Zou, L.; Liu, W.; Liu, C.; Liang, R.; Chen, J. Storage stability and skin permeation of vitamin C liposomes improved by pectin coating. Colloids Surf. B 2014, 117, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Li, X.; Yuan, F. The stabilization and release performances of curcumin-loaded liposomes coated by high and low molecular weight chitosan. Food Hydrocoll. 2020, 99, 105355. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Han, F.; Han, J. Advances and challenges in liposome digestion: Surface interaction, biological fate, and GIT modeling. Adv. Colloid Interface Sci. 2019, 263, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, M.; Knez, Z.; Primozic, M. Sustainable technologies for liposome preparation. J. Supercrit. Fluid 2020, 165, 104984. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar]
- Mortazavi, S.M.; Mohammadabadi, M.R.; Khosravi-Darani, K.; Mozafari, M.R. Preparation of liposomal gene therapy vectors by a scalable method without using volatile solvents or detergents. J. Biotechnol. 2007, 129, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Peschka, R.; Purmann, T.; Schubert, R. Cross-flow filtration—An improved detergent removal technique for the preparation of liposomes. Int. J. Pharm. 1998, 162, 177–183. [Google Scholar] [CrossRef]
- Shan, H.; Lin, Q.; Wang, D.; Sun, X.; Quan, B.; Chen, X.; Chen, Z. 3D Printed Integrated Multi-Layer Microfluidic Chips for Ultra-High Volumetric Throughput Nanoliposome Preparation. Front. Bioeng. Biotechnol. 2021, 9, 773705. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Ma, C.; Li, C.; Lin, L. Effect of nianoliposome-encapsulated thyme oil on growth of Salmonella enteritidis in chicken. J. Food Process. Preserv. 2017, 41, e13299. [Google Scholar] [CrossRef]
- Wu, J.; Guan, R.; Cao, G.; Liu, Z.; Wang, Z.; Shen, H.; Xia, Q. Antioxidant and Antimicrobial Effects of Catechin Liposomes on Chinese Dried Pork. J. Food Prot. 2018, 81, 827–834. [Google Scholar] [CrossRef]
- Pothakamury, U.R.; Barbosa-Cánovas, G.V. Fundamental aspects of controlled release in foods. Trends Food Sci. Technol. 1995, 6, 397–406. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. Chapter Two-Controlled release of nanoencapsulated food ingredients. In Release and Bioavailability of Nanoencapsulated Food Ingredients; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 5, pp. 27–78. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Sun, Y.; Cui, H. Characterization of chrysanthemum essential oil triple-layer liposomes and its application against Campylobacter jejuni on chicken. LWT-Food Sci. Technol. 2019, 107, 16–24. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bai, M.; Chen, X.; Hu, W.; Cui, H.; Lin, L. Controlled release and antibacterial activity of nanofibers loaded with basil essential oil-encapsulated cationic liposomes against Listeria monocytogenes. Food Biosci. 2022, 46, 101578. [Google Scholar] [CrossRef]
- Lin, L.; Dai, Y.; Cui, H. Antibacterial poly (ethylene oxide) electrospun nanofibers containing cinnamon essential oil/beta-cyclodextrin proteoliposomes. Carbohydr. Polym. 2017, 178, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, Y.; Chen, J.; Lai, J.; Sun, J.; Hu, F.; Wu, W. Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. Int. J. Pharm. 2009, 376, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, Y.; Zhang, Y.; Dang, B.; Liu, Y.; Feng, N. Enhanced oral bioavailability of silymarin using liposomes containing a bile salt: Preparation by supercritical fluid technology and evaluation in vitro and in vivo. Int. J. Nanomed. 2015, 10, 6633–6644. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ye, A.; Liu, C.; Liu, W.; Singh, H. Structure and integrity of liposomes prepared from milk- or soybean-derived phospholipids during in vitro digestion. Food Res. Int. 2012, 48, 499–506. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Navas-Carretero, S.; Perez-Granados, A.M.; Sarria, B.; Schoppen, S.; Vaquero, M.P. Iron Bioavailability from pate enriched with encapsulated ferric pyrophosphate or ferrous gluconate in rats. Food Sci. Technol. Int. 2007, 13, 159–163. [Google Scholar] [CrossRef]
- Navas-Carretero, S.; Perez-Granados, A.M.; Sarria, B.; Vaquero, M.P. Iron absorption from meat pate fortified with ferric pyrophosphate in iron-deficient women. Nutrition 2009, 25, 20–24. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Hongmei, L.; Xin, L.; Yanwei, M.; Yimin, Z.; Lixian, Z.; Xiaoyin, Y.; Rongrong, L.; Wenxuan, L.; Hui, C. A review of the functions and application of natural antioxidants in meat and meat products. Food Sci. China 2020, 41, 267–277. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Hernández Blanco, I.; Hoffmann, T.; Martin, H.D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M. Quantitative assessment of antioxidant properties of natural colorants and phytochemicals: Carotenoids, flavonoids, phenols and indigoids. The role of β-carotene in antioxidant functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. The inhibitory effect of plant essential oils on foodborne pathogenic bacteria in food. Crit. Rev. Food Sci. Nutr. 2019, 59, 3281–3292. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Seo, Y.-H.; Oh, S.-W. Antibacterial activities of polyphenols against foodborne pathogens and their application as antibacterial agents. Food Sci. Biotechnol. 2022, 31, 985–997. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Y.; Zhang, Z.; Wang, X.; Niu, Y.; Zhang, S.; Xu, W.; Ren, C. Advances of peptides for antibacterial applications. Colloids Surf. B 2021, 202, 111682. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as Vehicles for Astaxanthin: Characterization, In vitro Release Evaluation and Structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, L.; Li, Y.; Tao, G.; Zhou, C.; Lin, L. Preparation of nutmeg oil encapsulated in liposome and its application in meat products. China Food Addit. 2016, 10, 107–111. [Google Scholar]
- Lin, L.; Zhang, X.; Zhao, C.; Cui, H. Liposome containing nutmeg oil as the targeted preservative against Listeria monocytogenes in dumplings. RSC Adv. 2016, 6, 978–986. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Plasma enhanced-nutmeg essential oil solid liposome treatment on the gelling and storage properties of pork meat batters. J. Food Eng. 2020, 266, 109696. [Google Scholar] [CrossRef]
- Torab, M.; Basti, A.A.; Khanjari, A. Effect of free and nanoencapsulated forms of Zataria multiflora Boiss. essential oil on some microbial and chemical properties of beef burger. Carpathian J. Food Sci. Technol. 2017, 9, 93–102. [Google Scholar]
- Lin, L.; Zhu, Y.; Cui, H. Inactivation of Escherichia coli O157:H7 treated by poly-L-lysine-coated bacteriophages liposomes in pork. J. Food Saf. 2018, 38, e12535. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef]
- Tometri, S.S.; Ahmady, M.; Ariaii, P.; Soltani, M.S. Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. J. Food Meas. Charact. 2020, 14, 3333–3344. [Google Scholar] [CrossRef]
- Khatib, N.; Varidi, M.J.; Mohebbi, M.; Varidi, M.; Hosseini, S.M.H. Co-encapsulation of lupulon and xanthohumol in lecithin-based nanoliposomes developed by sonication method. J. Food Process. Preserv. 2019, 43, e14075. [Google Scholar] [CrossRef]
- Khatib, N.; Varidi, M.J.; Mohebbi, M.; Varidi, M.; Hosseini, S.M.H. Replacement of nitrite with lupulon-xanthohumol loaded nanoliposome in cooked beef-sausage: Experimental and model based study. J. Food Sci Technol. 2020, 57, 2629–2639. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, H.; Guan, R.; Wang, Y.; Fang, J.; Zhang, Y. The effect of catechin nanoliposomes on sauce duck preservation. J. Chin. Inst. Food Sci. Technol. 2013, 13, 109–114. [Google Scholar] [CrossRef]
- Wu, J.; Guan, R.; Huang, H.; Liu, Z.; Shen, H.; Xia, Q. Effect of catechin liposomes on the nitrosamines and quality of traditional Chinese bacon. Food Funct. 2019, 10, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Yekta, M.M.; Rezaei, M.; Nouri, L.; Azizi, M.H.; Jabbari, M.; Es, I.; Khaneghah, A.M. Antimicrobial and antioxidant properties of burgers with quinoa peptide-loaded nanoliposomes. J. Food Saf. 2020, 40, e12753. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Xiao, Z.; Lin, L.; Li, C. Antibacterial Properties of Nutmeg Oil in Pork and Its Possible Mechanism. J. Food Saf. 2015, 35, 370–377. [Google Scholar] [CrossRef]
- Nikolic, V.; Nikolic, L.; Dinic, A.; Gajic, I.; Urosevic, M.; Stanojevic, L.; Stanojevic, J.; Danilovic, B. Chemical Composition, Antioxidant and Antimicrobial Activity of Nutmeg (Myristica fragrans Houtt.) Seed Essential Oil. J. Essent. Oil Bear. Plants 2021, 24, 218–227. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M.; Pettinari, M.J. Liposome-Encapsulated Bacteriophages for Enhanced Oral Phage Therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yanagida, A.; Komeya, S.; Kawana, M.; Honma, D.; Tagashira, M.; Kanda, T.; Shibusawa, Y. Comprehensive Separation and Structural Analyses of Polyphenols and Related Compounds from Bracts of Hops (Humulus lupulus L.). J. Agric. Food Chem. 2014, 62, 2198–2206. [Google Scholar] [CrossRef]
- Folmer Correa, A.P.; Bertolini, D.; Lopes, N.A.; Veras, F.F.; Gregory, G.; Brandelli, A. Characterization of nanoliposomes containing bioactive peptides obtained from sheep whey hydrolysates. LWT-Food Sci. Technol. 2019, 101, 107–112. [Google Scholar] [CrossRef]
- Taylor, T.M.; Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Characterization of Antimicrobial-bearing Liposomes by ζ-Potential, Vesicle Size, and Encapsulation Efficiency. Food Biophys. 2007, 2, 1–9. [Google Scholar] [CrossRef]
- da Silva Malheiros, P.; Daroit, D.J.; Brandelli, A. Food applications of liposome-encapsulated antimicrobial peptides. Trends Food Sci. Technol. 2010, 21, 284–292. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021, 48, 107727. [Google Scholar] [CrossRef] [PubMed]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Kamkar, A.; Molaee-aghaee, E.; Khanjari, A.; Akhondzadeh-basti, A.; Noudoost, B.; Shariatifar, N.; Sani, M.A.; Soleimani, M. Nanocomposite active packaging based on chitosan biopolymer loaded with nano-liposomal essential oil: Its characterizations and effects on microbial, and chemical properties of refrigerated chicken breast fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Cheraghi, N.; Khanjari, A.; Rezaeigolestani, M.; Basti, A.A.; Kamkar, A.; Aghaee, E.M. Incorporation of nanoencapsulated garlic essential oil into edible films: A novel approach for extending shelf life of vacuum-packed sausages. Meat Sci. 2020, 166, 108135. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, L.; Li, W.; Lin, L. Antioxidant property of SiO2-eugenol liposome loaded nanofibrous membranes on beef. Food Packag. Shelf Life 2017, 11, 49–57. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT-Food Sci. Technol. 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Amjadi, S.; Nazari, M.; Alizadeh, S.A.; Hamishehkar, H. Multifunctional betanin nanoliposomes-incorporated gelatin/chitosan nanofiber/ZnO nanoparticles nanocomposite film for fresh beef preservation. Meat Sci. 2020, 167, 108161. [Google Scholar] [CrossRef]
- Esposto, B.S.; Jauregi, P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. Liposomes vs. chitosomes: Encapsulating food bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Mechanical and barrier properties of chitosan combined with other components as food packaging film. Environ. Chem. Lett. 2020, 18, 257–267. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, P.; Zou, Y.-X.; Luo, Z.-G.; Tamer, T.M. Co-encapsulation of Vitamin C and β-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.-J.; Lee, J.-G.; Lee, S.-Y.; Kim, S.; Choi, M.-J.; Cho, Y. Changes in Quality Characteristics of Pork Patties Containing Antioxidative Fish Skin Peptide or Fish Skin Peptide-loaded Nanoliposomes during Refrigerated Storage. Korean J. Food Sci. Anim. 2017, 37, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Natale, D.; Gibis, M.; Rodriguez-Estrada, M.T.; Weiss, J. Inhibitory Effect of Liposomal Solutions of Grape Seed Extract on the Formation of Heterocyclic Aromatic Amines. J. Agri. Food Chem. 2014, 62, 279–287. [Google Scholar] [CrossRef] [PubMed]




| Method | Advantages | Disadvantages | Type of Vesicles | |
|---|---|---|---|---|
| Conventional method | Thin film hydration | Simple process. | Low EE; organic solvent residue; small-scale production. | MLVs, GUVs |
| Reverse phase evaporation | Simple process; suitable EE. | Organic solvent residue; time-consuming. | MLVs, LUVs | |
| Solvent Injection | Simple, rapid, and reproducible process. | Organic solvent residue; time-consuming; possible nozzle blockage (ether system). | SMVs, SUVs | |
| Detergent removal | Good particle size control; simple process. | Organic solvent and detergent residue; time-consuming; poor EE. | MLVs, LUVs | |
| Emulsion method | Simple process. | Low yield; organic solvent residue. | MVVs | |
| Heating method | Simple and fast process; no organic solvent; no sterilization is needed. | Degradation of bioactive compounds. | MLVs, SUVs | |
| Advanced method | Cross-flow filtration | Rapid, scalable, sterile process; homogeneous size with high stability; easy removal of detergent. | Understudy method | SUVs, LUVs |
| Modified ethanol injection | Simple, rapid, scalable, and continuous process; homogenous liposomes. | Organic solvent residue; high-cost material. | SUVs, LUVs | |
| Dual asymmetric centrifugation | Simple, rapid, and reproducible process; homogeneous and small liposomes; high EE for hydrophilic compounds. | Only laboratory-scale; high pressure with agitation. | SUVs, LUVs | |
| Microfluidic method | Good particle size control; scalable process and used for biological samples | Organic solvent residue; high cost and complex equipment. | SUVs, LUVs, GUVs | |
| Supercritical fluids | Control of particle size, possible in situ sterilization, low organic solvent consumption | High cost, high pressure, usage of sophisticated instruments. | LUVs | |
| Encapsulated Compounds | Meat/Meat Products | Effects | References | ||
|---|---|---|---|---|---|
| Antimicrobial | Antioxidant | ||||
| Essential oils | Nutmeg essential oil | Pork, chicken, beef, mutton | Inhibit the growth of microorganisms (E. coli and L. monocytogenes) Have long-term acting antibacterial effect | / | [63] |
| Dumplings | Improve the antibacterial effect on L. monocytogenes in dumplings. Extend the treatment time. | / | [64] | ||
| Pork meat batters | Inhibit the growth of microorganisms (total viable counts) | Inhibit oxidation and decomposition of lipid and proteins (TBA, TVB-N, and carbonyl content) | [65] | ||
| Thyme essential oil | Chicken | Improve the antibacterial effect on S. enteritidis Extend the treatment time. | / | [37] | |
| Zataria multiflora Boiss. essential oil | Beef burger | Inhibit the growth of microorganisms (total mesophilic and psychrotrophic bacteria, molds/yeast) | Inhibit oxidation and decomposition of lipid and proteins (peroxide, TVB-N) | [66] | |
| Bacteriophages | Bacteriophage | Pork | Improve the antibacterial activity against E. coli O157:H7 in pork | / | [67] |
| Beef | Inhibit E. coli O157:H7 growth in beef | / | [68] | ||
| Bioactive compounds | Laurus nobilis leaf extract | Minced beef | Inhibit the growth of microorganisms (total viable counts and psychrotrophic count, E. coli and S. aureus) | Inhibit oxidation and decomposition of lipid and proteins (peroxide and TBA value, free fatty acid value, TVB-N) | [69] |
| Lupulon–xanthohumol | Cooked beef sausage | Inhibit the growth of microorganisms (total viable counts, Clostridium perfringens, coliforms, and molds/yeast) (nitrite + nanoliposome combination presented the best results). | Addition of liposome + nitrite successfully prevented lipid oxidation (TBARS) | [70,71] | |
| Catechin | Chinese dried pork | Inhibit the growth of microorganisms (total viable counts) | Inhibit lipid oxidation (peroxide, TBARS, pH value) | [38] | |
| Sauce duck | Inhibit the growth of microorganisms (total viable counts) | Inhibit oxidation and decomposition of proteins (TVB-N, pH value) | [72] | ||
| Traditional Chinese bacon | / | Reduce the nitrosamines contents in fried bacon | [73] | ||
| Peptides | Quinoa peptide | Burger | Inhibit the growth of microorganisms (total viable counts, S. aureus, and molds/yeast) | Inhibit oxidation and decomposition of lipid and proteins (peroxide, TBARS value, TVB-N) | [74] |
| Biopolymer | Loaded Compounds | Meat/Meat Products | Effects | References |
|---|---|---|---|---|
| Chitosan coating | Laurel essential oils + nanosilver | Pork | Protected the quality of pork at 4 °C for 15 days | [36] |
| Satureja plant essential oil | Lamb meat | Effectively retarded microbial growth and chemical spoilage | [83] | |
| Chitosan and pectin coating | Chrysanthemum essential oil | Chicken | Showed high antibacterial activity against C. jejuni on chicken, while did not affect its quality | [41] |
| Chitosan films | Garlic essential oil | Chicken breast fillet | Showed significant synergistic effects in chemical and bacterial preservation of chicken fillet samples | [84] |
| Chitosan and whey protein films | Garlic essential oil | Sausage | Retarded lipid oxidation and the growth of main spoilage bacterial groups | [85] |
| PEO nanofibers | SiO2-eugenol | Beef | Higher antioxidant activity on beef | [86] |
| PEO/soybean lecithin-based nanofibers | Basil essential oil | Chilled pork | Help maintain the quality of chilled pork during 4-day storage | [43] |
| Chitosan/PEO nanofibers | Tea tree oil | Chicken | High antibacterial activity against Salmonella | [87] |
| Gelatin/chitosan nanofiber/ZnO nanoparticles nanocomposite film | Betanin | Beef | The growth of inoculated bacteria, lipid oxidation, and the changes in the pH and color quality of the beef samples were controlled by packaging with the fabricated film | [88] |
| CEO/β-CD proteoliposomes nanofibers | Cinnamon essential oil | Beef | The satisfactory antibacterial efficiency against B. cereus on beef was achieved without any impact on sensory quality of beef | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Teng, W.; Cao, J.; Wang, J. Liposomes as Delivery System for Applications in Meat Products. Foods 2022, 11, 3017. https://doi.org/10.3390/foods11193017
Huang L, Teng W, Cao J, Wang J. Liposomes as Delivery System for Applications in Meat Products. Foods. 2022; 11(19):3017. https://doi.org/10.3390/foods11193017
Chicago/Turabian StyleHuang, Li, Wendi Teng, Jinxuan Cao, and Jinpeng Wang. 2022. "Liposomes as Delivery System for Applications in Meat Products" Foods 11, no. 19: 3017. https://doi.org/10.3390/foods11193017
APA StyleHuang, L., Teng, W., Cao, J., & Wang, J. (2022). Liposomes as Delivery System for Applications in Meat Products. Foods, 11(19), 3017. https://doi.org/10.3390/foods11193017








