Microorganisms—An Effective Tool to Intensify the Utilization of Sulforaphane
Abstract
1. Introduction
2. Intestinal Microorganisms Can Enhance the Utilization of SFN
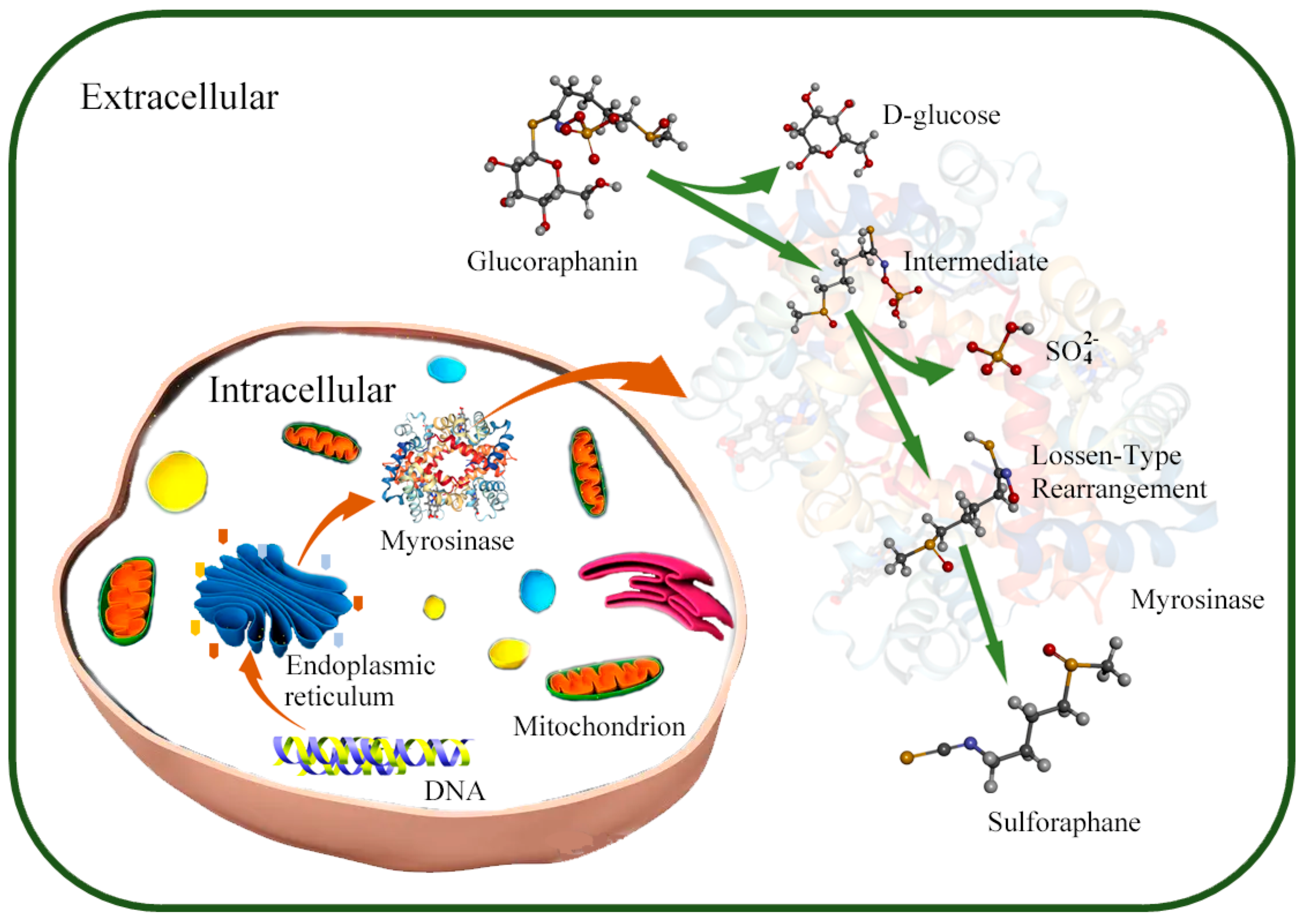
2.1. Structure and Biochemical Characteristics of SFN, Glucoraphanin, and Myrosinase
2.2. Increasing the Intestinal SFN Production Rate Is a Scientific Approach to Enhance SFN Utilization
2.3. Microorganisms Converted Glucoraphanin into SFN Using Myrosinase Synthesis
3. Improving the Myrosinase-Synthesizing Abilities of Microorganisms Is a Critical Approach to Increase SFN Production
3.1. Culture Conditions Influence the Efficiency of Myrosinase-Synthesizing Microorganisms
3.2. Modified Microorganisms with Higher Myrosinase-Synthesizing Abilities Can Promote SFN Production
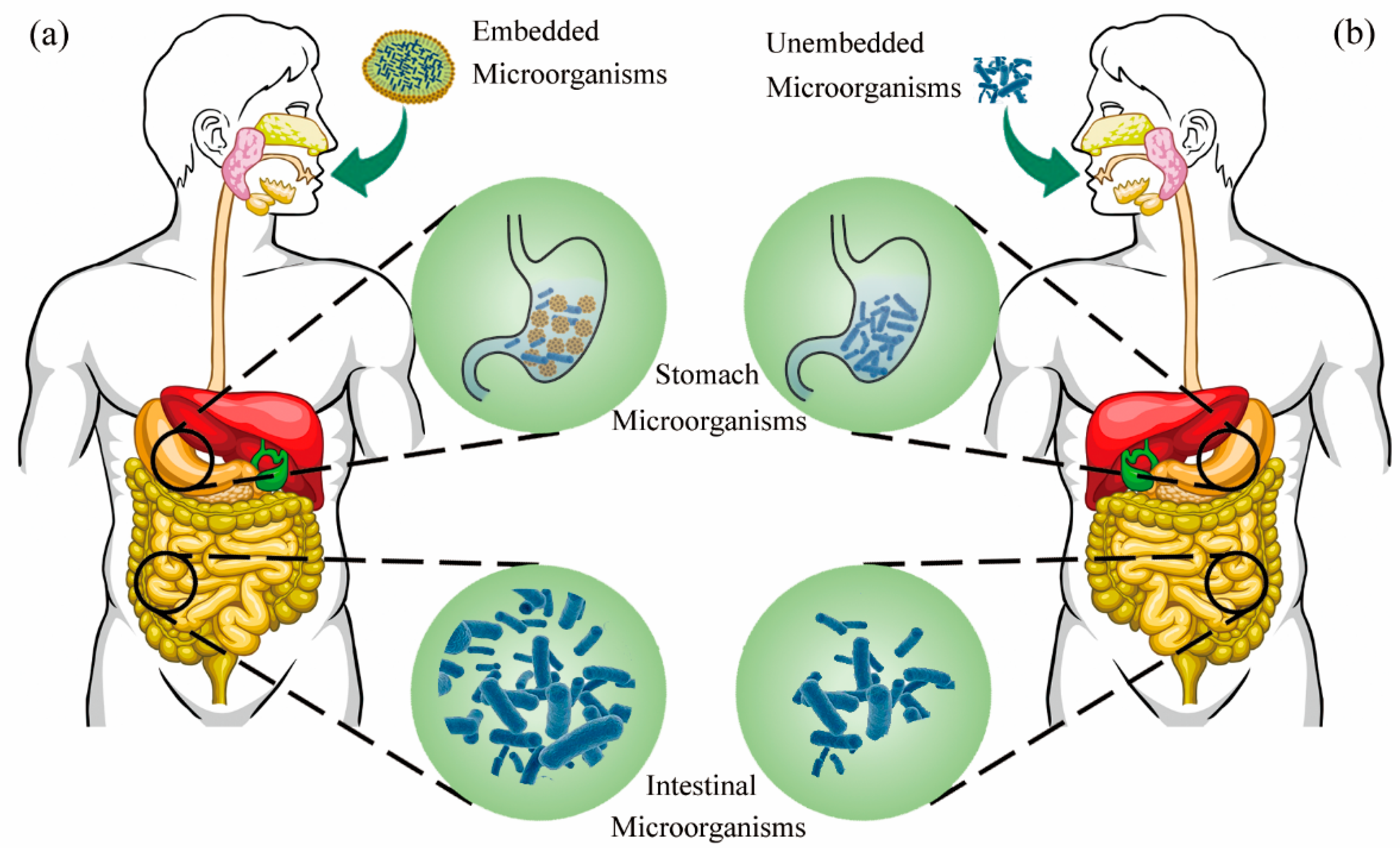
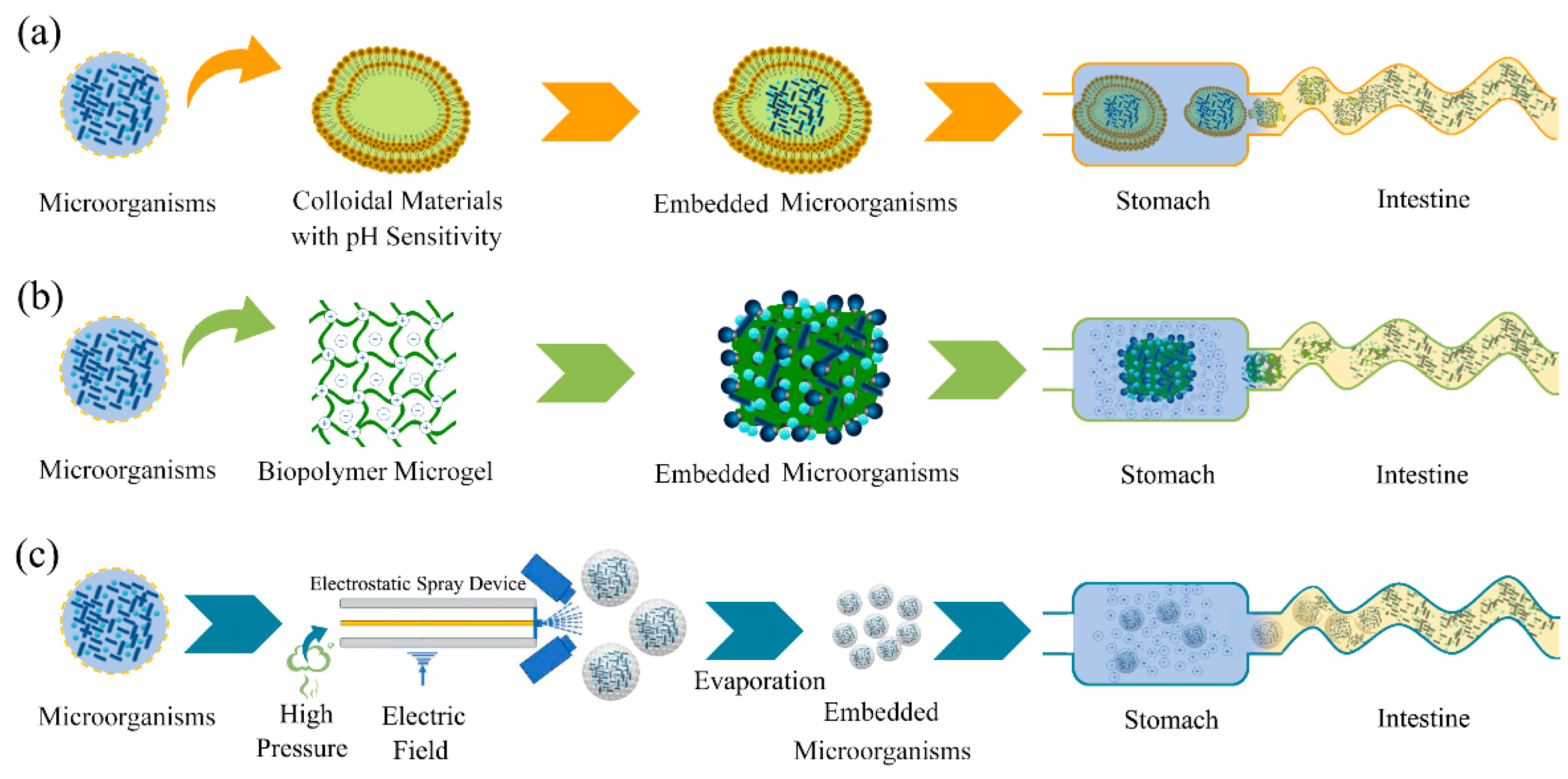
4. A Microencapsulated Delivery System Can Protect Microorganisms into Intestines
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 1029. [Google Scholar] [CrossRef]
- Abukhabta, S.; Ghawi, S.K.; Karatzas, K.A.; Charalampopoulos, D.; McDougall, G.J.; Allwood, J.W.; Verrall, S.R.; Lavery, S.; Latimer, C.; Pourshahidi, L.K.; et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur. J. Nutr. 2021, 60, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Hafezian, S.M.; Azizi, S.N.; Biparva, P.; Bekhradni, A. High-efficiency purification of sulforaphane from the broccoli extract by nanostructured SBA-15 silica using solid-phase extraction method. J. Chromatogr. B 2019, 1108, 1–10. [Google Scholar] [CrossRef]
- Janczewski, L. Sulforaphane and Its Bifunctional Analogs: Synthesis and Biological Activity. Molecules 2022, 27, 125. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 2019, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Wu, Q. Sulforaphane protects intestinal epithelial cells against lipopolysaccharide-induced injury by activating the AMPK/SIRT1/PGC-1alpha pathway. Bioengineered 2021, 12, 4349–4360. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Wozniak, K.; Daglia, M.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 5618. [Google Scholar] [CrossRef]
- Daily, J.W.; Byoung-Seob, K.; Jina, R.; Meiling, L.; Weijun, Z.; Park, S. Isothiocyanate from broccoli, sulforaphane, and its properties. J. Med. Food 2019, 22, 121–126. [Google Scholar]
- Kim, J. Pre-Clinical Neuroprotective Evidences and Plausible Mechanisms of Sulforaphane in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 201. [Google Scholar] [CrossRef]
- Ho-Sub, P.; Eun-Sang, H.; Ga-Young, C.; Hyun-Bum, K.; Kyun-Seob, P.; Jai-Yoon, S.; Yoonjin, H.; Geun Wook, C.; Byung Il, K.; Hyunwoo, P.; et al. Sulforaphane enhances long-term potentiation and ameliorate scopolamine-induced memory impairment. Physiol. Behav. 2021, 238, 113467. [Google Scholar]
- Bobermin, L.D.; Weber, F.B.; dos Santos, T.M.; Bello-Klein, A.; Wyse, A.T.S.; Goncalves, C.-A.; Quincozes-Santos, A. Sulforaphane Induces Glioprotection After LPS Challenge. Cell Mol. Neurobiol. 2022, 42, 829–846. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Bullen, C.K.; Villabona-Rueda, A.F.; Thompson, E.A.; Turner, M.L.; Merino, V.F.; Yan, Y.; Kim, J.; Davis, S.L.; Komm, O.; et al. Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice. Commun. Biol. 2022, 5, 1666. [Google Scholar] [CrossRef]
- Sangkret, S.; Pongmalai, P.; Devahastin, S.; Chiewchan, N. Enhanced production of sulforaphane by exogenous glucoraphanin hydrolysis catalyzed by myrosinase extracted from Chinese flowering cabbage (Brassica rapa var. parachinensis). Sci. Rep. 2019, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ying, D.Y.; Cheng, L.J.; Bayrak, M.; Jegasothy, H.; Sanguansri, L.; Augustin, M.A. Sulforaphane in broccoli-based matrices: Effects of heat treatment and addition of oil. LWT Food Sci. Technol. 2020, 128, 1464. [Google Scholar] [CrossRef]
- Li, C.; Song, S.; He, Y.; Zhang, X.; Liu, H. CaCl2-HCl electrolyzed water affects glucosinolate metabolism and improves the quality of broccoli sprouts. Food Res. Int. 2021, 150, 1901. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Xu, L.; Dong, J.; Zhu, X.; Ying, J.; Wang, Q.; Fan, L.; Li, C.; Liu, L. Methyl jasmonate, salicylic acid and abscisic acid enhance the accumulation of glucosinolates and sulforaphane in radish (Raphanus sativus L.) taproot. Sci. Hortic. 2019, 250, 159–167. [Google Scholar] [CrossRef]
- Triska, J.; Balik, J.; Houska, M.; Novotna, P.; Magner, M.; Vrchotova, N.; Hic, P.; Jilek, L.; Thorova, K.; Snurkovic, P.; et al. Factors Influencing Sulforaphane Content in Broccoli Sprouts and Subsequent Sulforaphane Extraction. Foods 2021, 10, 745. [Google Scholar] [CrossRef]
- Goharrizi, K.J.; Fatehi, F.; Nazari, M.; Salehi, F.; Maleki, M. Assessment of changes in the content of sulforaphane and expression levels of CYP79F1 and myrosinase genes and proteomic profile of Lepidium draba plant under water-deficit stress induced by polyethylene glycol. Acta Physiol. Plant 2020, 42, 991. [Google Scholar]
- Oloyede, O.O.; Wagstaff, C.; Methven, L. Influence of Cabbage (Brassica oleracea) Accession and Growing Conditions on Myrosinase Activity, Glucosinolates and Their Hydrolysis Products. Foods 2021, 10, 717. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Sun, X.; Ma, H.; Chen, X.; Ren, J.; Hu, K. New Method for the Synthesis of Sulforaphane and Related Isothiocyanates. Synthesis 2011, 1, 3991–3996. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1789. [Google Scholar] [CrossRef] [PubMed]
- Ren-Hau, L.; Miller, M.J.; Jeffery, E. Glucoraphanin hydrolysis by microbiota in the rat cecum results in sulforaphane absorption. Food Funct. 2010, 1, 161–166. [Google Scholar]
- Li, F.; Hullar, M.A.J.; Beresford, S.A.A.; Lampe, J.W. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br. J. Nutr. 2011, 106, 408–416. [Google Scholar] [CrossRef]
- Angelino, D.; Jeffery, E. Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: Focus on glucoraphanin. J. Funct. Foods 2014, 7, 67–76. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Li, H.; Liu, X. The potential of proteins, hydrolysates and peptides as growth factors for Lactobacillus and Bifidobacterium: Current research and future perspectives. Food Funct. 2020, 11, 1946–1957. [Google Scholar] [CrossRef]
- Xinzhou, W.; Peng, Z.; Xin, Z. Probiotics regulate gut microbiota: An effective method to improve immunity. Molecules 2021, 26, 6076. [Google Scholar]
- Monika, Y.; Mandeep; Pratyoosh, S. Probiotics of diverse origin and their therapeutic applications: A review. J. Am. Coll. Nutr. 2020, 39, 469–479. [Google Scholar]
- Gang, T.; Linyu, Z. Update on strategies of probiotics for the prevention and treatment of colorectal cancer. Nutr. Cancer 2022, 74, 27–38. [Google Scholar]
- How, Y.; Pui, L. Effect of prebiotics encapsulated with probiotics on encapsulation efficiency, microbead size, and survivability: A review. J. Food. Meas. Charac. 2021, 15, 4899–4916. [Google Scholar] [CrossRef]
- Monika, Y.; Pratyoosh, S. Efficient engineered probiotics using synthetic biology approaches: A review. Biotechnol. Appl. Biochem. 2020, 67, 22–29. [Google Scholar]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Yan Xue, C.; Ji Hui, W.; McAuley, C.; Augustin, M.A.; Terefe, N.S. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods 2019, 61, 103461. [Google Scholar]
- Jian-Hui, Y.; Long-Yue, H.; Terefe, N.S.; Augustin, M.A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 2019, 286, 616–623. [Google Scholar]
- Boehm, J.; Davis, R.; Murar, C.E.; Li, T.; McCleland, B.; Dong, S.; Yan, H.; Kerns, J.; Moody, C.J.; Wilson, A.J.; et al. Discovery of a crystalline sulforaphane analog with good solid-state stability and engagement of the Nrf2 pathway in vitro and in vivo. Bioorg. Med. Chem. 2019, 27, 579–588. [Google Scholar] [CrossRef]
- Zambrano, V.; Bustos, R.; Mahn, A. Insights about stabilization of sulforaphane through microencapsulation. Heliyon 2019, 5, 2899. [Google Scholar] [CrossRef]
- Jeon, M.; Lee, J.; Lee, H.K.; Cho, S.; Lim, J.H.; Choi, Y.; Pak, S.; Jeong, H.J. Sulforaphane mitigates mast cell-mediated allergic inflammatory reactions in in silico simulation and in vitro models. Immunopharmacol. Immunotoxicol. 2020, 42, 74–83. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, L.; Cao, L.; Xie, C.; Li, X.; Li, Y.; Meng, Y.; Chen, Y.; Wang, X.; Chen, J.; et al. Sulforaphane inhibits gastric cancer stem cells via suppressing sonic hedgehog pathway. Int. J. Food Sci. Nutr. 2019, 70, 570–578. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Redeker, K.; Kittipol, V.; Bancroft, I.; Hartley, S.E. Development of an efficient glucosinolate extraction method. Plant Methods 2017, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Favela-Gonzalez, K.M.; Hernandez-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 3, e13414. [Google Scholar] [CrossRef]
- Yang, H.; Qin, J.; Wang, X.; Ei-Shora, H.M.; Yu, B. Production of plant-derived anticancer precursor glucoraphanin in chromosomally engineered Escherichia coli. Microbiol. Res. 2020, 238, 126484. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.; Lognay, G.; Wathelet, J.P.; Haubruge, E. Characterisation of aphid myrosinase and degradation studies of glucosinolates. Arch. Insect Biochem. Physiol. 2002, 50, 173–182. [Google Scholar] [CrossRef]
- Roman, J.; Castillo, A.; Cottet, L.; Mahn, A. Kinetic and structural study of broccoli myrosinase and its interaction with different glucosinolates. Food Chem. 2018, 254, 87–94. [Google Scholar] [CrossRef]
- Lei, W.; Rose, D.; Pingfan, R.; Yue, Z. Development of prolamin-based composite nanoparticles for controlled release of sulforaphane. J. Agric. Food Chem. 2020, 68, 13083–13092. [Google Scholar]
- Wei, L.; Liu, C.; Zheng, H.; Zheng, L. Melatonin treatment affects the glucoraphanin-sulforaphane system in postharvest fresh-cut broccoli (Brassica oleracea L.). Food Chem. 2020, 307, 299. [Google Scholar] [CrossRef]
- Wu, Y.; Lv, C.; Zou, L.; Sun, J.; Song, X.; Zhang, Y.; Mao, J. Approaches for enhancing the stability and formation of sulforaphane. Food Chem. 2021, 345, 128771. [Google Scholar]
- Xinxing, X.; Mei, D.; Fei, L.; Fang, C.; Xiaosong, H.; Yuping, L.; Jihong, W. Effect of glucoraphanin from broccoli seeds on lipid levels and gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2020, 68, 103858. [Google Scholar]
- Budnowski, J.; Hanske, L.; Schumacher, F.; Glatt, H.; Platz, S.; Rohn, S.; Blaut, M. Glucosinolates Are Mainly Absorbed Intact in Germfree and Human Microbiota-Associated Mice. J. Agric. Food Chem. 2015, 63, 8418–8428. [Google Scholar] [CrossRef]
- Sun, J.; Charron, C.S.; Novotny, J.A.; Peng, B.; Yu, L.; Chen, P. Profiling glucosinolate metabolites in human urine and plasma after broccoli consumption using non-targeted and targeted metabolomic analyses. Food Chem. 2020, 4, 309. [Google Scholar] [CrossRef]
- Martijn Vermeulen, I.W.M.; Armstrong, K.P.-K.; Van Den Berg, R.; Vaes, W.H.J. Bioavailability and Kinetics of Sulforaphane in Humans after Consumption of Cooked versus Raw Broccoli. J Agric Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Chen, J.G.; Wang, J.B.; Wu, Y.; Sun, Y.; Lu, J.H.; Zhu, J.; Zhang, Y.H.; Chen, Y.S.; Friesen, M.D.; et al. Bioavailability of Sulforaphane from two broccoli sprout beverages: Results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev. Res. 2011, 4, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, T.A.; Jhones, W.F.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective Glucosinolates and Isothiocyanates of Broccoli Sprouts: Metabolism and Excretion in Humans1. Cancer Epidemiol. Biomark. Prev. 2001, 10, 501–508. [Google Scholar]
- Kuljarachanan, T.; Fu, N.; Chiewchan, N.; Devahastin, S.; Chen, X.D. In vitro digestion using dynamic rat stomach-duodenum model as an alternative means to assess bioaccessibility of glucosinolates in dietary fiber powder from cabbage. LWT Food Sci. Technol. 2021, 2, 151. [Google Scholar] [CrossRef]
- Yuanfeng, W.; Yuke, S.; Ye, Z.; Mupunga, J.; Ligen, Z.; Chao, L.; Shiwang, L.; Jianwei, M. Broccoli ingestion increases the glucosinolate hydrolysis activity of microbiota in the mouse gut. Int. J. Food Sci. Nutr. 2019, 70, 585–594. [Google Scholar]
- Hwang, E.-S.; Bornhorst, G.M.; Oteiza, P.I.; Mitchell, A.E. Assessing the Fate and Bioavailability of Glucosinolates in Kale (Brassica oleracea) Using Simulated Human Digestion and Caco-2 Cell Uptake Models. J. Agric. Food Chem. 2019, 67, 9492–9500. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Fang, Y.; Li, M.; Mi, H.; Liu, S.; Yang, G.; Lu, J.; Zhao, Y.; Liu, Q.; Zhang, W.; et al. Characterization of a Novel Myrosinase with High Activity from Marine Bacterium Shewanella baltica Myr-37. Int. J. Mol. Sci. 2022, 5, 23. [Google Scholar] [CrossRef]
- Mao, S.; Wang, J.; Wu, Q.; Liang, M.; Yuan, Y.; Wu, T.; Liu, M.; Wu, Q.; Huang, K. Effect of selenium-sulfur interaction on the anabolism of sulforaphane in broccoli. Phytochemistry 2020, 179, 112499. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Ron, S.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E.H. Epithiospecifier Protein from Broccoli (Brassica oleracea L. ssp. italica) Inhibits Formation of the Anticancer Agent Sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [CrossRef]
- Sporer, T.; Koernig, J.; Wielsch, N.; Gebauer-Jung, S.; Reichelt, M.; Hupfer, Y.; Beran, F. Hijacking the Mustard-Oil Bomb: How a Glucosinolate-Sequestering Flea Beetle Copes With Plant Myrosinases. Front. Plant Sci. 2021, 12, 1009. [Google Scholar] [CrossRef]
- Feng, Q.; Li, L.; Liu, Y.; Shao, X.; Li, X. Jasmonate regulates the FAMA/mediator complex subunit 8-THIOGLUCOSIDE GLUCOHYDROLASE 1 cascade and myrosinase activity. Plant Physiol. 2021, 187, 963–980. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Ryde, U.; Irani, M. QM/MM Study of the Catalytic Reaction of Myrosinase; Importance of Assigning Proper Protonation States of Active-Site Residues. J. Chem. Theory Comput. 2021, 17, 1822–1841. [Google Scholar] [CrossRef] [PubMed]
- Sicong, T.; Xiaodong, L.; Peng, L.; Xiaohong, Z.; Yujuan, S. Microbiota: A mediator to transform glucosinolate precursors in cruciferous vegetables to the active isothiocyanates. J. Sci. Food Agric. 2018, 98, 1255–1260. [Google Scholar]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2556. [Google Scholar] [CrossRef]
- Fahey, J.W.; Kensler, T.W. The Challenges of Designing and Implementing Clinical Trials With Broccoli Sprouts horizontal ellipsis and Turning Evidence Into Public Health Action. Front. Nutr. 2021, 8, 7290. [Google Scholar] [CrossRef]
- Elfoul, L.; Rabot, S.; Khelifa, N.; Quinsac, A.; Duguay, A.; Rimbault, A. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol. Lett. 2001, 197, 99–103. [Google Scholar] [CrossRef]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl- glucosinolates by a selection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, S.; Sun, J.; Song, X.; Jiang, C.; Wu, Y. Isolation and Characterization of Glucosinolate-Hydrolysis Enterococcus gallinarum HG001 and Escherichia coli HG002 from C57BL/6 Mouse Microbiota. Indian J. Microbiol. 2022, 62, 273–279. [Google Scholar] [CrossRef]
- Mullaney, J.A.; Kelly, W.J.; McGhie, T.K.; Ansell, J.; Heyes, J.A. Lactic Acid Bacteria Convert Glucosinolates to Nitriles Efficiently Yet Differently from Enterobacteriaceae. J. Agric. Food Chem. 2013, 61, 3039–3046. [Google Scholar] [CrossRef]
- Yan Xue, C.; Augustin, M.A.; Jegasothy, H.; Ji Hui, W.; Terefe, N.S. Mild heat combined with lactic acid fermentation: A novel approach for enhancing sulforaphane yield in broccoli puree. Food Funct. 2020, 11, 779–786. [Google Scholar]
- Watanabe, H.; Usami, R.; Kishino, S.; Osada, K.; Aoki, Y.; Morisaka, H.; Takahashi, M.; Izumi, Y.; Bamba, T.; Aoki, W.; et al. Enzyme systems involved in glucosinolate metabolism in Companilactobacillus farciminis KB1089. Sci. Rep. 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Cebeci, F.; Mayer, M.J.; Rossiter, J.T.; Mithen, R.; Narbad, A. Molecular Cloning, Expression and Characterisation of a Bacterial Myrosinase from Citrobacter Wye1. Protein J. 2022, 41, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Zhu, W.; Zhang, C.; Yin, L.; Zhang, Y.; Liu, L.; Yuan, H. Identification of Two Myrosinases from a Leclercia adecarboxylata Strain and Investigation of Its Tolerance Mechanism to Glucosinolate Hydrolysate. J. Agric. Food Chem. 2021, 69, 14151–14164. [Google Scholar] [CrossRef]
- Rakariyatham, N.; Butrindr, B.; Niamsup, H.; Shank, L. Screening of filamentous fungi for production of myrosinase. Braz. J. Microbiol. 2005, 36, 242–245. [Google Scholar] [CrossRef]
- Xu, X.; Bi, S.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Induced changes in bioactive compounds of broccoli juices after fermented by animal- and plant-derived Pediococcus pentosaceus. Food Chem. 2021, 357, 144. [Google Scholar] [CrossRef]
- Rakariyatham, N.; Sakorn, P. Biodegradation of glucosinolates in brown mustard seed meal (Brassica juncea) by Aspergillus sp. NR-4201 in liquid and solid-state cultures. Biodegradation 2002, 13, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Llanos Palop, M.; Smiths, J.P.; Brink, B.T. Degradation of sinigrin by Lactobacillus agilis strain R16. Int. J. Food Microbiol. 1995, 26, 219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Chen, H.; Lei, N.; Zhang, M.; Wan, H.; Shu, G. Effect of Prebiotics, Inorganic Salts and Amino Acids for Cell Envelope Proteinase Production from Lactobacillus Plantarum Lp69. Acta Sci. Pol. Technol. Aliment. 2019, 18, 269–278. [Google Scholar] [PubMed]
- Min, Z.; Jia, Z.; Jun, X.; Dong, Z.; Zong-Wei, Q.; Dan, H.; Hui-Bo, L.; Hui-Bo, L. Effects of environmental factors on the microbial community changes during medium-high temperature Daqu manufacturing. Food Res. Int. 2022, 153, 110955. [Google Scholar]
- Rama, G.R.; Kuhn, D.; Beux, S.; Maciel, M.J.; Volken de Souza, C.F. Potential applications of dairy whey for the production of lactic acid bacteria cultures. Int. Dairy J. 2019, 98, 25–37. [Google Scholar] [CrossRef]
- Dahiya, D.; Manuel, J.V.; Nigam, P.S. An Overview of Bioprocesses Employing Specifically Selected Microbial Catalysts for gamma-Aminobutyric Acid Production. Microorganisms 2021, 9, 6628. [Google Scholar] [CrossRef]
- Nabot, M.; Guerin, M.; Sivakumar, D.; Remize, F.; Garcia, C. Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing. Biology 2022, 11, 9762. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, W.A. Bacterium Hafnia alvei secretes L-methioninase enzyme: Optimization of the enzyme secretion conditions. Saudi J. Biol. Sci. 2020, 27, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Shah, N.P. Lactic acid produced by Streptococcus thermophilus activated glutamate decarboxylase (GadA) in Lactobacillus brevis NPS-QW 145 to improve gamma-amino butyric acid production during soymilk fermentation. LWT Food Sci. Technol. 2021, 137, 652. [Google Scholar] [CrossRef]
- Raj, T.S.; Athimoolam, S.; Vijayaraghavan, P. Biosynthesis and Characterization of a Novel Fibrinolytic Alkaline Serine Protease from Newly Isolated Bacillus flexus BF12 for Biomedical Applications. Curr. Pharm. Biotechnol. 2021, 22, 698–709. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Production of the Polysaccharide Curdlan by Agrobacterium species on Processing Coproducts and Plant Lignocellulosic Hydrolysates. Fermentation 2020, 6, 52. [Google Scholar] [CrossRef]
- Jing, Y.; Li, F.; Li, Y.; Jin, P.; Zhu, S.; He, C.; Zhao, J.; Zhang, Z.; Zhang, Q. Statistical optimization of simultaneous saccharification fermentative hydrogen production from corn stover. Bioengineered 2020, 11, 428–438. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Liu, Z.; Pu, Y.; Xia, L. Optimization of simultaneous saccharification and fermentation for ethanol production from steam-exploded cotton stalk. In Proceedings of the 2nd International Conference on Energy and Environmental Protection (ICEEP 2013), Guilin, China, 19–21 April 2013; Volume 724–725, p. 391. [Google Scholar]
- Guo, J.; Wei, J.; Huang, F.; Massey, I.Y.; Luo, J.; Yang, F. Optimization of microcystin biodegradation by bacterial community YFMCD4 using response surface method. Chemosphere 2021, 274, 7721. [Google Scholar] [CrossRef]
- Haile, S.; Ayele, A. Pectinase from Microorganisms and Its Industrial Applications. Sci. World J. 2022, 2022, 1881305. [Google Scholar] [CrossRef]
- Sondhi, S.; Saini, K. Response surface based optimization of laccase production from Bacillus sp. MSK-01 using fruit juice waste as an effective substrate. Heliyon 2019, 5, 811. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 562. [Google Scholar] [CrossRef]
- Ren, J.; Na, D.; Yoo, S.M. Optimization of chemico-physical transformation methods for various bacterial species using diverse chemical compounds and nanomaterials. J. Biotechnol. 2018, 288, 55–60. [Google Scholar] [CrossRef]
- Ren, J.; Karna, S.; Lee, H.-M.; Yoo, S.M.; Na, D. Artificial transformation methodologies for improving the efficiency of plasmid DNA transformation and simplifying its use. Appl. Microbiol. Biotechnol. 2019, 103, 9205–9215. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.A.; Guss, A.M. Approaches to genetic tool development for rapid domestication of non-model microorganisms. Biotechnol. Biofuels 2021, 14, 6144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Yu, L.; Tian, Y.; Li, S.; Leng, F.; Ma, J.; Chen, J. Effects of Sr2+ on the preparation of Escherchia coli DH5 alpha competent cells and plasmid transformation. Peerj 2020, 8, e8929. [Google Scholar]
- Deshmukh, K.; Ramanan, S.R.; Kowshik, M. Novel one step transformation method for Escherichia colt and Staphylococcus aureus using arginine-glucose functionalized hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2019, 96, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Devkota, A.; Sigdel, A.; Yadegari, Z.; Dumenyo, K.; Taheri, A. Citric acid/beta-alanine carbon dots as a novel tool for delivery of plasmid DNA into E. coli cells. Sci. Rep. 2021, 11, 1553. [Google Scholar] [CrossRef]
- Rosano, G.L.; Morales, E.S.; Ceccarelli, E.A. New tools for recombinant protein production in Escherichia coli: A 5-year update. Protein Sci. 2019, 28, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Wood, E.A.; Romero, Z.J.; Cox, M.M. RecA-independent recombination: Dependence on the Escherichia coli RarA protein. Mol. Microbiol. 2021, 115, 1122–1137. [Google Scholar] [CrossRef]
- Hao, N.; Chen, Q.; Dodd, I.B.; Shearwin, K.E. The pIT5 Plasmid Series, an Improved Toolkit for Repeated Genome Integration in E. coli. ACS Synth. Biol. 2021, 10, 1633–1639. [Google Scholar] [CrossRef]
- Chen, K.; Xie, M.; Chan, E.W.-C.; Chen, S. Delineation of ISEcp1 and IS26-Mediated Plasmid Fusion Processes by MinION Single-Molecule Long-Read Sequencing. Front. Microbiol. 2022, 12, 277. [Google Scholar] [CrossRef]
- Than, M.M.; Ruangsuriya, J.; Uthaipibull, C.; Srichairatanakool, S. Expression of fluorescent tagged recombinant erythroferrone protein. Asian Pac. J. Trop. Biomed. 2018, 8, 360–364. [Google Scholar]
- Wang, L.; Jiang, H.; Qiu, Y.; Dong, Y.; Hamouda, H.I.; Balah, M.A.; Mao, X. Biochemical Characterization of a Novel Myrosinase Rmyr from Rahnella inusitata for High-Level Preparation of Sulforaphene and Sulforaphane. J. Agric. Food Chem. 2022, 70, 2303–2311. [Google Scholar] [CrossRef]
- Curiqueo, C.; Mahn, A.; Castillo, A. Broccoli Myrosinase cDNA Expression in Escherichia coli and Saccharomyces cerevisiae. Biomolecules 2022, 12, 1872. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, H.; Liang, X.; Zhou, W.; Qiu, Y.; Xue, C.; Sun, J.; Mao, X. Preparation of Sulforaphene from Radish Seed Extracts with Recombinant Food-Grade Yarrowia lipolytica Harboring High Myrosinase Activity. J. Agric. Food Chem. 2021, 69, 5363–5371. [Google Scholar] [CrossRef]
- Rosenbergova, Z.; Kantorova, K.; Simkovic, M.; Breier, A.; Rebros, M. Optimisation of Recombinant Myrosinase Production in Pichia pastoris. Int. J. Mol. Sci. 2021, 22, 627. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Hoang, L.; Sporsheim, B.; Halle, K.K.; Wolff, S.A.; Ahmad, S.J.N.; Ahmad, J.N.; Bones, A.M. The Imaging of Guard Cells of thioglucosidase (tgg) Mutants of Arabidopsis Further Links Plant Chemical Defence Systems with Physical Defence Barriers. Cells 2021, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, R.; Zhang, J.; Liu, Z.; Fan, S.; Yang, G.; Jin, Z.; Pei, Y. Improving sulforaphane content in transgenic broccoli plants by overexpressing MAM1, FMOGS–OX2, and Myrosinase. Plant Cell Tissue Organ Cult. 2021, 146, 461–471. [Google Scholar] [CrossRef]
- Azizoglu, U.; Jouzani, G.S.; Yilmaz, N.; Baz, E.; Ozkok, D. Genetically modified entomopathogenic bacteria, recent developments, benefits and impacts: A review. Sci. Total Environ. 2020, 1, 734. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Luo, L.; Jiang, Y.; Zhang, J.; Tang, X.; Jiang, M.; Wu, Z.; Li, Y. A high amylase-producing bacterial strain exhibiting the phosphorus-dissolving ability, isolated from freshwater aquaculture pond. Aquacult. Res. 2020, 51, 4315–4326. [Google Scholar] [CrossRef]
- Mingfei, Y.; Jiaojiao, X.; Hengjun, D.; McClements, D.J.; Hang, X.; Lanjuan, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar]
- Lohrasbi, V.; Abdi, M.; Asadi, A.; Rohani, M.; Esghaei, M.; Talebi, M.; Amirmozafari, N. The effect of improved formulation of chitosan-alginate microcapsules of Bifidobacteria on serum lipid profiles in mice. Microb. Pathog. 2020, 149, 8821. [Google Scholar] [CrossRef]
- Zacchetti, B.; Andrianos, A.; van Dissel, D.; de Ruiter, E.; van Wezel, G.P.; Claessen, D. Microencapsulation extends mycelial viability of Streptomyces lividans 66 and increases enzyme production. BMC Biotechnol. 2018, 18, 7018. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-P.; Lee, C.-C.; Lee, J.-C.; Tsai, P.-J.; Hsueh, P.-R.; Ko, W.-C. The Potential of Probiotics to Eradicate Gut Carriage of Pathogenic or Antimicrobial-Resistant Enterobacterales. Antibiotics 2021, 10, 1662. [Google Scholar] [CrossRef]
- Enck, K.; Banks, S.; Yadav, H.; Welker, M.E.; Opara, E.C. Development of a Novel Oral Delivery Vehicle for Probiotics. Curr. Pharm. Des. 2020, 26, 3134–3140. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, L.; Xie, M.; Li, H.; Ma, D.; Xue, W. A Colon-Targeted Oral Probiotics Delivery System Using an Enzyme-Triggered Fuse-Like Microcapsule. Adv. Healthc. Mater. 2021, 10, e19913. [Google Scholar] [CrossRef]
- Liu, H.; Cai, Z.; Wang, F.; Hong, L.; Deng, L.; Zhong, J.; Wang, Z.; Cui, W. Colon-Targeted Adhesive Hydrogel Microsphere for Regulation of Gut Immunity and Flora. Adv. Sci. 2021, 8, e17662. [Google Scholar] [CrossRef]
- Kamguyan, K.; Torp, A.M.; Christfort, J.F.; Guerra, P.R.; Licht, T.R.; Nielsen, L.H.; Zor, K.; Boisen, A. Colon-Specific Delivery of Bioactive Agents Using Genipin-Cross-Linked Chitosan Coated Microcontainers. ACS Appl. Bio Mater. 2021, 4, 752–762. [Google Scholar] [CrossRef]
- Masoomi Dezfooli, S.; Gutierrez-Maddox, N.; Alfaro, A.C.; Seyfoddin, A. Development of a microencapsulated probiotic delivery system for New Zealand black-footed abalone (Haliotis iris). Pharm. Dev. Technol. 2021, 26, 390–402. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, M.; You, X.; Sela, D.A.; Xiao, H.; McClements, D.J. Encapsulation of bifidobacterium in alginate microgels improves viability and targeted gut release. Food Hydrocoll. 2021, 116, 2672. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, B.; Li, D.; Hu, Y.; Zhao, L.; Zhang, M.; Ge, S.; Pang, J.; Li, Y.; Wang, R.; et al. Improved Gastric Acid Resistance and Adhesive Colonization of Probiotics by Mucoadhesive and Intestinal Targeted Konjac Glucomannan Microspheres. Adv. Funct. Mater. 2020, 30, 214. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Guerra, P.R.; Bahl, M.I.; Torp, A.M.; Te Hwu, E.; Licht, T.R.; Boisen, A. Multi-layer PLGA-pullulan-PLGA electrospun nanofibers for probiotic delivery. Food Hydrocoll. 2022, 123, 2886. [Google Scholar] [CrossRef]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem. 2021, 339, 2872. [Google Scholar]
- Premjit, Y.; Mitra, J. Optimization of Electrospray-Assisted Microencapsulation of Probiotics (Leuconostoc lactis) in Soy Protein Isolate-Oil Particles Using Box-Behnken Experimental Design. Food Bioprocess Technol. 2021, 14, 1712–1729. [Google Scholar] [CrossRef]
- Huang, R.-M.; Feng, K.; Li, S.-F.; Zong, M.-H.; Wu, H.; Han, S.-Y. Enhanced survival of probiotics in the electrosprayed microcapsule by addition of fish oil. J. Food Eng. 2021, 307, 2613. [Google Scholar] [CrossRef]
- Oberoi, K.; Tolun, A.; Sharma, K.; Sharma, S. Microencapsulation: An overview for the survival of probiotic bacteria. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 280–287. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M. Maillard conjugate-based delivery systems for the encapsulation, protection, and controlled release of nutraceuticals and food bioactive ingredients: A review. Food Hydrocoll. 2020, 100, 2670. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Nanomaterial-based encapsulation for controlled gastrointestinal delivery of viable probiotic bacteria. Nanoscale Adv. 2021, 3, 2699–2709. [Google Scholar] [CrossRef]

| Strain | Source | Substrate | Products | Transformation Ability | References |
|---|---|---|---|---|---|
| Lactobacillus agilis R16 | NS | Glucoiberin/glucoraphanin | NS | 10% | [68] |
| Enterococcus casseliflavus CP1 | Human feces | Glucoiberin/glucoraphanin | Iberin/SFN | 40–50% | |
| Escherichia coli VL8 | Human feces | Glucoiberin/glucoraphanin | Glucoiberverin/glucoerucin | 80–90% | |
| Enterococcus gallinarum HG001 | Mouse feces | Glucosinolate | Isothiocyanate | 39.54% | [69] |
| Escherichia coli HG002 | Mouse feces | Glucosinolate | Isothiocyanate | 29.17% | |
| L. plantarum KW30 | NS | Glucoraphanin, etc. | SFN, etc. | 30–33% | [70] |
| Lactococcus lactis subsp. lactis KF147 | NS | Glucoraphanin, etc. | SFN, etc. | 30–33% | |
| E. coli Nissle 1917 | NS | Glucoraphanin, etc. | Glucoerucin, etc. | 65–78% | |
| Bacteroides thetaiotaomicron | Human feces | Sinigrin | Allyl isothiocyanate | NS | [71] |
| Companilactobacillus farciminis KB1089 | Pickles | Sinigrin | Allyl isothiocyanate | NS | [72] |
| Citrobacter Wye1 | Soil | Sinigrin | Allylcyanide | NS | [73] |
| Leclercia adecarboxylata | Soil | Sinigrin | Allylcyanide | NS | [74] |
| Aspergillus sp. NR46F13 | Soil | Sinigrin | NS | [75] | |
| LAB | Broccoli | Glucoraphanin | SFN | NS | [37] |
| LAB | Broccoli | Glucoraphanin | SFN | NS | [38] |
| Pediococcus pentosaceus | Natural fermented cherry juice | Glucoraphanin | SFN | NS | [76] |
| Aspergillus sp. NR-4201 | NS | Glucosinolate | Allylcyanide | NS | [77] |
| Lactobacillus agilis R16 | NS | Sinigrin | Allyl isothiocyanate | NS | [70] |
| Bacterial Strains | Compositions | Delivery Systems | Functions | References |
|---|---|---|---|---|
| Lactobaccillus casei NCDC 298 | Modified alginate | Hydrogel | Protects probiotics from enzymatic hydrolysis | [106] |
| E. coli MG1655 | Alginate and protamine | Microcapsule | Protects probiotics from acidity and bile salts | [107] |
| NS | Alginate | Microsphere | Antiacid and colon targeting | [108] |
| L. rhamnosus | Chitosan | Microcontainers | Targeted delivery of probiotics | [109] |
| Exiguobacterium | Chitosan and alginate | Microparticles | Targeted delivery of probiotics | [110] |
| Bifidobacterium pseudocatenulatum | Calcium alginate | Microgels | Improve the stability of probiotics | [43] |
| Bifidobacterium | Thiolated oxidized konjac glucomannan | Microspheres | Improves intestinal colonization of probiotics | [111] |
| Lactobacillus rhamnosus GG | Amylopectin | Nanofibers | Enhanced probiotic delivery capabilities | [112] |
| Bifidobacterium | Alginate | Microcapsules | Improve the survival rate of probiotics | [114] |
| Streptomyces lividans 66 | Alginate | Micro-encapsulation | Improve the enzyme production capacity of probiotics | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Y.; Zhao, G.; Liu, G.; Wang, P.; Li, J. Microorganisms—An Effective Tool to Intensify the Utilization of Sulforaphane. Foods 2022, 11, 3775. https://doi.org/10.3390/foods11233775
Li X, Wang Y, Zhao G, Liu G, Wang P, Li J. Microorganisms—An Effective Tool to Intensify the Utilization of Sulforaphane. Foods. 2022; 11(23):3775. https://doi.org/10.3390/foods11233775
Chicago/Turabian StyleLi, Xiude, Yihan Wang, Guoping Zhao, Guangmin Liu, Pengjie Wang, and Jinwang Li. 2022. "Microorganisms—An Effective Tool to Intensify the Utilization of Sulforaphane" Foods 11, no. 23: 3775. https://doi.org/10.3390/foods11233775
APA StyleLi, X., Wang, Y., Zhao, G., Liu, G., Wang, P., & Li, J. (2022). Microorganisms—An Effective Tool to Intensify the Utilization of Sulforaphane. Foods, 11(23), 3775. https://doi.org/10.3390/foods11233775







