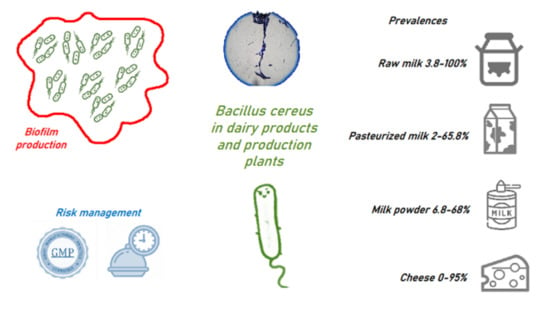
Bacillus cereus in Dairy Products and Production Plants
Abstract
1. Bacillus Cereus Characteristics
1.1. Taxonomy and Growth Requirements
1.2. Bacillus Cereus Culture and Isolation
1.3. Disease Caused by B. cereus
1.4. Reservoir and Contamination Routes
2. Role of B. cereus in the Dairy Supply Chain
2.1. Presence of B. cereus along the Dairy Production Chain
2.2. Spoilage and Pathogenic Potential of B. cereus
2.3. Growth Potential of B. cereus in Dairy Products
2.4. Biofilm Formation
3. Contamination Control Strategies Applicable to the Dairy Supply Chain
3.1. Reduction in Contamination in Raw Materials
3.2. Removal of Spores from Surfaces and Production Equipment
3.3. Product Conditioning
3.4. Food Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Messelhäußer, U.; Ehling-Schulz, M. Bacillus cereus—A Multifaceted Opportunistic Pathogen. Curr. Clin. Microbiol. Rep. 2018, 5, 120–125. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol. Spectr. 2019, 7, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.M.; Wiedmann, M.; Kovac, J. Proposal of a taxonomic nomenclature for the Bacillus cereus group which reconciles genomic definitions of bacterial species with clinical and industrial phenotypes. mBio 2020, 11, e00034-20. [Google Scholar] [CrossRef] [PubMed]
- Guinebretiere, M.-H.; Thompson, F.L.; Sorokin, A.; Normand, P.; Dawyndt, P.; Ehling-Schulz, M.; Svensson, B.; Sanchis, V.; Nguyen-The, C.; Heyndrickx, M.; et al. Ecological diversification in the Bacillus cereus Group. Environ. Microbiol. 2008, 10, 851–865. [Google Scholar] [CrossRef]
- Food Standard Australia New Zealand (FSANZ). Bacillus cereus. 2013. Available online: https://www.foodstandards.gov.au/publications/Documents/Bacillus%20cereus.pdf. (accessed on 14 July 2022).
- Oliveira Silva, H.; Santos Lima, J.A.; Gamero Aguilar, C.E.; Marques Rossi, G.A.; Mathias, L.A.; Centola Vidal, A.M. Efficiency of different disinfectants on Bacillus cereus sensu stricto biofilms on stainless-steel surfaces in contact with milk. Front. Microbiol. 2018, 9, 3924. [Google Scholar] [CrossRef]
- Reyes, J.E.; Bastias, J.M.; Gutiérrez, M.R.; Rodríguez, M. Prevalence of Bacillus cereus in dried milk products used by Chilean School Feeding Program. Food Microbiol. 2007, 24, 1–6. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Alnakip, M.E.A.; Abd-El Aal, S.F. Occurrence of Bacillus cereus in raw milk and some dairy products in Egypt. Jpn. J. Vet. Res. 2016, 64, S95–S102. [Google Scholar]
- Spanu, C.; Scarano, C.; Spanu, V.; Pala, C.; Casti, D.; Lamon, S.; Cossu, F.; Ibba, M.; Nieddu, G.; De Santis, E.P.L. Occurrence and behavior of Bacillus cereus in naturally contaminated ricotta salata cheese during refrigerated storage. Food Microbiol. 2016, 58, 135–138. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Granum, P.E.; Märtlbauer, E. The Bacillus cereus food infection as multifactorial process. Toxins 2020, 12, 701. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Hu, Q.; Xu, F.; Ding, S.-Y.; Zhu, K. Characterization of Bacillus cereus in dairy products in China. Toxins 2020, 12, 454. [Google Scholar] [CrossRef]
- Tuipulotu, D.E.; Mathur, A.; Ngo, C.; Man, S.M. Bacillus cereus: Epidemiology, virulence factors, and host-pathogen interactions. Trends Microbiol. 2021, 29, 458–471. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, 4524. [Google Scholar]
- Granum, P.E.; Andersson, A.; Gayther, C.; Te Giffel, M.; Larsen, H.; Lund, T.; O’Sullivan, K. Evidence for a further enterotoxin complex produced by Bacillus cereus. FEMS Microbiol. Lett. 1996, 141, 145–149. [Google Scholar] [CrossRef][Green Version]
- Granum, P.E.; Baird-Parker, T.C. Bacillus spp. In The Microbiological Safety and Quality of Food; Lund, B., Baird-Parker, T.C., Gould, G., Eds.; Aspen Publishers: Gaithersburg, MD, USA, 2000; pp. 1029–1039. [Google Scholar]
- Lund, T.; De Buyser, M.L.; Granum, P.E. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef]
- Johnson, K.M. Bacillus cereus in foodborne illness—An update. J. Food Prot. 1984, 47, 145–153. [Google Scholar] [CrossRef]
- Te Giffel, M.C.; Beumer, R.R.; Leijendekkers, S.; Rombouts, F.M. Incidence of Bacillus cereus and Bacillus subtilis in foods in the Netherlands. Food Microbiol. 1996, 13, 53–58. [Google Scholar] [CrossRef]
- Hong, H.A.; To, E.; Fakhry, S.; Baccigalupi, L.; Ricca, E.; Cutting, S.M. Defining the natural habitat of Bacillus spore-formers. Res. Microbiol. 2009, 160, 375–379. [Google Scholar] [CrossRef]
- Lin, S.; Schraft, H.; Odumeru, J.A.; Griffiths, M.W. Identification of contamination sources of Bacillus cereus in pasteurised milk. Int. J. Food Microbiol. 1998, 43, 159–171. [Google Scholar] [CrossRef]
- Wirtanen, G.; Husmark, U.; Mattila-Sandholm, T. Microbial evaluation of the biotransfer potential from surfaces with Bacillus biofilms after rinsing and cleaning procedures in closed food-processing systems. J. Food Prot. 1996, 59, 727–733. [Google Scholar] [CrossRef]
- Mettler, E.; Carpentier, B. Location, enumeration and identification of the microbial contamination after cleaning of EPDM gaskets introduced into a milk pasteurization line. Le Lait Rev. Int. De Sci. Et Technol. Du Lait 1997, 4, 489. [Google Scholar]
- Christiansson, A.; Ekelund, K.; Ogura, H. Membrane filtration method for enumeration and isolation of spores of Bacillus cereus from milk. Int. Dairy J. 1997, 7, 743–748. [Google Scholar] [CrossRef]
- Donovan, K.O. The occurrence of Bacillus cereus in milk and on dairy equipment. J. Appl. Bacteriol. 1959, 22, 131–137. [Google Scholar] [CrossRef]
- Svensson, B.; Eneroth, Å.; Brendehaug, J.; Molin, G.; Christiansson, A. Involvement of a pasteurizer in the contamination of milk by Bacillus cereus in a commercial dairy plant. J. Dairy Res. 2000, 67, 455–460. [Google Scholar] [CrossRef]
- Eneroth, S.A.; Svensson, B.; Molin, G.; Christiansson, A. Contamination of pasteurised milk by Bacillus cereus in the filling machine. J. Dairy Res. 2001, 68, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-S.; Tsai, W.-C.; Chou, C.-C. Surface characteristics of Bacillus cereus and its adhesion to stainless steel. Int. J. Food Microbiol. 2001, 65, 105–111. [Google Scholar] [CrossRef]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef]
- Lücking, G.; Stoeckel, M.; Atamer, Z.; Hinrichs, J.; Ehling-Schulz, M. Characterization of aerobic spore-forming bacteria associated with industrial dairy processing environments and product spoilage. Int. J. Food Microbiol. 2013, 166, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Dufrenne, J.; Soentoro, P.; Tatini, S.; Day, T.; Notermann, S. Characteristics of Bacillus cereus related to safe food production. Int. J. Food Microbiol. 1994, 23, 99–109. [Google Scholar] [CrossRef]
- Samaržija, D.; Zamberlin, Š.; Pogači, T. Psychrotrophic bacteria and milk and dairy products quality. Mljekarstvo 2012, 62, 77–95. [Google Scholar]
- Yibar, A.; Çetinkaya, F.; Soyutemiz, E.; Yaman, G. Prevalence, enterotoxin production and antibiotic resistance of Bacillus cereus İsolated from milk and cheese. Kafkas Univ. Vet. Fak. Derg 2017, 23, 635–642. [Google Scholar]
- Osama, R.; Ahmed, M.; Abdulmawjood, M.; Al-Ashmawy, M. Prevalence and antimicrobial resistance of Bacillus cereus in milk and dairy products. Mansoura Vet. Med. J. 2020, 21, 11–18. [Google Scholar] [CrossRef]
- Chang, Y.; Xie, Q.; Yang, J.; Ma, L.; Feng, H. The prevalence and characterization of Bacillus cereus isolated from raw and pasteurised buffalo milk in southwestern China. J. Dairy Sci. 2021, 104, 3980–3989. [Google Scholar] [CrossRef]
- Larsen, H.D.; Jørgensen, K. The occurrence of Bacillus cereus in Danish pasteurised milk. Int. J. Food Microbiol. 1997, 34, 179–186. [Google Scholar] [CrossRef]
- Wong, H.C.; Chang, M.H.; Fan, J.Y. Incidence and characterization of Bacillus cereus isolates contaminating dairy products. Appl. Environ. Microbiol. 1988, 54, 699–702. [Google Scholar] [CrossRef]
- Kumari, S.; Sarkar, P.K. Prevalence and characterization of Bacillus cereus group from various marketed dairy products in India. Dairy Sci. Technol. 2014, 94, 483–497. [Google Scholar] [CrossRef]
- Saleh-Lakha, S.; Leon-Velarde, C.G.; Chen, S.; Lee, S.; Shannon, K.; Fabri, M.; Downing, G.; Keown, B. A study to assess the numbers and prevalence of Bacillus cereus and its toxins in pasteurised fluid milk. J. Food Prot. 2017, 80, 1085–1089. [Google Scholar] [CrossRef]
- Gao, T.; Ding, Y.; Wu, Q.; Wang, J.; Zhang, J.; Yu, S.; Yu, P.; Liu, P.; Kong, L.; Feng, Z.; et al. Prevalence, virulence genes, antimicrobial susceptibility, and genetic diversity of Bacillus cereus isolated from pasteurised milk in China. Front. Microbiol. 2018, 9, 533. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Pluta, A.; Garbowska, M.; Stefańska, I. Prevalence and toxicity characterization of Bacillus cereus in food products from Poland. Foods 2019, 8, 269. [Google Scholar] [CrossRef]
- Shimojima, Y.; Kodo, Y.; Soeda, K.; Koike, H.; Kanda, M.; Hayashi, H.; Nishino, Y.; Fukui, R.; Kuroda, S.; Hirai, A.; et al. Prevalence of cereulide-producing Bacillus cereus in pasteurised milk. J. Food Hyg. Soc. Jpn. 2020, 61, 178–182. [Google Scholar] [CrossRef]
- Messelhäusser, U.; Kämpf, P.; Fricker Ehling-Schulz, M.; Zucker, M.R.; Wagner, B.; Busch, U.; Höller, C. Prevalence of emetic Bacillus cereus in different ice creams in Bavaria. J. Food Prot. 2010, 73, 395–399. [Google Scholar] [CrossRef]
- Di Pinto, A.; Bonerba, E.; Bozzo, G.; Ceci, E.; Terio, V.; Tantillo, G. Occurence of potentially enterotoxigenic Bacillus cereus in infant milk powder. Eur. Food Res. Technol. 2013, 237, 275–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Feng, C.; Zhan, L.; Zhang, J.; Li, Y.; Yang, Y.; Chen, H.; Zhang, Z.; Zhang, Y.; et al. Quantitative prevalence, phenotypic and genotypic characteristics of Bacillus cereus isolated from retail infant foods in China. Foodborne Pathog. Dis. 2017, 14, 564–572. [Google Scholar] [CrossRef]
- Pei, X.; Yang, S.; Zhu, J.; Song, X.; Hu, X.; Liu, G.; Ma, G.; Li, N.; Yang, D. Prevalence of Bacillus cereus in powdered infant and powdered follow-up formula in China. Food Control 2018, 93, 101–105. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Wuni, A.; Akabanda, F.; Tano-Debrah, K.; Jespersen, L. Prevalence, virulence factor genes and antibiotic resistance of Bacillus cereus sensu lato isolated from dairy farms and traditional dairy products. BMC Microbiol. 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Iurlina, M.O.; Saiz, A.I.; Fuselli, S.R.; Fritz, R. Prevalence of Bacillus spp. in different food products collected in Argentina. LWT-Food Sci. Technol. 2016, 39, 105–110. [Google Scholar] [CrossRef]
- Proroga, Y.T.R.; Capuano, F.; Castellano, S.; Giordano, A.; Mancusi, A.; Delibato, E.; Dumontet, S.; Pasquale, V. Occurrence and toxin gene profile of Bacillus cereus in dairy products. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 58–62. [Google Scholar] [CrossRef]
- Adame-Gómez, R.; Muñoz-Barrios, S.; Castro-Alarcón, N.; Leyva-Vázquez, M.A.; Toribio-Jiménez, J.; Ramírez-Peralta, A. Prevalence of the strains of Bacillus cereus group in artisanal Mexican cheese. Foodborne Pathog. Dis. 2020, 17, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Montone, A.M.I.; Capuano, F.; Mancusi, A.; Di Maro, O.; Peruzy, M.F.; Proroga, Y.T.R.; Cristiano, D. Exposure to Bacillus cereus in water buffalo mozzarella cheese. Foods 2020, 9, 1899. [Google Scholar] [CrossRef]
- Amor, M.G.-B.; Siala, M.; Zayani, M.; Grosset, N.; Smaoui, S.; Messadi-Akrout, F.; Baron, F.; Jan, S.; Gautier, M.; Gdour, R. Isolation, identification, prevalence, and genetic diversity of Bacillus cereus group bacteria from different foodstuffs in Tunisia. Front. Microbiol. 2018, 9, 447. [Google Scholar] [CrossRef]
- Watterson, M.J.; Kent, D.J.; Boor, K.J.; Wiedmann, M.; Martin, N.H. Evaluation of dairy powder products implicates thermophilic sporeformers as the primary organisms of interest. J. Dairy Sci. 2014, 97, 2487–2497. [Google Scholar] [CrossRef]
- European Commission (EC). Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005. Available online: https://www.legislation.gov.uk/eur/2005/2073/contents (accessed on 14 July 2022).
- Borge, G.I.A.; Skeie, M.; Sørhaug, T.; Langsru, T.; Granum, P.E. Growth and toxin profiles of Bacillus cereus isolated from different food sources. Int. J. Food Microbiol. 2001, 69, 237–246. [Google Scholar] [CrossRef]
- Svensson, B.; Monthán, A.; Shaheen, R.; Andersson, M.A.; Salkinoja-Salonen, M.; Christiansson, A. Occurrence of emetic toxin producing Bacillus cereus in the dairy production chain. Int. Dairy J. 2006, 16, 740–749. [Google Scholar] [CrossRef]
- Tirloni, E.; Bernardi, C.; Ghelardi, E.; Celandroni, F.; Andrighetto, C.; Rota, N.; Stella, S. Biopreservation as a potential hurdle for Bacillus cereus growth in fresh cheese. J. Dairy Sci. 2020, 103, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Catania, A.M.; Civera, T.; DICiccio, P.A.; Grassi, M.A.; Morra, P.; Dalmasso, A. Characterization of Vegetative Bacillus cereus and Bacillus subtilis Strains Isolated from Processed Cheese Products in an Italian Dairy Plant. Foods 2021, 10, 2876. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Park, J.-H. Characteristics of enterotoxin distribution, hemolysis, lecithinase, and starch hydrolysis of Bacillus cereus isolated from infant formulas and ready-to-eat foods. J. Dairy Sci. 2015, 98, 1652–1660. [Google Scholar] [CrossRef]
- Muñoz, A.; Maqueda, M.; Gálvez, A.; Martínez-Bueno, M.; Rodríguez, A.; Valdivia, E. Biocontrol of psychrotrophic enterotoxigenic Bacillus cereus in a nonfat hard cheese by an enterococcal strain–producing enterocin AS-48. J. Food Prot. 2004, 67, 1517–1521. [Google Scholar] [CrossRef]
- Tirloni, E.; Ghelardi, E.; Celandroni, F.; Bernardi, C.; Casati, R.; Rosshaug, P.S.; Stella, S. Bacillus cereus in fresh ricotta: Comparison of growth and Haemolysin BL production after artificial contamination during production or post processing. Food Control 2017, 79, 272–278. [Google Scholar] [CrossRef]
- Ellouze, M.; Buss Da Silva, N.; Rouzeau-Szynalski, K.; Cantergiani, F.; Baranyi, J. Modeling Bacillus cereus growth and cereulide formation in cereal-, dairy-, meat-, vegetable-based food and culture medium. Front. Microbiol. 2021, 12, 639546. [Google Scholar] [CrossRef]
- Tirloni, E.; Ghelardi, E.; Celandroni, F.; Bernardi, C.; Stella, S. Effect of dairy product environment on the growth of Bacillus cereus. J. Dairy Sci. 2017, 100, 7026–7034. [Google Scholar] [CrossRef]
- Tirloni, E.; Bernardi, C.; Celandroni, F.; Ghelardi, E.; Stella, S. Effectiveness of lactic and acetic acids on the growth of Listeria monocytogenes and Bacillus cereus in primo sale fresh cheese. LWT 2021, 151, 112170. [Google Scholar] [CrossRef]
- Little, C.L.; Knøchel, S. Growth and survival of Yersinia enterocolitica, Salmonella and Bacillus cereus in Brie stored at 4, 8 and 20 °C. Int. J. Food Microbiol. 1994, 24, 137–145. [Google Scholar] [CrossRef]
- Rukure, G.; Bester, B.H. Survival and growth of Bacillus cereus during Gouda cheese manufacturing. Food Control 2001, 12, 31–36. [Google Scholar] [CrossRef]
- Crielly, E.M.; Logan, N.A.; Anderton, A. Studies on the Bacillus flora of milk and milk products. J. Appl. Bacteriol. 1994, 77, 256–263. [Google Scholar] [CrossRef]
- Postollec, F.; Mathot, A.G.; Bernard, M.; Divanac’h, M.L.; Pavan, S.; Sohier, D. Tracking spore-forming bacteria in food: From natural biodiversity to selection by processes. Int. J. Food Microbiol. 2012, 158, 1–8. [Google Scholar] [CrossRef]
- Shaheen, R.; Svensson, B.; Andersson, M.A.; Christiansson, A.; Salkinoja-Salonen, M. Persistence strategies of Bacillus cereus spores isolated from dairy silo tanks. Food Microbiol. 2010, 27, 347–355. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Faille, C.; Bénézech, T.; Midelet-Bourdin, G.; Lequette, Y.; Clarisse, M.; Ronse, G.; Ronse, A.; Slomianny, C. Sporulation of Bacillus spp. within biofilms: A potential source of contamination in food processing environments. Food Microbiol. 2014, 40, 64–74. [Google Scholar]
- Davey, M.E.; O’toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef]
- Majed, R.; Faille, C.; Kallassy, M.; Gohar, M. Bacillus cereus Biofilms—Same, Only Different. Front. Microbiol. 2016, 7, 1054. [Google Scholar] [CrossRef]
- Pereira Perez Alonso, V.; Morishita, M.; Harada, M.; Dirce, M.; Kabuki, Y. Competitive and/or cooperative interactions of Listeria monocytogenes with Bacillus cereus in dual-species biofilm formation. Front. Microbiol. 2020, 11, 177. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Vilain, S.; Pretorius, J.M.; Theron, J.; Brozel, V.S. DNA as an adhesin: Bacillus cereus requires extracellular DNA to form biofilms. Appl. Environ. Microbiol. 2009, 75, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Hussain, M.S.; Oh, D.H. Biofilm formation of Bacillus cereus under food-processing-related conditions. Food Sci. Biotechnol. 2017, 26, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Wijman, J.G.E.; de Leeuw, P.P.L.A.; Moezelaar, R.; Zwietering, M.H.; Abee, T. Air–liquid interface biofilms of Bacillus cereus: Formation, sporulation, and dispersion. Appl. Environ. Microbiol. 2007, 73, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Hayrapetyan, H.; Muller, L.; Tempelaars, M.; Abee, T.; Groot, M.N. Comparative analysis of biofilm formation by Bacillus cereus reference strains and undomesticated food isolates and the effect of free iron. Int. J. Food Microbiol. 2015, 200, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.P.P.; Oliveira, J.; Dirce, M.; Kabuki, Y. Incidence of Bacillus cereus, Bacillus sporothermodurans and Geobacillus stearothermophilus in ultra-high temperature milk and biofilm formation capacity of isolates. Int. J. Food Microbiol. 2021, 354, 109318. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; Xie, Q.; Jiang, Y.; Feng, H.; Chang, Y.; Kang, H.; Xing, M.; Chen, J. Genotyping, antimicrobial susceptibility and biofilm formation of Bacillus cereus isolated from powdered food products in China. Foodborne Pathog. Dis. 2021, 18, 8–15. [Google Scholar] [CrossRef]
- Radmehr, B.; Zaferanloo, B.; Tran, T.; Beale, D.J.; Palombo, E.A. Prevalence and characteristics of Bacillus cereus group isolated from raw and pasteurised milk. Curr. Microbiol. 2020, 77, 3065–3075. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, F.; Zhao, L.; Guo, H. Genotypes and the persistence survival phenotypes of Bacillus cereus isolated from UHT milk processing lines. Food Control 2017, 82, 48–56. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Wu, X.; Sun, Y.; Liu, Z. Effect of thyme essential oil against Bacillus cereus planktonic growth and biofilm formation. Appl. Microbiol. Biotechnol. 2018, 102, 10209–10218. [Google Scholar] [CrossRef]
- Kumari, S.; Sarkar, P.K. Bacillus cereus hazard and control in industrial dairy processing environment. Food Control 2016, 69, 20–29. [Google Scholar] [CrossRef]
- Andersson, A.; Ronner, U.; Granum, P.E. What problem does the food industry have with the spore-forming pathogens Bacillus cereus and Clostridium perfringens? Int. J. Food Microbiol. 1995, 28, 145–155. [Google Scholar] [CrossRef]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus spores in the food industry: A review on resistance and response to novel inactivation technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef]
- Schneider, K.R.; Goodrich Schneider, R.; Silverberg, R.; Kurdmongkoltham, P.; Bertoldi, B. Preventing foodborne Illness: Bacillus cereus. 2017. Available online: http://edis.ifas.ufl.edu. (accessed on 14 July 2022).
| Diarrheal Syndromes | Emetic Syndrome | |
|---|---|---|
| Type of toxin | Proteins (HBL, NHE, CytK) | Cyclic peptide (cereulide) |
| Site of production | Small intestine | Preformed in food |
| Incubation period | 8–16 h (up to 24 h) | 0–5 h |
| Disease duration | 12–24 h (sometimes > 24 h) | 6–24 h |
| Infectious dose | 105–107 ingested total cfu | 105–108 cfu/g of contaminated food |
| Resistance to heat | Weak | Highly stable (up to 121 °C for 90 min) |
| Symptoms | Abdominal pain, watery diarrhea, nausea (sometimes) | Nausea, vomit, general weakness, diarrhea (sometimes) |
| Involved foods | Meat and derived foods, vegetables, sauces, soups | Rice, pasta, pastry products |
| Reference | Products * | Prevalence (Positive/Total Samples Analyzed) | Counts (cfu/g) | Country |
|---|---|---|---|---|
| [8] | raw milk (s) | 60% (2/53) | 6.3 × 102–2.4 × 103 | Egypt |
| [32] | raw milk (v + s) | 3.8% (11/106) | 4 × 101–3.8 × 105 | Turkey |
| [33] | raw milk (v + s) | 100% (25/25) | 101–2.2 × 102 | Egypt |
| [34] | raw buffalo milk (v + s) | 33.3% (50/150) | Data not present | China |
| [18] | pasteurized milk (v + s) | 33% (56/157) | 100–104 | Netherlands |
| [35] | pasteurized milk (v + s) | 56% (257/458) | 101–3 × 105 | Denmark |
| [36] | pasteurized milk (v + s) | 2% (1/200) | 2.8 × 102 | Taiwan |
| [37] | pasteurized milk (v + s) | 55% (30/55) | 101–104 | India |
| [8] | pasteurized milk (s) | 15% (3/20) | 2.0 × 101–2.0 × 102 | Egypt |
| [32] | pasteurized milk (v + s) | 26% (13/50) | 101–1.1 × 103 | Turkey |
| [38] | pasteurized milk (v + s) | 41% (104/254) | Up to 105 | Canada |
| [39] | pasteurized milk (v + s) | 27% (70/258) | Mean: 11 MPN/g | China |
| [40] | pasteurized milk (v + s) | 30% (18/60) | 2 × 100 | Poland |
| [41] | pasteurized milk (v + s) | 65.3% (66/101) | Data not present | Japan |
| [34] | pasteurized buffalo milk (v + s) | 15.3% (46/300) | Data not present | China |
| [33] | uht milk (v + s) | 13.3% (2/15) | 102 | Egypt |
| [33] | condensed milk (v + s) | 33.3% (3/10) | 102–4.0 × 102 | Egypt |
| [36] | fruit-flavored reconstituted milk (v + s) | 2% (1/200) | 1.5 × 101 | Taiwan |
| [36] | fermented milk (v + s) | 17% (34/200) | 5.0 × 100–1.2 × 102 | Taiwan |
| [36] | ice-cream (v + s) | 52% (104/200) | 5 × 100–2.5 × 102 | Taiwan |
| [36] | soft ice-cream (v + s) | 35% (70/200) | 5 × 100–8.0 × 102 | Taiwan |
| [42] | ice-cream (v + s) | 62.8% (508/809) | 0.1–2 × 101 | Germany |
| [8] | ice-cream (s) | 25% (10/40) | 5.2 × 102–1.5 × 103 | Egypt |
| [37] | ice-cream (v + s) | 40% (10/25) | 102–108 | India |
| [36] | milk powder (v + s) | 27% (54/200) | 5 × 100–4.5 × 102 | Taiwan |
| [7] | dried milk products (s) | 46% (175/381) | 3 × 100–104 | Chile |
| [43] | infant formula (v + s) | 18.3% (11/60) | <106 | Italy |
| [37] | milk powder (v + s) | 52% (18/35) | 102–103 | India |
| [8] | full-fat milk powder (s) | 15% (3/20) | 101–3.9 × 102 | Egypt |
| [8] | infant formula (s) | 10% (2/20) | 4.0 × 101–2.1 × 102 | Egypt |
| [44] | infant formula (v + s) | 6.8% (40/587) | 103–104 | China |
| [45] | infant formula (v + s) | 7.53% (501/6656) | 101–5 × 103 | China |
| [33] | milk powder (v + s) | 68% (17/25) | 102–3.5 × 103 | Egypt |
| [8] | yoghurt (s) | 0% (0/20) | - | Egypt |
| [46] | Fermented milk (nunu) (v + s) | 35.7% (10/28) | Mean: 6.5 × 10 | Ghana |
| [37] | butter (v + s) | 20% (5/25) | 103–104 | India |
| [47] | port salut argentino cheeses (v + s) | 50% (15/30) | Data not present | Argentina |
| [47] | quartirolo cheeses (v + s) | 0% (0/20) | - | Argentina |
| [37] | cheese (paneer) (v + s) | 4% (1/25) | 2 × 101–4 × 101 | India |
| [37] | cheese (khoa) (v + s) | 0% (0/20) | - | India |
| [37] | cheese (v + s) | 33% (8/25) | 102–106 | India |
| [9] | ricotta salata cheese (s) | 33.3% (48/144) | 2 × 101–2.6 × 102 | Italy |
| [46] | cheese (west african soft) (v + s) | 38.7% (12/31) | Mean: 4 × 102 | Ghana |
| [32] | cheese (v + s) | 10.4% (11/106) | 4 × 101–3.8 × 105 | Turkey |
| [40] | fresh acid cheeses (v + s) | 8.6% (3/35) | 101–1.6 × 102 | Poland |
| [40] | mold cheeses (v + s) | 52.5% (42/80) | 102–2.0 × 103 | Poland |
| [40] | ripening rennet cheeses (v + s) | 43.4% (76/175) | 101–6.5 × 103 | Poland |
| [48] | soft stretched curd cheeses (v + s) | 24.5% (81/331) | <103–106 | Italy |
| [48] | fresh cheeses (soft cheese) (v + s) | 30.5% (18/59) | <103–104 | Italy |
| [48] | fresh ricotta (v + s) | 33.3% (11/33) | <103–103 | Italy |
| [48] | salted ricotta (v + s) | 26.9% (14/52) | <103–106 | Italy |
| [48] | seasoned cheeses (v + s) | 35% (14/40) | <103–106 | Italy |
| [49] | artisanal mexican cheese (v + s) | 29.48% (23/78) | - | Mexico |
| [50] | buffalo mozzarella (v + s) | 26.2% (89/340) | 2.2 × 102–3.6 × 106 | Italy |
| [33] | cheese (Damietta, Ras and Kariesh) (v + s) | 95% (71/75) | 6.0 × 103–4.2 × 106 | Egypt |
| [51] | dairy products (milk, butter and cheese) (v + s) | 6% (5/85) | <103 | Morocco |
| Dairy Product | Temperature | Increase (log cfu/g) | Time | Reference |
|---|---|---|---|---|
| Raw milk | 15 °C | <0.5 | 5 days | [60] |
| Pasteurized milk | 15 °C | 3.08–3.11 | 5 days | [60] |
| Pasteurized milk | 30 °C | >6.0 | 4 days | [57] |
| Reconstituted milk | 12 °C | >4.0 | 12.5 days | [61] |
| Reconstituted milk | 22 °C | >4.0 | 2.5 days | [61] |
| Reconstituted milk | 30 °C | >4.0 | 1.6 days | [61] |
| Reconstituted milk | 42 °C | >4.0 | 1.6 days | [61] |
| Yoghurt | 15 °C | 0.40–0.90 | 5 days | [60] |
| Ricotta | 15 °C | 5.46–6.52 | 5 days | [62] |
| Ricotta | 10 °C | 2.98–3.91 | 7 days | [62] |
| Ricotta salata | 4 °C | <0.5 | 30, 60, 90 days | [9] |
| Brie | 4 °C | <0.5 | - | [64] |
| Brie | 8 °C | <0.5 | - | [64] |
| Gouda cheese | 30 °C (During production) | 2.2 | 4 h | [65] |
| Gouda cheese | 4–7 °C (During ripening) | <0.5 | From 1.6 to 42 days | [65] |
| Nonfat hard cheese | 30 °C | >0.5 | 30 days | [57] |
| Mascarpone cheese | 15 °C | 4.11–4.23 | 5 days | [60] |
| Primo sale cheese | 15 °C | 2.55–4.11 | 3 days | [56] |
| Taleggio cheese | 15 °C | <0.5 | 5 days | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirloni, E.; Stella, S.; Celandroni, F.; Mazzantini, D.; Bernardi, C.; Ghelardi, E. Bacillus cereus in Dairy Products and Production Plants. Foods 2022, 11, 2572. https://doi.org/10.3390/foods11172572
Tirloni E, Stella S, Celandroni F, Mazzantini D, Bernardi C, Ghelardi E. Bacillus cereus in Dairy Products and Production Plants. Foods. 2022; 11(17):2572. https://doi.org/10.3390/foods11172572
Chicago/Turabian StyleTirloni, Erica, Simone Stella, Francesco Celandroni, Diletta Mazzantini, Cristian Bernardi, and Emilia Ghelardi. 2022. "Bacillus cereus in Dairy Products and Production Plants" Foods 11, no. 17: 2572. https://doi.org/10.3390/foods11172572
APA StyleTirloni, E., Stella, S., Celandroni, F., Mazzantini, D., Bernardi, C., & Ghelardi, E. (2022). Bacillus cereus in Dairy Products and Production Plants. Foods, 11(17), 2572. https://doi.org/10.3390/foods11172572