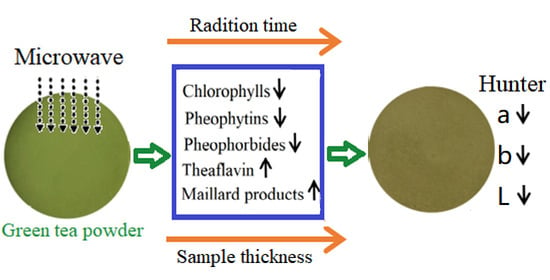
The Effect of Microwave Radiation on the Green Color Loss of Green Tea Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Microwave Treatment of Green Tea Powder
2.3. Temperature Determination of Green Tea Powder in Microwave Oven
2.4. Color Analysis
2.5. Kinetic Analysis of Color Loss by Microwave Radiation
2.6. Analytical Methods
2.7. Statistical Analyses
3. Results and Discussions
3.1. Kinetic of Color Loss in Green Tea Powder under Different Microwave Powers
3.2. Low Apparent Activation Energy Required for Color Loss of Thick GTP Sample
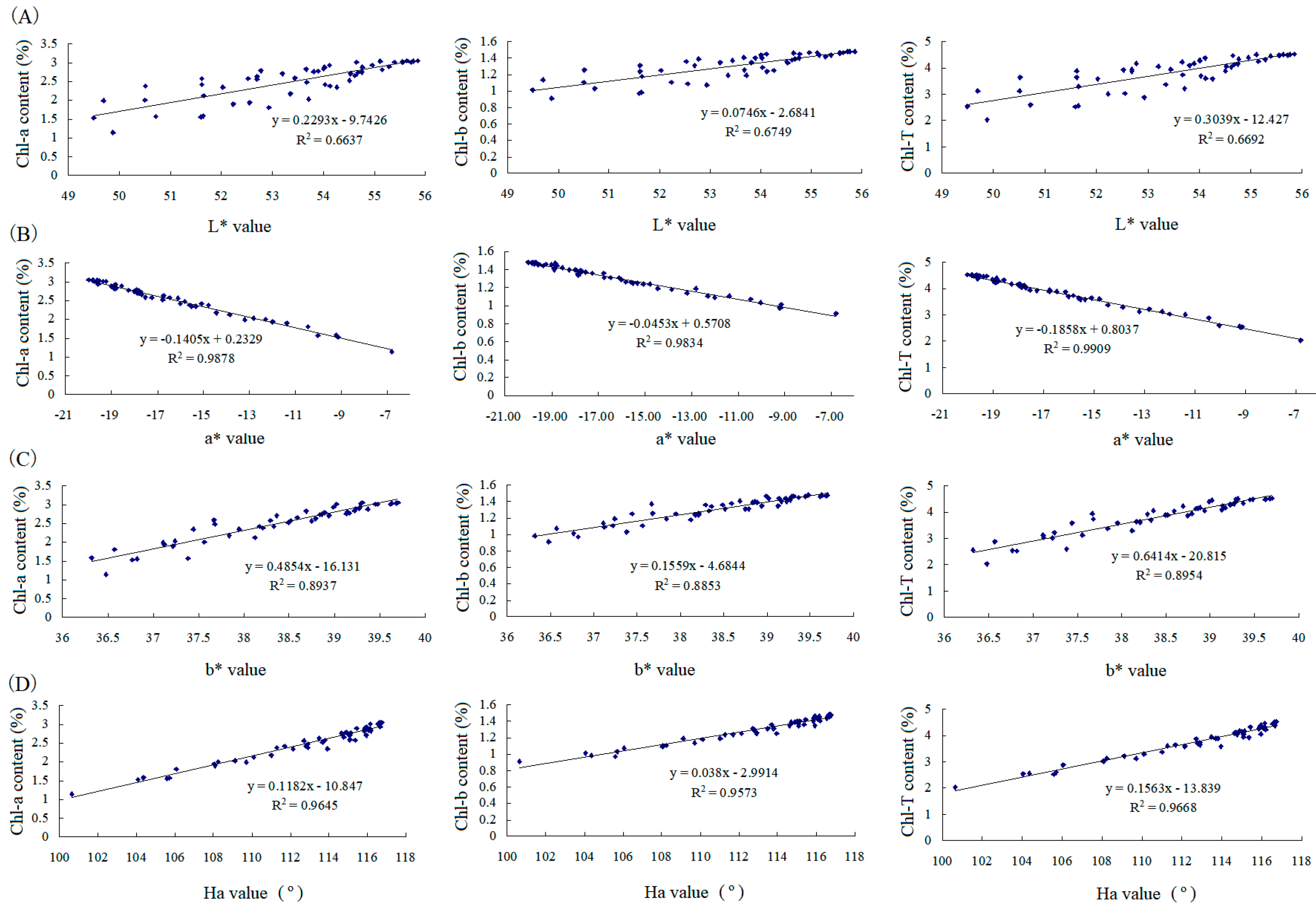
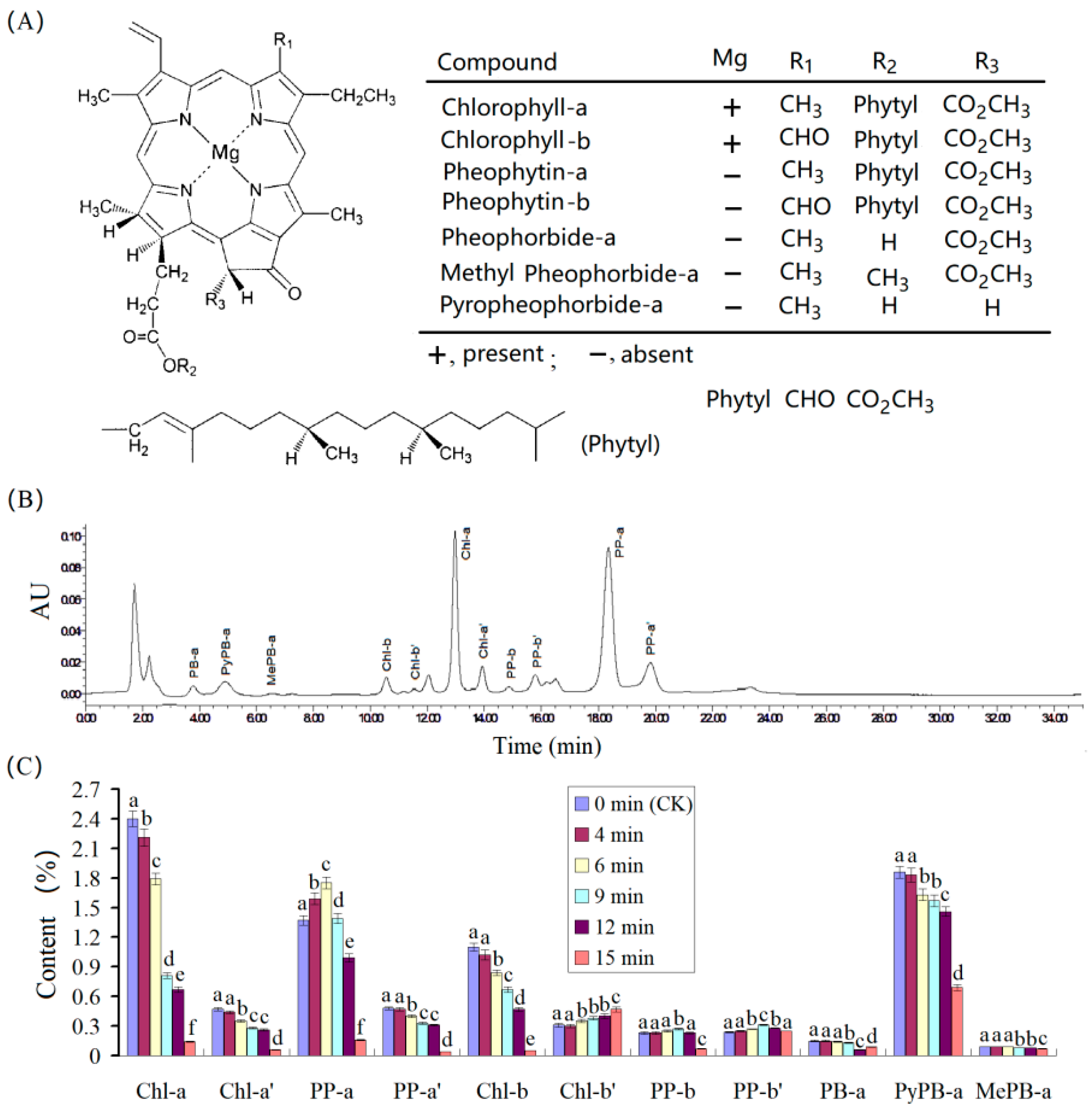
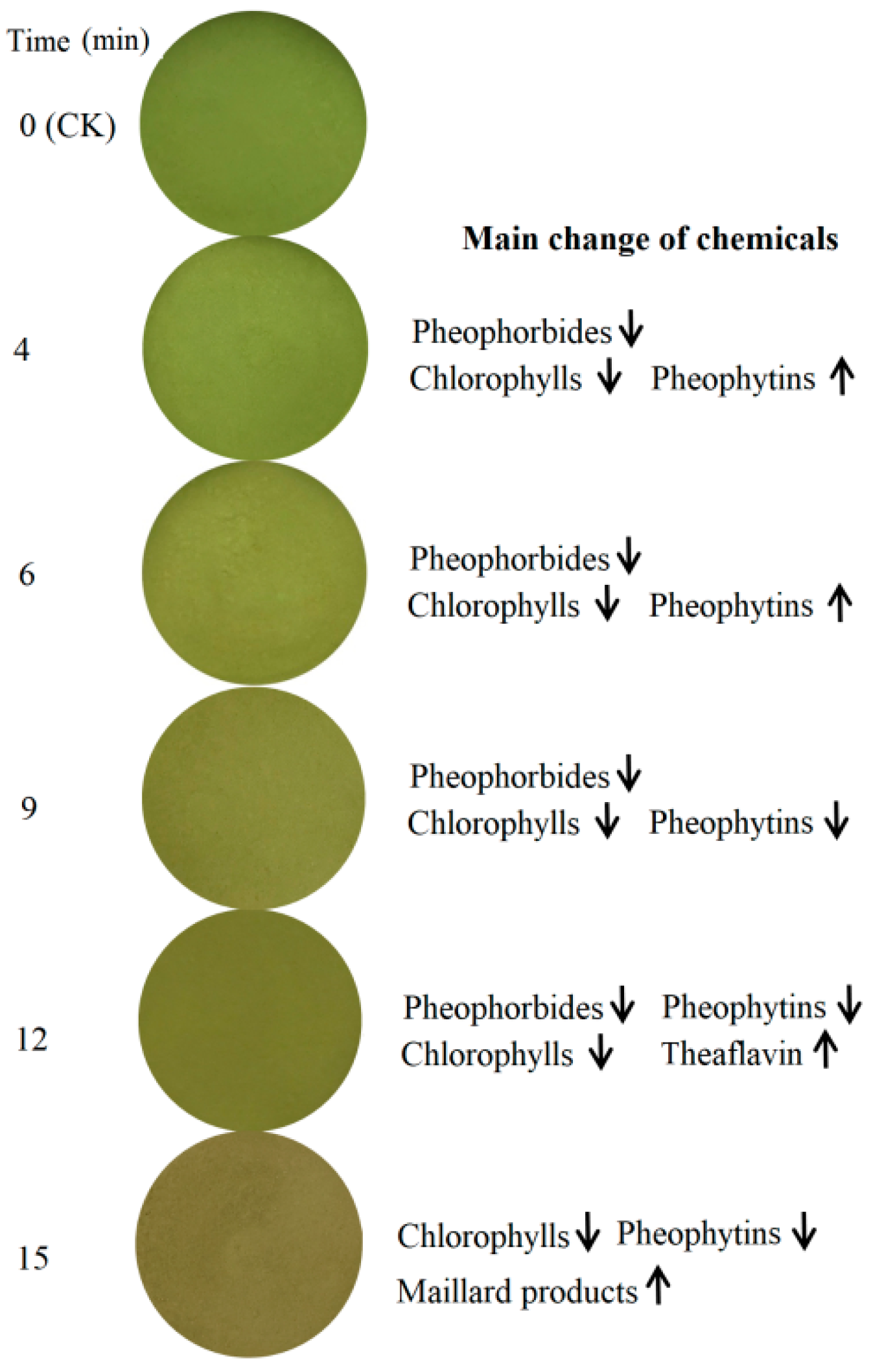
3.3. Chemicals to Contribute Color Loss in Green Tea Powder under Microwave Radiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurauchi, Y.; Devkota, H.P.; Hori, K.; Nishihara, Y.; Hisatsune, A.; Seki, T.; Katsuki, H. Anxiolytic activities of matcha tea powder, extracts, and fractions in mice: Contribution of dopamine D1 receptor- and serotonin 5-HT1A receptor-mediated mechanisms. J. Funct. Foods 2019, 59, 301–308. [Google Scholar] [CrossRef]
- Baba, R.; Amano, Y.; Wada, Y.; Kumazawa, K. Characterization of the potent odorants contributing to the characteristic aroma of matcha by gas chromatography- olfactometry techniques. J. Agric. Food Chem. 2017, 65, 2984–2989. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Ryu, H.; Zhang, T.; Lee, C.H.; Seo, H.G.; Han, S.G. Green tea powder supplementation enhances fermentation and antioxidant activity of set-type yogurt. Food Sci. Biotechnol. 2018, 27, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Dönmez, Ö.; Mogol, B.A.; Gökmen, V. Syneresis and rheological behaviors of set yogurt containing green tea and green coffee powders. J. Dairy Sci. 2017, 100, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Dekker, M.; Piqueras-Fiszman, B. An intervention study on the effect of matcha tea, in drink and snack bar formats, on mood and cognitive performance. Food Res. Int. 2017, 99, 72–83. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health benefits and chemical composition of matcha green tea: A Review. Molecules 2021, 26, 85. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wang, Y.; Hu, Q.H. Development of maccha ice-cream, maccha milk and maccha noodle. Food Sci. 2004, 25, 122–124. [Google Scholar] [CrossRef]
- Park, D.J.; Imm, J.Y.; Ku, K.H. Improved dispersibility of green tea powder by microparticulation and formulation. J. Food Sci. 2010, 66, 793–798. [Google Scholar] [CrossRef]
- Shen, W.; Yin, J.; Chen, G. The current situation and innovation development of Chinese matcha industry. China Tea 2020, 42, 9–13. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Guzik, P.; Kulawik, P.; Zając, M.; Migda, W. Microwave applications in the food industry: An overview of recent developments. Crit. Rev. Food Sci. Nutr. 2021, 5, 1–20. [Google Scholar] [CrossRef]
- Soni, A.; Smith, J.; Thompson, A.; Brightwell, G. Microwave-induced thermal sterilization-A review on history, technical Progress, advantages and challenges as compared to the conventional methods. Trends Food Sci. Technol. 2020, 97, 433–442. [Google Scholar] [CrossRef]
- Demirhan, E.; Özbek, B. Color change kinetics of tea leaves during microwave drying. Int. J. Food Eng. 2015, 11, 255–263. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerković, I.; Sarais, G.; Congiu, F.; Marijanović, Z.; Kuś, P.M. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE L*Cab*h(ab)o chromaticity coordinates. Food Chem. 2014, 145, 284–291. [Google Scholar] [CrossRef]
- Wu, J.; Ouyang, Q.; Park, B.; Kang, R.; Wang, Z.; Wang, L.; Chen, Q. Physicochemical indicators coupled with multivariate analysis for comprehensive evaluation of matcha sensory quality. Food Chem. 2022, 371, 131100. [Google Scholar] [CrossRef]
- Mclellan, M.R.; Lind, L.R.; Kime, R.W. Hue angle determinations and statistical analysis for multiquadrant Hunter L, a, b data. J. Food Qual. 1995, 18, 235–240. [Google Scholar] [CrossRef]
- Rudra, S.G.; Sarkar, B.C.; Shivhare, U.S. Thermal degradation kinetics of chlorophyll in pureed coriander leaves. Food Bioprocess Technol. 2008, 1, 91–99. [Google Scholar] [CrossRef]
- Huang, Y.; Sheng, J.; Yang, F.; Hu, Q. Effect of enzyme inactivation by microwave and oven heating on preservation quality of green tea. J. Food Eng. 2007, 78, 687–692. [Google Scholar] [CrossRef]
- Kohata, K.; Hanada, K.; Horie, H. High performance liquid chromatographic determination of pheophorbide-a and its related chlorophyll derivatives in tea leaves. Food Sci. Technol. Int. Tokyo 1998, 4, 80–84. [Google Scholar] [CrossRef]
- Donlao, N.; Ogawa, Y. The influence of processing conditions on catechin, caffeine and chlorophyll contents of green tea (Camelia sinensis) leaves and infusions. LWT-Food Sci. Technol. 2019, 116, 108567. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Choi, S.Y.; Kozukue, E.; Kozukue, N. HPLC analysis of catechins, theaflavins, and alkaloids in commercial teas and green tea dietary supplements: Comparison of water and 80% ethanol/water extracts. J. Food Sci. 2006, 71, C328–C337. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef]
- Gamli, Ö. Kinetic study of color changes of tomato purees with microwave and conventional drying. J. Food Sci. Eng. 2011, 1, 366–373. [Google Scholar]
- Demirhan, E.; Özbek, B. Color change kinetics of microwave-dried basil. Dry. Technol. 2009, 27, 156–166. [Google Scholar] [CrossRef]
- Demırhan, E.; Özbek, B. Color change kinetics of celery leaves undergoing microwave heating. Chem. Eng. Commun. 2011, 198, 1189–1205. [Google Scholar] [CrossRef]
- Dadali, G.; Demirhan, E.; Özbek, B. Microwave heat treatment of spinach: Drying kinetics and effective moisture diffusivity. Dry. Technol. 2007, 25, 1703–1712. [Google Scholar] [CrossRef]
- Li, J. Research of matcha cake production technology. Food Eng. 2015, 4, 18–21. [Google Scholar] [CrossRef]
- Lu, T.M.; Lee, C.C.; Mau, J.L.; Lin, S.D. Quality and antioxidant property of green tea sponge cake. Food Chem. 2010, 119, 1090–1095. [Google Scholar] [CrossRef]
- Huang, Z. Study on processing technology of matcha cake with low sugar. Mod. Flour Mill. Indust. 2019, 33, 32–37. (In Chinese) [Google Scholar]
- Huang, X.; Qiu, H.; Wang, J.; Zhang, H.; Zhao, K.; Liu, Z. Optimization of processing technology for matcha roulade cake with response surface methodology. J. Anhui Agric. Univ. 2017, 44, 973–979. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, C.; Sun, J.; Wang, X.; Zhao, X.; Mao, Y.; Li, X.; Song, Z. Quantitative measurement of energy utilization efficiency and study of influence factors in typical microwave heating process. Energy 2015, 87, 678–685. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.I.; Gandul-Rojas, B. Thermal degradation kinetics of chlorophyll pigments in virgin olive oils. 1. Compounds of series a. J. Agric. Food Chem. 2010, 58, 6200–6208. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Roca, M. Cooking effects on chlorophyll profile of the main edible seaweeds. Food Chem. 2018, 266, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Shivhare, U.S.; Sarkar, B.C.; Ahmed, J. Thermal chlorophyll degradation kinetics of mint leaves puree. Int. J. Food Prop. 2007, 10, 853–865. [Google Scholar] [CrossRef]
- Mehdipoor Damiri, G.R.; Motamedzadegan, A.; Safari, R.; Shahidi, S.A.; Ghorbani, A. Evaluation of stability, physicochemical and antioxidant properties of extracted chlorophyll from Persian clover (Trifolium resupinatum L.). J. Food Meas. Charact. 2021, 15, 327–340. [Google Scholar] [CrossRef]
- Matsuo, Y.; Oowatashi, R.; Saito, Y.; Tanaka, T. Nonenzymatic biomimetic synthesis of black tea pigment theaflavins. Synlett 2017, 28, 2505–2508. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]

| Parameter | Power (w) | Y0 | A | K (m−1) | R2 | |||
|---|---|---|---|---|---|---|---|---|
| Value | σ | Value | σ | Value | σ | |||
| L* | 200 | 55.94 | 0.040 | −0.096 | 0.0297 | 0.0578 | 0.00836 | 0.9964 |
| 400 | 57.02 | 0.180 | −1.128 | 0.1635 | 0.0399 | 0.02062 | 0.9835 | |
| 600 | 57.83 | 0.248 | −2.001 | 0.2044 | 0.0493 | 0.01213 | 0.9967 | |
| 800 | 57.48 | 0.042 | −1.609 | 0.0411 | 0.1085 | 0.02204 | 0.9955 | |
| 1000 | 57.86 | 0.989 | −1.968 | 0.9148 | 0.1681 | 0.0426 | 0.9936 | |
| a* | 200 | −20.22 | 0.206 | 0.220 | 0.1139 | 0.0393 | 0.01691 | 0.9834 |
| 400 | −20.37 | 0.483 | 0.425 | 0.4224 | 0.1063 | 0.01007 | 0.9974 | |
| 600 | −22.23 | 0.247 | 2.007 | 0.1050 | 0.0995 | 0.02409 | 0.9896 | |
| 800 | −21.60 | 0.801 | 1.303 | 0.7495 | 0.1893 | 0.04443 | 0.9865 | |
| 1000 | −23.15 | 1.424 | 3.014 | 1.0645 | 0.2100 | 0.04088 | 0.9945 | |
| b* | 200 | 39.91 | 0.682 | −0.201 | 0.6160 | 0.0491 | 0.02570 | 0.9649 |
| 400 | 40.38 | 1.206 | −0.644 | 0.7847 | 0.0516 | 0.01915 | 0.9865 | |
| 600 | 41.41 | 0.689 | −1.651 | 0.5929 | 0.0603 | 0.01357 | 0.9960 | |
| 800 | 42.43 | 1.268 | −2.699 | 0.7908 | 0.0683 | 0.01665 | 0.9972 | |
| 1000 | 42.05 | 1.028 | −2.320 | 0.9718 | 0.1182 | 0.03778 | 0.9947 | |
| Ha | 200 | 116.97 | 1.286 | −0.229 | 0.6394 | 0.0195 | 0.00263 | 0.9995 |
| 400 | 116.89 | 0.973 | −0.255 | 0.8587 | 0.1284 | 0.01605 | 0.9947 | |
| 600 | 118.55 | 1.394 | −1.461 | 1.1385 | 0.1204 | 0.02589 | 0.9893 | |
| 800 | 118.03 | 1.163 | −0.880 | 1.0976 | 0.2310 | 0.05563 | 0.9823 | |
| 1000 | 119.08 | 1.088 | −2.171 | 0.7835 | 0.2635 | 0.04254 | 0.9946 | |
| Sample Thickness (TTh, mm) | Radiation Time (TI, s) | Radiation Energy (W/g) | L* | a* | b* | Hue Angle a (Hab, ◦) | GTP Final Temp. (Tm, °C) |
|---|---|---|---|---|---|---|---|
| 20 | 180 | 0.34 | 49.87 ± 0.85 | −7.26 ± 0.27 | 36.48 ± 0.44 | 100.65 | 132.8 ± 4.73 |
| 150 | 0.28 | 50.71 ± 0.71 | −10.91 ± 0.34 | 37.38 ± 0.49 | 105.70 | 113.6 ± 5.62 | |
| 120 | 0.22 | 51.61 ± 0.85 | −13.45 ± 0.33 | 38.12 ± 0.61 | 110.10 | 95.3 ± 4.86 | |
| 90 | 0.17 | 52.58 ± 0.79 | −16.42 ± 0.38 | 38.75 ± 0.54 | 112.71 | 78.2 ± 2.55 | |
| 60 | 0.11 | 53.61 ± 0.93 | −18.45 ± 0.34 | 39.13 ± 0.57 | 114.64 | 62.7 ± 4.26 | |
| 16 | 230 | 0.54 | 49.41 ± 0.71 | −8.81 ± 0.24 | 36.77 ± 0.54 | 104.06 | 119.5 ± 4.16 |
| 190 | 0.44 | 50.35 ± 0.67 | −12.18 ± 0.27 | 37.59 ± 0.74 | 108.24 | 101.2 ± 3.08 | |
| 150 | 0.35 | 51.35 ± 0.80 | −15.01 ± 0.26 | 38.27 ± 0.69 | 111.69 | 84.1 ± 2.03 | |
| 110 | 0.26 | 52.44 ± 0.92 | −17.14 ± 0.48 | 38.81 ± 0.53 | 113.49 | 69.5 ± 2.59 | |
| 70 | 0.16 | 53.63 ± 0.98 | −18.77 ± 0.54 | 39.17 ± 0.49 | 115.13 | 55.3 ± 3.10 | |
| 12 | 280 | 0.87 | 49.69 ± 1.08 | −13.49 ± 0.34 | 37.29 ± 0.57 | 109.70 | 96.7 ± 3.56 |
| 230 | 0.72 | 50.29 ± 0.98 | −15.75 ± 0.41 | 37.91 ± 0.65 | 112.91 | 80.5 ± 2.52 | |
| 180 | 0.56 | 51.37 ± 0.99 | −16.83 ± 0.54 | 38.48 ± 0.51 | 113.80 | 75.6 ± 3.90 | |
| 130 | 0.41 | 52.46 ± 1.21 | −18.17 ± 0.53 | 38.89 ± 0.79 | 114.89 | 62.8 ± 3.10 | |
| 80 | 0.25 | 53.68 ± 1.01 | −19.01 ± 0.45 | 39.23 ± 0.56 | 115.85 | 52.7 ± 2.72 | |
| 8 | 330 | 1.54 | 52.15 ± 1.01 | −17.47 ± 0.38 | 37.44 ± 0.71 | 113.94 | 76.2 ± 3.00 |
| 270 | 1.26 | 52.47 ± 1.04 | −18.51 ± 0.45 | 38.46 ± 0.58 | 115.36 | 65.4 ± 2.95 | |
| 210 | 0.98 | 53.06 ± 0.83 | −19.07 ± 0.28 | 39.08 ± 0.62 | 115.94 | 56.2 ± 3.44 | |
| 150 | 0.70 | 53.79 ± 0.83 | −19.47 ± 0.31 | 39.3 ± 0.65 | 116.07 | 49.2 ± 2.78 | |
| 90 | 0.42 | 54.59 ± 0.71 | −19.71 ± 0.38 | 39.49 ± 0.68 | 116.16 | 45.7 ± 2.29 | |
| 4 | 380 | 3.55 | 53.44 ± 0.84 | −18.61 ± 0.42 | 38.81 ± 0.61 | 115.09 | 53.7 ± 3.95 |
| 310 | 2.90 | 53.51 ± 0.82 | −19.32 ± 0.38 | 39.24 ± 0.71 | 116.19 | 45.3 ± 2.25 | |
| 240 | 2.24 | 53.94 ± 0.76 | −19.68 ± 0.49 | 39.44 ± 0.80 | 116.66 | 39.7 ± 1.84 | |
| 170 | 1.59 | 54.46 ± 0.91 | −19.86 ± 0.31 | 39.59 ± 0.45 | 116.66 | 35.2 ± 1.73 | |
| 100 | 0.93 | 55.01 ± 0.91 | −19.95 ± 0.38 | 39.68 ± 0.60 | 116.70 | 31.5 ± 1.37 | |
| CK | 0 | 0.00 | 55.85 ± 0.80 | −20 ± 0.45 | 39.7 ± 0.72 | 116.74 |
| Color Parameters | Ea (KJ/mol) at Different HTh (mm) | Fitting | |||||
|---|---|---|---|---|---|---|---|
| 20 | 16 | 12 | 8 | 4 | Model | R2 | |
| a* | 12.15 | 16.32 | 25.95 | 38.23 | 56.24 | Ea = −0.044 + 83.6 exp(−0.011 HTh) | 0.9989 |
| b* | 12.67 | 16.32 | 32.98 | 53.98 | 99.63 | Ea = 4.32 + 179.7 exp(−0.159 HTh) | 0.9981 |
| Ha | 0.84 | 6.18 | 22.51 | 56.22 | 65.59 | Ea = −80.4 + 164.1 exp(−0.041 HTh) | 0.9514 |
| L* | 6.59 | 10.09 | 29.55 | 47.28 | 82.67 | Ea = −8.43 +146.3 exp(−0.119 HTh) | 0.9943 |
| Time (min) | EC (%) | EGC (%) | ECG (%) | EGCG (%) | FAA (%) | RS (%) | TF (%) |
|---|---|---|---|---|---|---|---|
| 0 | 0.68 ± 0.008 a | 2.86 ± 0.041 a | 0.89 ±0.016 a | 5.24 ± 0.074 a | 5.01 ± 0.052 a | 1.04 ± 0.021 a | 0.00 |
| 4 | 0.67 ± 0.012 a | 2.86 ± 0.052 a | 0.89 ±0.018 a | 5.24 ± 0.091 a | 4.91 ± 0.067 a | 1.03 ± 0.024 a | 0.00 |
| 6 | 0.67 ± 0.018 a | 2.86 ± 0.061 a | 0.89 ±0.021 a | 5.24 ± 0.084 a | 4.81 ± 0.071 a | 1.04 ± 0.016 a | 0.00 |
| 9 | 0.68 ± 0.017 a | 2.86 ± 0.063 a | 0.89 ±0.022 a | 5.24 ± 0.098 a | 4.62 ± 0.063 ab | 1.04 ± 0.019 a | 0.00 |
| 12 | 0.60 ± 0.014 b | 2.56 ± 0.057 b | 0.91± 0.018 a | 5.02 ± 0.110 a | 4.44 ± 0.056 b | 1.02 ± 0.018 a | 0.011 ± 0.008 a |
| 15 | 0.37 ± 0.008 c | 1.79 ± 0.036 c | 0.75 ±0.017 b | 3.74 ± 0.075 b | 4.04 ± 0.052 c | 0.88 ± 0.017 b | 0.008 ± 0.003 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhu, Y.; Xie, D.; Zhang, H.; Zhang, Y.; Jin, P.; Du, Q. The Effect of Microwave Radiation on the Green Color Loss of Green Tea Powder. Foods 2022, 11, 2540. https://doi.org/10.3390/foods11162540
Wang H, Zhu Y, Xie D, Zhang H, Zhang Y, Jin P, Du Q. The Effect of Microwave Radiation on the Green Color Loss of Green Tea Powder. Foods. 2022; 11(16):2540. https://doi.org/10.3390/foods11162540
Chicago/Turabian StyleWang, Huijuan, Yan Zhu, Dongchao Xie, Haihua Zhang, Yahui Zhang, Peng Jin, and Qizhen Du. 2022. "The Effect of Microwave Radiation on the Green Color Loss of Green Tea Powder" Foods 11, no. 16: 2540. https://doi.org/10.3390/foods11162540
APA StyleWang, H., Zhu, Y., Xie, D., Zhang, H., Zhang, Y., Jin, P., & Du, Q. (2022). The Effect of Microwave Radiation on the Green Color Loss of Green Tea Powder. Foods, 11(16), 2540. https://doi.org/10.3390/foods11162540