Toxoplasma gondii in Foods: Prevalence, Control, and Safety
Abstract
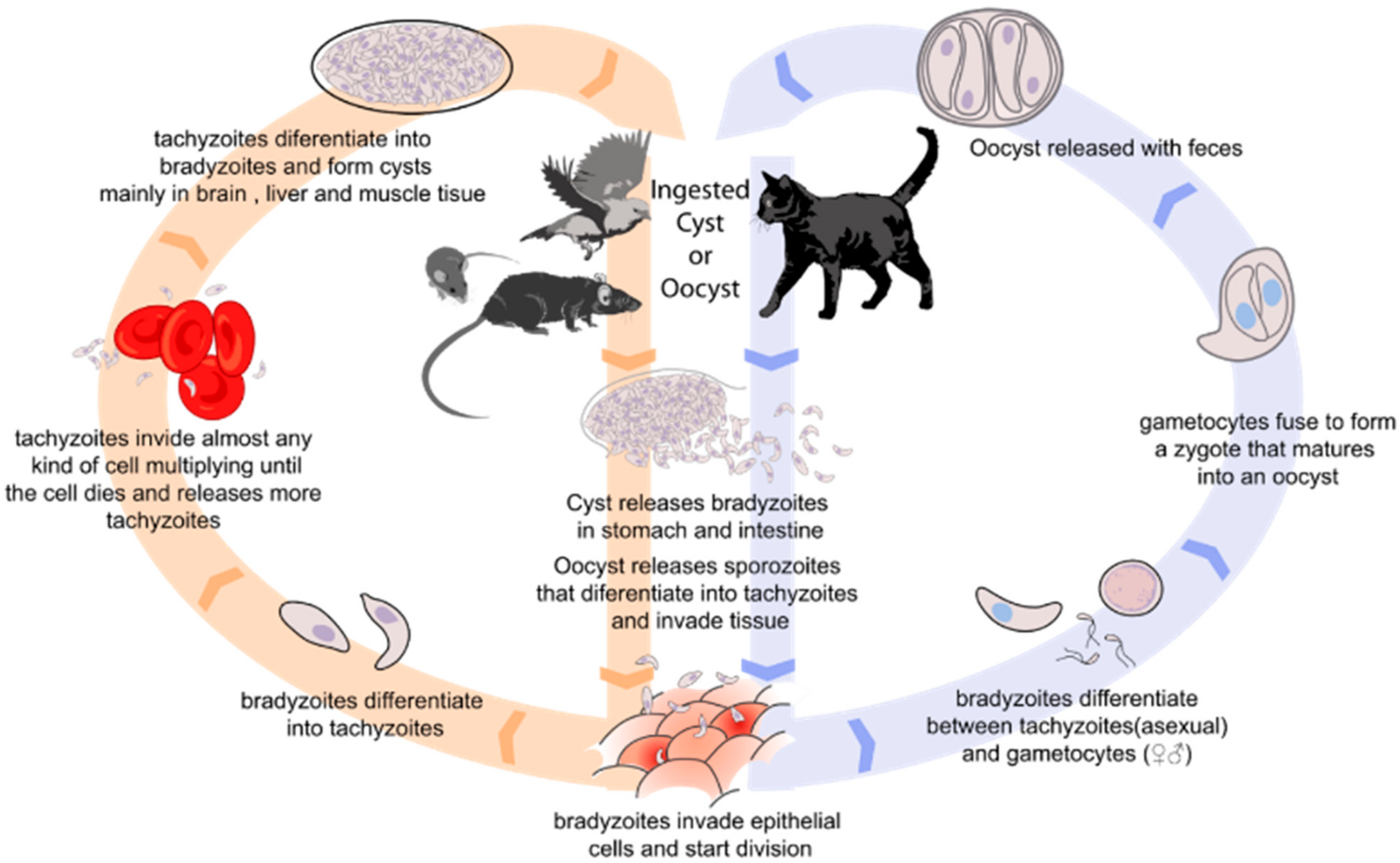
1. Introduction
2. Methods for T. gondii Detection in Food Products
2.1. Animal Model Bioassay
2.2. Cell Culture
2.3. Microscopic Methods
2.4. Molecular Methods
2.5. Serological Methods
2.6. New Methods of Detection
3. Prevalence of Toxoplasma gondii in Food Products
3.1. Meat and Meat Products
3.2. Milk and Dairy Products
3.3. Fresh Products and Vegetables
3.4. Marine Products
4. Control and Food Safety
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabilloud, M.; Wallon, M.; Peyron, F. In Utero and at Birth Diagnosis of Congenital Toxoplasmosis: Use of Likelihood Ratios for Clinical Management. Pediatr. Infect. Dis. J. 2010, 29, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, Y.A.; Read, J.S.; Committee on Infectious Diseases. Diagnosis, Treatment, and Prevention of Congenital Toxoplasmosis in the United States. Pediatrics 2017, 139, e20163860. [Google Scholar] [CrossRef]
- Wallon, M.; Peyron, F. Effect of Antenatal Treatment on the Severity of Congenital Toxoplasmosis. Clin. Infect Dis. 2016, 62, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Conceição, A.R.; Belucik, D.N.; Missio, L.; Gustavo Brenner, L.; Henrique Monteiro, M.; Ribeiro, K.S.; Costa, D.F.; Valadão, M.C.d.S.; Commodaro, A.G.; de Oliveira Dias, J.R.; et al. Ocular Findings in Infants with Congenital Toxoplasmosis after a Toxoplasmosis Outbreak. Ophthalmology 2021, 128, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Daher, D.; Shaghlil, A.; Sobh, E.; Hamie, M.; Hassan, M.E.; Moumneh, M.B.; Itani, S.; El Hajj, R.; Tawk, L.; El Sabban, M.; et al. Comprehensive Overview of Toxoplasma Gondii-Induced and Associated Diseases. Pathogens 2021, 10, 1351. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Rajapakse, S.; Weeratunga, P.; Rodrigo, C.; de Silva, N.L.; Fernando, S.D. Prophylaxis of Human Toxoplasmosis: A Systematic Review. Pathog. Glob. Health 2017, 111, 333–342. [Google Scholar] [CrossRef]
- Yazdani, M.R.; Mehrabi, Z.; Ataei, B.; Ghahfarokhi, A.B.; Moslemi, R.; Pourahmad, M. Frequency of Sero-Positivity in Household Members of the Patients with Positive Toxoplasma Serology. Rev. Esp. Quim. 2018, 31, 506–510. [Google Scholar]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Matta, S.K.; Rinkenberger, N.; Dunay, I.R.; Sibley, L.D. Toxoplasma gondii Infection and Its Implications within the Central Nervous System. Nat. Rev. Microbiol. 2021, 19, 467–480. [Google Scholar] [CrossRef]
- Hermes, G.; Ajioka, J.W.; Kelly, K.A.; Mui, E.; Roberts, F.; Kasza, K.; Mayr, T.; Kirisits, M.J.; Wollmann, R.; Ferguson, D.J.; et al. Neurological and Behavioral Abnormalities, Ventricular Dilatation, Altered Cellular Functions, Inflammation, and Neuronal Injury in Brains of Mice Due to Common, Persistent, Parasitic Infection. J. Neuroinflammation 2008, 5, 48. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Gressitt, K.L.; He, H.; Kannan, G.; Schultz, T.L.; Svezhova, N.; Carruthers, V.B.; Pletnikov, M.V.; Yolken, R.H.; et al. Cerebral Complement C1q Activation in Chronic Toxoplasma Infection. Brain Behav. Immun. 2016, 58, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.J.; Koshy, A.A. Latent Toxoplasmosis Effects on Rodents and Humans: How Much Is Real and How Much Is Media Hype? mBio 2020, 11, e02164-19. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.K.; Johnson, P.T.J. Toxoplasmosis: Recent Advances in Understanding the Link Between Infection and Host Behavior. Annu. Rev. Anim. Biosci. 2021, 9, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Vallochi, A.L.; Goldberg, A.C.; Falcai, A.; Ramasawmy, R.; Kalil, J.; Silveira, C.; Belfort, R.; Rizzo, L.V. Molecular Markers of Susceptibility to Ocular Toxoplasmosis, Host and Guest Behaving Badly. Clin. Ophthalmol. 2008, 2, 837–848. [Google Scholar]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States--Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Chaichan, P.; Mercier, A.; Galal, L.; Mahittikorn, A.; Ariey, F.; Morand, S.; Boumédiène, F.; Udonsom, R.; Hamidovic, A.; Murat, J.B.; et al. Geographical Distribution of Toxoplasma gondii Genotypes in Asia: A Link with Neighboring Continents. Infect. Genet. Evol. 2017, 53, 227–238. [Google Scholar] [CrossRef]
- Almeria, S.; Dubey, J.P. Foodborne Transmission of Toxoplasma gondii Infection in the Last Decade. An Overview. Res. Vet. Sci. 2021, 135, 371–385. [Google Scholar] [CrossRef]
- Shapiro, K.; Kim, M.; Rajal, V.B.; Arrowood, M.J.; Packham, A.; Aguilar, B.; Wuertz, S. Simultaneous Detection of Four Protozoan Parasites on Leafy Greens Using a Novel Multiplex PCR Assay. Food Microbiol. 2019, 84, 103252. [Google Scholar] [CrossRef]
- Luna, J.C.; Zamora, A.; Hernández-Arango, N.; Muñoz-Sánchez, D.; Pinzón, M.I.; Cortés-Vecino, J.A.; Lora-Suarez, F.; Gómez-Marín, J.E. Food Safety Assessment and Risk for Toxoplasmosis in School Restaurants in Armenia, Colombia. Parasitol. Res. 2019, 118, 3449–3457. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Public Health Risks Associated with Food-Borne Parasites. EFSA J. 2018, 16, e05495. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Opsteegh, M.; Dam-Deisz, C.; de Boer, P.; DeCraeye, S.; Faré, A.; Hengeveld, P.; Luiten, R.; Schares, G.; van Solt-Smits, C.; Verhaegen, B.; et al. Methods to Assess the Effect of Meat Processing on Viability of Toxoplasma Gondii: Towards Replacement of Mouse Bioassay by in Vitro Testing. Int. J. Parasitol. 2020, 50, 357–369. [Google Scholar] [CrossRef]
- Duong, H.D.; Taniguchi, Y.; Takashima, Y.; Sekiguchi, S.; Aye, K.M.; Ahmadi, P.; Bui, L.K.; Irie, T.; Nagayasu, E.; Yoshida, A. Diagnostic Value of Recombinant Nanoluciferase Fused Toxoplasma gondii Antigens in Luciferase-Linked Antibody Capture Assay (LACA) for Toxoplasma Infection in Pigs. J. Vet. Med. Sci. 2022, 84, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Suwan, E.; Chalermwong, P.; Rucksaken, R.; Sussadee, M.; Kaewmongkol, S.; Udonsom, R.; Jittapalapong, S.; Mangkit, B. Development and Evaluation of Indirect Enzyme-Linked Immunosorbent Assay Using Recombinant Dense Granule Antigen 7 Protein for the Detection of Toxoplasma gondii Infection in Cats in Thailand. Vet. World 2022, 15, 602–610. [Google Scholar] [CrossRef]
- Mancusi, A.; Giordano, A.; Bosco, A.; Girardi, S.; Proroga, Y.T.R.; Morena, L.; Pinto, R.; Sarnelli, P.; Cringoli, G.; Rinaldi, L.; et al. Development of a Droplet Digital Polymerase Chain Reaction Tool for the Detection of Toxoplasma gondii in Meat Samples. Parasitol. Res. 2022, 121, 1467–1473. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Devleesschauwer, B.; Graham, H.; Robertson, L.J.; van der Giessen, J.W. The Euro-Fbp Workshop Participants Prioritisation of Food-Borne Parasites in Europe, 2016. Eurosurveillance 2018, 23, 17–00161. [Google Scholar] [CrossRef]
- Dubey, J.P.; Verma, S.K.; Ferreira, L.R.; Oliveira, S.; Cassinelli, A.B.; Ying, Y.; Kwok, O.C.H.; Tuo, W.; Chiesa, O.A.; Jones, J.L. Detection and Survival of Toxoplasma gondii in Milk and Cheese from Experimentally Infected Goats. J. Food Prot. 2014, 77, 1747–1753. [Google Scholar] [CrossRef]
- Dehkordi, F.S.; Borujeni, M.R.H.; Rahimi, E.; Abdizadeh, R. Detection of Toxoplasma gondii in Raw Caprine, Ovine, Buffalo, Bovine, and Camel Milk Using Cell Cultivation, Cat Bioassay, Capture ELISA, and PCR Methods in Iran. Foodborne Pathog. Dis. 2013, 10, 120–125. [Google Scholar] [CrossRef]
- Opsteegh, M.; Maas, M.; Schares, G.; van der Giessen, J. Relationship between Seroprevalence in the Main Livestock Species and Presence of Toxoplasma gondii in Meat (GP/EFSA/BIOHAZ/2013/01) An Extensive Literature Review. Final Report. EFSA Support. Publ. 2016, 13, 996E. [Google Scholar] [CrossRef]
- El-Tras, W.F.; Tayel, A.A.; El-Kady, N.N. Source Diversity of Toxoplasma gondii Infection During Meal Preparation. J. Food Saf. 2012, 32, 1–5. [Google Scholar] [CrossRef]
- Esmerini, P.O.; Gennari, S.M.; Pena, H.F.J. Analysis of Marine Bivalve Shellfish from the Fish Market in Santos City, São Paulo State, Brazil, for Toxoplasma gondii. Vet. Parasitol. 2010, 170, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Toxoplasma gondii Oocyst Survival under Defined Temperatures. J. Parasitol. 1998, 84, 862–865. [Google Scholar] [CrossRef]
- De Oliveira Mendonça, A.; Domingues, P.F.; Vieira Da Silva, A.; Bergamaschi Pezerico, S.; Langoni, H. Detection of Toxoplasma gondii in Swine Sausages. Parasitol. Latinoam. 2004, 59, 42–45. [Google Scholar] [CrossRef][Green Version]
- Reiling, S.J.; Merks, H.; Zhu, S.; Boone, R.; Corneau, N.; Dixon, B.R. A Cloth-Based Hybridization Array System for Rapid Detection of the Food- and Waterborne Protozoan Parasites Giardia duodenalis, Cryptosporidium spp. and Toxoplasma gondii. Food Waterborne Parasitol. 2021, 24, e00130. [Google Scholar] [CrossRef]
- Marques, C.S.; Sousa, S.; Castro, A.; da Costa, J.M.C. Detection of Toxoplasma gondii Oocysts in Fresh Vegetables and Berry Fruits. Parasites Vectors 2020, 13, 180. [Google Scholar] [CrossRef]
- Deljavan, N.; Moosavy, M.-H.; Hajipour, N. Molecular Detection of Toxoplasma gondii DNA in Goats (Capra hircus), Sheep (Ovis aries), and Donkey (Equus asinus) Milk Using PCR in East Azerbaijan Province, Iran. Res. Vet. Sci. 2022, 152, 58–60. [Google Scholar] [CrossRef]
- Aigner, C.P.; da Silva, A.V.; Sandrini, F.; Osório, P.d.S.; Poiares, L.; Largura, A. Real-Time PCR-Based Quantification of Toxoplasma gondii in Tissue Samples of Serologically Positive Outdoor Chickens. Mem. Inst. Oswaldo Cruz 2010, 105, 935–937. [Google Scholar] [CrossRef]
- Marino, A.M.F.; Percipalle, M.; Giunta, R.P.; Salvaggio, A.; Caracappa, G.; Alfonzetti, T.; Aparo, A.; Reale, S. Development and Validation of a Real-Time PCR Assay for the Detection of Toxoplasma gondii DNA in Animal and Meat Samples. J. Vet. Diagn. Investig. 2017, 29, 203–207. [Google Scholar] [CrossRef]
- Temesgen, T.T.; Robertson, L.J.; Tysnes, K.R. A Novel Multiplex Real-Time PCR for the Detection of Echinococcus multilocularis, Toxoplasma gondii, and Cyclospora cayetanensis on Berries. Food Res. Int. 2019, 125, 108636. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Ma, L.; Kontogeorgos, I.; Zhang, X.; Li, X.; Karanis, P. First Molecular Detection of Toxoplasma gondii in Vegetable Samples in China Using Qualitative, Quantitative Real-Time PCR and Multilocus Genotyping. Sci. Rep. 2019, 9, 17581. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shapiro, K.; Rajal, V.B.; Packham, A.; Aguilar, B.; Rueda, L.; Wuertz, S. Quantification of Viable Protozoan Parasites on Leafy Greens Using Molecular Methods. Food Microbiol. 2021, 99, 103816. [Google Scholar] [CrossRef] [PubMed]
- Marangi, M.; Giangaspero, A.; Lacasella, V.; Lonigro, A.; Gasser, R.B. Multiplex PCR for the Detection and Quantification of Zoonotic Taxa of Giardia, Cryptosporidium and Toxoplasma in Wastewater and Mussels. Mol. Cell. Probes 2015, 29, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Thekisoe, O.M.M.; Aboge, G.O.; Kyan, H.; Yamagishi, J.; Inoue, N.; Nishikawa, Y.; Zakimi, S.; Xuan, X. Toxoplasma gondii: Sensitive and Rapid Detection of Infection by Loop-Mediated Isothermal Amplification (LAMP) Method. Exp. Parasitol. 2009, 122, 47–50. [Google Scholar] [CrossRef]
- Durand, L.; La Carbona, S.; Geffard, A.; Possenti, A.; Dubey, J.P.; Lalle, M. Comparative Evaluation of Loop-Mediated Isothermal Amplification (LAMP) vs QPCR for Detection of Toxoplasma gondii Oocysts DNA in Mussels. Exp. Parasitol. 2020, 208, 107809. [Google Scholar] [CrossRef]
- Lalle, M.; Possenti, A.; Dubey, J.P.; Pozio, E. Loop-Mediated Isothermal Amplification-Lateral-Flow Dipstick (LAMP-LFD) to Detect Toxoplasma gondii Oocyst in Ready-to-Eat Salad. Food Microbiol. 2018, 70, 137–142. [Google Scholar] [CrossRef]
- Mahmoudi, M.R.; Kazemi, B.; Haghighi, A.; Karanis, P. Detection of Acanthamoeba and Toxoplasma in River Water Samples by Molecular Methods in Iran. Iran. J. Parasitol. 2015, 10, 250–257. [Google Scholar]
- Fallahi, S.; Mazar, Z.A.; Ghasemian, M.; Haghighi, A. Challenging Loop-Mediated Isothermal Amplification (LAMP) Technique for Molecular Detection of Toxoplasma gondii. Asian Pac. J. Trop. Med. 2015, 8, 366–372. [Google Scholar] [CrossRef]
- Glor, S.B.; Edelhofer, R.; Grimm, F.; Deplazes, P.; Basso, W. Evaluation of a Commercial ELISA Kit for Detection of Antibodies against Toxoplasma gondii in Serum, Plasma and Meat Juice from Experimentally and Naturally Infected Sheep. Parasites Vectors 2013, 6, 85. [Google Scholar] [CrossRef]
- Schares, G.; Koethe, M.; Bangoura, B.; Geuthner, A.-C.; Randau, F.; Ludewig, M.; Maksimov, P.; Sens, M.; Bärwald, A.; Conraths, F.J.; et al. Toxoplasma gondii Infections in Chickens–Performance of Various Antibody Detection Techniques in Serum and Meat Juice Relative to Bioassay and DNA Detection Methods. Int. J. Parasitol. 2018, 48, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Mancianti, F.; Nardoni, S.; D’Ascenzi, C.; Pedonese, F.; Mugnaini, L.; Franco, F.; Papini, R. Seroprevalence, Detection of DNA in Blood and Milk, and Genotyping of Toxoplasma gondii in a Goat Population in Italy. Biomed. Res. Int. 2013, 2013, 905326. [Google Scholar] [CrossRef] [PubMed]
- Fabian, B.T.; Hedar, F.; Koethe, M.; Bangoura, B.; Maksimov, P.; Conraths, F.J.; Villena, I.; Aubert, D.; Seeber, F.; Schares, G. Fluorescent Bead-Based Serological Detection of Toxoplasma gondii Infection in Chickens. Parasites Vectors 2020, 13, 388. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H. Epidemiologic and Public Health Significance of Toxoplasma gondii Infections in Venison: 2009–2020. J. Parasitol. 2021, 107, 309–319. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Long-Term Survival of Toxoplasma gondii Sporulated Oocysts in Seawater. J. Parasitol. 2009, 95, 1019–1020. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Dubey, J.P. Foodborne Toxoplasmosis. Clin. Infect. Dis. 2012, 55, 845–851. [Google Scholar] [CrossRef]
- Kniel, K.E.; Lindsay, D.S.; Sumner, S.S.; Hackney, C.R.; Pierson, M.D.; Dubey, J.P. Examination of Attachment and Survival of Toxoplasma gondii Oocysts on Raspberries and Blueberries. J. Parasitol. 2002, 88, 790–793. [Google Scholar] [CrossRef]
- El-Nawawi, F.A.; Tawfik, M.A.; Shaapan, R.M. Methods for Inactivation of Toxoplasma gondii Cysts in Meat and Tissues of Experimentally Infected Sheep. Foodborne Pathog. Dis. 2008, 5, 687–690. [Google Scholar] [CrossRef]
- Juránková, J.; Basso, W.; Neumayerová, H.; Frencová, A.; Baláž, V.; Deplazes, P.; Koudela, B. Predilection Sites for Toxoplasma gondii in Sheep Tissues Revealed by Magnetic Capture and Real-Time PCR Detection. Food Microbiol. 2015, 52, 150–153. [Google Scholar] [CrossRef]
- Genchi, M.; Vismarra, A.; Mangia, C.; Faccini, S.; Vicari, N.; Rigamonti, S.; Prati, P.; Marino, A.M.; Kramer, L.; Fabbi, M. Lack of Viable Parasites in Cured “Parma Ham” (PDO), Following Experimental Toxoplasma gondii Infection of Pigs. Food Microbiol. 2017, 66, 157–164. [Google Scholar] [CrossRef]
- da Costa-Silva, T.A.; da Silva Meira, C.; Frazzatti-Gallina, N.; Pereira-Chioccola, V.L. Toxoplasma gondii Antigens: Recovery Analysis of Tachyzoites Cultivated in Vero Cell Maintained in Serum Free Medium. Exp. Parasitol. 2012, 130, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Zintl, A.; Halova, D.; Mulcahy, G.; O’Donovan, J.; Markey, B.; DeWaal, T. In Vitro Culture Combined with Quantitative TaqMan PCR for the Assessment of Toxoplasma gondii Tissue Cyst Viability. Vet. Parasitol. 2009, 164, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Toxoplasma gondii Infections in Chickens (Gallus domesticus): Prevalence, Clinical Disease, Diagnosis and Public Health Significance. Zoonoses Public Health 2010, 57, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Santoro, A.; Milardi, G.L.; Diaferia, M.; Branciari, R.; Miraglia, D.; Cioffi, A.; Gabrielli, S.; Ranucci, D. Comparison of PCR Assays Targeting the Multi-Copy Targets B1 Gene and 529 Bp Repetitive Element for Detection of Toxoplasma gondii in Swine Muscle. Food Microbiol. 2017, 63, 213–216. [Google Scholar] [CrossRef]
- Bayarri, S.; Gracia, M.J.; Lázaro, R.; Pe Rez-Arquillué, C.; Barberán, M.; Herrera, A. Determination of the Viability of Toxoplasma gondii in Cured Ham Using Bioassay: Influence of Technological Processing and Food Safety Implications. J. Food Prot. 2010, 73, 2239–2243. [Google Scholar] [CrossRef]
- Hohweyer, J.; Cazeaux, C.; Travaillé, E.; Languet, E.; Dumètre, A.; Aubert, D.; Terryn, C.; Dubey, J.P.; Azas, N.; Houssin, M.; et al. Simultaneous Detection of the Protozoan Parasites Toxoplasma, Cryptosporidium and Giardia in Food Matrices and Their Persistence on Basil Leaves. Food Microbiol. 2016, 57, 36–44. [Google Scholar] [CrossRef]
- Lemmon, G.H.; Gardner, S.N. Predicting the Sensitivity and Specificity of Published Real-Time PCR Assays. Ann. Clin. Microbiol. Antimicrob. 2008, 7, 18. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated Reaction by Loop-Mediated Isothermal Amplification Using Loop Primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Qu, D.; Zhou, H.; Han, J.; Tao, S.; Zheng, B.; Chi, N.; Su, C.; Du, A. Development of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) as a Diagnostic Tool of Toxoplasma gondii in Pork. Vet. Parasitol. 2013, 192, 98–103. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Schares, G.; Maksimov, P.; Joeres, M.; Ortega-Mora, L.M.; Calero-Bernal, R. Toxoplasma gondii Genotyping: A Closer Look Into Europe. Front. Cell. Infect. Microbiol. 2022, 12, 842595. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.; Villena, I.; Dumètre, A.; Escotte-Binet, S.; Favennec, L.; Dubey, J.P.; Aubert, D.; La Carbona, S. Evaluation of Propidium Monoazide-Based QPCR to Detect Viable Oocysts of Toxoplasma Gondii. Parasitol. Res. 2019, 118, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Dubey, J.P.; Hill, D.; Buchanan, R.L.; Gamble, H.R.; Jones, J.L.; Pradhan, A.K. Prevalence and Risk Factors for Toxoplasma gondii Infection in Meat Animals and Meat Products Destined for Human Consumption. J. Food Prot. 2015, 78, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Gamble, H.R.; Dubey, J.P.; Lambillotte, D.N. Comparison of a Commercial ELISA with the Modified Agglutination Test for Detection of Toxoplasma Infection in the Domestic Pig. Vet. Parasitol. 2005, 128, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Benedetto, S.M.C.; Coss, C.; McCrary, J.L.; Fournet, V.M.; Dubey, J.P. Effects of Time and Temperature on the Viability of Toxoplasma gondii Tissue Cysts in Enhanced Pork Loin. J. Food Prot. 2006, 69, 1961–1965. [Google Scholar] [CrossRef]
- Lalonde, L.F.; Gajadhar, A.A. Optimization and Validation of Methods for Isolation and Real-Time PCR Identification of Protozoan Oocysts on Leafy Green Vegetables and Berry Fruits. Food Waterborne Parasitol. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Slana, I.; Bier, N.; Bartosova, B.; Marucci, G.; Possenti, A.; Mayer-Scholl, A.; Jokelainen, P.; Lalle, M. Molecular Methods for the Detection of Toxoplasma gondii Oocysts in Fresh Produce: An Extensive Review. Microorganisms 2021, 9, 167. [Google Scholar] [CrossRef]
- Giangaspero, A.; Marangi, M.; Latrofa, M.S.; Annoscia, G.; Putignani, L.; Capelli, G.; Bonassisa, L.; Normanno, G.; Otranto, D.; Cereda, M.; et al. Efficiency of the Q3 Lab-on-Chip Real Time-PCR Platform for Detecting Protozoan Pathogens in Bivalve Mollusks. J. Food Sci. Technol. 2019, 56, 5000–5008. [Google Scholar] [CrossRef]
- Cazeaux, C.; Lalle, M.; Durand, L.; Aubert, D.; Favennec, L.; Dubey, J.P.; Geffard, A.; Villena, I.; La Carbona, S. Evaluation of Real-Time QPCR-Based Methods to Detect the DNA of the Three Protozoan Parasites Cryptosporidium Parvum, Giardia Duodenalis and Toxoplasma gondii in the Tissue and Hemolymph of Blue Mussels (M. edulis). Food Microbiol. 2022, 102, 103870. [Google Scholar] [CrossRef]
- Azimpour-Ardakan, T.; Fotouhi-Ardakani, R.; Hoghooghi-Rad, N.; Rokni, N.; Motallebi, A. Designing and Developing of High-Resolution Melting Technique for Separating Different Types of Toxoplasma gondii by Analysis of B1 and ROP8 Gene Regions. J. Microbiol. Methods 2021, 184, 106188. [Google Scholar] [CrossRef]
- Temesgen, T.T.; Barlaam, A.; Tysnes, K.R.; Robertson, L.J. Comparative Evaluation of UNEX-Based DNA Extraction for Molecular Detection of Cyclospora cayetanensis, Toxoplasma gondii, and Cryptosporidium parvum as Contaminants of Berries. Food Microbiol. 2020, 89, 103447. [Google Scholar] [CrossRef] [PubMed]
- Gisbert Algaba, I.; Geerts, M.; Jennes, M.; Coucke, W.; Opsteegh, M.; Cox, E.; Dorny, P.; Dierick, K.; De Craeye, S. A More Sensitive, Efficient and ISO 17025 Validated Magnetic Capture Real Time PCR Method for the Detection of Archetypal Toxoplasma gondii Strains in Meat. Int. J. Parasitol. 2017, 47, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Zhang, W.; Zhang, X.-Z.; Yao, X.-R.; Huang, W.; Jia, H.; Liu, X.-L.; Hou, S.-H.; Wang, X.-J. Development of a Real-Time Recombinase-Aided Amplification (RT-RAA) Molecular Diagnosis Assay for Sensitive and Rapid Detection of Toxoplasma Gondii. Vet. Parasitol. 2021, 298, 109489. [Google Scholar] [CrossRef] [PubMed]
- Loreck, K.; Mitrenga, S.; Meemken, D.; Heinze, R.; Reissig, A.; Mueller, E.; Ehricht, R.; Engemann, C.; Greiner, M. Development of a Miniaturized Protein Microarray as a New Serological IgG Screening Test for Zoonotic Agents and Production Diseases in Pigs. PLoS ONE 2019, 14, e0217290. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.D.; Appiah-Kwarteng, C.; Takashima, Y.; Aye, K.M.; Nagayasu, E.; Yoshida, A. A Novel Luciferase-Linked Antibody Capture Assay (LACA) for the Diagnosis of Toxoplasma gondii Infection in Chickens. Parasitol. Int. 2020, 77, 102125. [Google Scholar] [CrossRef]
- DeMone, C.; Hwang, M.-H.; Feng, Z.; McClure, J.T.; Greenwood, S.J.; Fung, R.; Kim, M.; Weese, J.S.; Shapiro, K. Application of next Generation Sequencing for Detection of Protozoan Pathogens in Shellfish. Food Waterborne Parasitol. 2020, 21, e00096. [Google Scholar] [CrossRef]
- Guggisberg, A.R.; Alvarez Rojas, C.A.; Kronenberg, P.A.; Miranda, N.; Deplazes, P. A Sensitive, One-Way Sequential Sieving Method to Isolate Helminths’ Eggs and Protozoal Oocysts from Lettuce for Genetic Identification. Pathogens 2020, 9, 624. [Google Scholar] [CrossRef]
- Opsteegh, M.; Prickaerts, S.; Frankena, K.; Evers, E.G. A Quantitative Microbial Risk Assessment for Meatborne Toxoplasma gondii Infection in The Netherlands. Int. J. Food Microbiol. 2011, 150, 103–114. [Google Scholar] [CrossRef]
- Yousefvand, A.; Mirhosseini, S.A.; Ghorbani, M.; Mohammadzadeh, T.; Moghaddam, M.M.; Mohammadyari, S. Molecular and Serological Detection and of Toxoplasma gondii in Small Ruminants of Southwest Iran and the Potential Risks for Consumers. J. Verbrauch. Lebensm. 2021, 16, 117–127. [Google Scholar] [CrossRef]
- Gharekhani, J.; Yakhchali, M.; Esmaeilnejad, B.; Mardani, K.; Majidi, G.; Sohrabi, A.; Berahmat, R.; Hazhir Alaei, M. Seroprevalence and Risk Factors of Neospora Caninum and Toxoplasma gondii in Small Ruminants in Southwest of Iran. Arch. Razi Inst. 2018, 73, 305–310. [Google Scholar] [CrossRef]
- Deksne, G.; Ligere, B.; Šneidere, A.; Jokelainen, P. Seroprevalence and Factors Associated with Toxoplasma gondii Infections in Sheep in Latvia: Latvian Dark Headed Sheep Breed Associated with Higher Seroprevalence. Vector Borne Zoonotic Dis. 2017, 17, 478–482. [Google Scholar] [CrossRef] [PubMed]
- El Safadi, D.; Abi Chahine, D.; Al Tarraf, A.; Raii, O.; Mesto, K.; Ismail, M.B.; Hamze, M. First Report on Seroprevalence and Risk Factors of Toxoplasma gondii Infection in Sheep and Goats in North Lebanon. J. Infect. Dev. Ctries. 2019, 13, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, B.; Kornacka, A.; Cybulska, A.; Cabaj, W.; Reiterova, K.; Bogdaszewski, M.; Steiner-Bogdaszewska, Z.; Bien, J. Seroprevalence of Toxoplasma gondii and Neospora caninum Infection in Sheep, Goats, and Fallow Deer Farmed on the Same Area. J. Anim. Sci. 2018, 96, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, D.; Kelifa, A.; Abdurahaman, M.; Yohannes, M. Seroepidemiology and Associated Risk Factors of Toxoplasma gondii in Sheep and Goats in Southwestern Ethiopia. BMC Vet. Res. 2016, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Bahreh, M.; Hajimohammadi, B.; Eslami, G. Toxoplasma gondii in Sheep and Goats from Central Iran. BMC Res. Notes 2021, 14, 46. [Google Scholar] [CrossRef]
- Satbige, A.S.; Sreekumar, C.; Rajendran, C.; Vijaya Bharathi, M. Isolation and Characterization of Toxoplasma gondii from Small Ruminants (Sheep and Goats) in Chennai City, South India. J. Parasit. Dis. 2017, 41, 869–873. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Zanzani, S.A.; Villa, L.; Manfredi, M.T. Toxoplasma gondii Infection in Meat-Producing Small Ruminants: Meat Juice Serology and Genotyping. Parasitol. Int. 2020, 76, 102060. [Google Scholar] [CrossRef]
- Jiang, N.; Su, R.; Jian, F.; Su, C.; Zhang, L.; Jiang, Y.; Yang, Y. Toxoplasma gondii in Lambs of China: Heart Juice Serology, Isolation and Genotyping. Int. J. Food Microbiol. 2020, 322, 108563. [Google Scholar] [CrossRef]
- Ai, K.; Huang, C.-Q.; Guo, J.-J.; Cong, H.; He, S.-Y.; Zhou, C.-X.; Cong, W. Molecular Detection of Toxoplasma gondii in the Slaughter Sheep and Goats from Shandong Province, Eastern China. Vector Borne Zoonotic Dis. 2020, 20, 193–196. [Google Scholar] [CrossRef]
- Amdouni, Y.; Rjeibi, M.R.; Rouatbi, M.; Amairia, S.; Awadi, S.; Gharbi, M. Molecular Detection of Toxoplasma gondii Infection in Slaughtered Ruminants (Sheep, Goats and Cattle) in Northwest Tunisia. Meat Sci. 2017, 133, 180–184. [Google Scholar] [CrossRef]
- Abdul Hamid, N.; Sadiq, M.B.; Ramanoon, S.Z.; Mansor, R.; Watanabe, M.; Md Isa, N.M.; Kamaludeen, J.; Syed-Hussain, S.S. Seroprevalence and Risk Factors of Toxoplasma gondii in Ruminant Meats from Wet Markets in Klang Valley and Abattoirs in Selangor, Malaysia. Animals 2020, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.C.; Ashander, L.M.; Appukuttan, B.; Woodman, R.J.; Dubey, J.P.; Whiley, H.; Smith, J.R. Lamb as a Potential Source of Toxoplasma gondii Infection for Australians. Aust. N. Z. J. Public Health 2020, 44, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Kalambhe, D.; Gill, J.P.S.; Singh, B.B. Molecular Detection of Toxoplasma gondii in the Slaughter Sheep and Goats from North India. Vet. Parasitol. 2017, 241, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Bachan, M.; Deb, A.R.; Maharana, B.R.; Sudhakar, N.R.; Sudan, V.; Saravanan, B.C.; Tewari, A.K. High Seroprevalence of Toxoplasma gondii in Goats in Jharkhand State of India. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 61–68. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Marino, A.M.F.; Garippa, G.; Rossi, L.; Mignone, W.; Dini, V.; Giunta, R.P.; Luini, M.; Villa, L.; Zanzani, S.A.; et al. Toxoplasma gondii Seroprevalence in Beef Cattle Raised in Italy: A Multicenter Study. Parasitol. Res. 2020, 119, 3893–3898. [Google Scholar] [CrossRef]
- Sroka, J.; Karamon, J.; Wójcik-Fatla, A.; Piotrowska, W.; Dutkiewicz, J.; Bilska-Zając, E.; Zając, V.; Kochanowski, M.; Dąbrowska, J.; Cencek, T. Toxoplasma gondii Infection in Slaughtered Pigs and Cattle in Poland: Seroprevalence, Molecular Detection and Characterization of Parasites in Meat. Parasites Vectors 2020, 13, 223. [Google Scholar] [CrossRef]
- Gharekhani, J.; Yakhchali, M. Risk Factors Associated to Toxoplasma gondii Infection in Dairy Farms in Hamedan Suburb, Iran. J. Parasit. Dis. 2020, 44, 116–121. [Google Scholar] [CrossRef]
- Langoni, H.; Generoso, D.; Hayasaka, Ê.Y.; Mantovan, K.B.; Menozzi, B.D.; Richini-Pereira, V.B.; da Silva, R.C. Molecular Characterization of Toxoplasma gondii and Sarcocystis spp. in Raw Kibbeh and Other Meat Samples Commercialized in Botucatu, Southeastern Brazil. Rev. Bras. Parasitol. Vet. 2021, 30, e029320. [Google Scholar] [CrossRef]
- Felin, E.; Hälli, O.; Heinonen, M.; Jukola, E.; Fredriksson-Ahomaa, M. Assessment of the Feasibility of Serological Monitoring and On-Farm Information about Health Status for the Future Meat Inspection of Fattening Pigs. Prev. Vet. Med. 2019, 162, 76–82. [Google Scholar] [CrossRef]
- Olsen, A.; Sandberg, M.; Houe, H.; Nielsen, H.V.; Denwood, M.; Jensen, T.B.; Alban, L. Seroprevalence of Toxoplasma gondii Infection in Sows and Finishers from Conventional and Organic Herds in Denmark: Implications for Potential Future Serological Surveillance. Prev. Vet. Med. 2020, 185, 105149. [Google Scholar] [CrossRef]
- Paştiu, A.I.; Cozma-Petruț, A.; Mercier, A.; Balea, A.; Galal, L.; Mircean, V.; Pusta, D.L.; Bogdan, L.; Györke, A. Prevalence and Genetic Characterization of Toxoplasma gondii in Naturally Infected Backyard Pigs Intended for Familial Consumption in Romania. Parasites Vectors 2019, 12, 586. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Cuenca, J.C.; Martínez-Moreno, Á.; Diaz-Cao, J.M.; Entrena-García, A.; Fraga, J.; Arias, P.C.; Almería, S.; García-Bocanegra, I. Seroprevalence of Toxoplasma gondii and Associated Risk Factors in Domestic Pigs Raised from Cuba. Parasitol. Res. 2021, 120, 2897–2903. [Google Scholar] [CrossRef] [PubMed]
- Gazzonis, A.L.; Marangi, M.; Villa, L.; Ragona, M.E.; Olivieri, E.; Zanzani, S.A.; Giangaspero, A.; Manfredi, M.T. Toxoplasma gondii Infection and Biosecurity Levels in Fattening Pigs and Sows: Serological and Molecular Epidemiology in the Intensive Pig Industry (Lombardy, Northern Italy). Parasitol. Res. 2018, 117, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, G.; Di Bella, S.; Purpari, G.; Giudice, E.; Mira, F.; Gucciardi, F.; Marino, A.M.F.; Russo, C.; Gómez-Morales, M.A.; Guercio, A. Evaluation of a Commercial Enzyme-Linked Immunosorbent Assay (ELISA) for Detecting Antibodies against Toxoplasma gondii from Naturally and Experimentally Infected Pigs. Infect. Dis. 2019, 51, 26–31. [Google Scholar] [CrossRef]
- Papini, R.; di Ciccio, P.; Marangi, M.; Ghidini, S.; Zanardi, E.; Vergara, A.; Giangaspero, A.; Nardoni, S.; Rocchigiani, G.; Mancianti, F.; et al. Occurrence of Toxoplasma gondii in Carcasses of Pigs Reared in Intensive Systems in Northern Italy. J. Food Prot. 2017, 80, 515–522. [Google Scholar] [CrossRef]
- Pipia, A.P.; Varcasia, A.; Dessì, G.; Panzalis, R.; Gai, C.; Nonnis, F.; Veronesi, F.; Tamponi, C.; Scala, A. Seroepidemiological and Biomolecular Survey on Toxoplasma gondii Infection on Organic Pig Farms. Parasitol. Res. 2018, 117, 1637–1641. [Google Scholar] [CrossRef]
- Kuruca, L.; Klun, I.; Uzelac, A.; Nikolić, A.; Bobić, B.; Simin, S.; Lalošević, V.; Lalošević, D.; Djurković-Djaković, O. Detection of Toxoplasma gondii in Naturally Infected Domestic Pigs in Northern Serbia. Parasitol. Res. 2017, 116, 3117–3123. [Google Scholar] [CrossRef]
- Oliveira, G.C.; de Souza Almeida, H.M.; Sartori, R.S.; Rossi, G.A.M.; de Oliveira, L.G.; Langoni, H. Prevalence of Toxoplasma gondii Infections in Swine of Non-Tecnified Rearing Farms of the Northeastern Region of the State of São Paulo, Brazil and Associated Risk Factors. Parasite Epidemiol. Control. 2019, 4, e00080. [Google Scholar] [CrossRef]
- Miura, A.C.; de Barros, L.D.; Ferreira, F.P.; Neto, J.M.F.; Sicupira Franco, P.M.L.; Su, C.; Vidotto, O.; Garcia, J.L. Genotyping of Toxoplasma gondii Isolated from Pigs for Human Consumption. Parasitol. Res. 2019, 118, 1593–1599. [Google Scholar] [CrossRef]
- Silva, E.M.C.; Sousa, P.D.S.; de Carvalho, S.K.G.S.; Marques, I.C.L.; Costa, F.B.; da Costa, A.P.; Santos, L.S.D.; Braga, M.d.S.C.O.; Abreu-Silva, A.L.; Machado, R.Z.; et al. High Level of Infection by Toxoplasma gondii in Pigs Slaughtered in the City of São Luís, Maranhão. Rev. Bras. Parasitol. Vet. 2021, 30, e008721. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Y.-L.; Yang, Z.; Li, X.-L.; Li, Z.-R.; Lin, Q. Seroprevalence and Risk Factors of Toxoplasma gondii in Slaughter Pigs in Shaanxi Province, Northwestern China. Vector Borne Zoonotic Dis. 2017, 17, 517–519. [Google Scholar] [CrossRef]
- Thakur, R.; Sharma, R.; Aulakh, R.S.; Gill, J.P.S.; Singh, B.B. Prevalence, Molecular Detection and Risk Factors Investigation for the Occurrence of Toxoplasma gondii in Slaughter Pigs in North India. BMC Vet. Res. 2019, 15, 431. [Google Scholar] [CrossRef]
- Gui, B.-Z.; Zheng, W.-B.; Zou, Y.; Lv, Q.-Y.; Liu, M.-T.; Li, F.; Yuan, A.-W.; Li, R.-C.; Liu, G.-H. Molecular Detection and Genotyping of Toxoplasma gondii in Pigs for Human Consumption in Hunan Province, China. Foodborne Pathog. Dis. 2018, 15, 809–813. [Google Scholar] [CrossRef]
- Vergara, A.; Marangi, M.; Caradonna, T.; Pennisi, L.; Paludi, D.; Papini, R.; Ianieri, A.; Giangaspero, A.; Normanno, G. Toxoplasma gondii Lineages Circulating in Slaughtered Industrial Pigs and Potential Risk for Consumers. J. Food Prot. 2018, 81, 1373–1378. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J.; Mi, R.; Ling, H.; Luo, L.; Jia, H.; Zhang, X.; Huang, Y.; Gong, H.; Han, X.; et al. Molecular Detection and Genetic Characterization of Toxoplasma gondii in Pork from Chongqing, Southwest China. Acta Trop. 2021, 224, 106134. [Google Scholar] [CrossRef]
- Costa, D.F.; Fowler, F.; Silveira, C.; Nóbrega, M.J.; Nobrega, H.A.J.; Nascimento, H.; Rizzo, L.V.; Commodaro, A.G.; Belfort, R. Prevalence of Toxoplasma gondii DNA in Processed Pork Meat. Foodborne Pathog. Dis. 2018, 15, 734–736. [Google Scholar] [CrossRef]
- Sroka, J.; Bilska-Zając, E.; Wójcik-Fatla, A.; Zając, V.; Dutkiewicz, J.; Karamon, J.; Piotrowska, W.; Cencek, T. Detection and Molecular Characteristics of Toxoplasma gondii DNA in Retail Raw Meat Products in Poland. Foodborne Pathog. Dis. 2019, 16, 195–204. [Google Scholar] [CrossRef]
- Dos Santos Silva, A.C.; de Barros, L.D.; Barros, V.M.C.; de Alcântara, A.M.; Andrade, M.R.; Garcia, J.L.; Mota, R.A.; Porto, W.J.N. Occurrence of Atypical and New Genotypes of Toxoplasma gondii in Free-Range Chickens Intended for Human Consumption in Brazil. Acta Parasitol. 2020, 65, 774–778. [Google Scholar] [CrossRef]
- Thakur, R.; Sharma, R.; Aulakh, R.S.; Singh, B.B. Toxoplasma gondii in Chickens (Gallus domesticus) from North India. Acta Parasitol. 2021, 66, 185–192. [Google Scholar] [CrossRef]
- Ying, Y.; Verma, S.K.; Kwok, O.C.H.; Alibana, F.; Mcleod, R.; Su, C.; Dubey, J.P.; Pradhan, A.K. Prevalence and Genetic Characterization of Toxoplasma gondii in Free-Range Chickens from Grocery Stores and Farms in Maryland, Ohio and Massachusetts, USA. Parasitol. Res. 2017, 116, 1591–1595. [Google Scholar] [CrossRef]
- Zou, Y.; Nie, L.-B.; Zhang, N.-Z.; Zou, F.-C.; Zhu, X.-Q.; Cong, W. First Genetic Characterization of Toxoplasma gondii Infection in Poultry Meat Intended for Human Consumption in Eastern China. Infect. Genet. Evol. 2017, 55, 172–174. [Google Scholar] [CrossRef]
- Qian, W.; Yan, W.; Lv, C.; Bai, R.; Wang, T. Occurrence and Genetic Characterization of Toxoplasma gondii and Neospora Caninum in Slaughtered Domestic Rabbits in Central China. Parasite 2019, 26, 36. [Google Scholar] [CrossRef]
- Bărburaș, D.; Gyӧrke, A.; Blaga, R.; Bărburaș, R.; Kalmár, Z.; Vişan, S.; Mircean, V.; Blaizot, A.; Cozma, V. Toxoplasma gondii in Water Buffaloes (Bubalus bubalis) from Romania: What Is the Importance for Public Health? Parasitol. Res. 2019, 118, 2695–2703. [Google Scholar] [CrossRef]
- Bártová, E.; Kobédová, K.; Budíková, M.; Račka, K. Serological and Molecular Detection of Toxoplasma gondii in Farm-Reared Ostriches (Struthio camelus) in the Czech Republic. Int. J. Food Microbiol. 2021, 356, 109333. [Google Scholar] [CrossRef]
- Cong, W.; Chi, W.-B.; Sun, W.-W.; Shan, X.-F.; Kang, Y.-H.; Meng, Q.-F.; Qian, A.-D. First Report of Toxoplasma gondii Infection in Common Quails (Coturnix coturnix) Intended for Human Consumption in Three Provinces of Northeastern China. Vector Borne Zoonotic Dis. 2017, 17, 351–353. [Google Scholar] [CrossRef]
- Cong, W.; Chen, L.; Shan, X.-F.; Qian, A.-D.; Meng, Q.-F. First Genetic Characterization of Toxoplasma gondii Infection in Donkey Meat Slaughtered for Human Consumption in Shandong Province, Eastern China. Infect. Genet. Evol. 2018, 61, 1–3. [Google Scholar] [CrossRef]
- Cong, W.; Zhou, C.-X.; Chen, L.; Zou, Y.; Wang, W.-L.; Meng, Q.-F.; Qian, A.-D. Toxoplasma gondii and Neospora Caninum in Tolai Hares (Lepus tolai) Intended for Human Consumption in China: Seroprevalence, DNA Detection, and Genotyping. Foodborne Pathog. Dis. 2018, 15, 544–547. [Google Scholar] [CrossRef]
- Pedersen, K.; Bauer, N.E.; Rodgers, S.; Bazan, L.R.; Mesenbrink, B.T.; Gidlewski, T. Antibodies to Various Zoonotic Pathogens Detected in Feral Swine (Sus scrofa) at Abattoirs in Texas, USA. J. Food Prot. 2017, 80, 1239–1242. [Google Scholar] [CrossRef]
- Bai, M.-J.; Zou, Y.; Elsheikha, H.M.; Ma, J.-G.; Zheng, W.-B.; Zhao, Q.; Zhang, X.-X.; Zhu, X.-Q. Toxoplasma gondii Infection in Farmed Wild Boars (Sus scrofa) in Three Cities of Northeast China. Foodborne Pathog. Dis. 2017, 14, 379–385. [Google Scholar] [CrossRef]
- Crotta, M.; Pellicioli, L.; Gaffuri, A.; Trogu, T.; Formenti, N.; Tranquillo, V.; Luzzago, C.; Ferrari, N.; Lanfranchi, P. Analysis of Seroprevalence Data on Hepatitis E Virus and Toxoplasma gondii in Wild Ungulates for the Assessment of Human Exposure to Zoonotic Meat-Borne Pathogens. Food Microbiol. 2022, 101, 103890. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M.; London, L.; Skrzypczak, T.; Kantala, T.; Laamanen, I.; Biström, M.; Maunula, L.; Gadd, T. Foodborne Zoonoses Common in Hunted Wild Boars. Ecohealth 2020, 17, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.M.R.; de Barros, L.D.; de Souza Lima Nino, B.; de Souza Pollo, A.; Dos Santos Silva, A.C.; Perles, L.; André, M.R.; Zacarias Machado, R.; Garcia, J.L.; Lux Hoppe, E.G. Toxoplasma gondii Infection in Wild Boars (Sus scrofa) from the State of São Paulo, Brazil: Serology, Molecular Characterization, and Hunter’s Perception on Toxoplasmosis. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100534. [Google Scholar] [CrossRef] [PubMed]
- Stollberg, K.C.; Schares, G.; Mayer-Scholl, A.; Hrushetska, I.; Diescher, S.; Johne, A.; Richter, M.H.; Bier, N.S. Comparison of Direct and Indirect Toxoplasma gondii Detection and Genotyping in Game: Relationship and Challenges. Microorganisms 2021, 9, 1663. [Google Scholar] [CrossRef]
- Santoro, M.; Viscardi, M.; Sgroi, G.; DʼAlessio, N.; Veneziano, V.; Pellicano, R.; Brunetti, R.; Fusco, G. Real-Time PCR Detection of Toxoplasma gondii in Tissue Samples of Wild Boars (Sus scrofa) from Southern Italy Reveals High Prevalence and Parasite Load. Parasites Vectors 2019, 12, 335. [Google Scholar] [CrossRef]
- Sgroi, G.; Viscardi, M.; Santoro, M.; Borriello, G.; D’Alessio, N.; Boccia, F.; Pacifico, L.; Fioretti, A.; Veneziano, V.; Fusco, G. Genotyping of Toxoplasma gondii in Wild Boar (Sus scrofa) in Southern Italy: Epidemiological Survey and Associated Risk for Consumers. Zoonoses Public Health 2020, 67, 805–813. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Villa, L.; Riehn, K.; Hamedy, A.; Minazzi, S.; Olivieri, E.; Zanzani, S.A.; Manfredi, M.T. Occurrence of Selected Zoonotic Food-Borne Parasites and First Molecular Identification of Alaria Alata in Wild Boars (Sus scrofa) in Italy. Parasitol. Res. 2018, 117, 2207–2215. [Google Scholar] [CrossRef]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Verma, S.K.; Kwok, O.C.H.; Pedersen, K.; Rosenthal, B.M.; Su, C. White-Tailed Deer (Odocoileus virginianus) Are a Reservoir of a Diversity of Toxoplasma gondii Strains in the USA and Pose a Risk to Consumers of Undercooked Venison. Parasitology 2020, 147, 775–781. [Google Scholar] [CrossRef]
- Castro-Scholten, S.; Cano-Terriza, D.; Jiménez-Ruiz, S.; Almería, S.; Risalde, M.A.; Vicente, J.; Acevedo, P.; Arnal, M.C.; Balseiro, A.; Gómez-Guillamón, F.; et al. Seroepidemiology of Toxoplasma gondii in Wild Ruminants in Spain. Zoonoses Public Health 2021, 68, 884–895. [Google Scholar] [CrossRef]
- Remes, N.; Kärssin, A.; Must, K.; Tagel, M.; Lassen, B.; Jokelainen, P. Toxoplasma gondii Seroprevalence in Free-Ranging Moose (Alces alces) Hunted for Human Consumption in Estonia: Indicator Host Species for Environmental Toxoplasma gondii Oocyst Contamination. Vet. Parasitol. Reg. Stud. Rep. 2018, 11, 6–11. [Google Scholar] [CrossRef]
- Skorpikova, L.; Reslova, N.; Lorencova, A.; Plhal, R.; Drimaj, J.; Kamler, J.; Slany, M. Molecular Detection of Toxoplasma gondii in Feathered Game Intended for Human Consumption in the Czech Republic. Int. J. Food Microbiol. 2018, 286, 75–79. [Google Scholar] [CrossRef]
- Bayarri, S.; Gracia, M.J.; Lázaro, R.; Pérez-Arquillué, C.; Herrera, A. Toxoplasma gondii in Meat and Food Safety Implications—A Review; IntechOpen: London, UK, 2012; ISBN 978-953-51-0479-7. [Google Scholar]
- Belluco, S.; Mancin, M.; Conficoni, D.; Simonato, G.; Pietrobelli, M.; Ricci, A. Investigating the Determinants of Toxoplasma gondii Prevalence in Meat: A Systematic Review and Meta-Regression. PLoS ONE 2016, 11, e0153856. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, E.L.; Morais, R.D.A.P.B.; Lima, M.d.S.; de Moraes, C.C.G.; Albuquerque, G.R.; da Silva, A.V.; Póvoa, M.M. Anti-Toxoplasma gondii Antibodies in Beef Cattle Slaughtered in the Metropolitan Region of Belém, Brazilian Amazon. Rev. Bras. Parasitol. Vet. 2017, 26, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Kijlstra, A.; Jongert, E. Control of the Risk of Human Toxoplasmosis Transmitted by Meat. Int. J. Parasitol. 2008, 38, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- van der Giessen, J.; Fonville, M.; Bouwknegt, M.; Langelaar, M.; Vollema, A. Seroprevalence of Trichinella Spiralis and Toxoplasma gondii in Pigs from Different Housing Systems in The Netherlands. Vet. Parasitol. 2007, 148, 371–374. [Google Scholar] [CrossRef]
- Batz, M.B.; Hoffmann, S.; Morris, J.G. Ranking the Disease Burden of 14 Pathogens in Food Sources in the United States Using Attribution Data from Outbreak Investigations and Expert Elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef]
- Sroka, J.; Kusyk, P.; Bilska-Zajac, E.; Karamon, J.; Dutkiewicz, J.; Wojcik-Fatla, A.; Zajac, V.; Stojecki, K.; Rozycki, M.; Cencek, T. Seroprevalence of Toxoplasma gondii Infection in Goats from the South-West Region of Poland and the Detection of T. Gondii DNA in Goat Milk. Folia Parasitol. 2017, 64, 023. [Google Scholar] [CrossRef]
- Felin, E.; Näreaho, A.; Fredriksson-Ahomaa, M. Comparison of Commercial ELISA Tests for the Detection of Toxoplasma Antibodies in the Meat Juice of Naturally Infected Pigs. Vet. Parasitol. 2017, 238, 30–34. [Google Scholar] [CrossRef]
- Gomez-Samblas, M.; Vilchez, S.; Ortega-Velázquez, R.; Fuentes, M.V.; Osuna, A. Absence of Toxoplasma gondii in 100% Iberian Products from Experimentally Infected Pigs Cured Following a Specific Traditional Process. Food Microbiol. 2021, 95, 103665. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, N.; Zhang, H.; Wang, F.; Li, H.; Liu, Y.; Zhao, X.; Zhang, X. Prevalence of Toxoplasma gondii Infections in Chicken Hearts from Farmers’ Markets and Supermarkets in the Tai’an Region of China. J. Food Prot. 2020, 3, 338–341. [Google Scholar] [CrossRef]
- Hiob, L.; Koethe, M.; Schares, G.; Goroll, T.; Daugschies, A.; Bangoura, B. Experimental Toxoplasma gondii and Eimeria tenella Co-Infection in Chickens. Parasitol. Res. 2017, 116, 3189–3203. [Google Scholar] [CrossRef]
- Schares, G.; Bangoura, B.; Randau, F.; Goroll, T.; Ludewig, M.; Maksimov, P.; Matzkeit, B.; Sens, M.; Bärwald, A.; Conraths, F.J.; et al. High Seroprevalence of Toxoplasma gondii and Probability of Detecting Tissue Cysts in Backyard Laying Hens Compared with Hens from Large Free-Range Farms. Int. J. Parasitol. 2017, 47, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Parker, S.; Al-Adhami, B.; Bachand, N.; Jenkins, E. Comparison of Tissues (Heart vs. Brain) and Serological Tests (MAT, ELISA and IFAT) for Detection of Toxoplasma gondii in Naturally Infected Wolverines (Gulo gulo) from the Yukon, Canada. Food Waterborne Parasitol. 2019, 15, e00046. [Google Scholar] [CrossRef]
- Plaza, J.; Dámek, F.; Villena, I.; Innes, E.A.; Katzer, F.; Hamilton, C.M. Detection of Toxoplasma gondii in Retail Meat Samples in Scotland. Food Waterborne Parasitol. 2020, 20, e00086. [Google Scholar] [CrossRef] [PubMed]
- Boughattas, S. Toxoplasma Infection and Milk Consumption: Meta-Analysis of Assumptions and Evidences. Crit. Rev. Food Sci. Nutr. 2017, 57, 2924–2933. [Google Scholar] [CrossRef]
- Jones, J.L.; Dargelas, V.; Roberts, J.; Press, C.; Remington, J.S.; Montoya, J.G. Risk Factors for Toxoplasma gondii Infection in the United States. Clin. Infect. Dis. 2009, 49, 878–884. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Z.-J.; Meng, Q.-F. Detection of Specific IgG-Antibodies Against Toxoplasma gondii in the Serum and Milk of Domestic Donkeys During Lactation in China: A Potential Public Health Concern. Front. Cell. Infect. Microbiol. 2021, 11, 760400. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, E.; Taus, N.S.; Ueti, M.W.; Sukhbaatar, L.; Bastsukh, Z.; Papageorgiou, S.; Fritz, H. Detection and Genotypic Characterization of Toxoplasma gondii DNA within the Milk of Mongolian Livestock. Parasitol. Res. 2019, 118, 2005–2008. [Google Scholar] [CrossRef] [PubMed]
- Luptakova, L.; Benova, K.; Rencko, A.; Petrovova, E. DNA Detection of Toxoplasma gondii in Sheep Milk and Blood Samples in Relation to Phase of Infection. Vet. Parasitol. 2015, 208, 250–253. [Google Scholar] [CrossRef]
- Vismarra, A.; Barilli, E.; Miceli, M.; Mangia, C.; Bacci, C.; Brindani, F.; Kramer, L. Toxoplasma gondii and Pre-Treatment Protocols for Polymerase Chain Reaction Analysis of Milk Samples: A Field Trial in Sheep from Southern Italy. Ital. J. Food Saf. 2017, 6, 6501. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Zanzani, S.A.; Stradiotto, K.; Olivieri, E.; Villa, L.; Manfredi, M.T. Toxoplasma gondii Antibodies in Bulk Tank Milk Samples of Caprine Dairy Herds. J. Parasitol. 2018, 104, 560–565. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Zanzani, S.A.; Villa, L.; Manfredi, M.T. Toxoplasma gondii in Naturally Infected Goats: Monitoring of Specific IgG Levels in Serum and Milk during Lactation and Parasitic DNA Detection in Milk. Prev. Vet. Med. 2019, 170, 104738. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.M.; Hussein, A.A.A.; Ewida, R.M. Occurrence of Toxoplasma gondii in Raw Goat, Sheep, and Camel Milk in Upper Egypt. Vet. World 2018, 11, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, J.; Yakhchali, M.; Afshari, A.; Adabi, M. Herd-Level Contamination of Neospora caninum, Toxoplasma gondii and Brucella in Milk of Iranian Dairy Farms. Food Microbiol. 2021, 100, 103873. [Google Scholar] [CrossRef] [PubMed]
- Koethe, M.; Schade, C.; Fehlhaber, K.; Ludewig, M. Survival of Toxoplasma gondii Tachyzoites in Simulated Gastric Fluid and Cow’s Milk. Vet. Parasitol. 2017, 233, 111–114. [Google Scholar] [CrossRef]
- Dumètre, A.; Dardé, M.L. How to Detect Toxoplasma gondii Oocysts in Environmental Samples? FEMS Microbiol. Rev. 2003, 27, 651–661. [Google Scholar] [CrossRef]
- Lass, A.; Pietkiewicz, H.; Szostakowska, B.; Myjak, P. The First Detection of Toxoplasma gondii DNA in Environmental Fruits and Vegetables Samples. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1101–1108. [Google Scholar] [CrossRef]
- Cabral Monica, T.; Pinto-Ferreira, F.; Martins, F.D.C.; de Matos, R.L.N.; de Matos, A.M.R.N.; Santos, A.C.; Nino, B.d.S.L.; Pereira, L.; Narciso, S.G.; Garcia, J.L.; et al. Epidemiology of a Toxoplasmosis Outbreak in a Research Institution in Northern Paraná, Brazil. Zoonoses Public Health 2020, 67, 760–764. [Google Scholar] [CrossRef]
- Ekman, C.C.J.; Chiossi, M.F.d.V.; Meireles, L.R.; de Andrade Júnior, H.F.; Figueiredo, W.M.; Marciano, M.A.M.; Luna, E.J.D.A. Case-Control Study of an Outbreak of Acute Toxoplasmosis in an Industrial Plant in the State of São Paulo, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2012, 54, 239–244. [Google Scholar] [CrossRef]
- Vieira, F.P.; Alves, M.d.G.; Martins, L.M.; Rangel, A.L.P.; Dubey, J.P.; Hill, D.; Bahia-Oliveira, L.M.G. Waterborne Toxoplasmosis Investigated and Analysed under Hydrogeological Assessment: New Data and Perspectives for Further Research. Mem. Inst. Oswaldo Cruz 2015, 110, 929–935. [Google Scholar] [CrossRef]
- Pinto-Ferreira, F.; Caldart, E.T.; Pasquali, A.K.S.; Mitsuka-Breganó, R.; Freire, R.L.; Navarro, I.T. Patterns of Transmission and Sources of Infection in Outbreaks of Human Toxoplasmosis. Emerg. Infect. Dis. 2019, 25, 2177–2182. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Karim, M.R.; Zhang, L. Detection of Human Intestinal Protozoan Parasites in Vegetables and Fruits: A Review. Parasites Vectors 2020, 13, 380. [Google Scholar] [CrossRef]
- Caradonna, T.; Marangi, M.; Del Chierico, F.; Ferrari, N.; Reddel, S.; Bracaglia, G.; Normanno, G.; Putignani, L.; Giangaspero, A. Detection and Prevalence of Protozoan Parasites in Ready-to-Eat Packaged Salads on Sale in Italy. Food Microbiol. 2017, 67, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Slany, M.; Dziedzinska, R.; Babak, V.; Kralik, P.; Moravkova, M.; Slana, I. Toxoplasma gondii in Vegetables from Fields and Farm Storage Facilities in the Czech Republic. FEMS Microbiol. Lett. 2019, 366, fnz170. [Google Scholar] [CrossRef] [PubMed]
- Berrouch, S.; Escotte-Binet, S.; Madline, A.; Aubert, D.; Nast, E.; La Carbona, S.; Hoummadi, L.; Hafid, J.; Villena, I. Protozoan Parasites and Leafy Greens in Marrakech: Study of Occurrence Using a Molecular Method. Acta Parasitol. 2021, 67, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Berrouch, S.; Escotte-Binet, S.; Amraouza, Y.; Flori, P.; Aubert, D.; Villena, I.; Hafid, J. Cryptosporidium spp., Giardia duodenalis and Toxoplasma gondii Detection in Fresh Vegetables Consumed in Marrakech, Morocco. Afr. Health Sci. 2020, 20, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Marchioro, A.A.; Tiyo, B.T.; Colli, C.M.; de Souza, C.Z.; Garcia, J.L.; Gomes, M.L.; Falavigna-Guilherme, A.L. First Detection of Toxoplasma gondii DNA in the Fresh Leafs of Vegetables in South America. Vector Borne Zoonotic Dis. 2016, 16, 624–626. [Google Scholar] [CrossRef]
- Barlaam, A.; Temesgen, T.T.; Tysnes, K.R.; Rinaldi, L.; Ferrari, N.; Sannella, A.R.; Normanno, G.; Cacciò, S.M.; Robertson, L.J.; Giangaspero, A. Contamination of Fresh Produce Sold on the Italian Market with Cyclospora cayetanensis and Echinococcus multilocularis. Food Microbiol. 2021, 98, 103792. [Google Scholar] [CrossRef]
- Aksoy, U.; Marangi, M.; Papini, R.; Ozkoc, S.; Bayram Delibas, S.; Giangaspero, A. Detection of Toxoplasma gondii and Cyclospora cayetanensis in Mytilus Galloprovincialis from Izmir Province Coast (Turkey) by Real Time PCR/High-Resolution Melting Analysis (HRM). Food Microbiol. 2014, 44, 128–135. [Google Scholar] [CrossRef]
- Ghozzi, K.; Marangi, M.; Papini, R.; Lahmar, I.; Challouf, R.; Houas, N.; Ben Dhiab, R.; Normanno, G.; Babba, H.; Giangaspero, A. First Report of Tunisian Coastal Water Contamination by Protozoan Parasites Using Mollusk Bivalves as Biological Indicators. Mar. Pollut. Bull. 2017, 117, 197–202. [Google Scholar] [CrossRef]
- Coupe, A.; Howe, L.; Shapiro, K.; Roe, W.D. Comparison of PCR Assays to Detect Toxoplasma gondii Oocysts in Green-Lipped Mussels (Perna canaliculus). Parasitol. Res. 2019, 118, 2389–2398. [Google Scholar] [CrossRef]
- Moratal, S.; Dea-Ayuela, M.A.; Cardells, J.; Marco-Hirs, N.M.; Puigcercós, S.; Lizana, V.; López-Ramon, J. Potential Risk of Three Zoonotic Protozoa (Cryptosporidium spp., Giardia duodenalis, and Toxoplasma gondii) Transmission from Fish Consumption. Foods 2020, 9, 1913. [Google Scholar] [CrossRef] [PubMed]
- Taghadosi, C.; Kojouri, G.A.; Taheri, M.A. Detection of Toxoplasma Antibodies in Sera of Salmonidae by ELISA. Comp. Clin. Pathol. 2010, 2, 203–206. [Google Scholar] [CrossRef]
- Cong, W.; Li, M.-Y.; Zou, Y.; Ma, J.-Y.; Wang, B.; Jiang, Z.-Y.; Elsheikha, H.M. Prevalence, Genotypes and Risk Factors for Toxoplasma gondii Contamination in Marine Bivalve Shellfish in Offshore Waters in Eastern China. Ecotoxicol. Environ. Saf. 2021, 213, 112048. [Google Scholar] [CrossRef] [PubMed]
- Coupe, A.; Howe, L.; Burrows, E.; Sine, A.; Pita, A.; Velathanthiri, N.; Vallée, E.; Hayman, D.; Shapiro, K.; Roe, W.D. First Report of Toxoplasma gondii Sporulated Oocysts and Giardia Duodenalis in Commercial Green-Lipped Mussels (Perna canaliculus) in New Zealand. Parasitol. Res. 2018, 117, 1453–1463. [Google Scholar] [CrossRef]
- Fung, R.; Manore, A.J.W.; Harper, S.L.; Sargeant, J.M.; Shirley, J.; Caughey, A.; Shapiro, K. Clams and Potential Foodborne Toxoplasma gondii in Nunavut, Canada. Zoonoses Public Health 2021, 68, 277–283. [Google Scholar] [CrossRef]
- Marino, A.M.F.; Giunta, R.P.; Salvaggio, A.; Castello, A.; Alfonzetti, T.; Barbagallo, A.; Aparo, A.; Scalzo, F.; Reale, S.; Buffolano, W.; et al. Toxoplasma gondii in Edible Fishes Captured in the Mediterranean Basin. Zoonoses Public Health 2019, 66, 826–834. [Google Scholar] [CrossRef]
- Putignani, L.; Mancinelli, L.; Del Chierico, F.; Menichella, D.; Adlerstein, D.; Angelici, M.C.; Marangi, M.; Berrilli, F.; Caffara, M.; di Regalbono, D.A.F.; et al. Investigation of Toxoplasma gondii Presence in Farmed Shellfish by Nested-PCR and Real-Time PCR Fluorescent Amplicon Generation Assay (FLAG). Exp. Parasitol. 2011, 127, 409–417. [Google Scholar] [CrossRef]
- Marquis, N.D.; Bishop, T.J.; Record, N.R.; Countway, P.D.; Fernández Robledo, J.A. Molecular Epizootiology of Toxoplasma gondii and Cryptosporidium parvum in the Eastern Oyster (Crassostrea virginica) from Maine (USA). Pathogens 2019, 8, 125. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Z.; Wang, S.; Tao, L.; Xu, L.; Yan, R.; Song, X.; Li, X. Detection of Toxoplasma gondii in Shellfish and Fish in Parts of China. Vet. Parasitol. 2014, 200, 85–89. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Rahimi, M.T.; Ghojoghi, A.; Rezaei, F.; Hatam-Nahavandi, K.; Oliveira, S.M.R.; de Lourdes Pereira, M.; Majidiani, H.; Siyadatpanah, A.; Elhamirad, S.; et al. Toxoplasma gondii Infection in Marine Animal Species, as a Potential Source of Food Contamination: A Systematic Review and Meta-Analysis. Acta Parasitol. 2022, 67, 592–605. [Google Scholar] [CrossRef]
- Jones, J.L.; Kruszon-Moran, D.; Wilson, M.; McQuillan, G.; Navin, T.; McAuley, J.B. Toxoplasma gondii Infection in the United States: Seroprevalence and Risk Factors. Am. J. Epidemiol. 2001, 154, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J.P. Toxoplasma Gondii: Transmission, Diagnosis and Prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, S.M.; Takeuchi, M.T.; Edwards, Z.M.; Edlefsen, M.; Kang, D.; Elaine Mayes, V.; Hillers, V.N. Food Safety Education Initiative to Increase Consumer Use of Food Thermometers in the United States. Br. Food J. 2006, 108, 775–794. [Google Scholar] [CrossRef]
- Hill, D.E.; Luchansky, J.; Porto-Fett, A.; Gamble, H.R.; Fournet, V.M.; Hawkins-Cooper, D.S.; Urban, J.F.; Gajadhar, A.A.; Holley, R.; Juneja, V.K.; et al. Rapid Inactivation of Toxoplasma gondii Bradyzoites during Formulation of Dry Cured Ready-to-Eat Pork Sausage. Food Waterborne Parasitol. 2018, 12, e00029. [Google Scholar] [CrossRef] [PubMed]
- Saridewi, R.; Lukman, D.; Sudarwanto, M.; Cahyanigsih, U.; Subekti, D. Survival of Toxoplasma gondii in Goat Milk after Pasteurization with Low Temperature and Long Time. Glob. Vet. 2013, 11, 789–793. [Google Scholar] [CrossRef]
- Rani, S.; Pradhan, A.K. Evaluating Uncertainty and Variability Associated with Toxoplasma gondii Survival during Cooking and Low Temperature Storage of Fresh Cut Meats. Int. J. Food Microbiol. 2021, 341, 109031. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Ferreira, F.; Paschoal, A.T.P.; Pasquali, A.K.S.; Bernardes, J.C.; Caldart, E.T.; Freire, R.L.; Mitsuka-Breganó, R.; Navarro, I.T. Techniques for Inactivating Toxoplasma gondii Oocysts: A Systematic Review. Rev. Bras. Parasitol. Vet. 2021, 30, e026420. [Google Scholar] [CrossRef]
- Djurković-Djaković, O.; Milenković, V. Effect of Refrigeration and Freezing on Survival of Toxoplasma gondii Tissue Cysts. Acta Vet. 2000, 50, 375–380. [Google Scholar]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High-Pressure Processing--Effects on Microbial Food Safety and Food Quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef]
- Kannan, G.; Prandovszky, E.; Steinfeldt, C.B.; Gressitt, K.L.; Yang, C.; Yolken, R.H.; Severance, E.G.; Jones-Brando, L.; Pletnikov, M.V. One Minute Ultraviolet Exposure Inhibits Toxoplasma gondii Tachyzoite Replication and Cyst Conversion without Diminishing Host Humoral-Mediated Immune Response. Exp. Parasitol. 2014, 145, 110–117. [Google Scholar] [CrossRef]
- Lacombe, A.; Breard, A.; Hwang, C.-A.; Hill, D.; Fan, X.; Huang, L.; Yoo, B.K.; Niemira, B.A.; Gurtler, J.B.; Wu, V.C.H. Inactivation of Toxoplasma gondii on Blueberries Using Low Dose Irradiation without Affecting Quality. Food Control 2017, 73, 981–985. [Google Scholar] [CrossRef]
- Mirza Alizadeh, A.; Jazaeri, S.; Shemshadi, B.; Hashempour-Baltork, F.; Sarlak, Z.; Pilevar, Z.; Hosseini, H. A Review on Inactivation Methods of Toxoplasma gondii in Foods. Pathog. Glob. Health 2018, 112, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Herrero, L.; Gracia, M.J.; Pérez-Arquillué, C.; Lázaro, R.; Herrera, A.; Bayarri, S. Toxoplasma gondii in Raw and Dry-Cured Ham: The Influence of the Curing Process. Food Microbiol. 2017, 65, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.S.; Collins, M.V.; Holliman, D.; Flick, G.J.; Dubey, J.P. Effects of High-Pressure Processing on Toxoplasma gondii Tissue Cysts in Ground Pork. J. Parasitol. 2006, 92, 195–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Innes, E.A.; Vermeulen, A.N. Vaccination as a Control Strategy against the Coccidial Parasites Eimeria, Toxoplasma and Neospora. Parasitology 2006, 133, S145–S168. [Google Scholar] [CrossRef]
- Katzer, F.; Canton, G.; Burrells, A.; Palarea-Albaladejo, J.; Horton, B.; Bartley, P.M.; Pang, Y.; Chianini, F.; Innes, E.A.; Benavides, J. Immunization of Lambs with the S48 Strain of Toxoplasma gondii Reduces Tissue Cyst Burden Following Oral Challenge with a Complete Strain of the Parasite. Vet. Parasitol. 2014, 205, 46–56. [Google Scholar] [CrossRef]
| Detection Method | Specific Method 1 | Type of Food Product | Detection Range (Sensitivity) 2 | References |
|---|---|---|---|---|
| Animal model bioassay | Cat | Milk | 25% | [29,30] |
| Meat | 100% | [31] | ||
| Mouse | Milk | 100% | [29] | |
| Meat | 100% (10 tachyzoites) | [24] | ||
| Fresh products | 13% | [32] | ||
| Bivalve mollusks | 2.5% | [33] | ||
| Water | 100% | [34] | ||
| Cell culture | Meat | 100% (10,000 tachyzoites) | [24] | |
| Milk | - | [30] | ||
| Microscopic method | Meat | - | [31] | |
| Molecular methods | PCR | Meat | 47.1% | [35] |
| Fresh products | 95–100% | [36,37] | ||
| Water | 100% | [36] | ||
| Milk | 100% | [29,38] | ||
| Cheese | 100% | [29] | ||
| qPCR | Meat | 92.3% (limit 0.01 pg) | [39,40] | |
| Fresh products | 100% (1 oocyst) | [41,42,43] | ||
| Bivalve mollusks | 100% | [44] | ||
| Water | 100% | [44] | ||
| LAMP | Lymph nodes | 85.7% | [45] | |
| Mussels | 5 oocyst/g | [46] | ||
| Fresh products | 25 oocyst/50 g | [47] | ||
| Water | 100% (1 fg) | [48,49] | ||
| Serological methods | IHA | Meat Juice | 100% (10,000 oocysts) | [50] |
| IFAT | Meat | 97% | [51] | |
| Meat Juice | 96.9% (10,000 oocysts) | [50] | ||
| MAT | Meat | 86.6% | [51] | |
| Milk | - | [52] | ||
| ELISA | Milk | - | [30] | |
| Meat | 91% | [51] | ||
| Meat Juice | 100% (10,000 oocysts) | [50] | ||
| BBMA | Meat | 98.5% | [53] |
| Animal | Sample Analyzed | Detection Method 1 | Number of Samples Tested | Number of Positive Samples (%) | Location | Reference |
|---|---|---|---|---|---|---|
| Sheep | Serum | ELISA | 150 | 26 (17.3%) | Iran | [89] |
| Serum | ELISA | 550 | 59 (10.8%) | Iran | [90] | |
| Serum | ELISA | 1039 | 179 (17.2%) | Latvia | [91] | |
| Serum | MAT | 100 | 42 (42%) | Lebanon | [92] | |
| Serum | ELISA | 64 | 30 (47%) | Slovakia | [93] | |
| Serum | DAT | 252 | 148 (58.2%) | Ethiopia | [94] | |
| Liver | PCR | 150 | 26 (17.3%) | Iran | [89] | |
| Liver | PCR | 90 | 13 (14.4%) | Iran | [95] | |
| Heart | PCR | 150 | 48 (32%) | Iran | [89] | |
| Brain and heart | MAT | 136 | 10 (7.4%) | India | [96] | |
| Meat juice | ELISA | 227 | 126 (28.6%) | Italy | [97] | |
| Meat juice | MAT | 166 | 11 (6.6%) | China | [98] | |
| Meat | PCR | 150 | 33 (22%) | Iran | [89] | |
| Meat | PCR | 438 | 43 (9.8) | China | [99] | |
| Meat | PCR | 150 | 50 (33.3) | Tunisia | [100] | |
| Meat | ELISA | 109 | 38 (34.9%) | Malaysia | [101] | |
| Meat | PCR | 79 | 34 (43%) | Australia | [102] | |
| Meat | PCR | 177 | 3 (1.7%) | India | [103] | |
| Goat | Serum | ELISA | 150 | 16 (10.7%) | Iran | [89] |
| Serum | ELISA | 185 | 37 (20%) | Iran | [90] | |
| Serum | ELISA | 445 | 189 (42.5%) | India | [104] | |
| Serum | MAT | 80 | 27 (34%) | Lebanon | [93] | |
| Serum | ELISA | 39 | 8 (21%) | Slovakia | [93] | |
| Serum | LAT | 116 | 64 (55.2%) | Ethiopia | [94] | |
| Liver | PCR | 150 | 24 (16%) | Iran | [89] | |
| Liver | PCR | 90 | 8 (8.8%) | India | [95] | |
| Heart | PCR | 150 | 36 (24%) | Iran | [89] | |
| Brain and heart | MAT | 57 | 4 (7%) | India | [96] | |
| Meat juice | ELISA | 51 | 14 (27.5%) | Italy | [97] | |
| Meat | PCR | 150 | 26 (17.3%) | Iran | [89] | |
| Meat | PCR | 254 | 27 (10.7) | China | [99] | |
| Meat | PCR | 120 | 39 (32.5) | Tunisia | [100] | |
| Meat | ELISA | 75 | 41 (54.7%) | Malaysia | [101] | |
| Meat | PCR | 223 | 3 (1.3%) | India | [104] | |
| Cattle | Serum | ELISA | 57 | 13 (22.8%) | Italy | [105] |
| Serum | DAT | 2411 | 313 (13%) | Poland | [106] | |
| Serum | ELISA | 400 | 52 (13%) | Iran | [107] | |
| Serum | IFAT | 500 | 2.3 (40.6%) | Brazil | [108] | |
| Meat | PCR | 150 | 29 (19.3) | Tunisia | [100] | |
| Meat | ELISA | 392 | 98 (25%) | Malaysia | [101] | |
| Meat | PCR | 48 | 5 (10.4%) | Brazil | [108] | |
| Pig | Serum | ELISA | 653 | 4 (0.6%) | Finland | [109] |
| Serum | ELISA | 447 | 73 (16.3%) | Denmark | [110] | |
| Serum | DAT | 3111 | 370 (11.9%) | Poland | [106] | |
| Serum | IFAT | 94 | 44 (46.8%) | Romania | [111] | |
| Serum | ELISA | 420 | 56 (23.3%) | Cuba | [112] | |
| Serum | ELISA | 370 | 14 (3.8%) | Italy | [113] | |
| Serum | ELISA and IFAT | 127 | 56 (44.1%) | Italy | [114] | |
| Serum | MAT | 375 | 8 (2.1%) | Italy | [115] | |
| Serum | ELISA | 414 | 214 (51.7%) | Italy | [116] | |
| Serum | MAT | 182 | 31 (17%) | Serbia | [117] | |
| Serum | MAT and IFAT | 356 | 25 (7%) and 48 (13.5%), respectively | Brazil | [118] | |
| Serum | MAT and IFAT | 400 | 26 (6.5%) | Brazil | [119] | |
| Serum | IFAT | 60 | 44 (77%) | Brazil | [120] | |
| Serum | IHA | 784 | 156 (19.9%) | China | [121] | |
| Tongue | PCR | 60 | 20 (33.3%) | Brazil | [120] | |
| Tongue and muscle | PCR | 810 | 54 (6.7%) | India | [122] | |
| Brain | PCR | 339 | 34 (10%) | China | [123] | |
| Brain | PCR | 107 | 51 (47.7%) | Italy | [116] | |
| Heart | PCR | 94 | 25 (26.6%) | Romania | [111] | |
| Heart | qPCR | 103 | 12 (11.6%) | Italy | [124] | |
| Diaphragm | PCR | 45 | 15 (33.3%) | Serbia | [117] | |
| Diaphragm | PCR | 1223 | 107 (8.7%) | China | [125] | |
| Diaphragm | PCR | 60 | 24 (40%) | Brazil | [120] | |
| Diaphragm | qPCR | 103 | 2 (1.9%) | Italy | [126] | |
| Tissue of seropositive animals | Mouse bioassay | 26 | 18 (69.2%) | Brazil | [119] | |
| Muscle | PCR | 60 | 23 (38.3%) | Brazil | [120] | |
| Meat juice | ELISA | 212 | 33 (15.6%) | Denmark | [110] | |
| Meat | qPCR | 118 | 46 (39%) | Brazil | [126] | |
| Meat | PCR | 498 | 165 (33.1%) | Italy | [64] | |
| Meat | PCR | 49 | 3 (6.1%) | Brazil | [108] | |
| Raw meat products | PCR | 3223 | 175 (5.4%) | Poland | [127] | |
| Chicken | Serum | IFAT | 200 | 72 (36%) | Brazil | [128] |
| Serum | ELISA | 522 | 34 (6.5%) | India | [129] | |
| Serum | LACA | 267 | 29 (10.9%) | Japan | [85] | |
| Brain | Mouse Bioassay | 14 | 2 (14.3%) | Brazil | [128] | |
| Heart juice | MAT | 1185 | 230 (19.4%) | USA | [130] | |
| Muscle and heart | PCR | 522 | 12 (2.3%) | India | [129] | |
| Meat | PCR | 257 | 21 (8.2%) | China | [131] | |
| Ducks | Meat | PCR | 115 | 9 (7.8%) | China | [131] |
| Geese | Meat | PCR | 42 | 2 (4.8%) | China | [131] |
| Rabbit | Brain and heart | PCR | 470 | 13 (2.8%) | China | [132] |
| Kibbeh | Meat | PCR | 44 | 1 (2.3%) | Brazil | [108] |
| Water Buffalo | Serum | MAT and ELISA | 197 | 16 (8.1%) and 13 (6.6%), respectively | Romania | [133] |
| Ostriches (farmed) | Serum | LAT | 409 | 149 (36%) | Czech Republic | [134] |
| Common quails (farmed) | Serum | MAT | 620 | 59 (9.5%) | China | [135] |
| Donkey (farmed) | Meat | PCR | 618 | 57 (9.2%) | China | [136] |
| Tolai hares (farmed) | Serum | PCR | 358 | 29 (8.1%) | China | [137] |
| Brain | PCR | 358 | 23 (6.4%) | China | [137] | |
| Feral swine | Serum | ELISA | 376 | 34 (9%) | USA | [138] |
| Wild boar (farmed) | Serum | LAT | 882 | 88 (10%) | China | [139] |
| Wild boar | Serum | ELISA | 331 | 164 (49%) | Italy | [140] |
| Serum | ELISA | 181 | 17 (9%) | Finland | [141] | |
| Serum | IFAT | 26 | 20 (76.9%) | Brazil | [142] | |
| Serum | ELISA | 306 | 61 (20%) | Germany | [143] | |
| Tissue | Mouse bioassay | 22 | 1 (4.5%) | Brazil | [142] | |
| Brain | qPCR | 141 | 44 (31.2%) | Italy | [144] | |
| Brain | PCR | 263 | 58 (22%) | Italy | [145] | |
| Heart | qPCR | 166 | 47 (28.3%) | Italy | [144] | |
| Heart | PCR | 310 | 70 (22.6%) | Italy | [145] | |
| Muscle | qPCR | 165 | 40 (24.2%) | Italy | [144] | |
| Muscle | PCR | 311 | 74 (23.8%) | Italy | [145] | |
| Meat juice | ELISA | 97 | 42 (43.3%) | Italy | [146] | |
| Meat | qPCR | 306 | 37 (12%) | Germany | [143] | |
| Venison | Serum | MAT | 914 | 329 (36%) | USA | [147] |
| Heart | Mouse bioassay | 36 | 11 (30.6%) | USA | [147] | |
| Roe deer | Serum | LAT | 356 | 141 (39.6%) | Spain | [148] |
| Serum | ELISA | 323 | 130 (40.2%) | Italy | [149] | |
| Serum | ELISA | 184 | 20 (11%) | Germany | [143] | |
| Meat | qPCR | 184 | 11 (6%) | Germany | [143] | |
| Fallow deer | Serum | LAT | 372 | 138 (37.1%) | Spain | [150] |
| Serum | ELISA | 167 | 17 (10%) | Slovakia | [93] | |
| Meat | qPCR | 80 | 2 (2%) | Germany | [143] | |
| Red deer | Serum | LAT | 553 | 92 (16.6%) | Spain | [148] |
| Serum | ELISA | 96 | 19 (19.8%) | Italy | [140] | |
| Serum | ELISA | 65 | 4 (6%) | Germany | [143] | |
| Meat | qPCR | 65 | 2 (2%) | Germany | [143] | |
| Southern chamois | Serum | LAT | 186 | 26 (14%) | Spain | [148] |
| Mouflon | Serum | LAT | 209 | 24 (11.5%) | Spain | [148] |
| Serum | ELISA | 50 | 12 (24%) | Italy | [140] | |
| Iberian wild goat | Serum | LAT | 346 | 27 (7.8%) | Spain | [148] |
| Chamois | Serum | ELISA | 104 | 4 (3.8%) | Italy | [140] |
| Barbary sheep | Serum | LAT | 18 | 1 (5.6%) | Spain | [148] |
| Moose | Serum | DAT | 463 | 111 (23.9%) | Estonia | [149] |
| Wild ducks | Brain | qPCR | 280 | 7 (2.5%) | Czech Republic | [150] |
| Heart | qPCR | 280 | 11 (3.9%) | Czech Republic | [150] | |
| Muscle | qPCR | 280 | 4 (1.4%) | Czech Republic | [150] | |
| Common pheasants | Brain | qPCR | 350 | 8 (2.3%) | Czech Republic | [150] |
| Heart | qPCR | 350 | 4 (1.1%) | Czech Republic | [150] | |
| Muscle | qPCR | 350 | 3 (0.9%) | Czech Republic | [150] |
| Animal | Sample Analyzed | Detection Method 1 | Number of Samples Tested | Number of Positive Samples (%) | Location | Reference |
|---|---|---|---|---|---|---|
| Donkey | Milk | ELISA | 418 | 41 (9.2%) | China | [167] |
| Goat | Milk | ELISA | 30 | 19 (63.3%) | Italy | [172] |
| Milk | PCR | 60 | 39 (65%) | Poland | [157] | |
| Milk | ELISA and qPCR | 30 | 27 (90%) and 1 (3.3%), respectively | Egypt | [173] | |
| Bulk tank milk | ELISA | 100 | 59 (59%) | Italy | [172] | |
| Sheep | Milk | PCR | 58 | 1 (1.7%) | Mongolia | [168] |
| Milk | ELISA and qPCR | 30 | 18 (60%) and 1 (3.3%), respectively | Egypt | [173] | |
| Camel | Milk | PCR | 9 | 8 (88.9%) | Mongolia | [168] |
| Milk | ELISA and qPCR | 30 | 1 (3.33%) and 0 (0%), respectively | Egypt | [173] | |
| Cattle | Bulk tank milk | ELISA | 149 | 8 (5.4%) | Iran | [174] |
| Product Analyzed | Detection Method 1 | Number of Samples Tested | Number of Positive Samples (%) | Location | Reference |
|---|---|---|---|---|---|
| Mixed-salad packages | qPCR | 648 packages | 5 (0.8%) | Italy | [183] |
| PCR | 90 packages | 8 (8.9%) | Czech Republic | [184] | |
| Leafy greens | qPCR | 152 | 45 (29.6%) | Morocco | [185] |
| Carrot | qPCR | 30 | 3 (10%) | Morocco | [186] |
| qPCR | 46 | 9 (19.5%) | Poland | [177] | |
| PCR | 93 | 7 (7.5%) | Czech Republic | [184] | |
| Chicory | PCR | 40 | 2 (5%) | Brazil | [187] |
| Red cabbage | qPCR | 8 | 1 (1.2%) | China | [42] |
| Coriander | qPCR | 29 | 8 (27.6%) | Morocco | [186] |
| Cucumber | PCR | 109 | 13 (11.9%) | Czech Republic | [184] |
| Lettuce | qPCR | 28 | 3 (10.7%) | Morocco | [186] |
| qPCR | 50 | 9 (18%) | Poland | [177] | |
| qPCR | 71 | 5 (7%) | China | [42] | |
| PCR | 168 | 5 (3%) | Brazil | [187] | |
| Spinach | qPCR | 50 | 2 (4%) | China | [42] |
| Parsley | qPCR | 29 | 13 (44.8%) | Morocco | [186] |
| PCR | 5 | 1 (20%) | Brazil | [187] | |
| Pak Choi | qPCR | 34 | 1 (2.9%) | China | [42] |
| Radish | qPCR | 16 | 1 (6.3%) | Morocco | [186] |
| qPCR | 60 | 3 (5%) | Poland | [42] | |
| Rape | qPCR | 22 | 1 (4.5%) | China | [42] |
| Rocket | PCR | 7 | 1 (14.3%) | Brazil | [187] |
| Animal | Sample Analyzed | Detection Method 1 | Number of Samples Tested | Number of Positive Samples (%) | Location | Reference |
|---|---|---|---|---|---|---|
| Bivalve shellfish | Tissue | PCR | 2907 | 82 (2.8%) | China | [194] |
| Green-lipped mussels | Tissue | PCR | 104 | 13 (16.4%) | New Zealand | [195] |
| Mediterranean mussel | Gills | qPCR | 53 pools at 795 specimens | 21 (39.6%) | Turkey | [189] |
| Clam | Tissue | qPCR | 61 pools at 1020 specimens | 4 (6.6%) | Tunisia | [190] |
| Digestive gland | PCR | 390 | 6 (1.5%) | Canada | [196] | |
| Haemolymph | PCR | 390 | 2 (0.6%) | Canada | [196] | |
| Mediterranean scald fish | Gills | PCR | 1 pool at 6 specimens | 1 (100%) | Italy | [197] |
| Pacific oyster | Gills | PCR | 6 pools at 109 specimens | 1 (16.67%) | Italy | [198] |
| Oyster | Mantle, gills, and rectum | qPCR | 1440 | 447 (31%) | USA | [199] |
| Bogue | Gills | PCR | 26 pools at 260 specimens | 4 (15.4%) | Italy | [197] |
| Intestine | PCR | 26 pools at 260 specimens | 3 (11.5%) | Italy | [197] | |
| Muscle | PCR | 26 pools at 260 fish | 6 (23.1%) | Italy | [197] | |
| White seabream | Muscle | PCR | 3 pools of 18 specimens | 1 (33.3%) | Italy | [197] |
| European anchovy | Gills | PCR | 35 pools at 350 specimens | 2 (5.7%) | Italy | [197] |
| Intestine | PCR | 35 pools at 350 specimens | 1 (2.9%) | Italy | [197] | |
| European hake | Gills | PCR | 15 pools at 90 specimens | 1 (6.7%) | Italy | [197] |
| Muscle | PCR | 15 pools at 90 specimens | 1 (6.7%) | Italy | [197] | |
| Red mullet | Intestine | PCR | 11 pools at 110 specimens | 3 (27.3%) | Italy | [197] |
| American prawn | Muscle | PCR | 618 | 4 | China | [197] |
| Nippon shrimp | Muscle | PCR | 813 | 1 | China | [200] |
| Axillary seabream | Gills | PCR | 8 pools at 80 specimens | 2 (25%) | Italy | [197] |
| Intestine | PCR | 8 pools at 80 specimens | 1 (12.5%) | Italy | [197] | |
| Muscle | PCR | 8 pools at 80 specimens | 1 (12.5%) | Italy | [197] | |
| Common pandora | Gills | PCR | 3 pools at 18 specimens | 1 (33.3%) | Italy | [197] |
| Intestine | PCR | 3 pools at 18 specimens | 2 (66.7%) | Italy | [197] | |
| Muscle | PCR | 3 pools at 18 specimens | 1 (33.3%) | Italy | [197] | |
| Thornback ray | Muscle | PCR | 1 fish | 1 (100%) | Italy | [198] |
| Red scorpionfish | Intestine | PCR | 1 pool at 3 specimens | 1 (100%) | Italy | [197] |
| Blotched picarel | Muscle | PCR | 4 pools at 24 specimens | 1 (25%) | Italy | [197] |
| Atlantic horse mackerel | Muscle | PCR | 15 pools at 120 specimens | 4 (26.7%) | Italy | [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-García, P.-J.; Planas, N.; Llobat, L. Toxoplasma gondii in Foods: Prevalence, Control, and Safety. Foods 2022, 11, 2542. https://doi.org/10.3390/foods11162542
Marín-García P-J, Planas N, Llobat L. Toxoplasma gondii in Foods: Prevalence, Control, and Safety. Foods. 2022; 11(16):2542. https://doi.org/10.3390/foods11162542
Chicago/Turabian StyleMarín-García, Pablo-Jesús, Nuria Planas, and Lola Llobat. 2022. "Toxoplasma gondii in Foods: Prevalence, Control, and Safety" Foods 11, no. 16: 2542. https://doi.org/10.3390/foods11162542
APA StyleMarín-García, P.-J., Planas, N., & Llobat, L. (2022). Toxoplasma gondii in Foods: Prevalence, Control, and Safety. Foods, 11(16), 2542. https://doi.org/10.3390/foods11162542








