Evaluation of Nutritional Quality and Sensory Parameters of Meat from Mallard and Four Species of Wild Goose
Abstract
1. Introduction
- What are the physical characteristics and nutritional values of the meat from the studied wildfowl species?
- To what extent are traces of heavy metals present in meat from the studied wildfowl species?
- What are the sensory characteristics of the meat from different goose species?
- Is it possible to discriminate between meat from mallards regarding gender and type?
2. Materials and Methods
2.1. Sampling
2.2. Instrumental Analyses
2.3. Chemical Analyses
2.4. Sensory Analyses
2.5. Statistical Analyses
2.6. Ethical Considerations
3. Results
3.1. Nutritional Content
3.2. Heavy Metal Content
3.3. Physical Characteristics
3.4. Sensory Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; The State of the World Series; The Food and Agriculture Organization (FAO): Rome, Italy, 2021. [Google Scholar]
- Ritchie, H.; Reay, D.S.; Higgins, P. Beyond Calories: A Holistic Assessment of the Global Food System. Front. Sustain. Food Syst. 2018, 2, 57. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Soriano, A.; Sánchez-García, C. Nutritional Composition of Game Meat from Wild Species Harvested in Europe, in Meat and Nutrition; Ranabhat, C.L., Ed.; IntechOpen: London, UK, 2021; 230p. [Google Scholar]
- Geldenhuys, G.; Muller, N.; Hoffman, L.C. The influence of season on the sensory profile of Egyptian goose (Alopochen aegyptiacus) meat. Poult. Sci. 2016, 95, 2174–2185. [Google Scholar] [CrossRef]
- Ottosson, U. Fåglarna i Sverige: Antal och Förekomst; Sveriges Ornitologiska Förening: Halmstad, Sweden, 2012. [Google Scholar]
- Fox, A.D.; Abraham, K.F. Why geese benefit from the transition from natural vegetation to agriculture. Ambio 2017, 46, 188–197. [Google Scholar] [CrossRef]
- Fox, A.D.; Madsen, J. Threatened species to super-abundance: The unexpected international implications of successful goose conservation. Ambio 2017, 46, 179–187. [Google Scholar] [CrossRef]
- Fox, A.D.; Elmberg, J.; Tombre, I.M.; Hessel, R. Agriculture and herbivorous waterfowl: A review of the scientific basis for improved management. Biol. Rev. Biol. Proc. Camb. Philos. Soc. 2017, 92, 854–877. [Google Scholar] [CrossRef]
- Liljebäck, N.; Bergqvist, G.; Elmberg, J.; Haas, F.; Nilsson, L.; Lindström, Å.; Månsson, J. Learning from long time series of harvest and population data: Swedish lessons for European goose management. Wildlife Biol. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Delacour, J. The Waterfowl of the World; Country Life Ltd.: London, UK, 1954. [Google Scholar]
- The Swedish Board of Agriculture (Jönköping, Sweden). Personal communication, 2022.
- Wetlands International. Waterbird Population Estimates. Available online: Wpe.wetlands.org (accessed on 28 February 2022).
- Guillemain, M.; Aubry, P.; Folliott, B.; Caizergues, A. Duck hunting bag estimates for the 2013/14 season in France. Wildfowl 2016, 66, 126–141. [Google Scholar]
- Solokha, A.; Gorokhovsky, K. Estimating waterbird harvest in Russia. In Proceedings of the 33rd International Union of Game Biologists Congress, Montpellier, France, 22–25 August 2017. [Google Scholar]
- Jägareförbundet. Viltdata.se. Available online: https://www.viltdata.se/ (accessed on 25 March 2022).
- Dalby, L.; Söderquist, P.; Christensen, T.K.; Clausen, P.; Einarsson, Á.; Elmberg, J.; Fox, A.D.; Holmqvist, N.; Langendoen, T.; Lehikoinen, A.; et al. The status of the Nordic populations of the Mallard (Anas platyrhynchos) in a changing world. Ornis Fenn. 2013, 90, 2–15. [Google Scholar]
- Moussy, C.; Quaintenne, G.; Gaudard, C. Comptage des Oiseaux d’eau à la mi-janvier en France. Résultats 2021 du comptage Wetlands International Ministère de la Transition écologique et solidaire. In LPO BirdLife France-Service Connaissance; Wetlands International: Ede, The Netherlands, 2021. [Google Scholar]
- Lincoln, F.C. Restocking of Marshes with Hand-Reared Mallards Not Proved Practicable. In Yearbook of Agriculture; U.S. Department of Agriculture: Washington, DC, USA, 1934. [Google Scholar]
- Madden, J.R. How many gamebirds are released in the UK each year? Eur. J. Wildl. Res. 2021, 67, 72. [Google Scholar] [CrossRef]
- Champagnon, J.; Gauthier-Clerc, M.; Lebreton, J.; Mouronval1, J.; Guillemain, M. Les canards colverts lâchés pour la chasse interagissent-ils avec les populations sauvages? Faune Sauvage 2013, 9. Available online: https://tourduvalat.org/wp-content/uploads/2017/11/article_fs_298_lacher_colverts_0.pdf (accessed on 14 August 2022).
- Söderquist, P. Large-Scale Releases of Native Species: The Mallard as a Predictive Model System; Sveriges Lantbruksuniversitet: Uppsala, Sweden, 2015; Available online: https://pub.epsilon.slu.se/11972/1/soderquist_p_150305.pdf (accessed on 14 August 2022).
- Guillemain, M.; Söderquist, P.; Champagnon, J.; Elmberg, J. Mallard (Anas platyrhynchos Linnaeus, 1758). In Invasive Birds: Global Trends and Impacts; Downs, C.T., Hart, L.A., Eds.; CAB Intemational: Wallingford, UK, 2020; pp. 194–199. [Google Scholar]
- Niewiadomska, K.; Kosicka-Gębska, M.; Gębski, J.; Gutkowska, K.; Jeżewska-Zychowicz, M.; Sułek, M. Game meat consumption-conscious choice or just a game? Foods 2020, 9, 1357. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Lewko, L.; Gornowicz, E.; Pietrzak, M.; Korol, W. The Effect of Origin, Sex and Feeding on Sensory Evaluation and Some Quality Characteristics of Goose Meat from Polish Native Flocks. Ann. Anim. Sci. 2017, 17, 1185–1196. [Google Scholar] [CrossRef][Green Version]
- Clinquart, A.; Ellies-Oury, M.; Hocquette, J.; Guillier, L.; Santé-Lhoutellier, V.; Prache, S. Review: On-farm and processing factors affecting bovine carcass and meat quality. Animal 2022, 16, 100426. [Google Scholar] [CrossRef]
- El-Deek, A.A.; Barakat, M.O.; Attia, Y.A.; El-Sebeay, A.S. Effect of feeding muscovy ducklings different protein sources: Performance, θ-3 fatty acid contents, and acceptability of their tissues. JAOCS 1997, 74, 999–1009. [Google Scholar] [CrossRef]
- Cramp, S.; Simmons, K.E.L. The Birds of the Western Palearctic; Clarendon Press: Oxford, UK, 1977. [Google Scholar]
- Dessborn, L.; Elmberg, J.; Nummi, P.; Pöysä, H.; Sjöberg, K. Hatching in dabbling ducks and emergence in chironomids: A case of predator–prey synchrony? Hydrobiologia 2009, 636, 319–329. [Google Scholar] [CrossRef]
- Pehrsson, O. Feeding behaviour, feeding habitat utilization, and feeding efficiency of mallard ducklings (Anas platyrhynchos L.) as guided by a domestic duck. Wildlife 1979, 10, 194–217. [Google Scholar]
- Song, Y.; Li, Y.; Zheng, S.; Dai, W.; Shen, X.; Zhang, Y.; Zhao, W.; Chang, G.; Xu, Q.; Chen, G. Effects of forage feeding versus grain feeding on the growth performance and meat quality of Yangzhou geese. Br. Poult. Sci. 2017, 58, 397–401. [Google Scholar] [CrossRef]
- Marjakangas, A.; Alhainen, M.; Fox, A.D.; Heinicke, T.; Madsen, J.; Nilsson, L.; Rozenfeld, S. International single species action plan for the conservation of the taiga bean goose Anser fabalis fabalis. AEWA Tech. Ser. 2015, 20, 1–76. [Google Scholar]
- Peterson, S.L.; Rockwell, R.F.; Witte, C.R.; Koons, D.N. The Legacy of Destructive Snow Goose Foraging on Supratidal Marsh Habitat in the Hudson Bay Lowlands. Arct. Antarct. Alp. Res. 2013, 45, 575–583. [Google Scholar] [CrossRef]
- Weng, K.; Huo, W.; Gu, T.; Bao, Q.; Hou, L.-E.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Effects of marketable ages on meat quality through fiber characteristics in the goose. Poult. Sci. 2021, 100, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Janiszewski, P.; Murawska, D.; Hanzal, V.; Gesek, M.; Michalik, D.; Zawacka, M. Carcass characteristics, meat quality, and fatty acid composition of wild-living mallards (Anas platyrhynchos L.). Poult. Sci. 2018, 97, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Boyd, H.; Harrison, J. First-autumn dispersal of hand-reared Mallard. Wildfowl Trust 1962, 12, 70–73. [Google Scholar]
- Olsson, V. Märkningar och återfynd av Svenska gräsänder, Anas platyrhynchos L. Swed. Wildl. 1960, 2, 87–152. [Google Scholar]
- Söderquist, P.; Gunnarsson, G.; Elmberg, J. Longevity and migration distance differ between wild and hand-reared mallards Anas platyrhynchos in Northern Europe. Eur. J. Wildl. Res. 2012, 59, 159–166. [Google Scholar] [CrossRef]
- Brakhage, G.K. Biology and behavior of tub-nesting Canada geese. J. Wildl. Manag. 1965, 29, 751–771. [Google Scholar] [CrossRef]
- Söderquist, P.; Gunnarsson, G.; Elmberg, J.; Dessborn, L. Survival of wild and farmed-released mallards: The Swedish example. Eur. J. Wildl. Res. 2021, 67, 19. [Google Scholar] [CrossRef]
- Kooloos, J.; Zweers, G. Mechanics of drinking in the mallard (Anas platyrhynchos, Anatidae). J. Morphol. 1989, 199, 327–347. [Google Scholar] [CrossRef]
- Söderquist, P.; Norrström, J.; Elmberg, J.; Guillemain, M.; Gunnarsson, G. Wild mallards have more “goose-like” bills than their ancestors: A case of anthropogenic influence? PLoS ONE 2014, 9, e115143. [Google Scholar] [CrossRef]
- Söderquist, P.; Elmberg, J.; Gunnarsson, G.; Thulin, C.-G.; Champagnon, J.; Guillemain, M.; Kreisinger, J.; Prins, H.H.T.; Crooijmans, R.P.M.A.; Kraus, R.H.S. Admixture between released and wild game birds: A changing genetic landscape in European mallards (Anas platyrhynchos). Eur. J. Wildl. Res. 2017, 63, 98. [Google Scholar] [CrossRef]
- Čížková, D.; Javůrková, V.; Champagnon, J.; Kreisinger, J. Duck’s not dead: Does restocking with captive bred individuals affect the genetic integrity of wild mallard (Anas platyrhynchos) population? Biol. Conserv. 2012, 152, 231–240. [Google Scholar] [CrossRef]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- Öhrvik, V.; Engman, J.; von Malmborg, A.; Wretling, S. Kött–Analys av Näringsämnen: Hjort, Lamm, Nötdjur, Ren, Rådjur, Vildsvin Och Kalkon; Swedish Food Agency: Uppsala, Sweden, 2013. [Google Scholar]
- Mateo, R.; Belliure, J.; Dolz, J.C.; Serrano, J.M.A.; Guitart, R. High prevalences of lead poisoning in wintering waterfowl in Spain. Arch. Environ. Contam. Toxicol. 1998, 35, 342–347. [Google Scholar] [CrossRef] [PubMed]
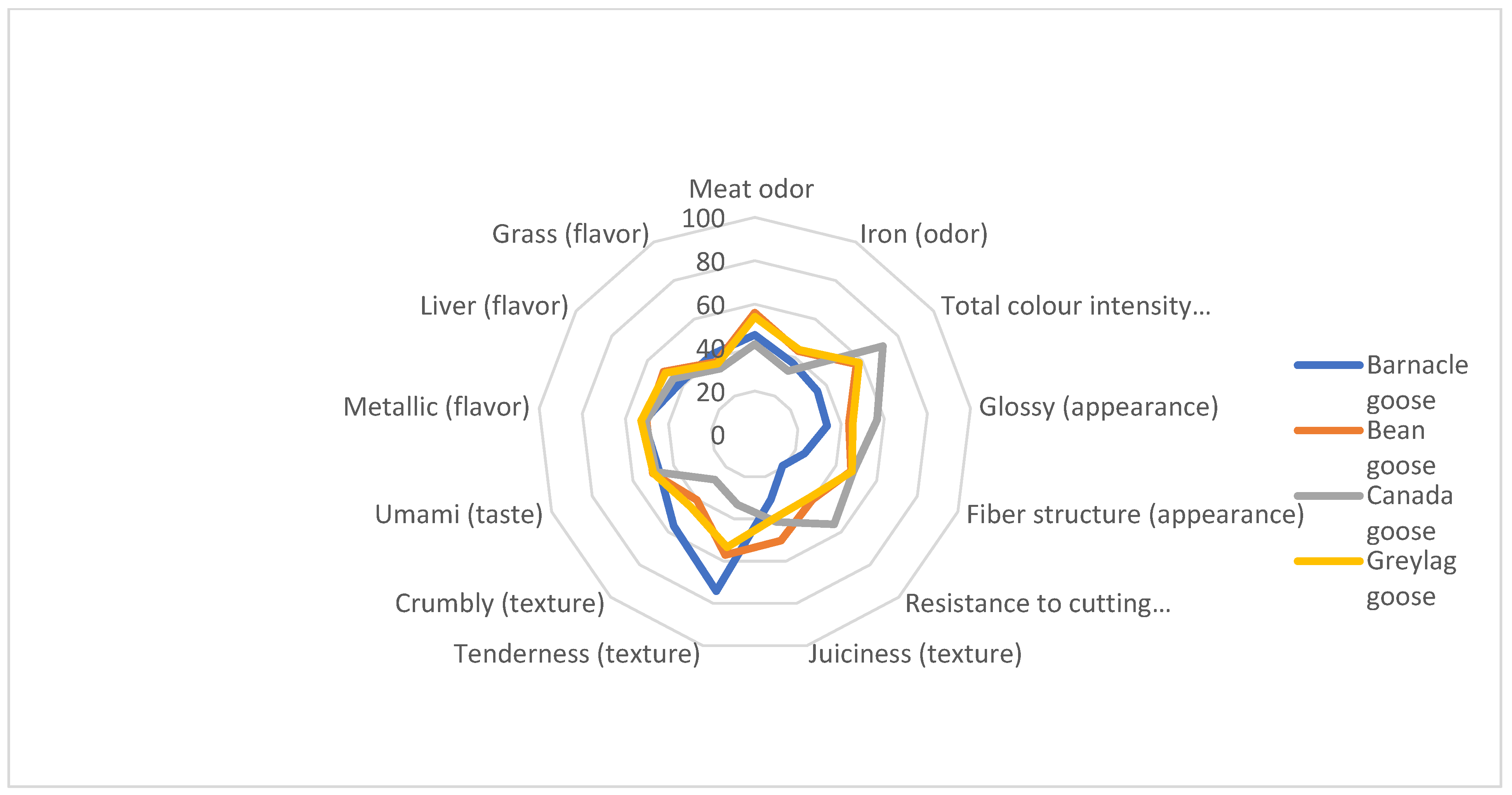
| Attribute | Type | Definition |
|---|---|---|
| Total color intensity | Appearance | The intensity of the red color (low-high) |
| Glossy | Appearance | Glossiness |
| Fiber structure | Appearance | 0 for fine fibers and 100 for coarse |
| Resistance to cutting | Texture | Resistance when using cutlery in a standardized manner, from low to high resistance |
| Juiciness | Texture | Use molar teeth. Assess texture in mouth with 1-2-3 chewings. Assess juiciness (low-high) at first bite. |
| Tenderness | Texture | Use molar teeth. Assess texture in mouth with 1-2-3 chewings. Assess tenderness (low-high) at second bite. |
| Crumbly | Texture | Use molar teeth. Assess texture in mouth with 1-2-3 chewings. Assess crumbliness/grainy texture (low-high) at third bite. |
| Meat odor | Odor | Rich meat odor, broth odor |
| Iron | Odor | Metallic odor from cooked liver |
| Umami | Taste | Basic taste |
| Metallic | Flavor | Flavor of iron and/or blood |
| Liver | Flavor | Flavor of cooked liver |
| Grass | Flavor | Green, a chlorophyllic sweetness. |
| Barnacle Goose | Bean Goose | Canada Goose | Greylag Goose | Farmed Male Mallard | Farmed Female Mallard | Wild Male Mallard | Wild Female Mallard | |
|---|---|---|---|---|---|---|---|---|
| Energy (kJ/kcal) | 517/124 | 525/125 | 580/139 | 531/127 | 539/129 | 525/125 | 517/124 | 521/125 |
| Carbohydrate (g) | 0.7 | <0.30 | 1.22 | 1.02 | <0.30 | <0.30 | <0.30 | 0.71 |
| Glucose (g) | <0.05 | 0.11 | 0.35 | 0.14 | <0.05 | 0.18 | 0.15 | 0.14 |
| Protein (g) | 23.6 | 24.3 | 23.8 | 24.6 | 24.2 | 23.4 | 24.3 | 24.4 |
| Fat (g) | 2.8 | 2.9 | 4.17 | 2.58 | 3.47 | 3.32 | 2.7 | 2.57 |
| Saturated fat (g) | 0.86 | 0.82 | 1.24 | 0.74 | 1.2 | 1.18 | 0.92 | 0.87 |
| Monounsaturated fat (g) | 0.77 | 1.21 | 1.59 | 1.06 | 1.28 | 1.16 | 0.87 | 0.76 |
| Polyunsaturated fat (g) | 1.05 | 0.74 | 1.15 | 0.67 | 0.83 | 0.83 | 0.8 | 0.82 |
| Omega-3 fatty acids, total (g) | 0.46 | 0.24 | 0.41 | 0.12 | <0.10 | <0.10 | <0.10 | <0.10 |
| Omega-6 fatty acids, total (g) | 0.59 | 0.5 | 0.75 | 0.54 | 0.83 | 0.83 | 0.74 | 0.8 |
| Salt (NaCl) (g) | 0.15 | 0.14 | 0.15 | 0.13 | 0.14 | 0.13 | 0.15 | 0.15 |
| Iron (mg) | 5.65 | 7.03 | 6.85 | 6.98 | 4.65 ± 0.55 a | 4.88 ± 0.72 a | 5.46 ± 0.35 b | 5.97 ± 0.77 b |
| Water (g) | 71.5 | 70.7 | 69.6 | 70.6 | 71.3 | 71.2 | 71.2 | 71.1 |
| Barnacle Goose | Bean Goose | Canada Goose | Greylag Goose | Farmed Male Mallard | Farmed Female Mallard | Wild Male Mallard | Wild Female Mallard | |
|---|---|---|---|---|---|---|---|---|
| C16:0 Palmitic acid | 16.4 | 17.2 | 18.6 | 18.0 | 21.1 | 21.7 | 18.8 | 18.3 |
| C16:1 Palmitoleic acid | 2.09 | 2.29 | 2.3 | 3.32 | 1.7 | 1.48 | 1.94 | 1.71 |
| C18:0 Stearic acid | 14.2 | 11.2 | 11.1 | 10.8 | 13.5 | 14.0 | 15.2 | 15.7 |
| C18:1n9c Oleic acid | 23.8 | 37.1 | 34,0 | 35.3 | 32.5 | 30.9 | 28.1 | 25.0 |
| C18:2n6c Linoleic acid | 14.2 | 11.1 | 13.1 | 14.2 | 14.0 | 13.6 | 14.8 | 16.9 |
| C18:3n3 Linolenic acid | 14.6 | 6.51 | 7.96 | 4.74 | <0.05 | <0.05 | 1.04 | 1.03 |
| C20:4n6 Arachidonic acid | 6.77 | 6.1 | 4.81 | 6.93 | 10.0 | 11.4 | 12.6 | 14.1 |
| C20:5n3 Cis-eicosapentaenoic acid | 1.92 | 0.94 | 1.02 | <0.05 | <0.05 | <0.05 | 1.02 | <0.05 |
| C22:6n3 Cis-docosahexaenoic acid | <0.05 | 0.7 | 0.76 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Omega-3 fatty acids, total | 16.6 | 8.16 | 9.74 | 4.74 | <0.20 | <0.20 | 2.06 | 1.03 |
| Omega-6 fatty acids, total | 21.0 | 17.2 | 17.9 | 21.1 | 24.0 | 25.0 | 27.4 | 31.0 |
| Barnacle Goose (n = 6) | Bean Goose (n = 5) | Canada Goose (n = 5) | Greylag Goose (n = 5) | Farmed Female Mallard (n = 5) | Farmed Male Mallard (n = 6) | Wild Female Mallard (n = 6) | Wild Male Mallard (n = 6) | |
|---|---|---|---|---|---|---|---|---|
| Hg * | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 ± 0.0 | 0.01 ± 0.00 | 0.019 ± 0.019 | 0.028 ± 0.025 |
| Pb ** | 1.85 | 0.02 | 0.089 | 0.148 | 0.035 ± 0.027 | 0.03 ± 0.012 | 0.31 ± 0.42 | 0.069 ± 0.090 |
| As *** | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Cd **** | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Barnacle Goose | Bean Goose | Canada Goose | Greylag Goose | Farmed Male Mallard | Farmed Female Mallard | Wild Male Mallard | Wild Female Mallard | |
|---|---|---|---|---|---|---|---|---|
| n | 6 | 5 | 5 | 5 | 6 | 6 | 6 | 6 |
| Raw weight (g) | 130.4 ± 18.7 a | 227.9 ± 26.5 b | 308.2 ± 61.7 c | 248.0 ± 35.6 b,c | 79.7 ± 11.7 i | 67.4 ± 9.9 i | 94.6 ± 8.8 j | 70.1 ± 12.7 i |
| Cooking weight (g) | 110.9 ± 17.3 d | 198.9 ± 23.5 e | 272.2 ± 58.0 f | 223.9 ± 21.8 e,f | 67.1 ± 9.9 k | 58.3 ± 9.1 k | 81.6 ± 9.0 l | 60.5 ± 10.0 k |
| cooking loss (%) | 17.1 ± 1.8 g | 12.7 ± 2.3 h | 12.0 ± 1.7 h | 14.5 ± 2.5 g,h | 15.7 ± 2.9 m | 13.6 ± 1.3 m | 13.7 ± 4.2 m | 13.5 ± 2.6 m |
| L* | 33.3 ± 3.0 n | 33.9 ± 2.2 n | 30.5 ± 2.6 n | 31.9 ± 2.0 n | 38.2 ± 8.8 q | 37.7 ± 3.5 q | 38.7 ± 3.0 q | 36.0 ± 2.5 q |
| a* | 12.2 ± 2.0 | 9.3 ± 1.2 o | 11.2 ± 2.0 o | 9.9 ± 2.3 o | 6.0 ± 1.3 r | 5.2 ± 1.5 r | 4.9 ± 2.1 r | 5.8 ± 1.4 r |
| b* | 8.6 ± 1.4 p | 6.3 ± 1.7 p | 7.8 ± 1.6 p | 6.9 ± 1.5 p | 2.6 ± 1.0 s | 1.9 ± 1.0 s | 2.4 ± 1.2 s | 1.6 ± 0.6 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Söderquist, P.; Olsson, C.; Birch, K.; Olsson, V. Evaluation of Nutritional Quality and Sensory Parameters of Meat from Mallard and Four Species of Wild Goose. Foods 2022, 11, 2486. https://doi.org/10.3390/foods11162486
Söderquist P, Olsson C, Birch K, Olsson V. Evaluation of Nutritional Quality and Sensory Parameters of Meat from Mallard and Four Species of Wild Goose. Foods. 2022; 11(16):2486. https://doi.org/10.3390/foods11162486
Chicago/Turabian StyleSöderquist, Pär, Camilla Olsson, Karina Birch, and Viktoria Olsson. 2022. "Evaluation of Nutritional Quality and Sensory Parameters of Meat from Mallard and Four Species of Wild Goose" Foods 11, no. 16: 2486. https://doi.org/10.3390/foods11162486
APA StyleSöderquist, P., Olsson, C., Birch, K., & Olsson, V. (2022). Evaluation of Nutritional Quality and Sensory Parameters of Meat from Mallard and Four Species of Wild Goose. Foods, 11(16), 2486. https://doi.org/10.3390/foods11162486






