Effect of Physical and Enzymatic Modifications on Composition, Properties and In Vitro Starch Digestibility of Sacred Lotus (Nelumbo nucifera) Seed Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Preparation of Native Lotus Seed Flour
2.2. Physical Modifications of Lotus Seed Flour
2.2.1. Partial Gelatinization
2.2.2. Heat–Moisture Treatment
2.3. Enzymatic Modification of Lotus Seed Flour
2.4. Characterization of Lotus Seed Flour
2.4.1. Chemical Composition
2.4.2. Amylose Content
2.4.3. Starch Fractions
2.4.4. Functional Properties
2.4.5. In Vitro Gastrointestinal Digestion and Estimated Glycemic Index (eGI)
2.5. Statistical Analyses
3. Results and Discussion
3.1. Chemical Composition of Native and Modified Lotus Seed Flour
3.2. Amylose Content and Starch Fractions of Native and Modified Lotus Seed Flour
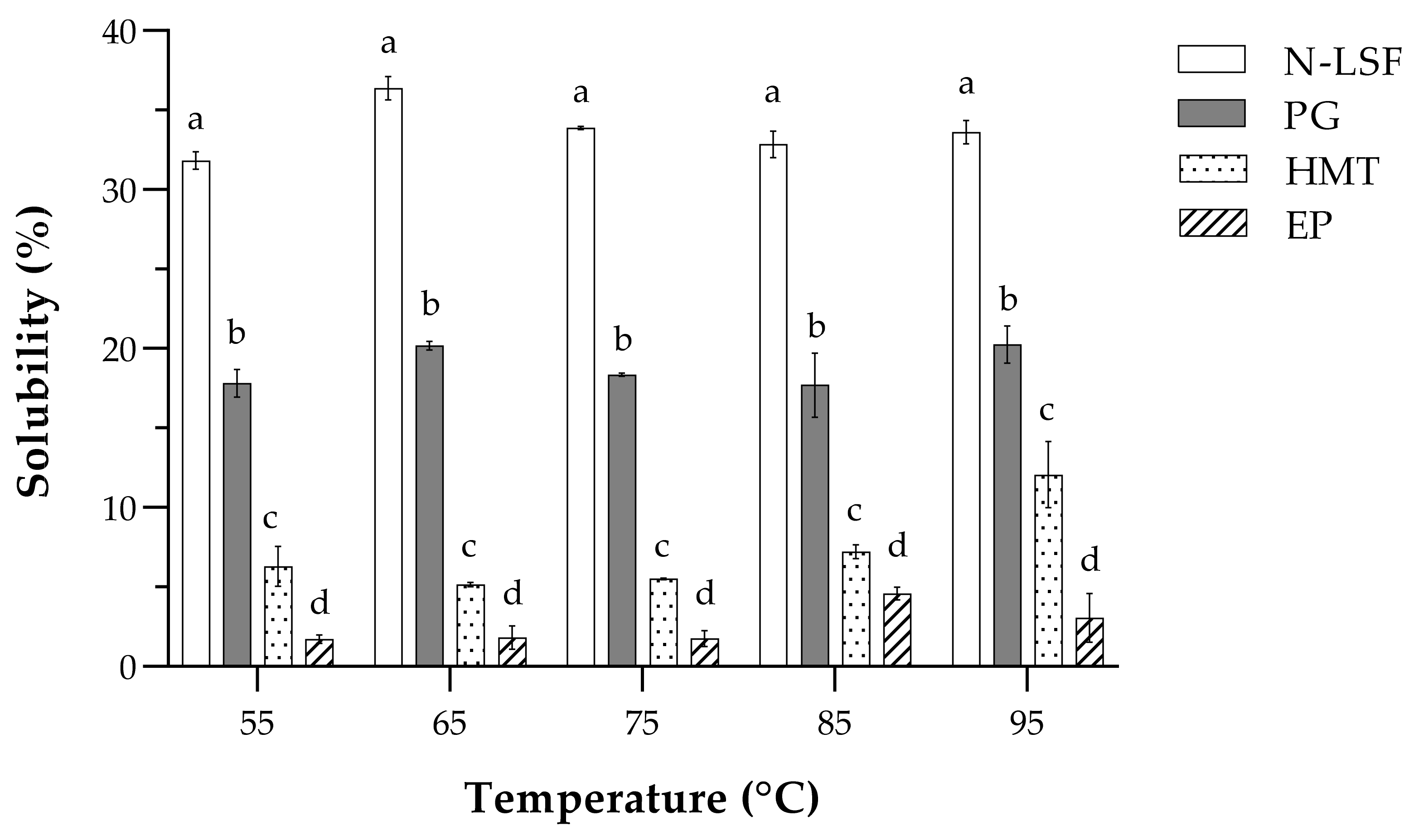
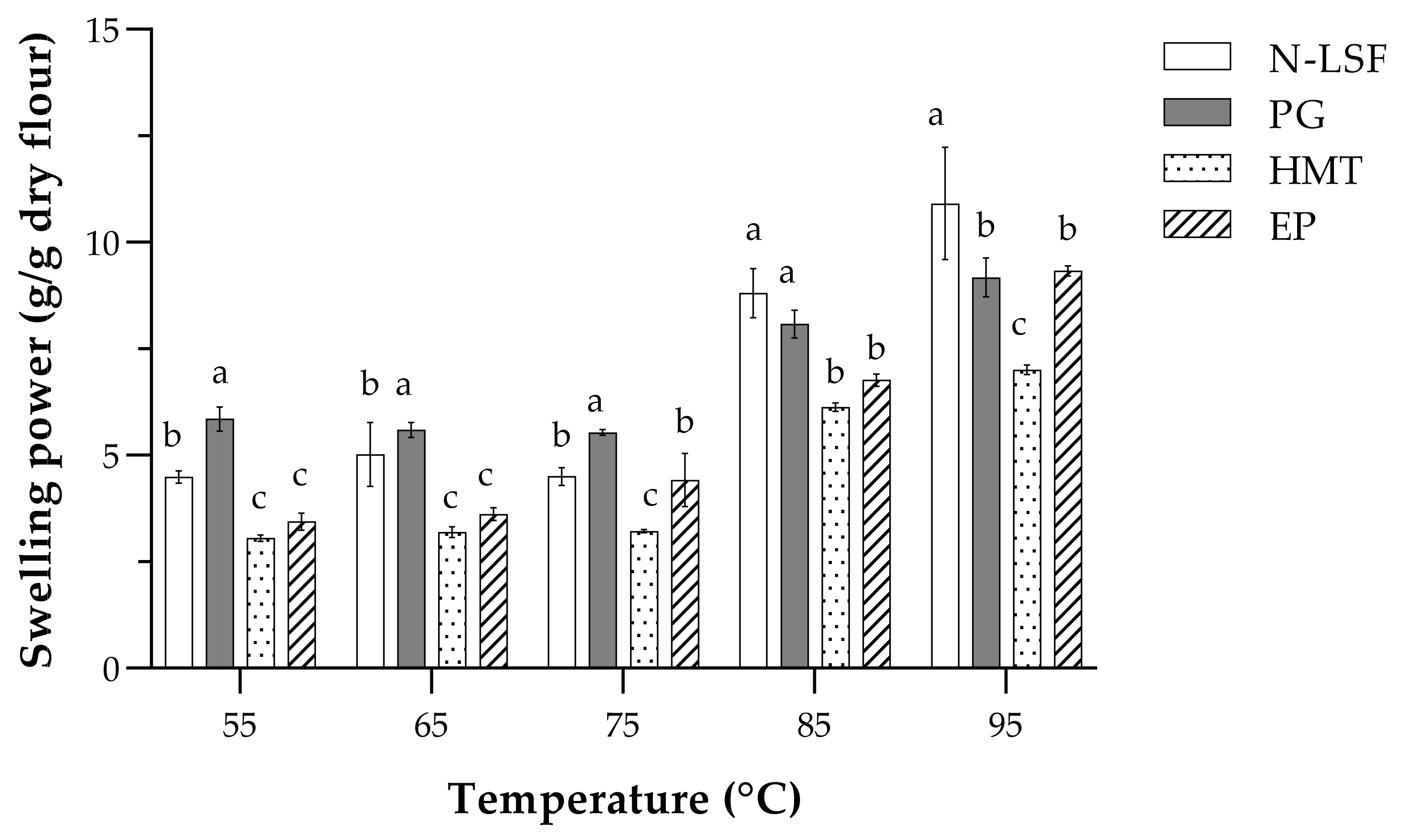
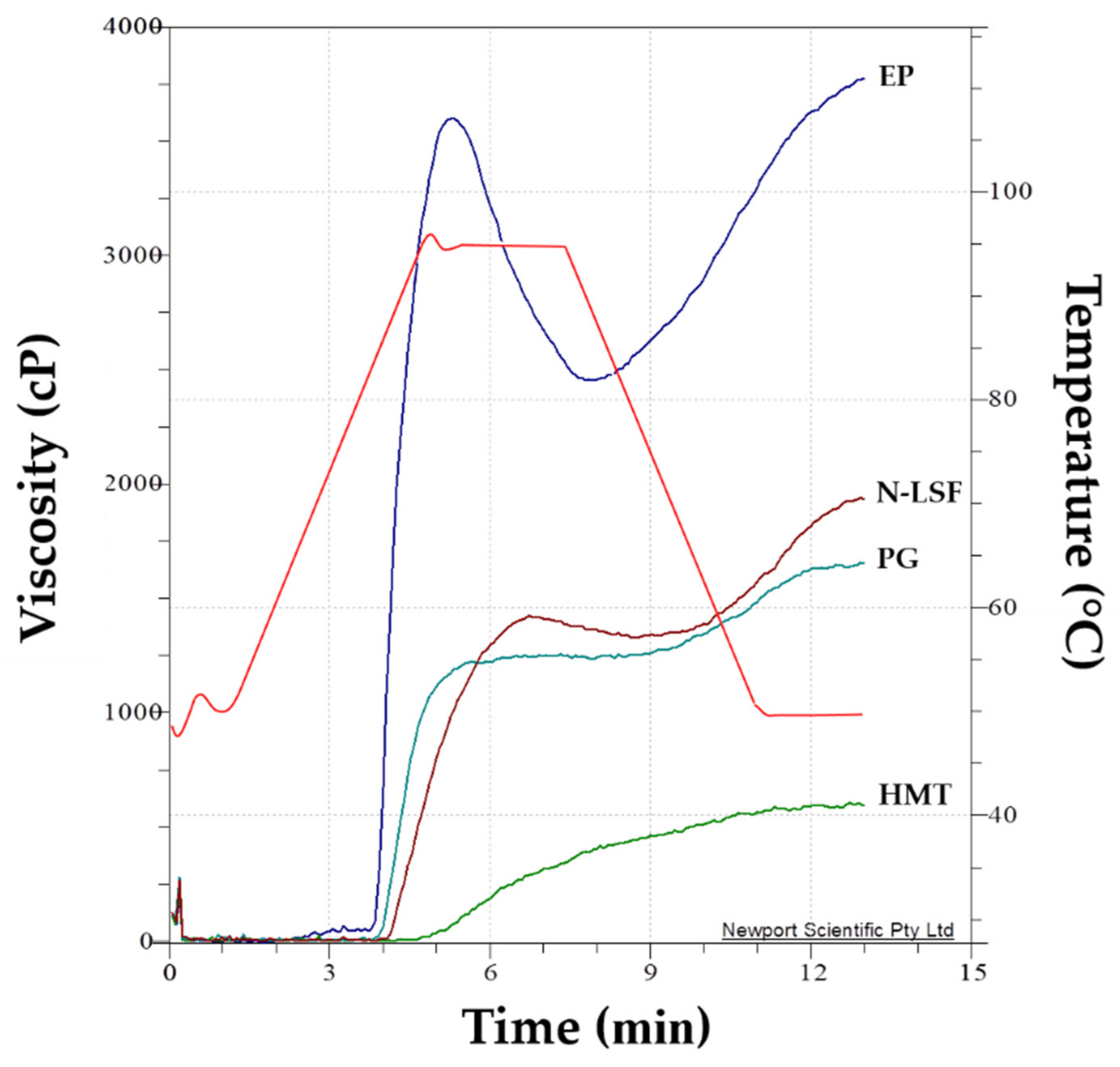
3.3. Functional Properties of Native and Modified Lotus Seed Flours
3.4. Estimated Glycemic Index of Native and Modified Lotus Seed Flours
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation (IDF). Summary. In IDF Diabetes Atlas, 10th ed.; International Diabetes Federation (IDF): Brussels, Belgium, 2021; Volume 10, pp. 4–5. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 31 May 2022).
- Chen, C.; Li, G.; Zhu, F. A novel starch from lotus (Nelumbo nucifera) seeds: Composition, structure, properties and modifications. Food Hydrocoll. 2021, 120, 106899. [Google Scholar] [CrossRef]
- Singthong, J.; Meesit, U. Characteristic and functional properties of Thai lotus seed (Nelumbo nucifera) flours. Int. Food Res. J. 2017, 24, 1414–1421. [Google Scholar]
- Zhang, Y.; Lu, X.; Zeng, S.; Huang, X.; Guo, Z.; Zheng, Y.; Tian, Y.; Zheng, B. Nutritional composition, physiological functions and processing of lotus (Nelumbo nucifera Gaertn.) seeds: A review. Phytochem. Rev. 2015, 14, 321–334. [Google Scholar] [CrossRef]
- Zhu, R.; Fan, Z.; Han, Y.; Li, S.; Li, G.; Wang, L.; Ye, T.; Zhao, W. Acute Effects of Three Cooked Non-Cereal Starchy Foods on Postprandial Glycemic Responses and In Vitro Carbohydrate Digestion in Comparison with Whole Grains: A Randomized Trial. Nutrients 2019, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.M.; Rosell, C.M.; Gomez, M. Modification of wheat flour functionality and digestibility through different extrusion conditions. J. Food Eng. 2014, 143, 74–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zheng, B.; Lu, X.; Zhuang, W. The in vitro effects of retrograded starch (resistant starch type 3) from lotus seed starch on the proliferation of Bifidobacterium adolescentis. Food Funct. 2013, 4, 1609–1616. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—A review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Sanchez, T.; Salcedo, E.; Ceballos, H.; Dufour, D.; Mafla, G.; Morante, N.; Calle, F.; Pérez, J.C.; Debouck, D.; Jaramillo, G.; et al. Screening of starch quality traits in cassava (Manihot esculenta Crantz). Starch Stärke 2009, 61, 12–19. [Google Scholar] [CrossRef]
- Puncha-arnon, S.; Uttapap, D. Rice starch vs. rice flour: Differences in their properties when modified by heat–moisture treatment. Carbohydr. Polym. 2013, 91, 85–91. [Google Scholar] [CrossRef]
- Punia, S.; Dhull, S.B.; Sandhu, K.S.; Kaur, M.; Purewal, S.S. Kidney bean (Phaseolus vulgaris) starch: A review. Legume Sci. 2020, 2, e52. [Google Scholar] [CrossRef]
- Charoenkul, N.; Uttapap, D.; Pathipanawat, W.; Takeda, Y. Physicochemical characteristics of starches and flours from Cassava varieties having different cooked root textures. LWT Food Sci. Technol. 2011, 44, 1774–1781. [Google Scholar] [CrossRef]
- Otegbayo, B.; Oguniyan, D.; Akinwumi, O. Physicochemical and functional characterization of yam starch for potential industrial applications. Starch Stärke 2014, 66, 235–250. [Google Scholar] [CrossRef]
- Paiyarach, D.; Punbusayakul, N. Nutritional quality and a prospected functional food ingredient of Thai lotus (Nelumbo nucifera) seed. In Proceedings of the 47th Kasetsart University Annual Conference, Bangkok, Thailand, 17–20 March 2009; pp. 671–679. [Google Scholar]
- Jirukkakul, N.; Sengkhamparn, N. Physicochemical properties and potential of lotus seed flour as wheat flour substitute in noodles. Songklanakarin J. Sci. Technol. 2018, 40, 1354–1360. Available online: https://rdo.psu.ac.th/sjstweb/journal/40-6/16.pdf (accessed on 20 November 2019).
- Kavlani, N.; Sharma, V.; Singh, L. Various techniques for modification of starch and applications of its derivatives. Int. Res. J. Pharm. 2012, 3, 25–31. [Google Scholar]
- Zia-ud-Din; Hanguo, X.; Peng, F. Physical and chemical modification of starches: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.; Guo, Z.; Chen, J.; Zheng, B. The characteristics of lotus seed starch modified by dry heating. Chin. J. Top. Crops 2012, 33, 364–369, (In Chinese with English abstract). [Google Scholar]
- Nawaz, H.; Shad, M.A.; Saleem, S.; Khan, M.U.A.; Nishan, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. Characteristics of starch isolated from microwave heat treated lotus (Nelumbo nucifera) seed flour. Int. J. Biol. Macromol. 2018, 113, 219–226. [Google Scholar] [CrossRef]
- Chen, C.; Fu, W.; Chang, Q.; Zheng, B.; Zhang, Y.; Zeng, H. Moisture distribution model describes the effect of water content on the structural properties of lotus seed resistant starch. Food Chem. 2019, 286, 449–458. [Google Scholar] [CrossRef]
- Ali, N.A.; Dash, K.K.; Routray, W. Physicochemical characterization of modified lotus seed starch obtained through acid and heat moisture treatment. Food Chem. 2020, 319, 126513. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, H.; Wang, Y.; Zeng, S.; Zheng, B. Structural characteristics and crystalline properties of lotus seed resistant starch and its prebiotic effects. Food Chem. 2014, 155, 311–318. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, X.; Lin, S.; Zeng, H.; Lu, X.; Zhang, Y.; Zheng, B. Structural characteristics and physicochemical properties of lotus seed resistant starch prepared by different methods. Food Chem. 2015, 186, 213–222. [Google Scholar] [CrossRef]
- Lin, X.; Sun, S.; Wang, B.; Zheng, B.; Guo, Z. Structural and physicochemical properties of lotus seed starch nanoparticles. Int. J. Biol. Macromol. 2020, 157, 240–246. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Q.; Gao, L.; Gong, X.; Qu, Y.; Feng, B. Functional and physicochemical properties of flours and starches from different tuber crops. Int. J. Biol. Macromol. 2020, 148, 324–332. [Google Scholar] [CrossRef]
- Whantongkhum, N.; Suwannaporn, P. Effect of rice flour treated with enzyme hydrolysis and heat-moisture treatment on texture quality and thermal properties of rice cookies. In Proceedings of the 54th Kasetsart University Annaual Conference, Bangkok, Thailand, 2–5 February 2016; pp. 947–953. [Google Scholar]
- Wattananapakasem, I.; Costabile, A.; Suwannaporn, P. Slow digestible colored rice flour as wall material for microencapsulation: Its impacts on gut bacterial population and metabolic activities. Int. Food Res. J. 2018, 103, 182–191. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Mahmood, T.; Turner, A.M.; Stoddard, L.F. Comparison of methods for colorimetric amylose determination in cereal grains. Starch Stärke 2007, 59, 357–365. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar]
- Goni, I.; Garcia-Diz, L.; Manas, E.; Saura-Calixto, F. Analysis of resistant starch: A method for foods and food products. Food Chem. 1996, 56, 445–449. [Google Scholar] [CrossRef]
- Guo, Z.; Zeng, S.; Lu, X.; Zhou, M.; Zheng, M.; Zheng, B. Structural and physicochemical properties of lotus seed starch treated with ultra-high pressure. Food Chem. 2015, 186, 223–230. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- Izydorczyk, M. Using RVA to Measure Pre-Germination in Barley and Predict Germination Energy after Storage; Canadian Grain Commission: Winnipeg, MB, Canada, 2008.
- Goni, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Jia, X.; Wang, J.; Lu, X.; Zheng, B.; Zheng, B.; Guo, Z. Structure and dilatational rheological behavior of heat-treated lotus (Nelumbo nucifera Gaertn.) seed protein. LWT Food Sci. Technol. 2019, 116, 108579. [Google Scholar] [CrossRef]
- Grassi de Alcântara, R.; Aparecida de Carvalho, R.; Maria Vanin, F. Evaluation of wheat flour substitution type (corn, green banana and rice flour) and concentration on local dough properties during bread baking. Food Chem. 2020, 326, 126972. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Zheng, Y.B.; Chen, T.Q.; Yi, J.; Qin, L.P.; Rahman, K.; Lin, W.X. Evaluation of the quality of lotus seed of Nelumbo nucifera Gaertn from outer space mutation. Food Chem. 2007, 105, 540–547. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Cai, L.H.; Cai, X.L.; Wang, Y.J.; Li, Y.Q. Amino acid profiles and quality from lotus seed proteins. J. Sci. Food Agric. 2012, 93, 1070–1075. [Google Scholar] [CrossRef]
- Dupuis, J.H.; Liu, Q.; Yada, R.Y. Methodologies for increasing the resistant starch content of food starches: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1219–1234. [Google Scholar] [CrossRef]
- Zhong, G.; Chen, Z.; We, Y. Physicochemical properties of lotus (Nelumbo nucifera Gaertn.) and kudzu (Pueraria hirsute Matsum.) starches. Int. J. Food Sci. 2007, 42, 1449–1455. [Google Scholar]
- Yeh, Y.; Lai, L.S. Effect of single and dual hydrothermal treatments on the resistant starch content and physicochemical properties of lotus rhizome starches. Molecules 2021, 26, 4339. [Google Scholar] [CrossRef]
- Liu, W.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. In structure and in vitro digestibility of waxy corn starch debranched by pullulanase. Food Hydrocoll. 2017, 67, 104–110. [Google Scholar] [CrossRef]
- Liu, G.; Hong, Y.; Gu, Z.; Li, Z.; Cheng, L. Pullulanase hydrolysis behaviors and hydrogel properties of debranched starches from different sources. Food Hydrocoll. 2015, 45, 351–360. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, B.; Zeng, H.; Guo, Z.; Lu, X.; Zhang, Y.; Zheng, B. Effect of microwave irradiation on the physicochemical and digestive properties of lotus seed starch. J. Agric. Food Chem. 2016, 64, 2442–2449. [Google Scholar] [CrossRef]
- Huang, T.T.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effect of repeated heat-moisture treatments on digestibility, physicochemical and structural properties of sweet potato starch. Food Hydrocoll. 2016, 54, 202–210. [Google Scholar] [CrossRef]
- Tiamiyu, H.K.; Babajide, J.M.; Adeyeye, S.A.O. Some quality attributes of heat-moisture treated water yam (Dioscorea alata) starch. Cogent. Food Agric. 2017, 3, 1–13. [Google Scholar] [CrossRef]
- Cahyana, Y.; Wijaya, E.; Halimah, T.S.; Marta, H.; Suryadi, E.; Kurniati, D. The effect of different thermal modifications on slowly digestible starch and physicochemical properties of green banana flour (Musa acuminata colla). Food Chem. 2019, 274, 274–280. [Google Scholar] [CrossRef]
- Chen, B.; Zeng, S.; Zeng, H.; Guo, Z.; Zhang, Y.; Zheng, B. Properties of lotus seed starch-glycerin monostearin complexes formed by high pressure homogenization. Food Chem. 2017, 226, 119–127. [Google Scholar] [CrossRef]
- Yadav, B.S.; Yadav, R.B.; Kumari, M.; Khatkar, B.S. Studies on suitability of wheat flour blends with sweet potato, Colocasia and water chestnut flours for noodle making. LWT Food Sci. Technol. 2014, 57, 352–358. [Google Scholar] [CrossRef]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid Visco Analyzer (RVA) as a tool for measuring starch-related physicochemical properties in cereals: A review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Delatte, S.; Doran, L.; Blecker, C.; De Mol, G.; Roiseux, O.; Gofflot, S.; Malumba, P. Effect of pilot-scale steam treatment and endogenous alpha-amylase activity on wheat flour functional properties. J. Cereal Sci. 2019, 88, 38–46. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, B.; Lu, X.; Zeng, S.; Zheng, B. Structural, pasting and thermal properties of ultra-high pressure treated lotus seed starch. Chin. J. Struct. Chem. 2014, 33, 647–653. [Google Scholar]
- Hu, Y.; Wang, L.; Zhu, H.; Li, Z. Modification of physicochemical properties and in vitro digestibility of wheat flour through superheated steam processing. J. Cereal Sci. 2017, 74, 231–237. [Google Scholar] [CrossRef]
- Neill, G.; Al-Muhtaseb, A.H.; Magee, T.R.A. Optimisation of time/temperature treatment, for heat treated soft wheat flour. J. Food Eng. 2012, 113, 422–426. [Google Scholar] [CrossRef]
- Kim, W.; Choi, S.G.; Kerr, W.L.; Johnson, J.W.; Gaines, C.S. Effect of heating temperature on particle size distribution in hard and soft wheat flour. J. Cereal Sci. 2004, 40, 9–16. [Google Scholar] [CrossRef]
- Vamademan, V.; Bertoft, E.; Seeratharaman, K. On the importance of organization of glucan chains on thermal properties of starch. Carbohydr. Polym. 2013, 92, 1653–1659. [Google Scholar] [CrossRef]
- Gunaratne, A. Heat-moisture treatment of starch. In Physical Modification of Starch; Sui, Z., Kong, X., Eds.; Springer Nature: Singapore, 2018; pp. 15–36. [Google Scholar]
- Ahn, J.H.; Baek, H.R.; Kim, K.M.; Han, G.J.; Choi, J.B.; Kim, Y.; Moon, T.W. Slowly digestible sweet potato flour: Preparation by heat-moisture treatment and characterization of physicochemical properties. Food Sci. Biotechnol. 2013, 22, 383–391. [Google Scholar] [CrossRef]
- Hoover, R. Composition, molecular structure, and physicochemical properties of tuber and root starches: A review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Dura, A.; Blaszczak, W.; Rosell, C.M. Functionality of porous starch obtained by amylase or amyloglucosidase treatments. Carbohydr. Polym. 2014, 101, 837–845. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Xi, H.; Xu, J.; Deng, D.; Huang, G. Effects of soluble dietary fiber on the crystallinity, pasting, rheological, and morphological properties of corn resistant starch. LWT Food Sci. Technol. 2019, 111, 632–639. [Google Scholar]
- Zheng, J.; Huang, S.; Zhao, R.; Wang, N.; Kan, J.; Zhang, F. Effect of four viscous soluble dietary fibers on the physicochemical, structural properties, and in vitro digestibility of rice starch: A comparison study. Food Chem. 2021, 362, 130181. [Google Scholar] [CrossRef]
- Marques, C.; D’Auria, L.; Cani, P.D.; Baccelli, C.; Rozenberg, R.; Ruibal-Mendieta, N.L.; Petitjean, G.; Delacroix, D.L.; Quetin-Leclercq, J.; Habib-Jiwan, J.L.; et al. Comparison of glycemic index of spelt and wheat bread in human volunteers. Food Chem. 2007, 100, 1265–1271. [Google Scholar] [CrossRef]
- Thorne, M.J.; Thompson, L.U.; Jenkins, D.J. Factors affecting starch digestibility and the glycemic response with special reference to legumes. Am. J. Clin. Nutr. 1983, 38, 481–488. [Google Scholar] [CrossRef]
| Sample | Moisture (g/100 g Flour) | Composition (g/100 g Dry Flour) | |||||
|---|---|---|---|---|---|---|---|
| Protein (N × 5.70) | Fat | Ash | CHO | Dietary Fiber | |||
| Soluble | Insoluble | ||||||
| N-LSF | 7.92 | 26.79 | 1.84 | 4.31 | 67.06 | 8.36 | 3.59 |
| PG | 12.48 | 25.81 | 2.00 | 4.34 | 67.85 | 7.28 | 3.55 |
| HMT | 11.87 | 11.99 | 1.99 | 2.33 | 83.70 | 5.25 | 9.88 |
| EP | 10.51 | 24.26 | 1.40 | 1.06 | 73.28 | 11.62 | <0.10 |
| Sample | Amylose (g/100 Dry Flour) | Starch Fraction (g/100 g Starch) | ||
|---|---|---|---|---|
| RDS | SDS | RS | ||
| N-LSF | 24.30 ± 0.33 c | 6.56 ± 0.31 b | 12.72 ± 1.71 b | 80.72 ± 1.46 c |
| PG | 26.49 ± 0.51 c | 19.53 ± 0.48 a | 16.00 ± 0.53 a | 64.46 ± 0.42 d |
| HMT | 39.23 ± 0.31 a | 4.33 ± 0.44 c | 3.14 ± 0.69 d | 92.52 ± 0.69 a |
| EP | 34.57 ± 0.48 b | 7.34 ± 0.20 b | 5.67 ± 0.37 c | 86.99 ± 0.49 b |
| Sample | Viscosity (cP) | Peak Time (min) | Pasting Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|
| Peak | Trough | Breakdown | Setback | Final | |||
| N-LSF | 1416.67 ± 8.14 b | 1294.67 ± 12.06 b | 122.00 ± 6.24 b | 638.33 ± 13.01 b | 1933.00 ± 13.11 b | 6.71 ± 0.10 b | 86.33 ± 1.22 b |
| PG | 1237.67 ± 16.92 c | 1217.00 ± 10.58 c | 20.67 ± 6.51 c | 431.00 ± 3.61 c | 1648.00 ± 7.00 c | 6.80 ± 0.13 ab | 84.67 ± 1.75 bc |
| HMT | 308.67 ± 5.51 d | 290.32 ± 17.03 d | 5.13 ± 3.57 d | 397.00 ± 1.73 d | 592.33 ± 2.08 d | 7.00 ± 0.00 a | 94.92 ± 0.14 a |
| EP | 3473.68 ± 49.52 a | 2372.96 ± 40.29 a | 1100.64 ± 29.76 a | 1231.32 ± 15.47 a | 3604.36 ± 30.59 a | 5.22 ± 0.08 c | 82.70 ± 0.98 c |
| Sample | AUC | HI | eGI | GI Category | GL | GL Category |
|---|---|---|---|---|---|---|
| N-LSF | 269.30 ± 23.62 b | 11.87 ± 1.04 b | 46.23 ± 0.57 b | Low | 8.50 ± 0.11 c | Low |
| PG | 841.13 ± 64.47 a | 37.08 ± 1.50 a | 60.07 ± 0.82 a | Medium | 12.91 ± 0.90 a | Low |
| HMT | 596.87 ± 23.05 ab | 17.63 ± 0.68 b | 49.39 ± 0.37 b | Low | 12.14 ± 0.21 a | Low |
| EP | 541.83 ± 10.33 ab | 16.00 ± 0.30 b | 48.50 ± 0.17 b | Low | 10.55 ± 0.05 b | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopawong, P.; Warodomwichit, D.; Srichamnong, W.; Methacanon, P.; Tangsuphoom, N. Effect of Physical and Enzymatic Modifications on Composition, Properties and In Vitro Starch Digestibility of Sacred Lotus (Nelumbo nucifera) Seed Flour. Foods 2022, 11, 2473. https://doi.org/10.3390/foods11162473
Sopawong P, Warodomwichit D, Srichamnong W, Methacanon P, Tangsuphoom N. Effect of Physical and Enzymatic Modifications on Composition, Properties and In Vitro Starch Digestibility of Sacred Lotus (Nelumbo nucifera) Seed Flour. Foods. 2022; 11(16):2473. https://doi.org/10.3390/foods11162473
Chicago/Turabian StyleSopawong, Pornnutcha, Daruneewan Warodomwichit, Warangkana Srichamnong, Pawadee Methacanon, and Nattapol Tangsuphoom. 2022. "Effect of Physical and Enzymatic Modifications on Composition, Properties and In Vitro Starch Digestibility of Sacred Lotus (Nelumbo nucifera) Seed Flour" Foods 11, no. 16: 2473. https://doi.org/10.3390/foods11162473
APA StyleSopawong, P., Warodomwichit, D., Srichamnong, W., Methacanon, P., & Tangsuphoom, N. (2022). Effect of Physical and Enzymatic Modifications on Composition, Properties and In Vitro Starch Digestibility of Sacred Lotus (Nelumbo nucifera) Seed Flour. Foods, 11(16), 2473. https://doi.org/10.3390/foods11162473






