Characteristics of Mussels-Derived Carbon Dots and Their Applications in Bio-Imaging and Detection of Riboflavin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
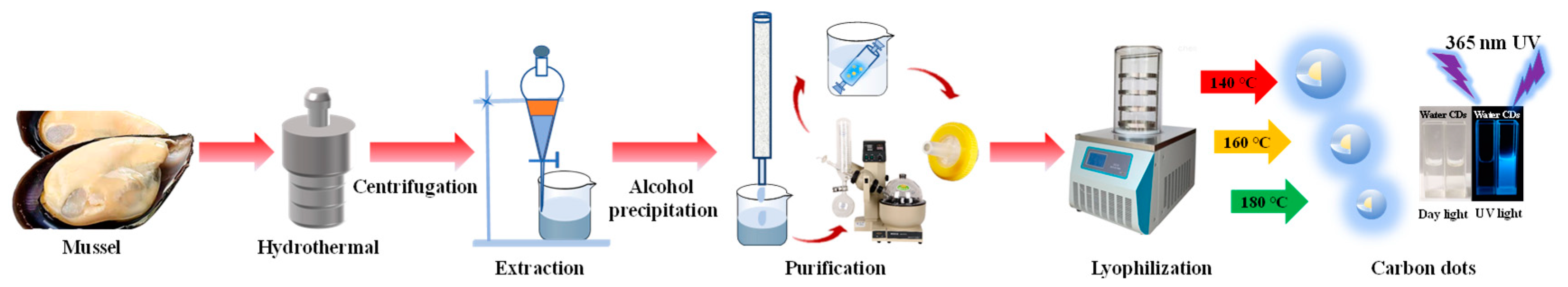
2.2. Synthesis of Mussels-Derived CDs
2.3. Structural and Optical Characterizations of CDs
2.4. Cytotoxicity and Bio-Imaging of CDs
2.5. Fluorescence Detection of Riboflavin by CDs
3. Results
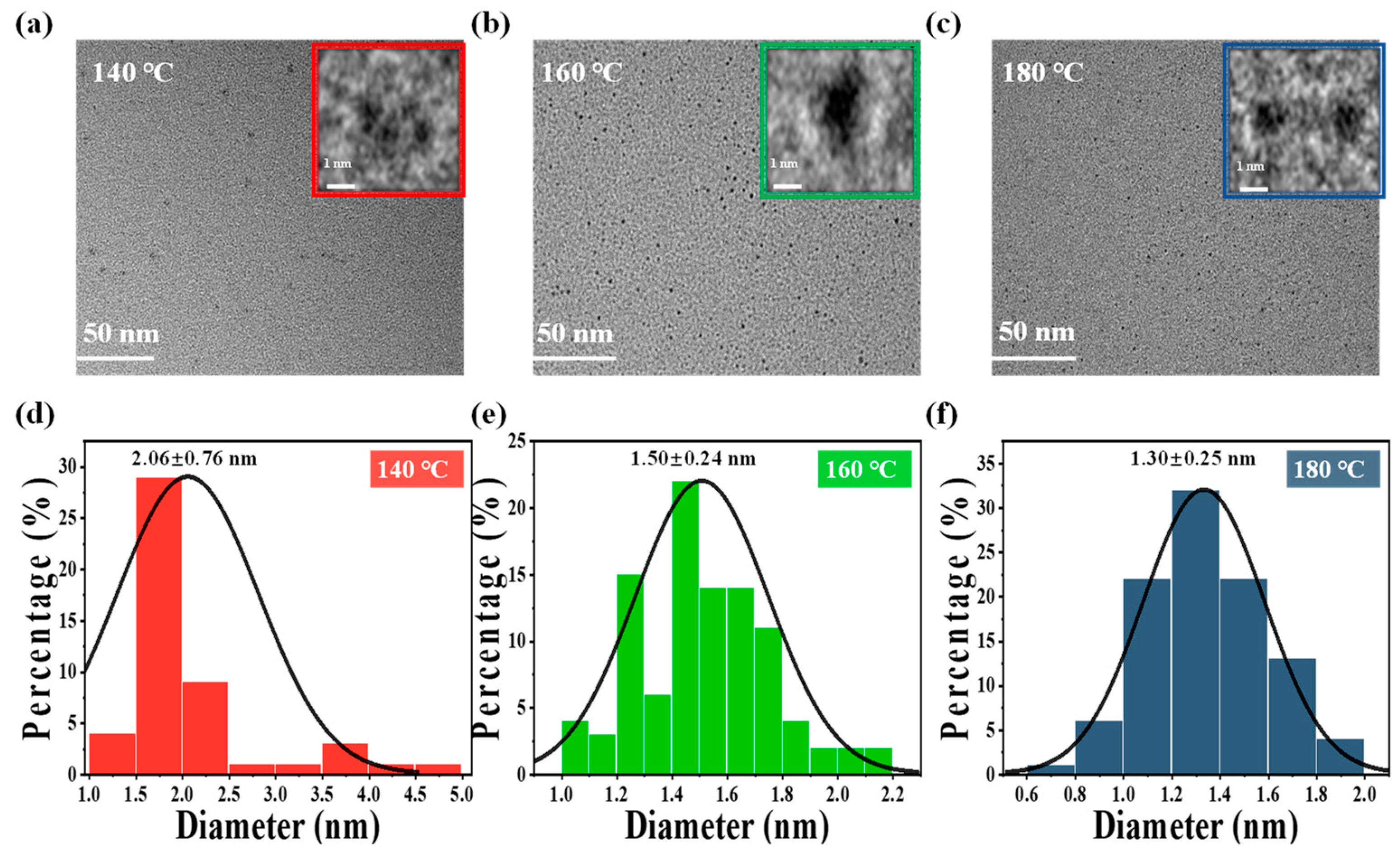
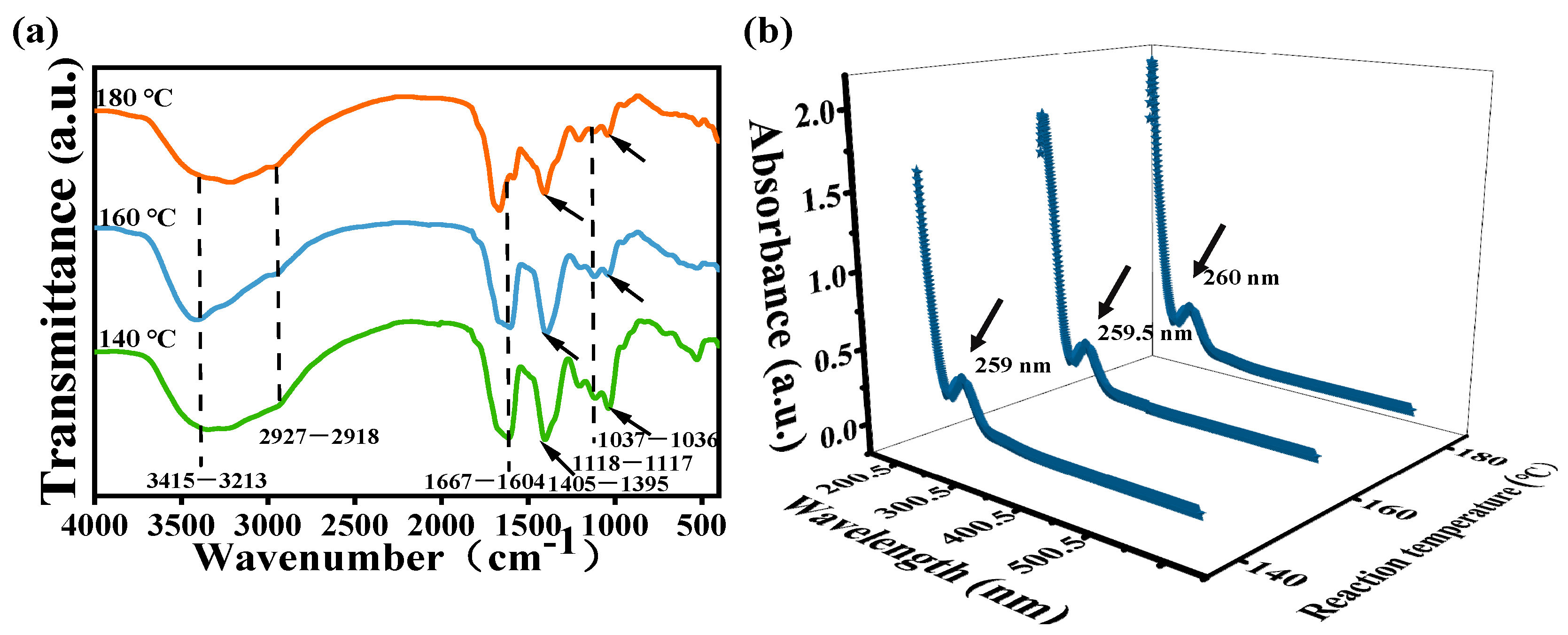
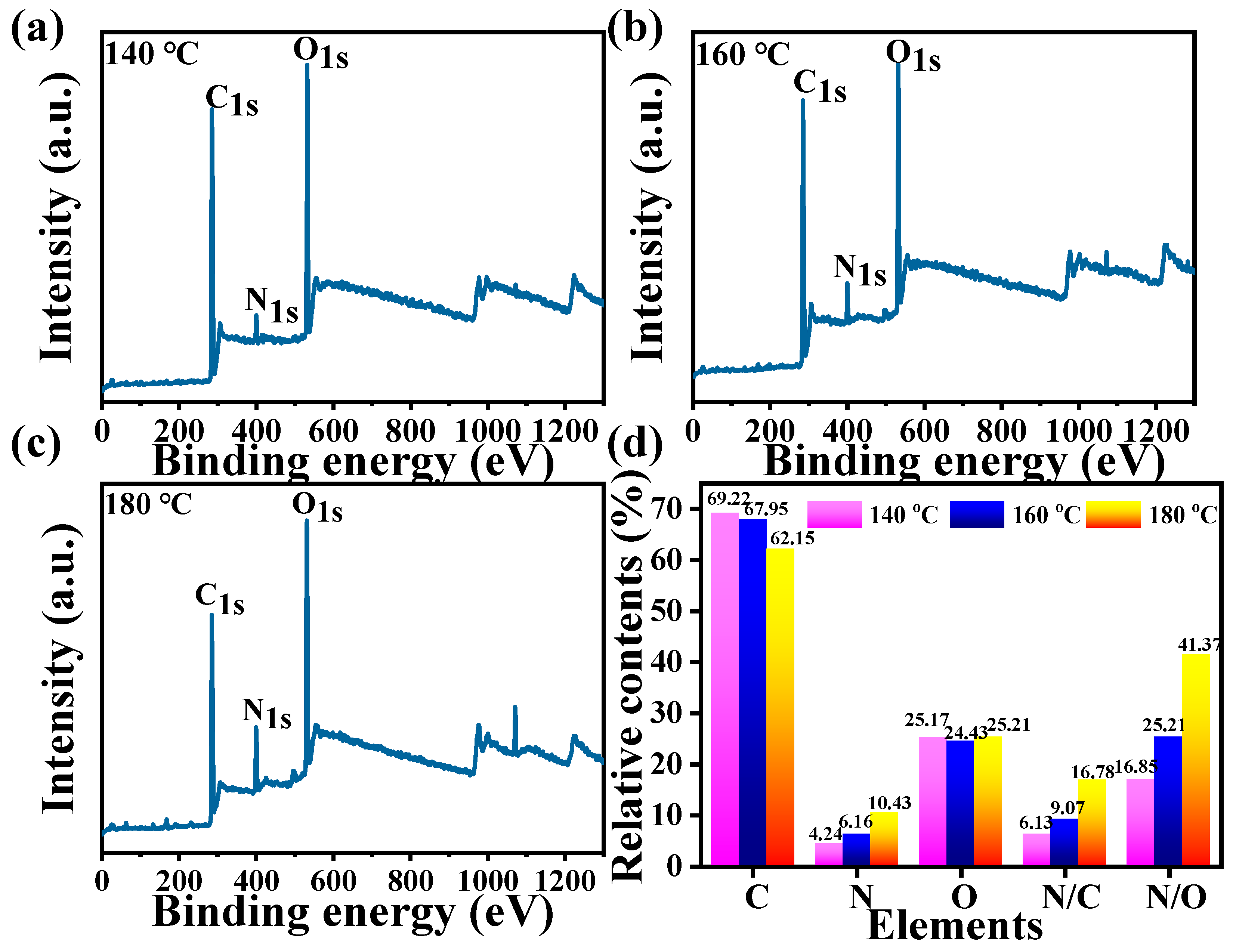
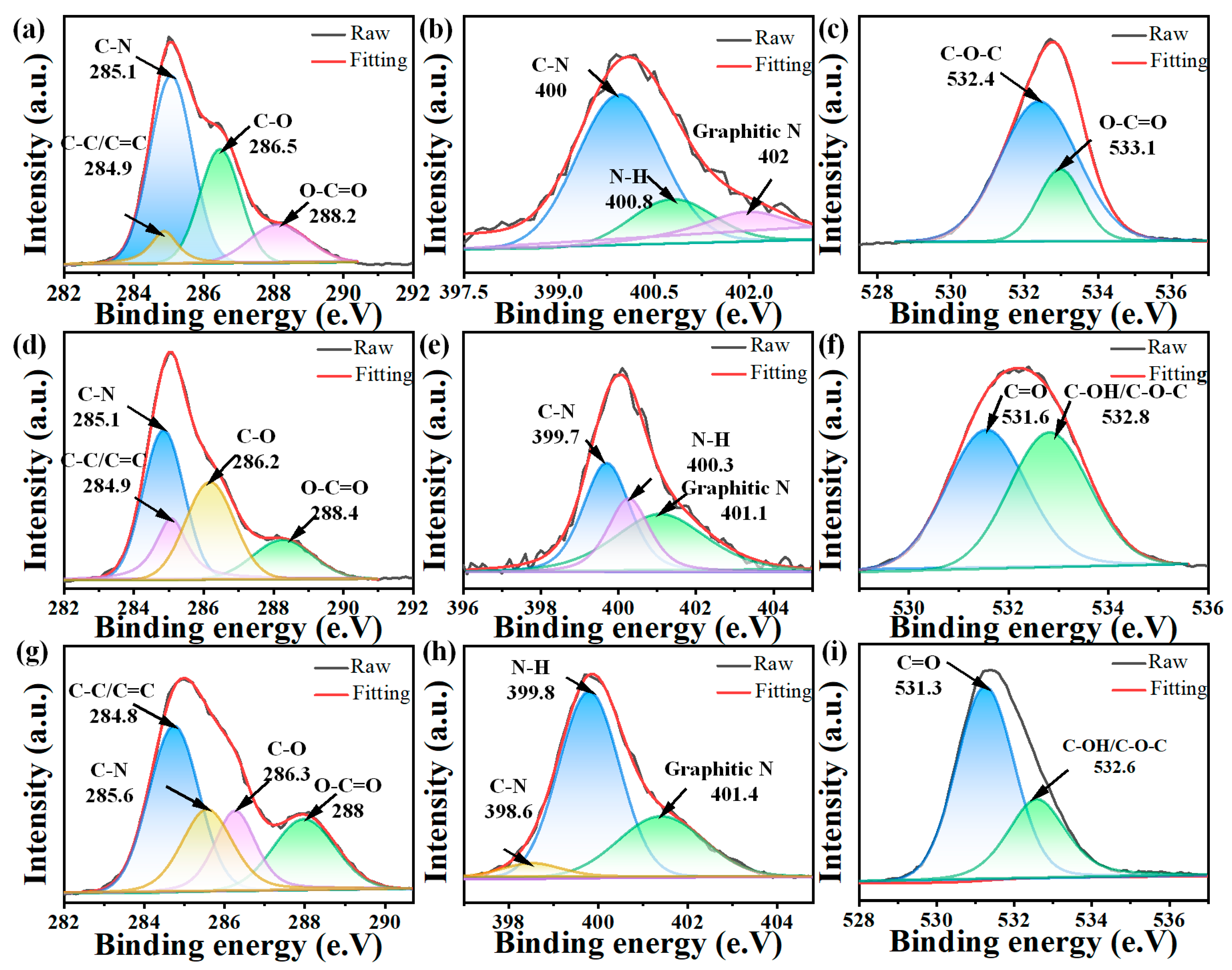
3.1. Synthesis and Structural Characterizations of CDs
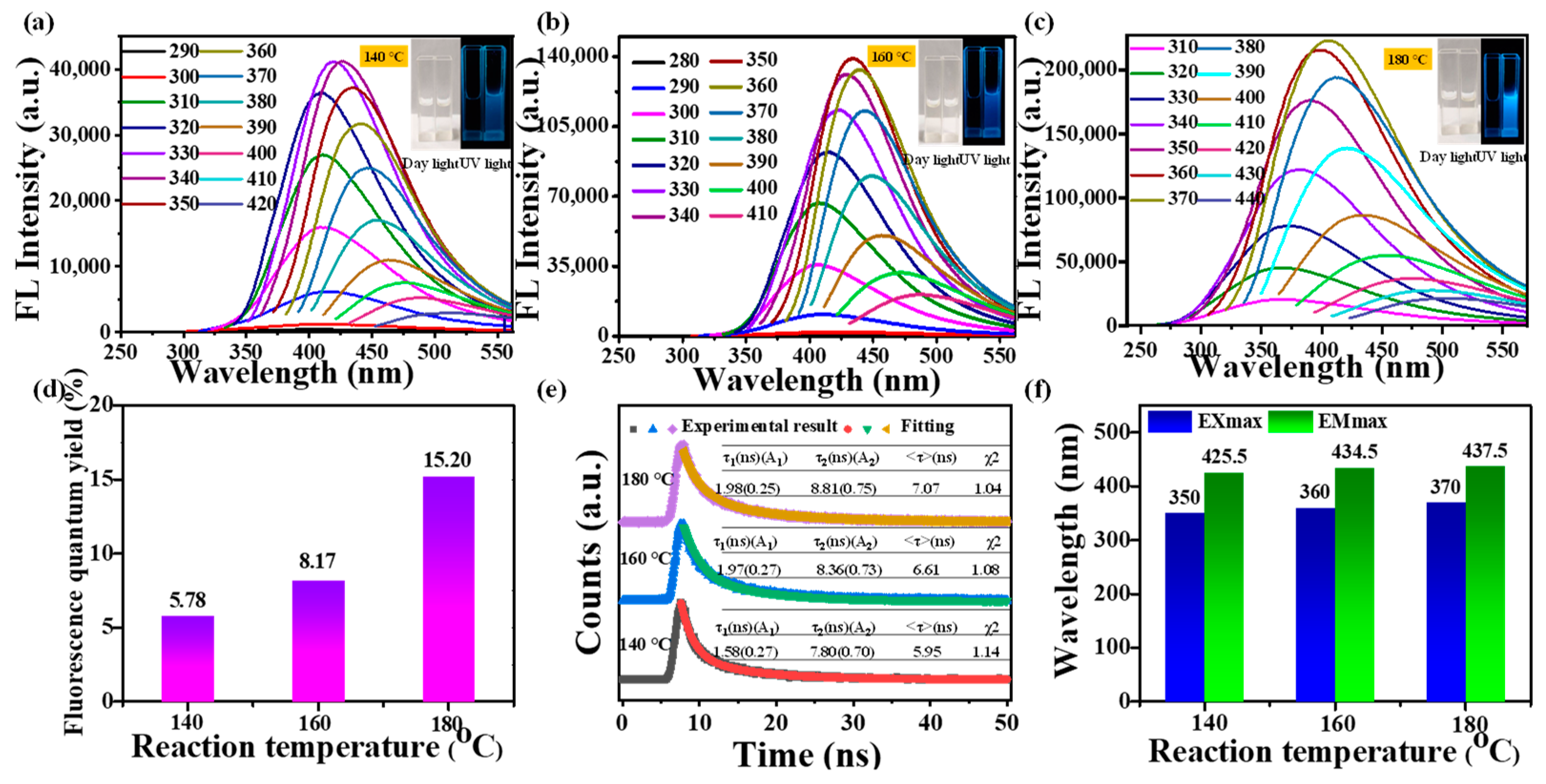
3.2. Optical Characteristics of CDs
3.3. Fluorescence Stability of Mussels-Derived CDs
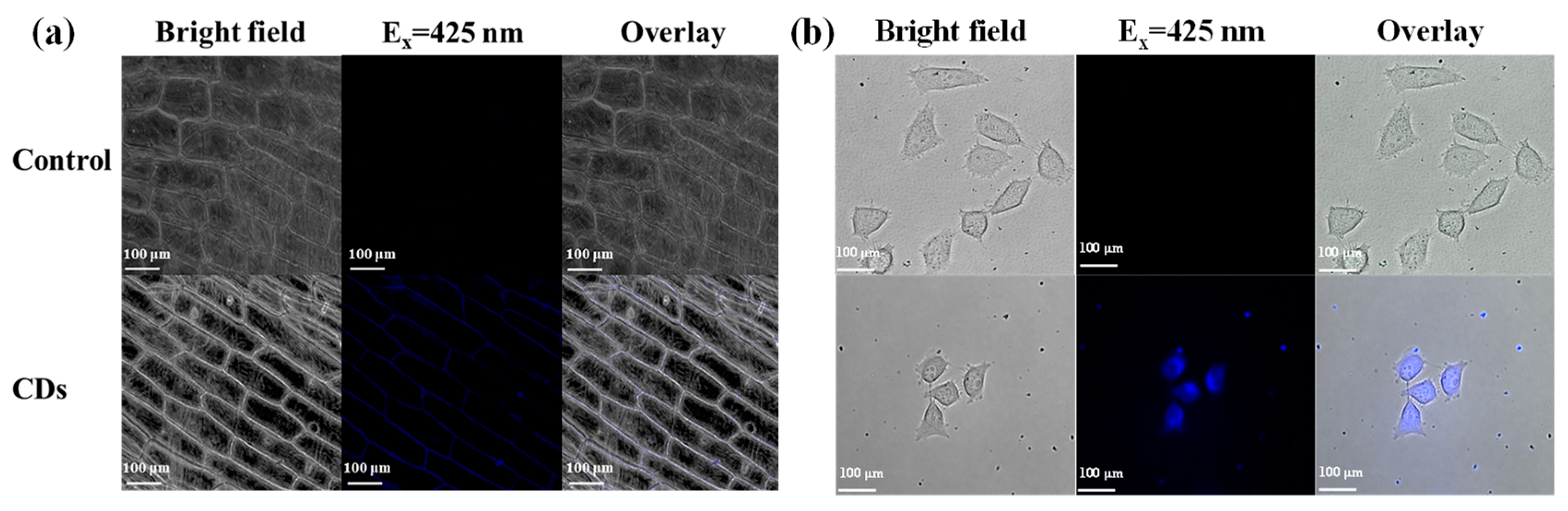
3.4. Bioimaging of CDs in Onion Epidermal Cells and HepG2 Cells
3.5. In Vivo Imaging of Zebrafish
3.6. Application of CDs for Riboflavin Detection
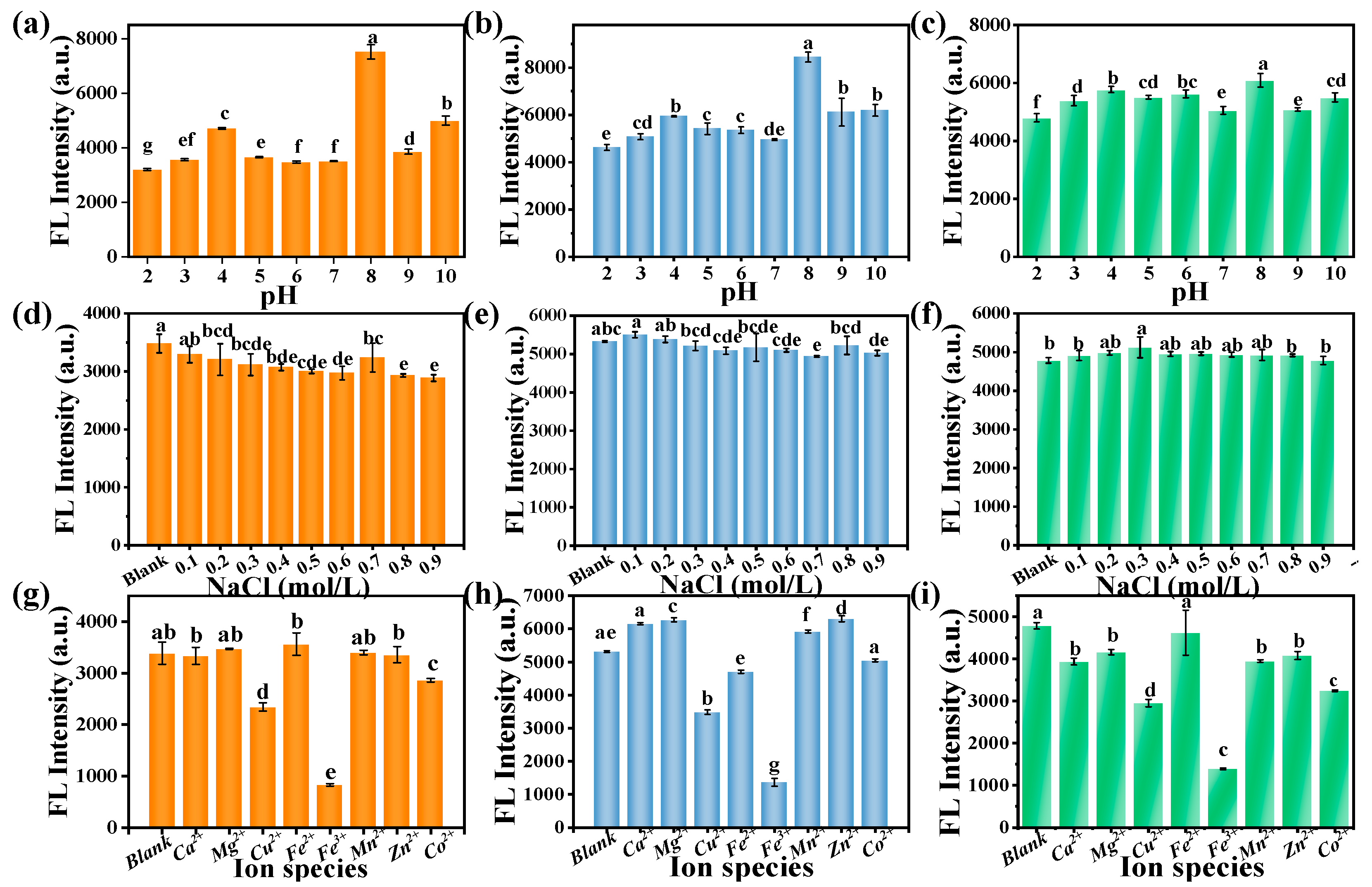
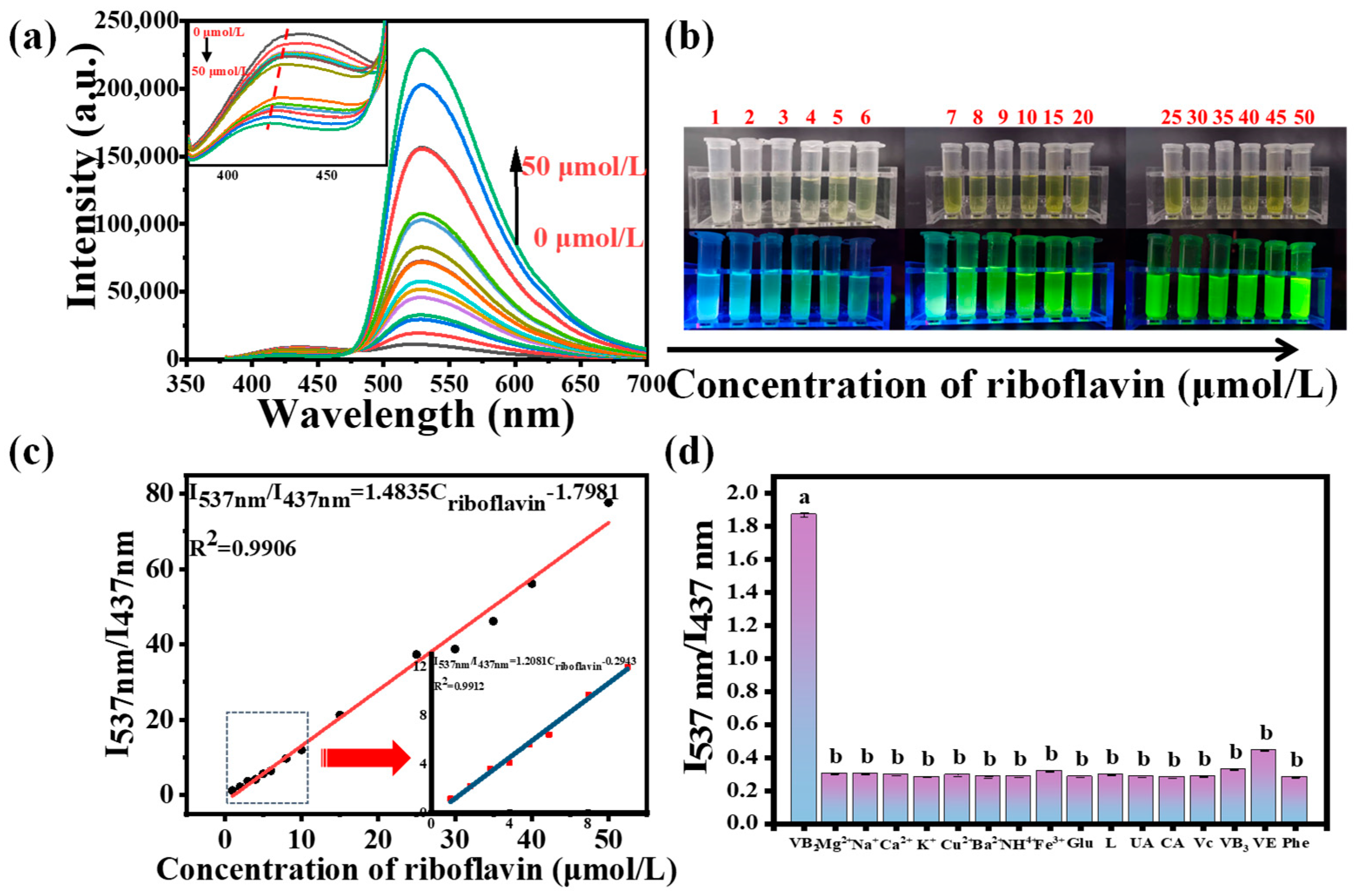
3.6.1. Optimization of Riboflavin Detection Conditions
3.6.2. Analytical Performance of CDs in the Detection of Riboflavin
3.6.3. Analysis of Riboflavin in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Niu, X.; Zhang, H.; Xu, L.; Zhao, S.; Chen, H.; Chen, X. Switch-on fluorescence sensing of glutathione in food samples based on a graphitic carbon nitride quantum dot (g-CNQD)-Hg2+ chemosensor. J. Agric. Food. Chem. 2015, 63, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, F.; Li, Y. Solid phase synthesis of nitrogen and phosphor co-doped carbon quantum dots for sensing Fe3+ and the enhanced photocatalytic degradation of dyes. Sens. Actuators B Chem. 2018, 255, 1105–1111. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Z.; Wang, Y.; Yang, Y.; Liu, X. Efficient resistance against solid-state quenching of carbon dots towards white light emitting diodes by physical embedding into silica. Carbon 2017, 126, 426–436. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Valcárcel, M. Reusable sensor based on functionalized carbon dots for the detection of silver nanoparticles in cosmetics via inner filter effect. Anal. Chim. Acta. 2015, 872, 70–76. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, Y.-S.; Weng, Y.-M.; Chen, W.; Lai, Y.-S. Sustainable development of carbon nanodots technology: Natural products as a carbon source and applications to food safety. Trends Food Sci. Technol. 2019, 86, 144–152. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Ma, C.; Niu, N.; Chen, Z.; Liu, S.; Li, J.; Li, S. Seeking value from biomass materials: Preparation of coffee bean shell-derived fluorescent carbon dots via molecular aggregation for antioxidation and bioimaging applications. Mater. Chem. Front. 2018, 2, 1269–1275. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Sustainable synthesis of multifunctional carbon dots using biomass and their applications: A mini-review. J. Environ. Chem. Eng. 2021, 9, 105802. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, Y.; Yu, Y.; Yan, Z.; Chen, J. Carbon quantum dots: Synthesis, characterization, and assessment of cytocompatibility. J. Mater. Sci. Mater. M. 2015, 26, 213. [Google Scholar] [CrossRef]
- Ding, C.; Deng, Z.; Chen, J.; Jin, Y. One-step microwave synthesis of N, S co-doped carbon dots from 1, 6-hexanediamine dihydrochloride for cell imaging and ion detection. Colloid. Surface. B 2020, 189, 110838. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liu, K.; Chen, M.; Peng, W.; Yang, Y.; Li, X. Laser fabricated carbon quantum dots in anti-solvent for highly efficient carbon-based perovskite solar cells. J. Colloid. Interf. Sci. 2021, 600, 691–700. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Li, L.S.; Qin, S.; Zhang, Y.; Huang, K.; Xu, L. The synthesis of fluorescent carbon dots from mango peel and their multiple applications. Colloid. Surface. A 2019, 577, 306–314. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosal, K.; Mohammad, S.A.; Sarkar, K. Dendrimer functionalized carbon quantum dot for selective detection of breast cancer and gene therapy. Chem. Eng. J. 2019, 373, 468–484. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, H.; Tang, J.; Deng, S.; Yan, F.; Li, W.; Qu, M. Carbon dots doped with heteroatoms for fluorescent bioimaging: A review. Microchim. Acta 2017, 184, 343–368. [Google Scholar] [CrossRef]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-derived carbon dots for toxic metal ion detection and as antibacterial and anticancer agents. ACS. Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Jayaweera, S.; Yin, K.; Ng, W.J. Nitrogen-doped durian shell derived carbon dots for inner filter effect mediated sensing of tetracycline and fluorescent ink. J. Fluoresc. 2018, 29, 221–229. [Google Scholar] [CrossRef]
- Wei, X.; Li, L.; Liu, J.; Yu, L.; Li, H.; Cheng, F.; Yi, X.; He, J.; Li, B. Green synthesis of fluorescent carbon dots from gynostemma for bioimaging and antioxidant in zebrafish. ACS. Appl. Mater. Inter. 2019, 11, 9832–9840. [Google Scholar] [CrossRef]
- Liu, Z.; Li, B.; Shi, X.; Li, L.; Feng, Y.; Jia, D.; Zhou, Y. Target-oriented synthesis of high synthetic yield carbon dots with tailored surface functional groups for bioimaging of zebrafish, flocculation of heavy metal ions and ethanol detection. Appl. Surf. Sci. 2021, 538, 148118–148128. [Google Scholar] [CrossRef]
- Dare, P.J.; Edwards, D.B. Seasonal changes in flesh weight and biochemical composition of mussels (Mytilus edulis L.) in the Conwy Estuary, North Wales. J. Exp. Mar. Bio. Ecol. 1975, 18, 89–97. [Google Scholar] [CrossRef]
- Supchocksoonthorn, P.; Hanchaina, R.; Sinoy, M.C.A.; de Luna, M.D.G.; Kangsamaksin, T.; Paoprasert, P. Novel solution- and paper-based sensors based on label-free fluorescent carbon dots for the selective detection of pyrimethanil. Appl. Surf. Sci. 2021, 564, 150372. [Google Scholar] [CrossRef]
- Cong, S.; Bi, J.; Song, X.; Yu, C.; Tan, M. Ultrasmall fluorescent nanoparticles derived from roast duck: Their physicochemical characteristics and interaction with human serum albumin. Food Funct. 2018, 9, 2490–2495. [Google Scholar] [CrossRef]
- Li, D.; Na, X.; Wang, H.; Xie, Y.; Cong, S.; Song, Y.; Zhu, B.W.; Tan, M. Fluorescent carbon dots derived from maillard reaction products: Their properties, biodistribution, cytotoxicity, and antioxidant activity. J. Agr. Food Chem. 2018, 66, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Li, Y.; Wang, H.; Song, Y.; Cong, S.; Yu, C.; Zhu, B.-W.; Tan, M. Presence and formation mechanism of foodborne carbonaceous nanostructures from roasted pike eel (Muraenesox cinereus). J. Agr. Food Chem. 2018, 66, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Boruah, A.; Saikia, M.; Das, T.; Goswamee, R.L.; Saikia, B.K. Blue-emitting fluorescent carbon quantum dots from waste biomass sources and their application in fluoride ion detection in water. J. Photoch. Photobio. B. 2020, 209, 111940. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Ren, Y.; Sun, X.; Jin, L.; Liu, X.; Chen, H.; Wang, K.; Yu, M.; Zhao, Y. Photoluminescent functionalized carbon quantum dots loaded electroactive silk fibroin/PLA nanofibrous bioactive scaffolds for cardiac tissue engineering. J. Photoch. Photobio. B. 2020, 202, 111680. [Google Scholar] [CrossRef] [PubMed]
- Malavika, J.P.; Shobana, C.; Ragupathi, M.; Kumar, P.; Lee, Y.S.; Govarthanan, M.; Selvan, R.K. A sustainable green synthesis of functionalized biocompatible carbon quantum dots from Aloe barbadensis miller and its multifunctional applications. Environ. Res. 2021, 200, 111414. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small 2020, 16, 76–83. [Google Scholar] [CrossRef]
- Supchocksoonthorn, P.; Thongsai, N.; Moonmuang, H.; Kladsomboon, S.; Jaiyong, P.; Paoprasert, P. Label-free carbon dots from black sesame seeds for real-time detection of ammonia vapor via optical electronic nose and density functional theory calculation. Colloid. Surf. A. 2019, 575, 118–128. [Google Scholar] [CrossRef]
- Cong, S.; Liu, K.; Qiao, F.; Song, Y.; Tan, M. Biocompatible fluorescent carbon dots derived from roast duck for in vitro cellular and in vivo C. elegans bio-imaging. Methods 2019, 168, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, J.; Zheng, M.; Xie, Z. Lysosome targeting carbon dots-based fluorescent probe for monitoring pH changes in vitro and in vivo. Chem. Eng. J. 2020, 381, 122665–122677. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharmaceut. 2019, 564, 308–317. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Zhang, R.; Yu, C.; Tan, M. Bio-distribution and interaction with dopamine of fluorescent nanodots from roasted chicken. Food Funct. 2018, 9, 6227–6235. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, J.; Li, W.; Zhang, L.; Yue, D. Synthesis of amphiphilic carbon quantum dots with phosphorescence properties and their multifunctional applications. J. Mater. Chem. C. 2016, 4, 10146–10153. [Google Scholar] [CrossRef]
- Sk, M.P.; Jaiswal, A.; Paul, A.; Ghosh, S.S.; Chattopadhyay, A. Presence of amorphous carbon nanoparticles in food caramels. Sci. Rep. UK 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Jiang, C.; Liu, W.; Vera, J.M.; Seni, O.D.; Demera, K.; Yu, C.; Tan, M. Fluorescent nanoparticles from several commercial beverages: Their properties and potential application for bioimaging. J. Agr. Food Chem. 2015, 63, 8527–8533. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wu, H.; Ye, Z.; Li, R.; Xu, T.; Zhang, B. Optical properties of pH-sensitive carbon-dots with different modifications. J. Lumin. 2014, 148, 238–242. [Google Scholar] [CrossRef]
- Liu, R.; Liu, K.; Tan, M. Nanocorona formation between foodborne nanoparticles extracted from roast squid and human serum albumin. J. Agr. Food Chem. 2019, 67, 10470–10480. [Google Scholar] [CrossRef]
- Shan, F.; Fu, L.; Chen, X.; Xie, X.; Liao, C.; Zhu, Y.; Xia, H.; Zhang, J.; Yan, L.; Wang, Z.; et al. Waste-to-wealth: Functional biomass carbon dots based on bee pollen waste and application. Chinese Chem. Lett. 2022, 33, 2942–2948. [Google Scholar] [CrossRef]
- Cong, S.; Wang, N.; Wang, K.; Wu, Y.; Li, D.; Song, Y.; Prakash, S.; Tan, M. Fluorescent nanoparticles in the popular pizza: Properties, biodistribution and cytotoxicity. Food Funct. 2019, 10, 2408–2416. [Google Scholar] [CrossRef]
- Zhou, N.; Zhu, S.; Maharjan, S.; Hao, H.; Song, Y.; Zhao, X.; Jiang, Y.; Yang, B.; Lu, L. Elucidating the endocytosis, intracellular trafficking, and exocytosis of carbon dots in neural cells. RSC Adv. 2014, 107, 62086–62095. [Google Scholar] [CrossRef]
- Wang, J.; Su, S.; Wei, J.; Bahgi, R.; Hope-Weeks, L.; Qiu, J.; Wang, S. Ratio-metric sensor to detect riboflavin via fluorescence resonance energy transfer with ultrahigh sensitivity. Phys. E. 2015, 72, 17–24. [Google Scholar] [CrossRef]
- Eitenmiller, R.R.; Landen, W.O., Jr.; Ye, L. Vitamin Analysis for the Health And food Sciences; CRC Press: Boca Raton, FL, USA, 2008; p. 2041. [Google Scholar]
- Han, J.; Zou, H.; Gao, M.; Huang, C. A graphitic carbon nitride based fluorescence resonance energy transfer detection of riboflavin. Talanta 2016, 148, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Song, J.; Zhang, S.; Wang, M.; Guo, Y.; Dong, C. Colorimetric detection of riboflavin by silver nanoparticles capped with β-cyclodextrin-grafted citrate. Colloid Surf. B. 2016, 148, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Pei, F.; Wu, Y.; Lv, J.; Hao, Q.; Yang, T.; Tong, Z.; Lei, W. A ratiometric fluorescent sensor based on g-CNQDs@Zn-MOF for the sensitive detection of riboflavin via FRET. Spectrochim Acta A. 2021, 246, 119004. [Google Scholar] [CrossRef] [PubMed]

| Sensors | Linear Range (μmol/L) | Detection Limit (nmol/L) | Reference |
|---|---|---|---|
| N, S-CDs | 0.56–7.44 | 1.9 | [40] |
| g-C3N4 nanosheets 1 | 0.4–10 | 170 | [42] |
| Ag nanoparticles | 0.17–4.67 | 3.7 | [43] |
| N, P-CDs | 0.5–50 | 170 | [23] |
| g-CNQDs@Zn-MOF 2 | 0.005–1.0 | 15 | [44] |
| Mussels-derived CDs | 1–50 | 6.06 | This work |
| Sample | Measured (μmol/L) | Added (μmol/L) | Found (μmol/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Milk | 0.45 | 40.00 | 40.50 | 100.1 | 1.3 |
| 20.00 | 20.41 | 99.8 | 0.7 | ||
| 4.00 | 4.38 | 98.3 | 1.1 | ||
| Milk powder | 0.98 | 40.00 | 38.18 | 93.0 | 0.7 |
| 20.00 | 19.98 | 95.0 | 0.5 | ||
| 4.00 | 4.87 | 97.3 | 1.2 | ||
| Riboflavin tablet | 5.12 | 40.00 | 45.52 | 101.0 | 0.9 |
| 20.00 | 24.99 | 99.4 | 0.6 | ||
| 4.00 | 9.05 | 98.3 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Zhang, Y.; Cao, B.; Li, Z.; Sun, C.; Cao, X.; Cong, S. Characteristics of Mussels-Derived Carbon Dots and Their Applications in Bio-Imaging and Detection of Riboflavin. Foods 2022, 11, 2451. https://doi.org/10.3390/foods11162451
Zhao W, Zhang Y, Cao B, Li Z, Sun C, Cao X, Cong S. Characteristics of Mussels-Derived Carbon Dots and Their Applications in Bio-Imaging and Detection of Riboflavin. Foods. 2022; 11(16):2451. https://doi.org/10.3390/foods11162451
Chicago/Turabian StyleZhao, Wenyu, Yi Zhang, Bin Cao, Zhuoyan Li, Chengfeng Sun, Xiaolin Cao, and Shuang Cong. 2022. "Characteristics of Mussels-Derived Carbon Dots and Their Applications in Bio-Imaging and Detection of Riboflavin" Foods 11, no. 16: 2451. https://doi.org/10.3390/foods11162451
APA StyleZhao, W., Zhang, Y., Cao, B., Li, Z., Sun, C., Cao, X., & Cong, S. (2022). Characteristics of Mussels-Derived Carbon Dots and Their Applications in Bio-Imaging and Detection of Riboflavin. Foods, 11(16), 2451. https://doi.org/10.3390/foods11162451





