Cereal Waste Valorization through Conventional and Current Extraction Techniques—An Up-to-Date Overview
Abstract
:1. Introduction
2. Conventional Extraction Methods
2.1. Acid and Alkaline Hydrolysis
2.2. Solvent Extraction
2.3. Soxhlet Extraction (SE)
3. Advanced Treatments and Extraction Techniques
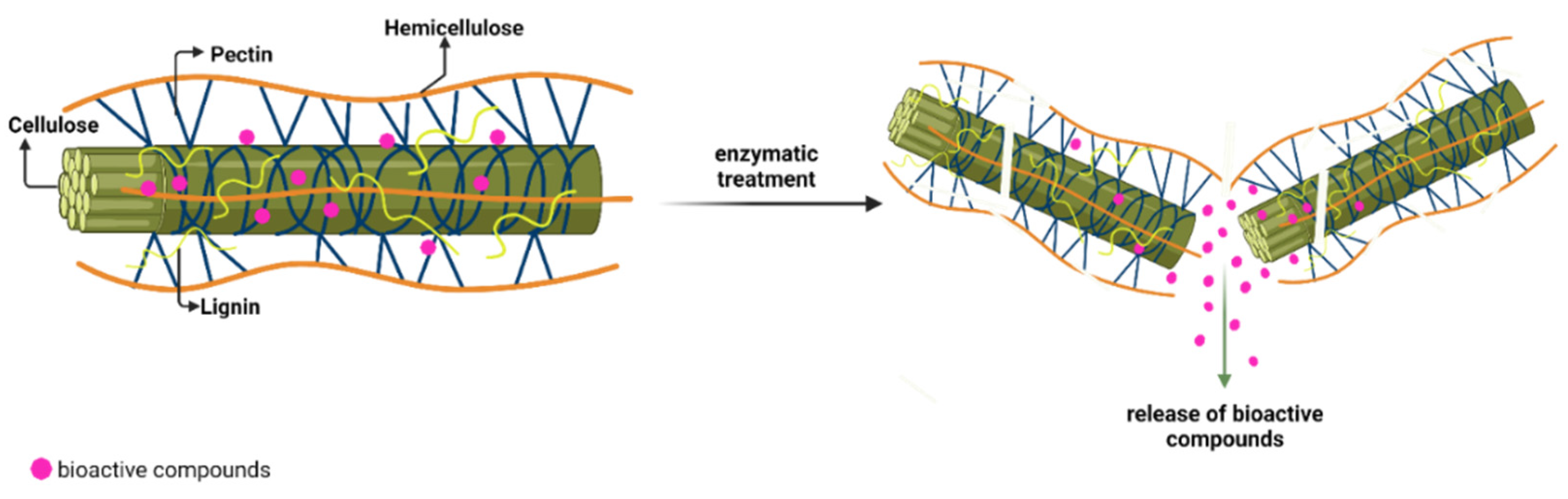
3.1. Enzymatic-Assisted Extraction (EAE)
| Source | Targeted Compound | Enzyme | Commercial Formulation | EAE Parameters | Results | Application | References |
|---|---|---|---|---|---|---|---|
| Rice bran | Fatty acids | Alcalase | Alcalase 2.4 L by Novozymes Bagsvaerd | Powdered rice bran was mixed with distilled water at a ratio of 1:7.5 (w/v); pH mixture: 9.0; Temperature: 57 °C; Time: 150 min; Enzyme quantity: 2 g/100 g | Higher content of unsaturated fatty acids: 76.31%; Tocopherols and tocotrienols: 1004 mg/kg; Sterols: 7749 mg/100 g; Squalene: 2962 mg/kg; Oryzanol: 2.43 g/100 g; Extracted oil has lower crystallization and melting points. The wax and phospholipid concentrations of the extracted oil were reduced. | Edible oil in food industry | [49] |
| Rice bran | Protein | Trypsin type I | Trypsin type I from bovine pancreas | 50 mL of protein solution was hydrolyzed using a ratio 1:100 enzyme-substrate; Temperature: 37 °C pH: 8 Time: 4 h; Enzyme activity: 10,000 BAEE units/mg of protein. The enzymatic activity was inactivated by reducing the final pH to 3. | Protein concentration (g of protein/100 g of extract): Albumin: 42.40 ± 1.70 Globulin: 41.14 ± 2.72 Glutelin: 69.01 ± 1.53 Total protein soluble: 63.20 ± 1.70 | Foods, cosmetics and pharmaceuticals | [50] |
| Sesame bran | Protein and phenolics | Alcalase | Alcalase 2.4 L by Novozymes | Enzyme concentration: 0.12–2.40 AU/100 g Ratio sesame bran and dH2O: 1:10 (w/v); pH: 9.8; Temperature: 45 °C; Time: 30 min; Vacuum time: 1–30 min; Vacuum pressure: 100–650 mmHg. | Combined enzymatic treatment resulted in 19.1% and 61.4% more protein yield; Increased protein yield, total phenolic content and antioxidant capacity values. | New extraction protocols including vacuum treatment. | [12] |
| Corn husks | Flavonoids | Cellulose | EC 3.2.1.4 by Macklin Biochemical Co., Ltd. | Extraction solvent: aqueous ethanol; Enzyme dosage: 0.3–0.5 g/100 g; Incubation time: 1.5–2.5 h Liquid-to-solid ratio: 30–40 mL g−1; Temperature: 40 °C; pH: 5.0; The enzyme was inactivated in boiling water for 5 min | 1.3 g/100 g of total flavonoids of dry waste were recovered; | Corn industry | [51] |
| Brewer’s spent grain | Arabinoxylans | Xylanases; Peptidase. | EC 3.2.1.8 by AB Enzymes; Clarex by DSM Food Specialties. | 2 and 5 units of xylanases; 25 µg of the peptidase; Temperature: 50 °C; Time: 15, 45, 90, 150 and 240 min. | Over 33% of Arabinoxylans was solubilized. | Food ingredient | [52] |
| Rye bran | Phenolic acids | Xylanase; Amylase. | Grindamyl A 1000 Depol 740 L | 200 nkat/g bran xylanase; 5 nkat/g bran amylase 65% water content; Temperature: 40 °C Time: 4 h | Ferulic acid production was greatly improved by the applied bioprocess. Reduced levels of phenylacetic acids was were identified. | Food product (bread) | [53] |
| Brewer’s spent grain | Dietary fiber, protein, unsaturated fats, and lignans | Xylanase; Alcalase | Depol740 L; Celluclast Alcalase 2.4 L by Novozymes | First hydrolysis: pH: 5.4 Time: 5 h Temperature: 50 °C; Second hydrolysis: pH: 10 Time: 4 h; Temperature: 60 °C. | Solubility rate: 66% of BSG; Lipids content: 11% The main fatty acids identified: linoleic, palmitic, and oleic acids; The most abundant lignans: syringaresinol and secoisolariciresinol. | Food ingredient | [47] |
| Brewer’s spent grain | Carbohydrates | Cellulase-hemicellulase mixtures | Econase; Spezyme CP; Depol 740 and 686. | Time: 5 h; pH: 5; Temperature: 50 °C; The enzymes were dosed according to their xylanase activity. | Carbohydrates solubilization: 26–28%; Arabinoxylans solubilization: 30–34%; Due to the presence of feruloyl esterase activity in the enzyme cocktail, released ferulic acid, arabinoxylan-oligosaccharides, and their monomers were produced; The unhydrolysed fraction contains over 40% of carbohydrates. | Food and non-food application | [54] |
| Brewer’s spent grain | Protein and lignin | Protease | Biotouch Roc 250 L | pH: 10; Time: 5 h; Temperature: 50 °C; Enzyme inactivation was performed by boiling the tubes for 10 min. | Increased protein solubilization from 15% to almost 100%. | Valorization of BSG into multi-use food ingredients | [45] |
| Rice bran | Antioxidant peptides | Proteases (papain, flavourzyme, neutrase, protamex, and trypsin) | Novo Nordisk Co | Time: 3 h; Temperature: 37–55 °C (optimal for each enzyme); pH: 6.5–8.0 (optimal for each enzyme). | Highest antioxidant activity was performed by papain and flavourzyme activity; | Suitable natural antioxidants for food processing and ingredient for functional foods. | [55] |
3.2. Ultrasound-Assisted Extraction (UAE)
3.2.1. Parameters That Influence UAE Process
3.2.2. UAE Applicability in Cereal Waste Valorization
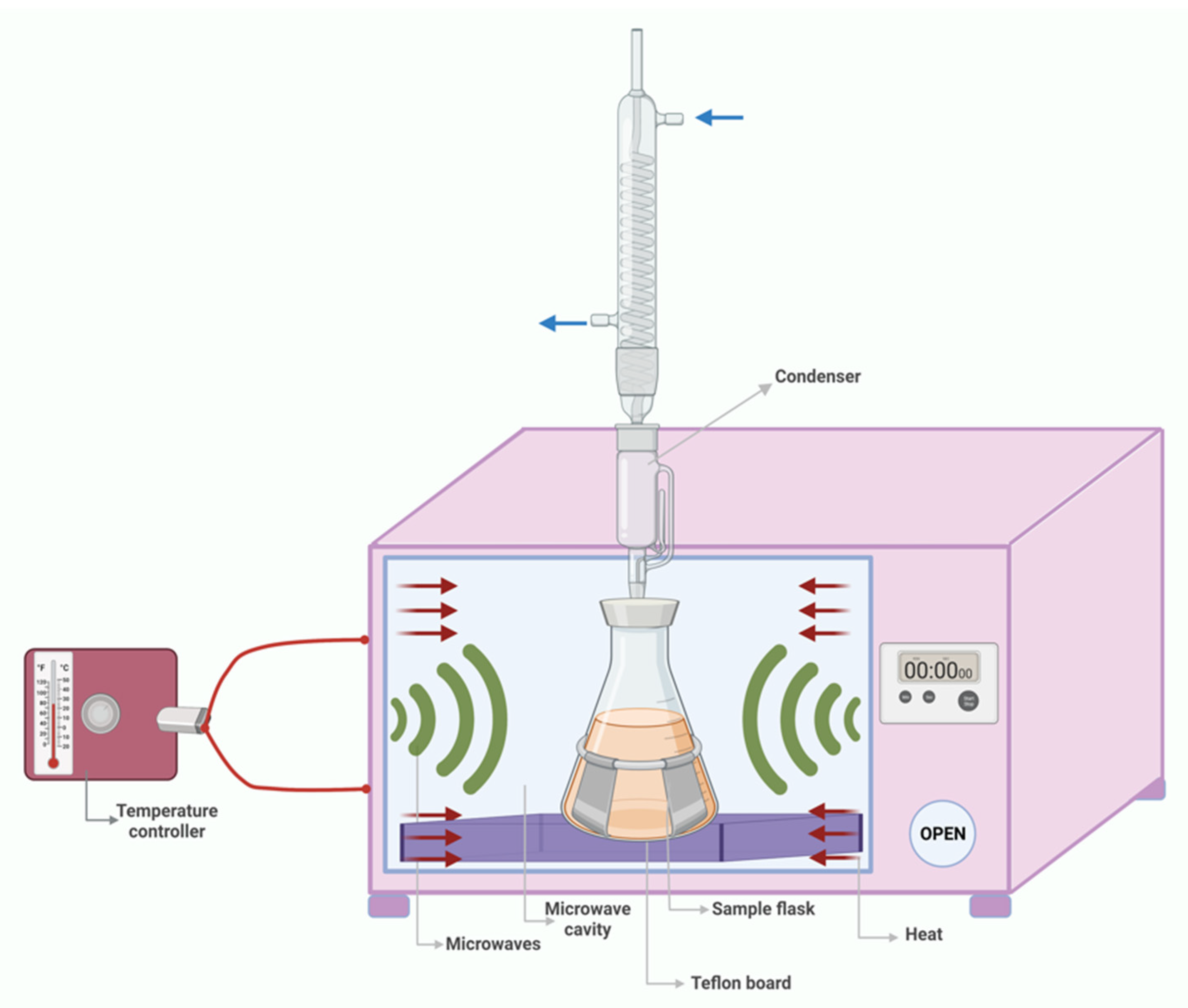
3.3. Microwave-Assisted Extraction (MAE)
3.3.1. Parameters That Influence MAE Performance
3.3.2. MAE Applicability in Cereal Waste Valorization
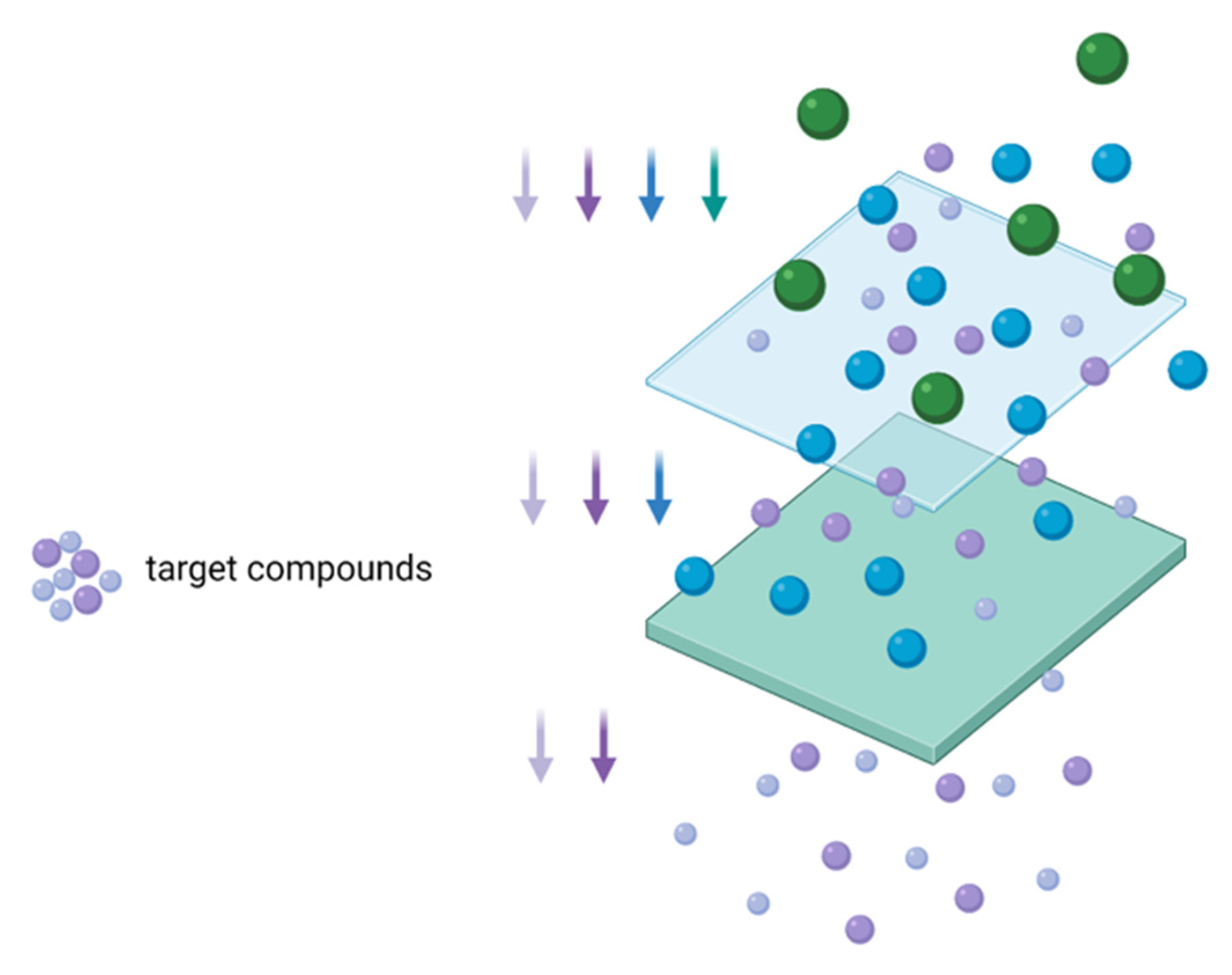
3.4. Membrane Fractionation of Different Compounds from Cereal Waste and by-Products
3.5. Pressure Based Extraction Techniques
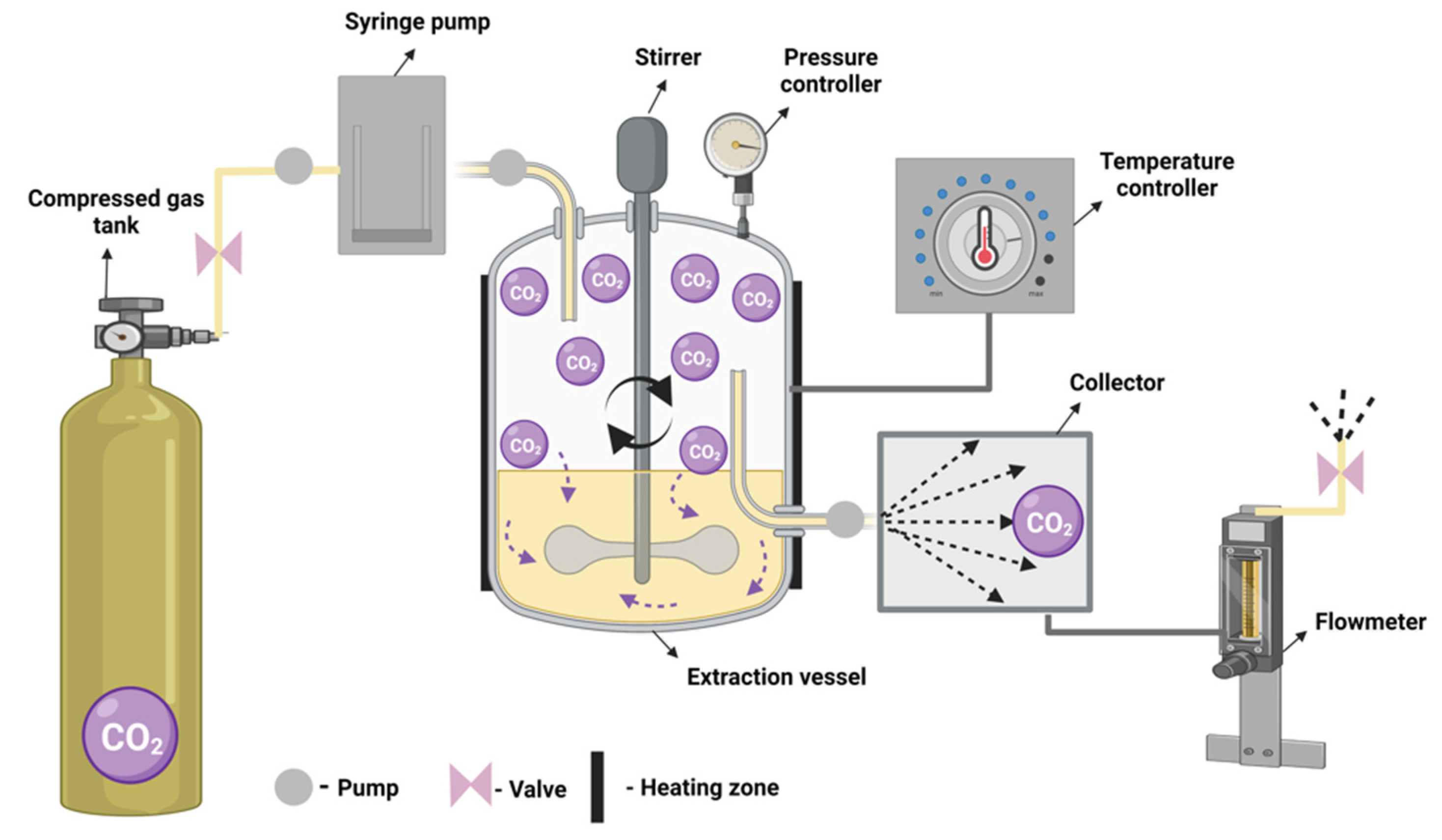
3.5.1. Supercritical Fluid Extraction Principle and Characteristics
3.5.2. Pressurized Liquid Extraction (PLE), Subcritical Water Extraction (SWE) and Steam Explosion (STE)
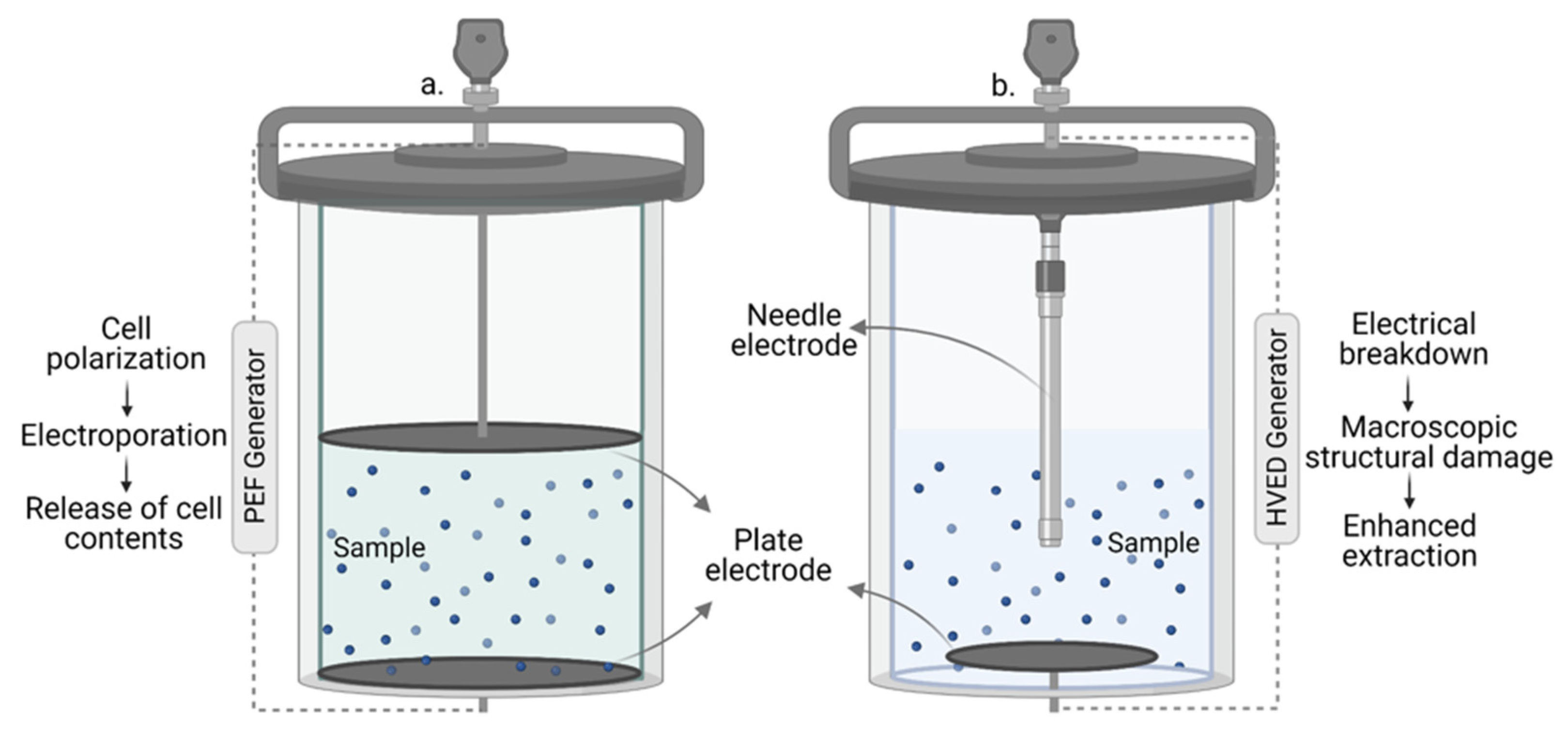
3.5.3. Pulsed-Electric Field Extraction (PEF) and High Voltage Electrical Discharge (HVED)
4. Environmental and Economic Sustainability Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Negreanu, G.P.; Belc, N.; Mustatea, G.; Apostol, L.; Iorga, S.; Vlăduţ, V.-N.; Mosoiu, C.; Vlăduţ, N.V. Cereal supply chain waste in the context of circular economy. In Proceedings of the E3S Web of Conferences, Targoviste, Romania, 6–8 June 2019; Volume 112, p. 03031. [Google Scholar] [CrossRef]
- Viganó, J.; Machado, A.P.D.F.; Martínez, J. Sub- and supercritical fluid technology applied to food waste processing. J. Supercrit. Fluids 2015, 96, 272–286. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Bilbao, A.; Vilches, P.; Angulo, I.; LLuis, L.L.; Fite, B.; Paseiro-Losada, P.; Cruz, J.M. Brewery waste as a potential source of phenolic compounds: Optimisation of the extraction process and evaluation of antioxidant and antimicrobial activities. Food Chem. 2014, 145, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Galanakis, C.M. Membrane technologies for the fractionation of compounds recovered from cereal processing by-products. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 159–187. [Google Scholar]
- Rojas-Pérez, L.C.; Narváez-Rincón, P.C.; Ballesteros, I. Improving sugar extraction from brewers’ spent grain using sequential deproteinization and acid-catalyzed steam explosion in a biorefinery context. Biomass Bioenergy 2022, 159, 106389. [Google Scholar] [CrossRef]
- Alonso-Riano, P.; Sanz Diez, M.T.; Blanco, B.; Beltran, S.; Trigueros, E.; Benito-Roman, O. Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants 2020, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Farcas, A.C.; Socaci, S.A.; Chiș, M.S.; Pop, O.L.; Fogarasi, M.; Păucean, A.; Igual, M.; Michiu, D. Reintegration of Brewers Spent Grains in the Food Chain: Nutritional, Functional and Sensorial Aspects. Plants 2021, 10, 2504. [Google Scholar] [CrossRef] [PubMed]
- Macias-Garbett, R.; Serna-Hernandez, S.O.; Sosa-Hernandez, J.E.; Parra-Saldivar, R. Phenolic Compounds From Brewer’s Spent Grains: Toward Green Recovery Methods and Applications in the Cosmetic Industry. Front. Sustain. Food Syst. 2021, 1–10, 5. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, E.; Marina, M.L.; Garcia, M.C. Impact of the use of pressurized liquids on the extraction and functionality of proteins and bioactives from brewer’s spent grain. Food Chem. 2021, 359, 129874. [Google Scholar] [CrossRef]
- Moirangthem, K.; Ramakrishna, P.; Amer, M.H.; Tucker, G.A. Bioactivity and anthocyanin content of microwave-assisted subcritical water extracts of Manipur black rice (Chakhao) bran and straw. Future Foods 2021, 3, 100030. [Google Scholar] [CrossRef]
- Mackėla, I.; Andriekus, T.; Venskutonis, P.R. Biorefining of buckwheat (Fagopyrum esculentum) hulls by using supercritical fluid, Soxhlet, pressurized liquid and enzyme-assisted extraction methods. J. Food Eng. 2017, 213, 38–46. [Google Scholar] [CrossRef]
- Gorguc, A.; Bircan, C.; Yilmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Campos, D.A.; Osorio, H.; Nerli, B.B.; Pintado, M. Enzymatic soy protein hydrolysis: A tool for biofunctional food ingredient production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Valério, R.; Serra, A.T.; Baixinho, J.; Cardeira, M.; Fernández, N.; Bronze, M.R.; Duarte, L.C.; Tavares, M.L.; Crespo, J.G.; Brazinha, C. Combined hydrothermal pre-treatment and enzymatic hydrolysis of corn fibre: Production of ferulic acid extracts and assessment of their antioxidant and antiproliferative properties. Ind. Crops Prod. 2021, 170, 113731. [Google Scholar] [CrossRef]
- Karimi, R.; Azizi, M.H.; Sahari, M.A.; Kazem, A.E. In vitro fermentation profile of soluble dietary fibers obtained by different enzymatic extractions from barley bran. Bioact. Carbohydr. Diet. Fibre 2020, 21, 100205. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Patil, P.D.; Patil, S.P.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Nadar, S.S. Enzyme-assisted supercritical fluid extraction: An integral approach to extract bioactive compounds. Trends Food Sci. Technol. 2021, 116, 357–369. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Bonifacio-Lopes, T.; Teixeira, J.A.; Pintado, M. Current extraction techniques towards bioactive compounds from brewer’s spent grain—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Ideia, P.; Sousa-Ferreira, I.; Castilho, P.C. A Novel and Simpler Alkaline Hydrolysis Methodology for Extraction of Ferulic Acid from Brewer’s Spent Grain and its (Partial) Purification through Adsorption in a Synthetic Resin. Foods 2020, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio-Lopes, T.; Vilas Boas, A.A.; Coscueta, E.R.; Costa, E.M.; Silva, S.; Campos, D.; Teixeira, J.A.; Pintado, M. Bioactive extracts from brewer’s spent grain. Food Funct. 2020, 11, 8963–8977. [Google Scholar] [CrossRef] [PubMed]
- Burlini, I.; Grandini, A.; Tacchini, M.; Maresca, I.; Guerrini, A.; Sacchetti, G. Different Strategies to Obtain Corn (Zea mays L.) Germ Extracts with Enhanced Antioxidant Properties. Nat. Prod. Commun. 2020, 15, 1934578X20903562. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct measurement of the total antioxidant capacity of cereal products. J. Cereal Sci. 2008, 48, 816–820. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. A novel application of microwave-assisted extraction of polyphenols from brewer’s spent grain with HPLC-DAD-MS analysis. Anal. Bioanal. Chem. 2012, 403, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Buranov, A.U.; Mazza, G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and p-coumaric acids extraction by alkaline hydrolysis of brewer’s spent grain. Ind. Crops Prod. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. Characterisation of protein-rich isolates and antioxidative phenolic extracts from pale and black brewers’ spent grain. Int. J. Food Sci. Technol. 2013, 48, 1670–1681. [Google Scholar] [CrossRef]
- Bacha, E.G. Response Surface Methodology Modeling, Experimental Validation, and Optimization of Acid Hydrolysis Process Parameters for Nanocellulose Extraction. South Afr. J. Chem. Eng. 2022, 40, 176–185. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Acid hydrolysis and fermentation of brewer’s spent grain to produce xylitol. J. Sci. Food Agric. 2005, 85, 2453–2460. [Google Scholar] [CrossRef]
- Arranz, S.; Calixto, F.S. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Bauer, J.L.; Harbaum-Piayda, B.; Schwarz, K. Phenolic compounds from hydrolyzed and extracted fiber-rich by-products. Lwt-Food Sci. Technol. 2012, 47, 246–254. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the extraction solvent on phenolic content, antioxidant, antimicrobial and antimutagenic activities of brewers’ spent grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- Smuda, S.S.; Mohsen, S.M.; Olsen, K.; Aly, M.H. Bioactive compounds and antioxidant activities of some cereal milling by-products. J. Food Sci. Technol. 2018, 55, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Birsan, R.I.; Wilde, P.; Waldron, K.W.; Rai, D.K. Recovery of Polyphenols from Brewer’s Spent Grains. Antioxidants 2019, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Rao, J.; Chen, B. Phenolic compounds in germinated cereal and pulse seeds: Classification, transformation, and metabolic process. Crit. Rev. Food Sci. Nutr. 2020, 60, 740–759. [Google Scholar] [CrossRef]
- Guido, L.F.; Moreira, M.M. Techniques for Extraction of Brewer’s Spent Grain Polyphenols: A Review. Food Bioprocess Technol. 2017, 10, 1192–1209. [Google Scholar] [CrossRef]
- Dar, B.N.; Sharma, S. Total Phenolic Content of Cereal Brans using Conventional and Microwave Assisted Extraction. Am. J. Food Technol. 2011, 6, 1045–1053. [Google Scholar] [CrossRef]
- López-Linares, J.C.; García-Cubero, M.T.; Lucas, S.; González-Benito, G.; Coca, M. Microwave assisted hydrothermal as greener pretreatment of brewer’s spent grains for biobutanol production. Chem. Eng. J. 2019, 368, 1045–1055. [Google Scholar] [CrossRef]
- Seidel, V. Initial and bulk extraction of natural products isolation. Methods Mol. Biol. 2012, 864, 27–41. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Popescu, A.; Matei, E.; Cozaru, G.C.; Schröder, V.; Ozon, E.A.; Fița, A.C.; Lupuliasa, D.; et al. Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea barbata (L.) Weber ex F.H.Wigg from Călimani Mountains, Romania. Pharmaceuticals 2022, 15, 829. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajar, S.; Devi, A.; Pant, D. An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour. Technol. Rep. 2021, 14, 100652. [Google Scholar] [CrossRef]
- Rommi, K.; Niemi, P.; Kemppainen, K.; Kruus, K. Impact of thermochemical pre-treatment and carbohydrate and protein hydrolyzing enzyme treatment on fractionation of protein and lignin from brewer’s spent grain. J. Cereal Sci. 2018, 79, 168–173. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Ruan, S.; Lv, R.; Zhou, J.; Tian, J.; Cheng, H.; Xu, E.; Liu, D. A comprehensive review of cereal germ and its lipids: Chemical composition, multi-objective process and functional application. Food Chem. 2021, 362, 130066. [Google Scholar] [CrossRef] [PubMed]
- Niemi, P.; Tamminen, T.; Smeds, A.; Viljanen, K.; Ohra-aho, T.; Holopainen-Mantila, U.; Faulds, C.B.; Poutanen, K.; Buchert, J. Characterization of lipids and lignans in brewer’s spent grain and its enzymatically extracted fraction. J. Agric. Food Chem. 2012, 60, 9910–9917. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, R.; Verma, S.; Kumar, A.; Rajesh, C.; Sharma, P.K. Structural and functional insights about unique extremophilic bacterial lipolytic enzyme from metagenome source. Int. J. Biol. Macromol. 2020, 152, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hao, J.; Wang, Z.; Liang, D.; Wang, J.; Ma, Y.; Zhang, M. Physicochemical properties, fatty acid compositions, bioactive compounds, antioxidant activity and thermal behavior of rice bran oil obtained with aqueous enzymatic extraction. LWT 2021, 149, 111817. [Google Scholar] [CrossRef]
- Rodríguez-Restrepo, Y.A.; Ferreira-Santos, P.; Orrego, C.E.; Teixeira, J.A.; Rocha, C.M.R. Valorization of rice by-products: Protein-phenolic based fractions with bioactive potential. J. Cereal Sci. 2020, 95, 103039. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; González-Delgado, Á.D.; García-Martinez, J.B.; L’Abbate, P. Optimization of Enzyme-Assisted Extraction of Flavonoids from Corn Husks. Processes 2019, 7, 804. [Google Scholar] [CrossRef]
- Severini, C.; Azzollini, D.; Jouppila, K.; Jussi, L.; Derossi, A.; De Pilli, T. Effect of enzymatic and technological treatments on solubilisation of arabinoxylans from brewer’s spent grain. J. Cereal Sci. 2015, 65, 162–166. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Nordlund, E.; Katina, K.; Mattila, I.; Poutanen, K.; Hanhineva, K.; Aura, A.M. Effect of Bioprocessing on the In Vitro Colonic Microbial Metabolism of Phenolic Acids from Rye Bran Fortified Breads. J. Agric. Food Chem. 2017, 65, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Forssell, P.; Kontkanen, H.; Schols, H.A.; Hinz, S.; Eijsink, V.G.H.; Treimo, J.; Robertson, J.A.; Waldron, K.W.; Faulds, C.B.; Buchert, J. Hydrolysis of Brewers’ Spent Grain by Carbohydrate Degrading Enzymes. J. Inst. Brew. 2008, 114, 306–314. [Google Scholar] [CrossRef]
- Mei, D.J.; Yu, G.P.; Sun, A.M. Preparation, purification and identification of antioxidant peptides with bienzyme hydrolysis from rice bran protein. Prog. Environ. Sci. Eng. Pts 1–4 2013, 610–613, 72–80. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Picó, Y. Ultrasound-assisted extraction for food and environmental samples. TrAC Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.K.; Fabiano-Tixier, A.S.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef]
- Rutkowska, M.; Namieśnik, J.; Konieczka, P. Ultrasound-Assisted Extraction. Appl. Green Solvents Sep. Processes 2017, 301–324. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Osorio, L.; Florez-Lopez, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Kottaras, P.; Koulianos, M.; Makris, D. Low-Transition Temperature Mixtures (LTTMs) Made of Bioorganic Molecules: Enhanced Extraction of Antioxidant Phenolics from Industrial Cereal Solid Wastes. Recycling 2017, 2, 3. [Google Scholar] [CrossRef]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recent Advances in the Recovery Techniques of Plant-Based Proteins from Agro-Industrial By-Products. Food Rev. Int. 2020, 37, 447–468. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valorization 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Li, Y.; Chen, Z. Ultrasound-assisted extraction from defatted oat (Avena sativa L.) bran to simultaneously enhance phenolic compounds and β-glucan contents: Compositional and kinetic studies. J. Food Eng. 2018, 222, 1–10. [Google Scholar] [CrossRef]
- Bedin, S.; Netto, F.M.; Bragagnolo, N.; Taranto, O.P. Reduction of the process time in the achieve of rice bran protein through ultrasound-assisted extraction and microwave-assisted extraction. Sep. Sci. Technol. 2019, 55, 300–312. [Google Scholar] [CrossRef]
- Qu, W.; Ma, H.; Jia, J.; He, R.; Luo, L.; Pan, Z. Enzymolysis kinetics and activities of ACE inhibitory peptides from wheat germ protein prepared with SFP ultrasound-assisted processing. Ultrason. Sonochem. 2012, 19, 1021–1026. [Google Scholar] [CrossRef]
- Li, W.Y.; Yang, H.R.; Coldea, T.E.; Zhao, H.F. Modification of structural and functional characteristics of brewer’s spent grain protein by ultrasound assisted extraction. Lwt-Food Sci. Technol. 2021, 139, 110582. [Google Scholar] [CrossRef]
- Luo, X.P.; Cui, J.M.; Zhang, H.H.; Duan, Y.Q.; Zhang, D.; Cai, M.H.; Chen, G.Y. Ultrasound assisted extraction of polyphenolic compounds from red sorghum (Sorghum bicolor L.) bran and their biological activities and polyphenolic compositions. Ind. Crops Prod. 2018, 112, 296–304. [Google Scholar] [CrossRef]
- Guerrini, A.; Burlini, I.; Huerta Lorenzo, B.; Grandini, A.; Vertuani, S.; Tacchini, M.; Sacchetti, G. Antioxidant and antimicrobial extracts obtained from agricultural by-products: Strategies for a sustainable recovery and future perspectives. Food Bioprod. Process. 2020, 124, 397–407. [Google Scholar] [CrossRef]
- Du, B.; Zhu, F.; Xu, B. β-Glucan extraction from bran of hull-less barley by accelerated solvent extraction combined with response surface methodology. J. Cereal Sci. 2014, 59, 95–100. [Google Scholar] [CrossRef]
- Zamorano Ulloa, R.; Guadalupe Hernandez Santiago, M.; Villegas Rueda, V.L. The Interaction of Microwaves with Materials of Different Properties. In Electromagnetic Fields and Waves; IntechOpen: London, UK, 2019; pp. 1–29. [Google Scholar]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-Assisted Extraction; The Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Uzel, R.A. Microwave-Assisted Green Extraction Technology for Sustainable Food Processing. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; BoD–Books on Demand: Norderstedt, Germany, 2018; pp. 159–178. [Google Scholar]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Angiolillo, L.; Del Nobile, M.A.; Conte, A. The extraction of bioactive compounds from food residues using microwaves. Curr. Opin. Food Sci. 2015, 5, 93–98. [Google Scholar] [CrossRef]
- Zuorro, A.; Iannone, A.; Lavecchia, R. Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain. Processes 2019, 7, 126. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Microwave-assisted enzymatic extraction of plant protein with antioxidant compounds from the food waste sesame bran: Comparative optimization study and identification of metabolomics using LC/Q-TOF/MS. J. Food Process. Preserv. 2019, 44, e14304. [Google Scholar] [CrossRef]
- Oufnac, D.S. Determination of Antioxidant Capacity in Corn Germ, Wheat Germ and Wheat Bran Using Solvent and Microwave-Assisted Solvent Extraction. Master’s Theses, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2006. Volume 78. [Google Scholar]
- Demirhan, H.; Fauzi, A.; Skoulou, V.K.; Haywood, S.H.; Zein, S.H. Wheat Straw Bio-refining. Part I: Optimization of the Microwave Radiation Process with Sulphuric Acid Pre-treatment. Curr. Microw. Chem. 2017, 4, 205–218. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Sánchez, R.; Díaz-Carrasco, P.; Espinosa, E.; García-Domínguez, M.T.; Rodríguez, A. Isolation and characterization of lignins from wheat straw: Application as binder in lithium batteries. Int. J. Biol. Macromol. 2017, 104, 909–918. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, M.A.; Saraiva, J.A.; Coimbra, M.A. Microwave superheated water and dilute alkali extraction of brewers’ spent grain arabinoxylans and arabinoxylo-oligosaccharides. Carbohydr. Polym. 2014, 99, 415–422. [Google Scholar] [CrossRef]
- Rocha, M.A.M.; Saraiva, J.A.; Coimbra, M.A. Microwave-assisted Extraction of Brewer’s Spent Grain Arabinoxylans. In Proceedings of the 7th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 2–4 November 2011; pp. 1–5. [Google Scholar]
- dos Santos, D.M.; Bukzem Ade, L.; Ascheri, D.P.; Signini, R.; de Aquino, G.L. Microwave-assisted carboxymethylation of cellulose extracted from brewer’s spent grain. Carbohydr. Polym. 2015, 131, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Rorke, D.C.S.; Suinyuy, T.N.; Gueguim Kana, E.B. Microwave-assisted chemical pre-treatment of waste sorghum leaves: Process optimization and development of an intelligent model for determination of volatile compound fractions. Bioresour. Technol. 2017, 224, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Tsubaki, S.; Teramoto, Y.; Azuma, J. Optimization of microwave-assisted extraction of carbohydrates from industrial waste of corn starch production using response surface methodology. Bioresour. Technol. 2010, 101, 7820–7826. [Google Scholar] [CrossRef]
- Jha, P.; Das, A.J.; Deka, S.C. Optimization of ultrasound and microwave assisted extractions of polyphenols from black rice (Oryza sativa cv. Poireton) husk. J. Food Sci. Technol. 2017, 54, 3847–3858. [Google Scholar] [CrossRef]
- Vollet Marson, G.; Belleville, M.-P.; Lacour, S.; Dupas Hubinger, M. Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts. Membranes 2021, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Markouli, E.; Gekas, V. Recovery and fractionation of different phenolic classes from winery sludge using ultrafiltration. Sep. Purif. Technol. 2013, 107, 245–251. [Google Scholar] [CrossRef]
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley Protein Properties, Extraction and Applications, with a Focus on Brewers’ Spent Grain Protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Sapkale, G.N.; Patil, S.M.; Surwase, U.S.; Bhatbhage, P.K. Supercritical Fluid Extraction. Int. J. Chem. Sci. 2010, 8, 729–743. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waskiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Vasheghani-Farahani, E. Application of supercritical fluid extraction in biotechnology. Crit. Rev. Biotechnol. 2005, 25, 231–242. [Google Scholar] [CrossRef]
- Campalani, C.; Amadio, E.; Zanini, S.; Dall’Acqua, S.; Panozzo, M.; Ferrari, S.; De Nadai, G.; Francescato, S.; Selva, M.; Perosa, A. Supercritical CO2 as a green solvent for the circular economy: Extraction of fatty acids from fruit pomace. J. CO2 Util. 2020, 41, 101259. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsi, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Pereira, C.G.; Nunes, S.B.; Padilha, F.F.; Umsza-Guez, M.A. Supercritical Fluid Extraction Using CO2: Main Applications and Future Perspectives. Sep. Sci. Technol. 2013, 48, 2741–2760. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Leyva-López, N.; Hernández, C.; Gutiérrez-Grijalva, E.P.; Salazar-Leyva, J.A.; Osuna-Ruíz, I.; Martínez-Montaño, E.; Arrizon, J.; Guerrero, A.; Benitez-Hernández, A.; et al. Antioxidant Molecules from Plant Waste: Extraction Techniques and Biological Properties. Processes 2020, 8, 1566. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Rebolleda, S.; Rubio, N.; Beltrán, S.; Sanz, M.T.; González-Sanjosé, M.L. Supercritical fluid extraction of corn germ oil: Study of the influence of process parameters on the extraction yield and oil quality. J. Supercrit. Fluids 2012, 72, 270–277. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Brignole, E.A. Supercritical Fluid Extraction. Fluid Phase Equilibria 1986, 29, 133–144. [Google Scholar] [CrossRef]
- Wrona, O.; Rafinska, K.; Mozenski, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Tyskiewicz, K.; Konkol, M.; Roj, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA—J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. Trac-Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrason. Sonochem. 2021, 74, 105584. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Arora, S.; Kumar, A. Industrial applications of supercritical fluid extraction: A review Industrial applications of supercritical fluid extraction: A review. Int. J. Chem. Stud. 2017, 5, 336–340. [Google Scholar]
- Povilaitis, D.; Venskutonis, P.R. Optimization of supercritical carbon dioxide extraction of rye bran using response surface methodology and evaluation of extract properties. J. Supercrit. Fluids 2015, 100, 194–200. [Google Scholar] [CrossRef]
- Gelmez, N.; Kincal, N.S.; Yener, M.E. Optimization of supercritical carbon dioxide extraction of antioxidants from roasted wheat germ based on yield, total phenolic and tocopherol contents, and antioxidant activities of the extracts. J. Supercrit. Fluids 2009, 48, 217–224. [Google Scholar] [CrossRef]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabral, F.A. Influence of ethanol, water, and their mixtures as co-solvents of the supercritical carbon dioxide in the extraction of phenolics from purple corn cob (Zea mays L.). J. Supercrit. Fluids 2016, 118, 11–18. [Google Scholar] [CrossRef]
- Jung, G.W.; Kang, H.M.; Chun, B.S. Characterization of wheat bran oil obtained by supercritical carbon dioxide and hexane extraction. J. Ind. Eng. Chem. 2012, 18, 360–363. [Google Scholar] [CrossRef]
- Kitrytė, V.; Šaduikis, A.; Venskutonis, P.R. Assessment of antioxidant capacity of brewer’s spent grain and its supercritical carbon dioxide extract as sources of valuable dietary ingredients. J. Food Eng. 2015, 167, 18–24. [Google Scholar] [CrossRef]
- Benito-Román, O.; Varona, S.; Sanz, M.T.; Beltrán, S. Valorization of rice bran: Modified supercritical CO2 extraction of bioactive compounds. J. Ind. Eng. Chem. 2019, 80, 273–282. [Google Scholar] [CrossRef]
- Spinelli, S.; Conte, A.; Lecce, L.; Padalino, L.; Del Nobile, M.A. Supercritical carbon dioxide extraction of brewer’s spent grain. J. Supercrit. Fluids 2016, 107, 69–74. [Google Scholar] [CrossRef]
- Fernández, M.P.; Rodriguez, J.F.; García, M.T.; de Lucas, A.; Gracia, I. Application of Supercritical Fluid Extraction to Brewer’s Spent Grain Management. Ind. Eng. Chem. Res. 2008, 47, 1614–1619. [Google Scholar] [CrossRef]
- Piras, A.; Rosa, A.; Falconieri, D.; Porcedda, S.; Dessi, M.A.; Marongiu, B. Extraction of oil from wheat germ by supercritical CO2. Molecules 2009, 14, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Rebolleda, S.; Beltrán, S.; Sanz, M.T.; González-Sanjosé, M.L.; Solaesa, Á.G. Extraction of alkylresorcinols from wheat bran with supercritical CO2. J. Food Eng. 2013, 119, 814–821. [Google Scholar] [CrossRef]
- Walters, M.; Lima Ribeiro, A.P.; Hosseinian, F.; Tsopmo, A. Phenolic acids, avenanthramides, and antioxidant activity of oats defatted with hexane or supercritical fluid. J. Cereal Sci. 2018, 79, 21–26. [Google Scholar] [CrossRef]
- Cobb, B.F.; Kallenbach, J.; Hall, C.A.; Pryor, S.W. Optimizing the Supercritical Fluid Extraction of Lutein from Corn Gluten Meal. Food Bioprocess Technol. 2018, 11, 757–764. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Chapter 13—Pressurized Liquid Extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z.S. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. Trac-Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Pazo-Cepeda, V.; Benito-Román, Ó.; Navarrete, A.; Alonso, E. Valorization of Wheat Bran: Ferulic Acid Recovery Using Pressurized Aqueous Ethanol Solutions. Waste Biomass Valorization 2019, 11, 4701–4710. [Google Scholar] [CrossRef]
- Dunford, N.T.; Zhang, M. Pressurized solvent extraction of wheat germ oil. Food Res. Int. 2003, 36, 905–909. [Google Scholar] [CrossRef]
- Povilaitis, D.; Šulniūtė, V.; Venskutonis, P.R.; Kraujalienė, V. Antioxidant properties of wheat and rye bran extracts obtained by pressurized liquid extraction with different solvents. J. Cereal Sci. 2015, 62, 117–123. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; de Carvalho Junior, R.N. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Rudjito, R.C.; Ruthes, A.C.; Jiménez-Quero, A.; Vilaplana, F. Feruloylated Arabinoxylans from Wheat Bran: Optimization of Extraction Process and Validation at Pilot Scale. ACS Sustain. Chem. Eng. 2019, 7, 13167–13177. [Google Scholar] [CrossRef]
- Pourali, O.; Asghari, F.S.; Yoshida, H. Production of phenolic compounds from rice bran biomass under subcritical water conditions. Chem. Eng. J. 2010, 160, 259–266. [Google Scholar] [CrossRef]
- Yilmaz-Turan, S.; Jiménez-Quero, A.; Moriana, R.; Arte, E.; Katina, K.; Vilaplana, F. Cascade extraction of proteins and feruloylated arabinoxylans from wheat bran. Food Chem. 2020, 333, 127491. [Google Scholar] [CrossRef] [PubMed]
- Raud, M.; Krennhuber, K.; Jäger, A.; Kikas, T. Nitrogen explosive decompression pre-treatment: An alternative to steam explosion. Energy 2019, 177, 175–182. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Boonmahitthisud, A. Study on structural and thermal properties of cellulose microfibers isolated from pineapple leaves using steam explosion. J. Environ. Chem. Eng. 2019, 7, 102836. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, M.; Liu, X.; Zhong, K.; Tong, L.; Zhou, X.; Zhou, S. Effect of steam explosion-assisted extraction on phenolic acid profiles and antioxidant properties of wheat bran. J. Sci. Food Agric. 2016, 96, 3484–3491. [Google Scholar] [CrossRef]
- Kong, F.; Wang, L.; Gao, H.; Chen, H. Process of steam explosion assisted superfine grinding on particle size, chemical composition and physico-chemical properties of wheat bran powder. Powder Technol. 2020, 371, 154–160. [Google Scholar] [CrossRef]
- Buchmann, L.; Brandle, I.; Haberkorn, I.; Hiestand, M.; Mathys, A. Pulsed electric field based cyclic protein extraction of microalgae towards closed-loop biorefinery concepts. Bioresour. Technol. 2019, 291, 121870. [Google Scholar] [CrossRef]
- Boussetta, N.; Soichi, E.; Lanoiselle, J.L.; Vorobiev, E. Valorization of oilseed residues: Extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind. Crops Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, Z.; Liu, J.; Yu, Y.; Yin, Y.; Lin, S.; Chen, F. Optimized extraction of polysaccharides from corn silk by pulsed electric field and response surface quadratic design. J. Sci. Food Agric. 2011, 91, 2201–2209. [Google Scholar] [CrossRef]
- Guderjan, M.; Topfl, S.; Angersbach, A.; Knorr, D. Impact of pulsed electric field treatment on the recovery and quality of plant oils. J. Food Eng. 2005, 67, 281–287. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Walsh, D.; Griffin, T.P.; Islam, N.; Lyng, J.G.; Brunton, N.P.; Rai, D.K. Impact of pulsed electric field pre-treatment on nutritional and polyphenolic contents and bioactivities of light and dark brewer’s spent grains. Innov. Food Sci. Emerg. Technol. 2019, 54, 200–210. [Google Scholar] [CrossRef]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed electric field (PEF) as pre-treatment to improve the phenolic compounds recovery from brewers’ spent grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Blouet, C.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Effect of pulsed electric fields and high voltage electrical discharges on polyphenol and protein extraction from sesame cake. Innov. Food Sci. Emerg. Technol. 2015, 29, 170–177. [Google Scholar] [CrossRef]
- Ghosh, S.; Gillis, A.; Sheviryov, J.; Levkov, K.; Golberg, A. Towards waste meat biorefinery: Extraction of proteins from waste chicken meat with non-thermal pulsed electric fields and mechanical pressing. J. Clean. Prod. 2019, 208, 220–231. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Maza, M.; Álvarez, I.; Raso, J. Utilising Pulsed Electric Field Processing to Enhance Extraction Processes. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Neetoo, H.; Chen, H. Alternative Food Processing Technologies. Food Processing: Princ. Appl. 2014, 1, 137–169. [Google Scholar]
- Žuntar, I.; Putnik, P.; Bursać Kovačević, D.; Nutrizio, M.; Šupljika, F.; Poljanec, A.; Dubrović, I.; Barba, F.J.; Režek Jambrak, A. Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obtained by High Voltage Electrical Discharges (HVED). Foods 2019, 8, 248. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Barba, F.J.; Boussetta, N.; Vorobiev, E. Emerging technologies for the recovery of isothiocyanates, protein and phenolic compounds from rapeseed and rapeseed press-cake: Effect of high voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2015, 31, 67–72. [Google Scholar] [CrossRef]
- Rocha, C.M.R.; Genisheva, Z.; Ferreira-Santos, P.; Rodrigues, R.; Vicente, A.A.; Teixeira, J.A.; Pereira, R.N. Electric field-based technologies for valorization of bioresources. Bioresour. Technol. 2018, 254, 325–339. [Google Scholar] [CrossRef]
- Xi, J.; He, L.; Yan, L.-G. Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem. 2017, 230, 354–361. [Google Scholar] [CrossRef]
- Brahim, M.; Checa Fernandez, B.L.; Regnier, O.; Boussetta, N.; Grimi, N.; Sarazin, C.; Husson, E.; Vorobiev, E.; Brosse, N. Impact of ultrasounds and high voltage electrical discharges on physico-chemical properties of rapeseed straw’s lignin and pulps. Bioresour. Technol. 2017, 237, 11–19. [Google Scholar] [CrossRef]
- Wood, I.P.; Cook, N.M.; Wilson, D.R.; Ryden, P.; Robertson, J.A.; Waldron, K.W. Ethanol from a biorefinery waste stream: Saccharification of amylase, protease and xylanase treated wheat bran. Food Chem. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Moncada, J.; Roberto, I.C.; Cardona, C.A. Techno-economic analysis for brewer’s spent grains use on a biorefinery concept: The Brazilian case. Bioresour. Technol. 2013, 148, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Duan, Y.; Zhang, H.; Ma, H. A Mini-Review on Brewer’s Spent Grain Protein: Isolation, Physicochemical Properties, Application of Protein, and Functional Properties of Hydrolysates. J. Food Sci. 2019, 84, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Anttonen, M.; Lammi, M.; Mykkanen, J.; Repo, P. Circular Economy in the Triple Helix of Innovation Systems. Sustainability 2018, 10, 2646. [Google Scholar] [CrossRef]
- Skendi, A.; Zinoviadou, K.G.; Papageorgiou, M.; Rocha, J.M. Advances on the Valorisation and Functionalization of By-Products and Wastes from Cereal-Based Processing Industry. Foods 2020, 9, 1243. [Google Scholar] [CrossRef] [PubMed]

| Raw Material | Target Compounds | Extraction Method | Extraction Conditions | Yield/Released | Reference |
|---|---|---|---|---|---|
| Brewers’ spent grains | Ferulic acid | Alkaline hydrolysis | Ratio of solvent to raw material (mL g−1): 20 Extraction time: 90 min Temp. (°C): 110 Solvent: NaOH 2% | 0.27 (% w/w) | [25] |
| Flax shoves | Ferulic acid p-coumaric acid | Alkaline hydrolysis | 0.5 M NaOH 4 h at 50 °C neutralized with 6 M HCl. | 25 mg/100 g 61 mg/100 g | [26] |
| Wheat bran | 391 mg/100 g 20 mg/100 g | ||||
| Corn bran | 2510 mg/100 g 350 mg/100 g | ||||
| Brewers’ spent grains | Ferulic acid p-coumaric acid | Alkaline hydrolysis | NaOH 2% 90 min at 120 °C | 145.3 mg/L 138.8 mg/L | [27] |
| Yellow maize germ | Ferulic acid | Alkaline hydrolysis | 2 M sodium hydroxide at room temperature for 1 h. The pH of the mixture was adjusted to 3 with 6N hydrochloric acid. | 461.89 (mg FA/g dried extract) | [23] |
| White maize germ | 522.99 (mg FA/g dried extract) | ||||
| Brewers’ spent grains | Ferulic acid | Alkaline hydrolysis | NaOH 2% (w/v) 20 mL NaOH/g 120 °C, 1.5 h | 476.99 mg/100 g | [21] |
| Black brewers’ spent grains Pale brewers’ spent grains | Phenolic compounds | Alkaline hydrolysis | 1 N NaOH for 16 h at room temperature in the dark under N2 | 5.29 (mg GAE/g dw) 3.75 (mg GAE/g dw) | [28] |
| Teff straw | Nanocellulose | Acid hydrolysis | Sulfuric acid concentration 44.4% v/v Time 32 min Temperature 40.5 °C | 62.2% nanocellulose | [29] |
| Brewers’ spent grains | Hemicellulosic fraction | Acid hydrolysis | Sulfuric acid concentration 100–140 mg g−1 dry matter; Reaction time 17–37 min; | 85.8% hydrolyzed xylan 95.7% hydrolyzed arabinan | [30] |
| Wheat flour and bran | Polyphenols | Acid hydrolysis | Methanol/H2SO4 90:10 (v/v); Time 20 h Temperature 85 °C | 200–1600 mg/100 g polyphenols in the acidic hydrolysates | [31] |
| Corn fiber and wheat bran | Phenolic compounds | Acid hydrolysis | 500 mL of 50 mmol trifluoroacetic acid; Time 3 h; In a boiling water bath under constant stirring. | Soluble ferulated oligosaccharides | [32] |
| Brewers’ spent grains | Ferulic acid | Soxhlet extraction | Ratio of solvent to raw material (mL g−1): 30 Extraction time: 4 h Temp. (°C): b.p. of solvent Solvent: Ethanol | 0.0014 (% w/w) | [25] |
| Black brewers’ spent grains Pale brewers’ spent grains | Lipid content | Soxhlet extraction | 70 mL analytical grade chloroform for 20 h. | 9.96 (g 100/g dw) 13.51 (g 100/g dw) | [28] |
| Brewers’ spent grains | Phenolic compounds | Solvent extraction | Methanol Ethanol Ethanol-water 60:40 v/v Ethanol-water 40:60 v/v Acetone-water 60:40 v/v Acetone-water 40:60 v/v | 110.58 mg GAE/100 g dw 40.97 mg GAE/100 g dw 112.04 mg GAE/100 g dw 100.38 mg GAE/100 g dw 114.23 mg GAE/100 g dw 97.38 mg GAE/100 g dw | [33] |
| Corn Bran | Total phenolic compounds | Solvent extraction | Water Ethanol Methanol Acetone in a water bath at 50 °C | 1925 mg GAE/100 g dw 1779.5 mg GAE/100 g dw 1814 mg GAE/100 g dw 1538 mg GAE/100 g dw | [34] |
| Rice Bran | Total phenolic compounds | Solvent extraction | Water Ethanol Methanol Acetone in a water bath at 50 °C | 1084.8 mg GAE/100 g dw 1335.9 mg GAE/100 g dw 1176.9 mg GAE/100 g dw 953.6 mg GAE/100 g dw | [34] |
| Brewer’s spent grains | Total phenolic compounds | Solvent extraction | Water Ethanol 80% ethanol–water 60% ethanol–water 2 min at 1900 rpm | 0.51 mg GAE/100 g 0.14 mg GAE/100 g 0.56 mg GAE/100 g 0.66 mg GAE/100 g | [22] |
| Brewer’s spent grains | Total phenolic compounds | Solvent extraction | Water 80% Methanol 60% Ethanol 60% Acetone Hexane Ethyl Acetate 30 min at 80 °C, respectively 60 °C | 3.59 (mg GAE/g BSG) 6.46 (mg GAE/g BSG) 7.13 (mg GAE/g BSG) 9.90 (mg GAE/g BSG) 4.44 (mg GAE/g BSG) 2.14 (mg GAE/g BSG) | [35] |
| Brewers’ spent grains light; Brewers’ spent grains dark; Brewers’ spent grains mix (light–dark, ~9:1 w/w) | Total phenolic compounds | Solvent extraction | 60% Acetone 30 min at 60 ° C | 2.84 (mg GAE/g BSG dw) 2.81 (mg GAE/g BSG dw) 3.85 (mg GAE/g BSG dw) | [36] |
| Waste | Recovered Fraction | Extraction Parameters | Yield | Applicability | Reference |
|---|---|---|---|---|---|
| Wheat bran | Polyphenols | Solvent: Aqueous solution of glycerol- based eutectic mixture Temperature: 80 °C Time: 90 min Power: 140 W Frequency: 37 kHz Acoustic energy density (AED): 35 W L−1 | 17.78% ± 1.50 | Antioxidant activity | [66] |
| Defatted oat bran | Phenolic compounds (TPC = total phenolic compounds) | Solvent: ethanol 80% Temperature: 70 °C Time: 25 min Power: 200–600 W Frequency: 40 kHz | TPC 184.16 mg/100 g | Antioxidant activity | [67] |
| Defatted oat bran | β-glucans | Solvent: ethanol 80% Temperature: 20 °C Time: 5.5 min Power: 200–600 W Frequency: 40 kHz | 5.73% | Food and pharmaceutical industry, Cosmetic industry as moisturizer | [67] |
| Rice bran | Lactose/gluten free protein | Solvent: water (sample–water—0.5:10) Pulse on/off in the emission of power (60 s on/30 s off) Time: 10 min Temperature: room temperature | 11.71% | Food formulation Protein supplementation | [68] |
| Wheat germ | ACE-inhibitor peptides | Solvent: water Pulsed on-time and off-time of 500 and 5 s Power: 24 W Frequency: 24 ± 2 kHz Time: 120 min | 65.9% | ACE-inhibitory activity | [69] |
| BSG | Proteins | Solvent: NaOH 110 mM Power: 250 W Duty cycle 60% (pulsed on/off time of 3/2 s) Time: 20 min Temperature: 25 °C | 86.16% | Plant-based protein source to the food industry | [70] |
| Red sorghum bran | Polyphenolic compounds | Solvent: ethanol 53% (52:1 mL/g of solvent to solid ratio) Time: 21 min Frequency: 25 kHz Power: 200 W | 49.743 mg GAE/g dw in total polyphenols | Antioxidant activity | [71] |
| Durum wheat bran | Free phenolics | Solvent: ethanol 65% Time: 25 min Frequency: 48 kHz | 17.29 ± 1.40 | Antiradical and antimicrobial activities | [72] |
| Rice bran | Free phenolics | Solvent: ethanol 65% Time: 45 min Frequency: 48 kHz | 19.73 ± 1.45 | Antiradical and antimicrobial activities | [72] |
| Hull-less barley bran | β-glucans | Solvent: ethanol 80% Time: 60 min Power: 100 W Temperature: 50 °C | 0.3% crude glucans | Food and pharmaceutical industry | [73] |
| Waste | Recovered Fraction | Extraction Parameters | Yield | Applicability | Reference |
|---|---|---|---|---|---|
| Wheat bran | Phenolic compounds (467.5 μg Catechin Equivalent/g) | Solvent: methanol Temperature: 60–120 °C Time: 20 min | 4.71% to 5.01% | Antioxidant capacity | [84] |
| Corn germ | Phenolic compounds (654 μg Catechin Equivalent/g of fresh corn germ) | Solvent: methanol Temperature: 60–120 °C Time: 20 min | 2.49% to 3.51% | Antioxidant capacity | [84] |
| Wheat germ | Phenolic compounds (1248 μg Catechin Equivalent/g) | Solvent: methanol Temperature: 60–120 °C Time: 20 min | 10.11% to 14.63% | Antioxidant capacity | [84] |
| BSG | Ferulic acid | Solvent: NaOH 0.75% Temperature:100 °C Time: 15 min | 1.3% | Antioxidant Antimicrobial agent Anti-inflammatory agent | [25] |
| Wheat straw | Lignin | Solvent: H2SO4 0.46 M Power: 602 W Stirring: yes Time: 39 min | 3.4 to 11.8% | Natural binder | [85,86] |
| BSG | Arabino-xylans and arabinoxylo-oligosaccharides | 3 sequence extraction Temperature: 180 °C Time: 2 min | 62% | Prebiotic effects Antioxidant activity | [87] |
| BSG | Arabino-xylans | Solvent: water Temperature: 210 °C Time: 2 min | 43% | Prebiotic effects | [88] |
| BSG | Hemicellulosic sugar | Solvent: water (5 g BSG, 50 mL water) Temperature: 192.7 °C Time: 5.4 min | 82% | Butanol production | [40] |
| BSG | Carboxymethylcellulose (CMC) | Etherification reaction of BSG to obtain CMC Solvent: 5 mL monochloroacetic acid and isopropanol Power: 200 W Temperature: 70 °C Time: 7.5 min | 1.46% | Cellulose isolation | [89] |
| Sorghum leaves | Reducing sugar acetic acid furfural 5-hydroxymethylfurfural (HMF) phenol | Pretreatment with NaOH and HCl solutions Power: 200–800 W Time: 2–10 min | Depends on the interested compound | Biofuels Biomaterials | [90] |
| Corn pericarp | Xylo-oligosaccharides | Solvent: water Temperature: 175 °C Stirring: yes Time: 18 min | 70.8% | Functional food Source of bioethanol production | [91] |
| Black rice husk | Phenolic compounds: flavonoid, anthocyanins | Solvent: ethanol Temperature: 175 °C Stirring: yes Time: 31.11 sec | Flavonoids (3.04 mg/100 g), anthocyanin (3.39 mg/100 g) | Functional food Antioxidant action | [92] |
| Waste | Recovered Fraction | Extraction Parameters | Yield | Applicability | Reference |
|---|---|---|---|---|---|
| Rye bran | Phenolic compounds (14.62 mg GAE/g) | Solvent: CO2 Temperature: 70 °C Pressure: 55 MPa Time: 120 min | 2.5% | Antioxidant capacity | [116] |
| Roasted wheat germ | Phenolic compounds (6 mg GAE phenolics/g) | Solvent: CO2 Temperature: 58 °C Pressure: 336 bar Time: 10 min | 5.3% | Antioxidant capacity | [117] |
| Roasted wheat germ | Tocopherol (6.7 mg/g) | Solvent: CO2 Temperature: 58 °C Pressure: 336 bar Time: 10 min | 100% | Antioxidant capacity, cosmetic and food industry | [117] |
| Purple corn cob | Phenolic compounds (290 mg EC/g) and especially anthocyanins 67 mg C3G/g) | Solvent: CO2 + EtOH (70%) Temperature: 50 °C Pressure: 400 bar Time: 10 min | 24.4% | Antioxidant capacity | [118] |
| Corn germ | Oil (tocopherols) | Solvent: CO2 Temperature: 35–86 °C Pressure: 20–53 MPa Solvent flow rate: 4–9 kg CO2/h | ND | Antioxidant capacity | [106] |
| Wheat bran | Oil | Solvent: CO2 Temperature: 313.15–333.15 K Pressure: 10–30 MPa | ND | Antioxidant capacity and radical scavenging activity | [119] |
| BSG | BSG lipophilic fractions | Solvent: CO2 Temperature: 40 °C, Pressure: 40 MPa Solvent flow rate: 2–3 Standard Liters/min Time: 70 min (including 10 min of static extraction time). | 5.49 ± 0.07 g/100 g | Antioxidant capacity | [120] |
| Rice bran | Oil (total phenolics content 3.42 mg GAE/g of oil and tocopherol 5.47 mg/g oil) | Solvent: CO2 + EtOH (5–10%) Temperature: 40 °C, Pressure: 40 MPa Time: 120 min | 14.4 g oil/100 g | Antioxidant capacity | [121] |
| BSG | Phenolic compounds (0.35 ± 0.01 mg/g BSG) Flavonoids (0.22 ± 0.01 mg/g BSG) | Solvent: CO2 + EtOH (60%) Temperature: 40 °C Pressure: 35 MPa Time: 240 min | ND | Antioxidant capacity | [122] |
| BSG | Tocopherols | Solvent: CO2 Temperature: 313 K Pressure: 35 MPa Pretreatment: milling (particle size 0.85 mm) | ND | Antioxidant capacity | [123] |
| Wheat germ | Tocopherols | Solvent: CO2 Temperature: 40 °C Pressure: 300 bar Time: 480 min | 9% | Antioxidant capacity | [124] |
| Wheat bran | Alkylresorcinols (1119 ± 42 lg AR/g dry bran) | Solvent: CO2 Temperature: 80 °C Pressure: 40 MPa Time: 120 min | 34.7 mg extract/g dry bran | Antioxidant capacity stimulant/inhibitory effects on some metabolic enzymes | [125] |
| Oat bran | Polyphenols: avenanthramides and phenolic acids | Solvent: CO2 Temperature: 50 °C Pressure: 350 bar Time: 300 min Solvent flow rate: 15 g/min | 4.6–5.3% oil from oat bran sample | Antioxidant capacity | [126] |
| Corn gluten meal (CGM) | Lutein | Solvent: CO2 + EtOH (15%) Temperature: 40 °C Pressure: 6820 psi | 84.7μg lutein/g CGM | Food and pharmaceutical industries | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Salanță, L.C.; Chiș, M.S.; Pop, C.R.; Borșa, A.; Diaconeasa, Z.; Vodnar, D.C. Cereal Waste Valorization through Conventional and Current Extraction Techniques—An Up-to-Date Overview. Foods 2022, 11, 2454. https://doi.org/10.3390/foods11162454
Fărcaș AC, Socaci SA, Nemeș SA, Salanță LC, Chiș MS, Pop CR, Borșa A, Diaconeasa Z, Vodnar DC. Cereal Waste Valorization through Conventional and Current Extraction Techniques—An Up-to-Date Overview. Foods. 2022; 11(16):2454. https://doi.org/10.3390/foods11162454
Chicago/Turabian StyleFărcaș, Anca Corina, Sonia Ancuța Socaci, Silvia Amalia Nemeș, Liana Claudia Salanță, Maria Simona Chiș, Carmen Rodica Pop, Andrei Borșa, Zorița Diaconeasa, and Dan Cristian Vodnar. 2022. "Cereal Waste Valorization through Conventional and Current Extraction Techniques—An Up-to-Date Overview" Foods 11, no. 16: 2454. https://doi.org/10.3390/foods11162454
APA StyleFărcaș, A. C., Socaci, S. A., Nemeș, S. A., Salanță, L. C., Chiș, M. S., Pop, C. R., Borșa, A., Diaconeasa, Z., & Vodnar, D. C. (2022). Cereal Waste Valorization through Conventional and Current Extraction Techniques—An Up-to-Date Overview. Foods, 11(16), 2454. https://doi.org/10.3390/foods11162454














