Abstract
In this study, the fermentation of Artemisia capillaris by probiotic Leuconostoc mesenteroides MKJW (MKJW) was optimized to increase the acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibitory and antioxidant activities using the response surface method (RSM). The independent variables were the contents of A. capillaris, Gryllus bimaculatus, and yeast extract, while the dependent variables were AChE inhibitory activity, BuChE inhibitory activity, and antioxidant activities such as FRAP, reducing power, and DPPH radical scavenging ability. Seventeen experimental runs were designed with RSM and analyzed after fermentation with MKJW. Quadratic models were used to analyze the inhibition of AChE and BuChE, and a linear model was used to analyze the FRAP. The three models were significantly appropriate (p < 0.0001). The highest optimal condition of the AChE inhibitory activity was derived by a multiple regression equation. When the optimum fermentation conditions were A. capillaris 6.75%, G. bimaculatus 0.18%, and yeast extract 1.27%, 91.1% was reached for AChE inhibitory, 74.0% for BuChE inhibitory, and 34.1 mM FeSO4 for FRAP. The predicted dependent variables were not significantly different from the experimental values (p > 0.05). In conclusion, the A. capillaris fermented by MKJW might be used as a natural antidementia improving agent with AChE inhibitory, BuChE inhibitory, and antioxidant activities.
1. Introduction
Neurological disorders are the leading causes of disability-adjusted life years (DALYs; the sum of years of life lost) and death, including chronic degenerative diseases, cancer, diabetes, and cardiovascular diseases [1,2]. The neurotransmitter acetylcholine is reduced in the brains of dementia patients compared to that in normal brains due to a decrease in acetylcholine synthesis and the breakdown of acetylcholine by cholinergic enzymes [3]. Symptoms of dementia include a decrease in memory and cognitive ability, among which Alzheimer’s disease shows decreased memory, depression, decreased judgment, and confusion. The main biological function of acetylcholinesterase (AChE) is to rapidly break down the neurotransmitter acetylcholine into acetic acid and choline [4]. Butyrylcholinesterase (BuChE) is also involved in neurological disorders as a surrogate hydrolase for acetylcholine [5]. Choline deficiency due to cholinergic enzymes is associated with the onset of Alzheimer’s disease, and the current drug approach to Alzheimer’s disease is based upon suppressing cholinergic enzymes [6]. However, common adverse effects of drugs include loss of appetite, gastrointestinal symptoms such as nausea, vomiting, diarrhea, and a lot of weight loss that appears when used for a long period [7,8]. Furthermore, no single-target drug has been successful in preventing dementia. Consequently, interest in natural plants or fermented natural products has emerged.
Artemisia sp., including A. princeps, A. argyi, A. iwayomogi, A. princeps, A. capillaris, etc. is a perennial herb with a wide distribution in Europe and Southeast Asia, including Korea [9]. Artemisia sp. has various physiological activities and is widely used for food or medicinal purposes [10]. In particular, many studies have been conducted on the extract of Artemisia sp., such as its antioxidant and anti-inflammatory effects and antibacterial, antiobesity, antileukemia, and anticancer properties [11,12,13,14]. In addition, the possibility of a natural antidementia treatment has been reported due to acetylcholinesterase inhibitory activity in the essential oil of Artemisia sp. [15,16].
Probiotics are living microbes that benefit consumer health by maintaining or improving the gut microbial balance [17]. Some studies on the relationship between gut microbiota and the brain have been reported [18,19]. The gut microbiota affects cognitive functions in neurodegenerative diseases related to aging and mood disorders such as anxiety and depression. The colonization of beneficial bacteria such as probiotics in the intestine catalyzes the biosynthesis of neurotransmitters and prevents neuronal damage and death [20].
Lactic acid bacteria (LAB) fermentation is a safe and green technology to preserve foods and develop novel functional foods that can modify the function of raw materials. Especially, Leuconostoc mesenteroides produces mannitol, dextran, and oligosaccharides as well as common LAB metabolites such as lactic acid, acetic acid, ethanol, and carbon dioxide during the fermentation process [21,22,23,24]. Leuconostoc mesenteroides MKJW (MKJW) is a probiotic with high resistance to digestive juices; antibacterial activity against pathogenic bacteria such as Staphylococcus aureus, Enterococcus faecalis, and Shigella flexneri; and high levels of antioxidant activities, such as the DPPH radical scavenging activity, superoxide dismutase (SOD)-like activity, and reducing power [24].
In this study, response surface methodology (RSM) was used to optimize the fermentation conditions (A. capillaris, Gryllus bimaculatus, and yeast extract contents) of A. capillaris by probiotic MKJW. Some biological activities such as AChE and BuChE inhibition and antioxidant activity of the A. capillaris after fermentation were investigated.
2. Materials and Methods
2.1. Materials
Hot-water-extracted A. capillaris (40 °brix) was obtained from DHbio (Anseong, Korea). Briefly, A. capillaris was extracted with ten-fold water for 6 h at 120 °C. Then, six-fold water was added, and boiled for 3 h at 105 °C. G. bimaculatus, obtained from Cricket Farm Co. (Hwaseong, Korea), was hot-air-dried at 70 °C for 10 h at the farm, and was ground with a mixer and sieved with an 18 mm mesh sieve. MKJW (KCTC14459BP) was used from our laboratory stock.
2.2. Preliminary Experiments for Fermentation Conditions
In order to determine the range of A. capillaris content to be input into the RSM, fermentation patterns according to A. capillaris at 4%, 5%, 6%, 8%, and 10% were investigated. In addition, three types of the combination of 5% A. capillaris and protein supplements (first, 2% (w/v) G. bimaculatus [Cricket Farm, Hwaseong, Korea]; second, 2% (w/v) yeast extract [Difco, Becton Dickinson and Co., Sparks, MD, USA]; and third, 1% (w/v) G. bimaculatus and 1% (w/v) yeast extract) were confirmed. After adding 2.5% (w/v) glucose to each preliminary test group, the mixture was sterilized for 20 min at 121 °C. After cooling, MKJW cultured for 18 h was inoculated to 5% (v/v) of the total medium volume. Then, shaking incubation (130 rpm, 30 °C) was performed for 24 h. After fermentation, the solution was pasteurized at 60 °C for 30 min and centrifuged to separate the supernatant and the precipitate.
2.3. Box–Behnken Experiment Design
The Box–Behnken design (BBD) can identify the linear influence, interactions between factors, and quadratic effects, and is used for RSM purposes. Response surface analysis was performed using the Stat-Ease program (Design-Expert® software, version 12, Stat-Ease, Inc., Minneapolis, MN, USA) to determine the optimal Artemisia fermentation conditions. The amount for A. capillaris was between 3% and 7% (X1), for G. bimaculatus was between 0% and 2% (X2), and for yeast extract, was between 0% and 2% (X3), which were set as independent variables (Table 1). As dependent variables, acetylcholinesterase and butyrylcholinesterase inhibition activities and ferric reducing antioxidant activity (FRAP) were selected.

Table 1.
Levels of independent variables in the Box–Behnken experimental design.
2.4. Fermentation
The BBD design planned 17 experiments with five replications of the center points (Table 2). The corresponding fermentation was prepared. Briefly, different contents of A. capillaris (3, 5, and 7%), G. bimaculatus powder (0, 1, and 2%), and yeast extract (0, 1, and 2%) provided from the BBD design were added into each tube, autoclaved at 121 °C for 20 min, and cooled. All fermentation broth contained sterilized 2.5% glucose. Three-times-subcultured MKJW on MRS broth (Difco, Becton Dickinson and Co., Sparks, MD, USA) was inoculated to 5% (v/v) of the total broth volume (8 Log CFU∙g−1). The fermentation was carried out with shaking incubation at 130 rpm and 30 °C for 12 h.

Table 2.
Change of the viable cell counts, pH value, and physiological activities of MKJW fermented A. capillaris.
The fermented samples were serially diluted with 0.1% peptone water and spread onto the MRS agar. MRS agar was incubated for 24 h at 30 °C to determine total viable bacterial counts. For other analysis, the sample tubes were heated at 60 °C for 30 min, followed by centrifugation to collect the supernatant for use in the experiment. The pH of the samples was measured with a pH meter (Fisher Science Education, Waltham, MA USA).
2.5. Acetylcholinesterase (AChE) and Butyrylcholinesterase (BuChE) Inhibitory Activity
AChE and BuChE inhibitory assays were carried out in a 96-well plate using a modified method, as described by Sharififar et al. [25]. The reagents (Sigma Aldrich, St. Louis, MO, USA) were injected into the well in the following procedure: 5 µL enzyme solution (AChE or BuChE, 2 U/mL), 40 µL 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB, 1 mM), and 125 µL diluted sample solution. The mixture was pre-incubated for 5 min at 25 °C. The reaction was initiated by adding 7.5 µL substrate (acetylcholine iodide or butyrylthiocholine iodide, 7.5 mM), and then the absorbance was measured at 412 nm for 15 min at 25 °C. The control consisted of adding the phosphate buffer (200 mM, pH 7.7) instead of the sample. All solutions were dissolved in 200 mM phosphate buffer (pH 7.7). Absorbance was plotted against time and enzyme activity was calculated from the slope of the line, obtained and expressed as a percentage compared with an assay using a buffer without inhibitor. Galantamine was used as the positive control.
% AChE and BuChE inhibitory activity = [1 − (Ssample/Sblank)] × 100
Ssample: Slope of the sample
Sblank: Slope of the control
Ssample: Slope of the sample
Sblank: Slope of the control
2.6. Antioxidant Activity
2.6.1. DPPH Radical Scavenging Activity
The activity to scavenge the free radical DPPH was measured according to the method of Park et al. [26], with modifications. Briefly, a volume of 300 µL of the sample solution was mixed with 200 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution dissolved in methanol, which was left to react for 30 min at room temperature in the dark. The amount of scavenged DPPH radical was determined by measuring the absorbance at 517 nm. Methanol was used as the control instead of the sample. Ascorbic acid was used as the positive control. The DPPH radical scavenging activity was calculated as follows:
% DPPH radical scavenging activity = [1 − (Acontrol − Asample)/Acontrol] × 100
Acontrol: Absorbance of the control
Asample: Absorbance of the sample
Acontrol: Absorbance of the control
Asample: Absorbance of the sample
2.6.2. Reducing Power
The method developed by Lin and Yen [27] was used with modifications to evaluate the reducing activity of fermented samples. Then, 250 µL sample was mixed with an equal volume of 200 mM sodium phosphate buffer (pH 6.6) and 1% (w/v) potassium ferricyanide solution. The reaction mixture was incubated at 50 °C for 20 min, then 250 µL of 10% (w/v) trichloroacetic acid was added to stop the reaction. Next, 500 µL the upper phase was obtained by centrifugation at 10,000 rpm for 5 min. Then, 500 µL of distilled water and 100 µL of 0.1% (w/v) FeCl3 was added. The absorbance was read at 700 nm. Ascorbic acid was used to the positive control. The reducing power was expressed as the absorbance value at Abs 700 nm.
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
The FRAP value of the fermented samples was determined using the method described by Cermeno et al. [28], with some modifications. The FRAP reagent was prepared by mixing 300 mM acetate buffer, 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ), and 20 mM FeCl3∙6H2O in a 10:1:1 (v/v/v) ratio and heating at 37 °C for 15 min. Then, 1 mL of FRAP reagent was added to 50 µL of the sample solution. Then, the absorbance was read at 590 nm after 30 min incubation at 37 °C. The FRAP value was expressed as the millimole (mM) of the FeSO4 equivalents.
2.7. Total Polyphenol Contents
The total polyphenol content (TPC) was determined by the Folin–Ciocalteu method, according to a method adapted from Anesini et al. [29]. 0.2 mL of the sample solution was mixed with 1.0 mL of Folin–Ciocalteu reagent, which was diluted 10 times in water. Then, 0.8 mL of 7.5% (w/v) Na2CO3 was added and the mixture was left to stand at room temperature for 1 hr. The absorbance was measured at 765 nm. The TPC was expressed as the mg of gallic acid equivalents per ml of sample (mg GAE/mL).
2.8. Statistical Analysis
The data were expressed as the means ± standard deviation of triplicates. Analysis of variance and Tukey’s means comparison tests were performed to determine the significant differences (p < 0.05) in the results using Minitab 16.0 software (Minitab Inc., State College, PA, USA). The influence of the independent variable on the dependent variable was quantified by deriving a quadratic regression equation, and the optimal condition was predicted by plotting a three-dimensional response surface diagram using the Stat-Ease program (Design-Expert® software, version 12, Stat-Ease, Inc., Minneapolis, MN, USA).
3. Results and Discussion
3.1. Preliminary Experiments for Fermentation Conditions
Figure 1 shows the number of viable bacteria when different concentrations of A. capillaris (4–10%) were fermented by MKJW for 24 h. The fermentation was successfully performed when 4% and 5% A. capillaris were used. However, more than 5% A. capillaris did not proliferate. Artemisia sp. has been reported to inhibit the growth of even lactic acid bacteria such as Lactobacillus plantarum and Leuconostoc mesenteroides, which are commonly used as fermentation starters [30,31], as well as pathogenic bacteria such as Bacillus subtilis [32,33], Escherichia coli [32,33], Listeria monocytogenes [34], Pseudomonas aeruginosa [32], Staphylococcus aureus [32,33,34], and Saccharomyces cerevisiae [35].
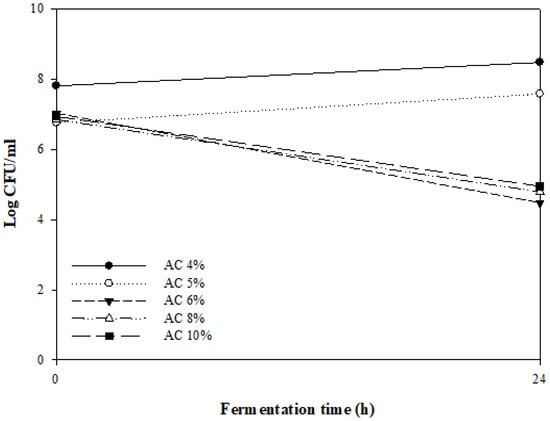
Figure 1.
Changes in the viable cell counts of MKJW-fermented A. capillaris (AC) during 24 h.
Figure 2 shows the number of viable bacteria and pH changes that occurred during 24 h with the addition of each G. bimaculatus and yeast extract to 5% A. capillaris. The addition of supplemental materials such as G. bimaculatus and yeast extract to A. capillaris enhanced the growth of MKJW to approximately 9 log CFU/mL compared to the 5% A. capillaris only (7.58 log CFU/mL) during 24 h fermentation. The pH of 5% A. capillaris decreased from 5.11 to 4.73 for 24 h; 5% A. capillaris supplemented with 2% G. bimaculatus decreased from 5.45 to 3.66 after fermentation; 5% A. capillaris supplemented with 2% yeast extract decreased from 5.32 to 4.00; and 5% A. capillaris supplemented with 1% G. bimaculatus and 1% yeast extract decreased from 5.36 to 3.83.
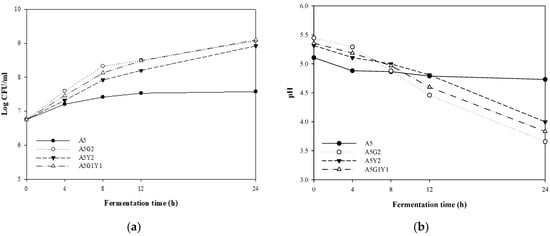
Figure 2.
Comparison of changes in the viable cell counts (a) and pH (b) of MKJW-fermented A. capillaris during 24 h. A5, A. capillaris 5%; A5G2, A. capillaris 5% + G. bimaculatus 2%; A5Y2, A. capillaris 5% + yeast extract 2%; A5G1Y1, A. capillaris 5% + G. bimaculatus 1% + yeast extract 1%.
As a result, MKJW did not grow well when fermented with only A. capillaris, but the bacteria grew well as a result of fermentation with the addition of the protein-rich G. bimaculatus and yeast extract. A. capillaris is thought to have poor growth due to a lack of nitrogen source necessary for the synthesis of protein, enzymes, nucleic acid, and other microbial components among the nutrients that are necessary for the growth of lactic acid bacteria. Park et al. [36] fermented glasswort witha mixture of rice bran and soybeans to supplement sugar and protein. When Yang et al. [37] mixed and fermented amaranth and quinoa to compensate for the protein source lacking in white rice, the number of viable bacteria increased compared to when only white rice was fermented.
Finally, the A. capillaris content was set to 5%, which allowed the bacteria to grow. The fermentation time was determined to be 12 h when the bacteria grew well, and the antioxidant activity was high.
3.2. Experiment Design for the Optimal Condition of A. capillaris Fermentation
3.2.1. Microbiological and Physiological Analysis of Fermented A. capillaris
Based on the preliminary experiments, the amounts of A. capillaris, G. bimaculatus, and yeast extract were selected as independent variables. Fermentation of A. capillaris was carried out under 17 conditions according to the Box–Behnken design. Table 2 shows the changes in the viable cell counts, pH value, and dependent variables during fermentation for the 17 experimental runs. All samples increased in viable cells and decreased in pH during fermentation. This result shows that MKJW eats nutrients such as glucose and yeast extracts, and as it grows, it produces fermentation products such as organic acids [38].
The substrate combination of 3% A. capillaris, 2% G. bimaculatus, and 1% yeast extract, had a significant increase in viable cell counts (8.99 Log CFU/mL), and a significant decrease in pH values. The fermentation with supplementation of G. bimaculatus or/and yeast extract to A. capillaris boosted the fermentation with increasing cell growth and lowering the pH values.
3.2.2. Suitability of the Regression Model
The results were analyzed using multiple regression, and the coefficients of each model were evaluated for their significance by regression analysis (Table 3). The model of the AChE inhibitory activity was adopted as a quadratic model in which independent variables interact. The F-value of 32.83 implied that the model was significant. p-values less than 0.05 indicated that model terms (factors X1, X2, X3, X1X2, X2X3, X12) were statistically significant. In addition, F-value (1.94) of the lack of fit was not significant (p = 0.26) relative to the pure error. A nonsignificant lack of fit is a good indication that the model fits the actual relationships of the response parameters within the chosen ranges. Likewise, the model of the BuChE inhibitory activity was adopted as a quadratic model. The F-value of 99.08 and p-values less than 0.05 showed that the model was significant. The factors, X1, X2, X1X2, X1X3, X2X3, X12, X22, and X32, were highly significant. The lack of fit was also not significant (F = 1.21, p = 0.4129) indicating fit goodness of the proposed model [34]. The values of the F-(77.12) and p-(<0.0001) was to indicate that FRAP (Y3) is suitable for the linear model. Furthermore, the p-value for all primary terms was less than 0.05 as a result of the analysis of variance. This means that each independent variable of all linear terms affects the dependent variable. F-value of the lack of fit was 1.77 and the p-value was 0.31, which was more than 0.1, thus indicating that the model is appropriate. The lack of fit of the reducing power (Y4) and DPPH radical scavenging ability (Y5), which are for the remaining antioxidant experiments, were 0.0983 and 0.0742, respectively. For each, the lack of fit was lower than 0.1, so the two experiments were determined to be inappropriate models.

Table 3.
ANOVA results for dependent variables.
Table 4 shows the actual and predicted values of each dependent variable (AChE inhibitory activity, BuChE inhibitory activity, and FRAP) based on the experimental design. The observed and predicted values matched reasonably well.

Table 4.
Observed and predicted values for AChE, BuChE inhibitory activity (%), and FRAP (mM FeSO4) of MKJW-fermented A. capillaris.
3.2.3. Model Verification
The final estimated response model equation for the AChE inhibitory activity was as follows:
Y1 = 74.60 + 15.13 X1 − 7.46 X2 + 5.16 X3 − 4.10 X1X2 − 0.6500 X1X3 + 4.42 X2X3 − 7.34 X12 + 0.2375 X22 − 2.36 X32
The coefficients of A. capillaris (X1) and the yeast extract (X3) were positive; G. bimaculatus (X2) was negative of the dependent variable Y1. This means that when the contents of A. capillaris (X1) and yeast extract (X3) increase and the content of G. bimaculatus (X2) decreases, the AChE inhibitory activity (Y1) increases. The response increased as the content of yeast extract (X3) increased, but the influence on the response was less than that of A. capillaris (X1). The adjusted coefficient of determination for the multiple regression equation was 0.9471 and the p-value was less than 0.0001.
Equation (3) was visualized as a 3D response surface and is shown in Figure 3. Figure 3a shows that the AChE inhibitory activity was rapidly increasing in a curved shape when the A. capillaris (X1) content increased, which then decreased in response to the increase in the G. bimaculatus (X2) content. Figure 3b shows the effect of the A. capillaris (X1) content and yeast extract (X3) content response, and as in 3a, it increased as the A. capillaris (X1) content increased, but with little effect on the increase of yeast extract (X3) content. Figure 3c shows that the lower the content of G. bimaculatus (X2), the lower the change in the AChE inhibitory activity as the yeast extract (X3) increased. Conversely, as the content of G. bimaculatus (X2) increased, the effect of the yeast extract (X3) on the AChE inhibitory activity increased.
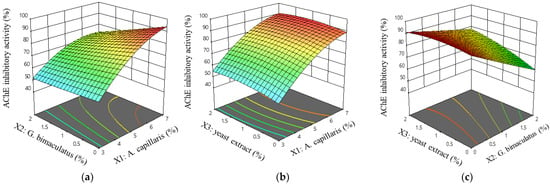
Figure 3.
Response surface and contour plots for the AChE inhibitory activity of MKJW-fermented A. capillaris. A. capillaris and G. bimaculatus (a); A. capillaris and yeast extract (b); G. bimaculatus and yeast extract (c).
The coefficient for BuChE inhibitory activity (Y2) was positive for A. capillaris (X1) and yeast extract (X3), and negative for G. bimaculatus (X2). This result was the same as Y1. As the contents of A. capillaris (X1) and yeast extract (X3) increased and the content of G. bimaculatus (X2) decreased, the BuChE inhibitory activity (Y2) value increased. The adjusted coefficient of determination was 0.9822 and the p-value was less than 0.0001. The final estimated response model equation for BuChE inhibitory activity is as follows:
Y2 = 50.10 + 16.13 X1 − 2.96 X2 + 0.3375 X3 − 6.52 X1X2 − 6.08 X1X3 + 3.65 X2X3 + 2.87 X12 + 4.15 X22 − 4.80 X32
Equation (4) was consistent with the three-dimensional graph that shows the interaction between factors, which is presented in Figure 4. Figure 4a,b show that the BuChE inhibitory activity increased rapidly as the A. capillaris (X1) increased. In addition, the response was decreased when the content of G. bimaculatus (X2) or yeast extract increased. Figure 4c shows that the response decreased as the yeast extract (X3) and G. bimaculatus (X2) increased.
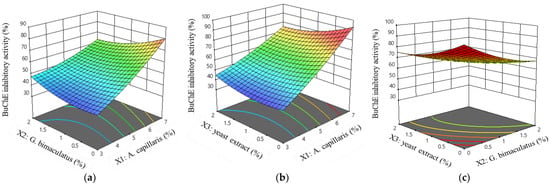
Figure 4.
Response surface and contour plots for the BuChE inhibitory activity of MKJW-fermented A. capillaris. A. capillaris and G. bimaculatus (a); A. capillaris and yeast extract (b); G. bimaculatus and yeast extract (c).
The coefficient for FRAP (Y3) was positive for A. capillaris (X1) and yeast extract (X3), whereas G. bimaculatus (X2) was negative. G. bimaculatus (X2) has less influence on the dependent variable than do X1 and X3. Its R2 was 0.9468. The final estimated response model equation for the FRAP value is as follows:
Y3 = 24.33 + 7.84 X1 − 1.68 X2 + 3.09 X3
Figure 5 shows the changes in the independent variables A. capillaris (X1) and G. bimaculatus (X2) when the independent variable yeast extract (X3) was fixed. FRAP was only significant for linear terms (p < 0.05). As with the regression equation, as A. capillaris (X1) increased, the FRAP value increased linearly.
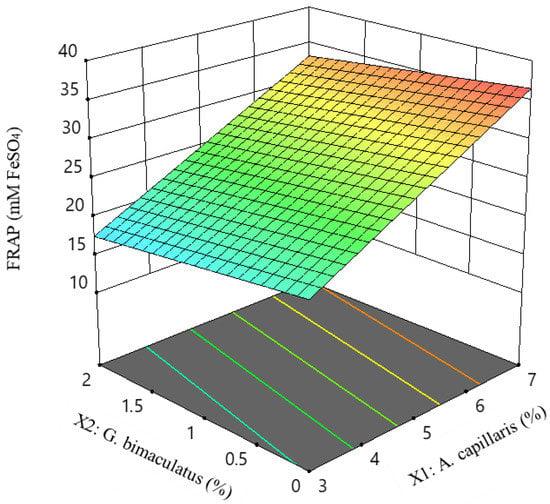
Figure 5.
Response surface and contour plots for FRAP value of MKJW-fermented A. capillaris.
Based on our results, the models for Y1, Y2, and Y3 can be useful tools to optimize the fermentation conditions because these are statistically significant and provide solid solutions as a function of independent variables.
3.3. Optimization of A. capillaris Fermented Product
The optimal fermentation conditions were determined by maximizing the desirability of the responses. The maximal desirability should be initially at the highest values for AChE and BuChE inhibitory activities, and FRAP, and then be at the higher concentration of A. capillaris. Finally, the optimal fermentation conditions for AChE and BuChE inhibitory activities are as follows: X1 = 6.75%, X2 = 0.18%, and X3 = 1.27%. Inoculum volume was 6.82 increased to 8.14 with a decrease in pH values from 5.16 to 4.93. Using the above conditions, the predicted values for each model are Y1 = 91.6%, Y2 = 74.5%, and Y3 = 33.3 mM FeSO4. After fermentation under optimal conditions, the predicted and observed values for the three dependent variables were analyzed (Figure 6). The observed values of each dependent variable were the AChE inhibitory activity (Y1) of 91.1 ± 4.75% (p = 0.864), BuChE inhibitory activity (Y2) of 74.0 ± 1.96% (p = 0.681), and FRAP (Y3) of 34.1 ± 0.43 mM FeSO4, (p = 0.060). There was no significant difference between the predicted value and the observed value. These results reveal that the model used in this study is valid.
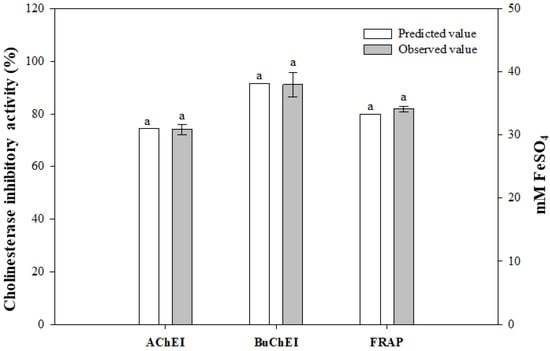
Figure 6.
Comparison of predicted and observed values of MKJW-fermented A. capillaris. Same letters between the predicted and observed value are not significantly different (p > 0.05).
The antioxidant activities and TPC of fermented A. capillaris under optimal conditions are shown in Table 5. The DPPH radical scavenging activity was 86.5 ± 0.89%. It was higher than Lactobacillus-fermented A. annua (80%) during 12 h, which was reported by Lee et al. [39]. The reducing power was 2.14 ± 0.02 at Abs 700 nm. The total polyphenol content of fermented A. capillaris was 1.52 ± 0.61 mg GAE/mL. It was a much higher content compared to other fermented Artemisia sp. The total polyphenol contents of A. annua L. fermented by Lactobacillus sp. for 12 h were 0.3 mg GAE/mL [39], and those of A. princeps Pamp. fermented by Monascus sp. were about 0.7 mg GAE/mL [40]. This is thought to be because the Artemisia sp. type and starter are different. A significant correlation between total polyphenol content and AChE inhibitory activity among the five Asteraceae sp. plants has been reported [41]. The aromatic phenyl groups (-C6H5) of polyphenols structurally interact well with the active site of AChE, which forms as an aromatic residue to inhibit the decomposition of acetylcholine [42]. Aside from the onset of Alzheimer’s disease by cholinesterase, another risk factor for Alzheimer’s disease is that oxidative stress increases in aging patients due to the accumulation of reactive oxygen species [43]. Oxidative stress can be eliminated by antioxidant active substances such as polyphenols and exopolysaccharides. Papandreou et al. [44] reported that when polyphenol-rich blueberry extracts were administered in the mouse brain, both oxidative stress and AChE activity reduced. The antioxidant active substances can be produced or increased as a result of fermentation using lactic acid bacteria [45]. Therefore, it is believed that the antioxidant activity of the raw A. capillaris increased further due to the fermentation of MKJW as well as AChE inhibitory activity.

Table 5.
Antioxidant activities and TPC of fermented A. capillaris under optimal conditions.
4. Conclusions
This study showed that optimized fermentation of A. capillaris by L. mesenteroides MKJW increased its cholinesterase inhibition activity and antioxidant activity. The maximal activity of acetylcholinesterase (91.6%) and butyrylcholinesterase (74.5%) inhibition and FRAP (33.3 mM FeSO4) was achieved when 6.75% A. capillaris was fermented by MKJW in the presence of 0.18% G. bimaculatus and 1.27% yeast extract. Therefore, it is thought that it can be used as probiotic A. capillaris possessing antidementia and antioxidant properties, but further research should be undertaken.
Author Contributions
Formal analysis, data curation, and writing—original draft, J.C.; formal analysis, J.Y.; funding acquisition, conceptualization, supervision, and writing—review and editing, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Research Foundation (NRF) of Korea, 2020R1I1A3072127.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This article is a part of Jina Choi’s Master’s Thesis completed at the Department of Food Science and Nutrition, Dankook University, Korea under the supervision of Misook Kim.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1996-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Santana-Galvez, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci. Technol. 2019, 88, 179–193. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Tõugu, V. Acetylcholinesterase: Mechanism of catalysis and inhibition. Curr. Med. Chem. 2001, 1, 155–170. [Google Scholar] [CrossRef]
- Masson, P.; Bec, N.; Froment, M.T.; Nachon, F.; Balny, C.; Lockridge, O.; Schopfer, L.M. Rate-determining step of butyrylcholinesterase-catalyzed hydrolysis of benzoylcholine and benzoylthiocholine. Eur. J. Biochem 2004, 271, 1980–1990. [Google Scholar] [CrossRef]
- McGleenon, B.M.; Dynan, K.B.; Passmore, A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999, 48, 471–480. [Google Scholar] [CrossRef]
- Mukhergee, P.K.; Kumar, V.K.; Mal, M.N.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2020, 190, 108352. [Google Scholar] [CrossRef]
- Choi, B.B.; Lee, H.J.; Bang, S.K. Studies on the amino acid, sugar analysis and antioxidative effect of extracts from Artemisia sp. Korean J. Food Nutr. 2004, 17, 86–91. [Google Scholar]
- Park, J.C.; Yu, Y.B.; Lee, J.H.; Kim, N.J. Studies on the chemical components and biological activities of edible plants in Korea. Korean J. Soc. Food Nutr. 1994, 23, 116–119. [Google Scholar]
- Kim, K.C.; Kim, J.S. Effect of varying ethanol concentrations on the extraction properties and physiological activity of Artemisia annua L. Korean J. Food Sci. Technol. 2020, 52, 130–137. [Google Scholar]
- Guo, S.; Ma, J.; Xing, Y.; Xu, Y.; Jin, X.; Yan, S.; Shi, B. Artemisia annua L. aqueous extract as an alternative to antibiotics improving growth performance and antioxidant function in broilers. Ital. J. Anim. Sci. 2020, 19, 399–409. [Google Scholar] [CrossRef]
- Protti, M.; Mandriolo, R.; Mandrone, M.; Cappadone, C.; Farruggia, G.; Chiocchio, I.; Malucelli, E.; Poli, F.; Mercolini, L. Analysis of Artemisia annua extracts and related products by high performance liquid chromatography-tandem mass spectrometry coupled to sample treatment miniaturization. J. Pharm. Biomed. Anal. 2019, 174, 81–88. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, K.L. Anti-oxidative and anti-inflammatory effects of Artemisia capillaris extract. Korean J. Aesthet. Cosmetol. 2015, 13, 805–812. [Google Scholar]
- Shoaib, M.; Shah, I.; Ali, N.; Shah, S.W.A. In vitro acetylcholinesterase and butyrylcholinesterase inhibitory potentials of essential oil of Artemisia macrocephala. Bangladesh J. Pharmaacol. 2015, 10, 87–91. [Google Scholar] [CrossRef]
- Choi, J.S.; Song, B.M.; Park, H.J. Identification of the component with anti-acetylcholinesterase activity from the essential oil of Artemisia iwayomogi. J. Plant Res. 2017, 30, 17–21. [Google Scholar]
- Saarela, M.; Mogensen, G.; Fonden, R.; Matto, J.; Sandholm, T.M. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, H.S. The roles of dietary polyphenols in brain neuromodulation. J. Life Sci. 2018, 28, 1386–1395. [Google Scholar]
- Choi, J.H.; Jin, Y.H.; Kim, J.H.; Hong, Y.G. Does the gut microbiota regulate a cognitive function? J. Life Sci. 2019, 29, 747–753. [Google Scholar]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhao, M.; Tang, J.; Deng, L.; Feng, F. Probiotics-fermented blueberry juices as potential antidiabetic product: Antioxidant, antimicrobial and antidiabetic potentials. J. Sci. Food Agric. 2021, 101, 4420–4427. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoon, J.; Seong, H.; Jeong, Y. Novel Leuconostoc Mesenteroides MKJW and Its Uses Thereof. KR Patent 102021001464, 2 February 2021. [Google Scholar]
- Sharififar, F.; Moshafi, M.H.; Shafazand, E.; Koohpayeh, A. Acetylcholinesterase inhibitory, antioxidant and cytotoxic activity of three dietary medicinal plants. Food Chem. 2012, 130, 20–23. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.K.; Park, S.I.; Kim, I.Y.; Jeong, Y.H.; Yu, S.Y.; Shin, S.C.; Kim, M.S. Antioxidant activity of Korea traditional soy sauce fermented in Korean earthenware, onggi, from different regions. J. Korean Soc. Food Sci. Nutr. 2015, 44, 847–853. [Google Scholar] [CrossRef]
- Lin, M.Y.; Yen, C.L. Antioxidative ability of lactic acid bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef]
- Cermeno, M.; FitzGerald, R.J.; O’Brien, N.M. In vitro antioxidant, and immunomodulatory activity of transglutaminase-treated sodium caseinate hydrolysates. Int. Dairy J. 2016, 63, 107–114. [Google Scholar] [CrossRef]
- Anesini, C.; Ferraro, G.E.; Filip, R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. 2008, 56, 9225–9229. [Google Scholar] [CrossRef]
- Isshiki, K.; Nishinomiya, T.; Nozaka, N.; Tokuoka, K. Growth inhibition of microorganisms by plant extracts. Nippon Shokuhin Kogyo Gakkaishi 1993, 40, 525–527. [Google Scholar] [CrossRef]
- Sagdic, O.; Karahan, A.G.; Ozcan, M.; Ozkan, G. Note: Effect of some spice extracts on bacterial inhibition. Food Sci. Technol. Int. 2003, 9, 353–356. [Google Scholar] [CrossRef]
- Naili, M.B.; Alghazeer, R.O.; Saleh, N.A.; Al-Najjar, A.Y. Evaluation of antibacterial and antioxidant activities of Artemisia campestris (Astraceae) and ZiZiphus lotus (Rhamnacea). Arab. J. Chem. 2010, 3, 79–84. [Google Scholar] [CrossRef]
- Mannan, H.A.; Ahmed, I.; Hussain, I.; Jamil, M.; Miza, B. Antibacterial activity and brine shrimp toxicity of Artemisia dubia extract. Pak. J. Bot. 2012, 44, 1487–1490. [Google Scholar]
- Ghlissi, Z.; Sayari, N.; Kallel, R.; Bougatef, A.; Sahnoun, Z. Antioxidant, antibacterial, anti-inflammatory, and wound healing effects of Artemisia campestris aqueous extract in rat. Biomed. Pharmacother. 2016, 84, 115–122. [Google Scholar] [CrossRef]
- Kyoung, S.S.; Hyung, J.J.; Kyeong, W.Y. Antimicrobial activity and chemical components of two plants. Artemisia capillaris and Artemisia iwayomogi, used as Korean herbal Injin. J. Ecol. Field Biol. 2010, 33, 141–147. [Google Scholar]
- Park, I.B.; Park, J.W.; Lee, Y.J.; Shin, G.W.; Kim, H.S.; Jo, Y.C. Quality characteristic of glasswort (Salicornia herbacea L.) fermented by Bacillus subtilis. Korean J. Soc. Food Sci. Nutr. 2009, 38, 902–908. [Google Scholar] [CrossRef]
- Yang, S.J.; Kim, Y.C.; Hong, J.H. Cultural characteristics and biological activities of cereals fermented by Bacillus subtilis CBD2. J. Chitin Chitosan 2014, 19, 277–284. [Google Scholar]
- Rheem, S.; Rheem, I.; Oh, S. Response surface methodology using a fullest balanced model: A re-analysis of a dataset in the Korean journal for food science of animal resources. Food Sci. Anim. Resour. 2017, 37, 139–146. [Google Scholar] [CrossRef]
- Lee, A.R.; Niu, K.M.; Kang, S.K.; Han, S.G.; Lee, J.B.; Kim, S.K. Antioxidant, and antibacterial activities of Lactobacillus-fermented Artemisia annua L. as a potential fish feed additive. J. Life Sci. 2017, 27, 652–660. [Google Scholar]
- Lee, D.S.; Lee, I.H. Development of Monacolin K-enriched Ganghwayakssuk (Artemisia princeps Pamp.) by fermentation with Monascus pilosus. J. Microbiol. Biotechnol. 2012, 22, 975–980. [Google Scholar] [CrossRef]
- Mekinic, I.G.; Burcul, F.; Blazevic, I.B.; Skroza, D.; Kerum, D.; Katalinic, V. Antioxidative/Acetylcholinesterase inhibitory activity of some Asteraceae Plants. Nat. Prod. Commun. 2013, 8, 471–474. [Google Scholar] [CrossRef]
- Mesquita, R.S.; Kyrylchuk, A.; Oliveira, R.C.; Sa’, I.S.C.; Camargo, G.C.B.; Pontes, G.S.; Silva, F.M.A.; Nunomura, R.C.S.; Grafov, A. Alkaloids of Abuta panurensis Eichler: In silico and in vitro study of acetylcholinesterase inhibition, cytotoxic and immunomodulatory activities. PLoS ONE 2020, 15, e0239364. [Google Scholar]
- Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000, 71, 621S–629S. [Google Scholar] [CrossRef]
- Papandreou, M.A.; Dimakopoulou, A.; Linardaki, Z.I.; Cordopatis, P.; Zacas, D.K.; Margarity, M.; Lamari, F.N. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav. Brain Res. 2009, 198, 352–358. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jeong, H.J.; Chung, H.S.; Seong, J.H.; Kim, H.S.; Kim, D.S.; Lee, Y.G. Anti-oxidative activity of the extracts from Houttuynia cordata Thunb. Fermented by lactic acid bacteria. J. Life Sci. 2016, 26, 468–474. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).