Effect of Different Degrees of Deep Freezing on the Quality of Snowflake Beef during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Frozen Storage Conditions
2.3. Freezing Temperature Profile
2.4. Microstructure
2.5. Drip Loss
2.6. Color
2.7. Shear Force Measurement
2.8. Thiobarbituric Acid Reactive Substances (TBARS)
2.9. Carbonyl Content Determination
2.10. Volatile Organic Compounds
2.11. Statistical Analysis
3. Results and Discussion
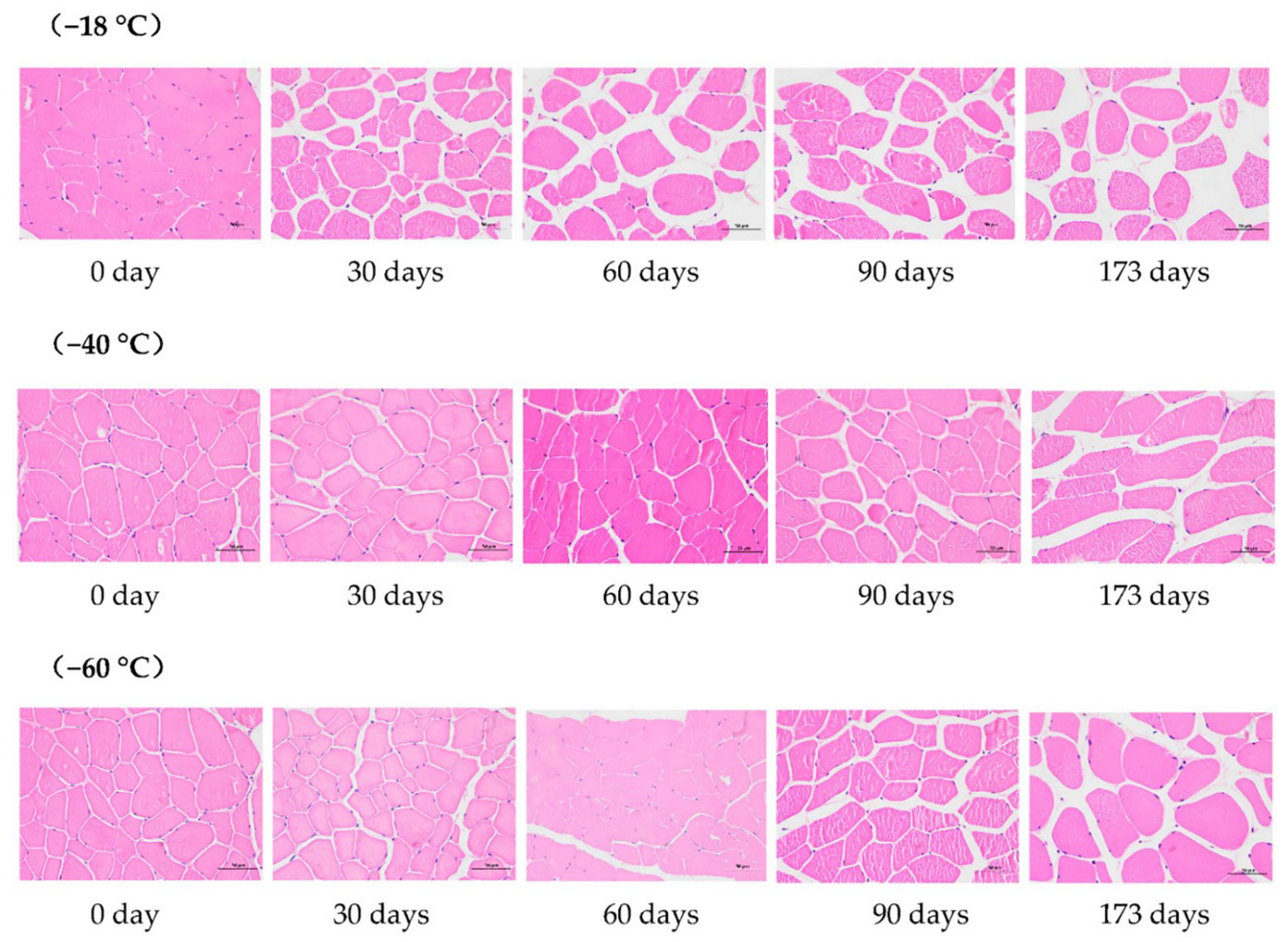
3.1. Microstructure
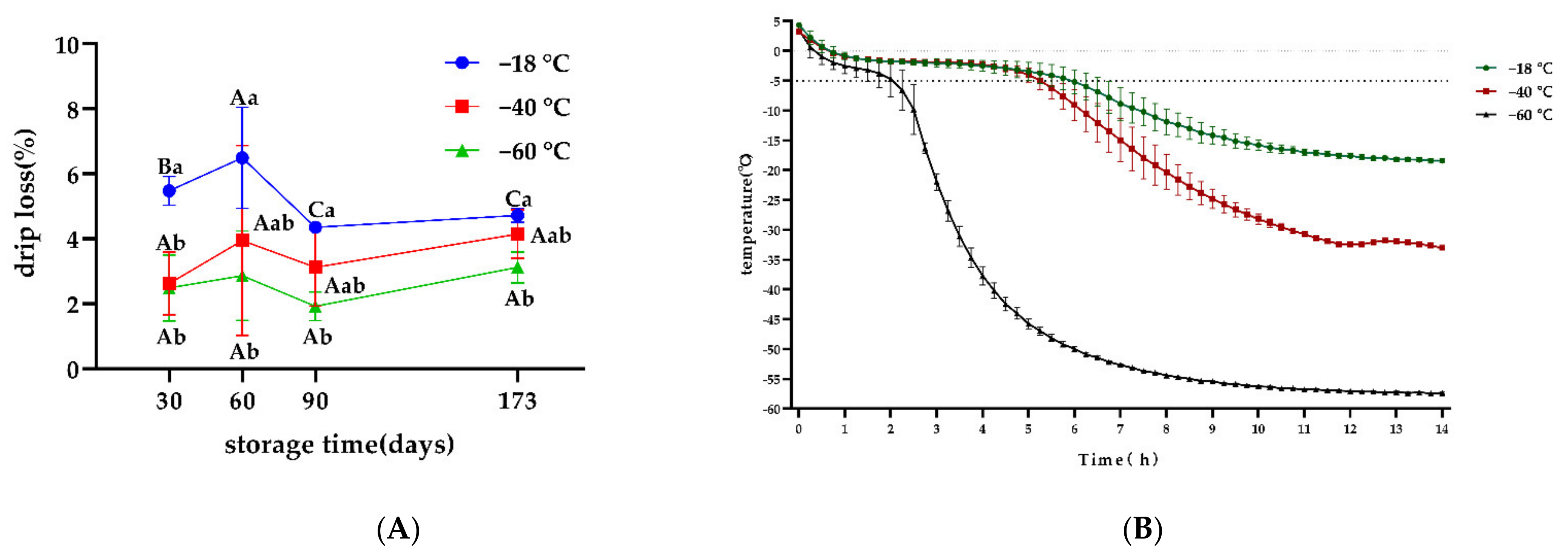
3.2. Freezing Temperature Profile and Drip Loss
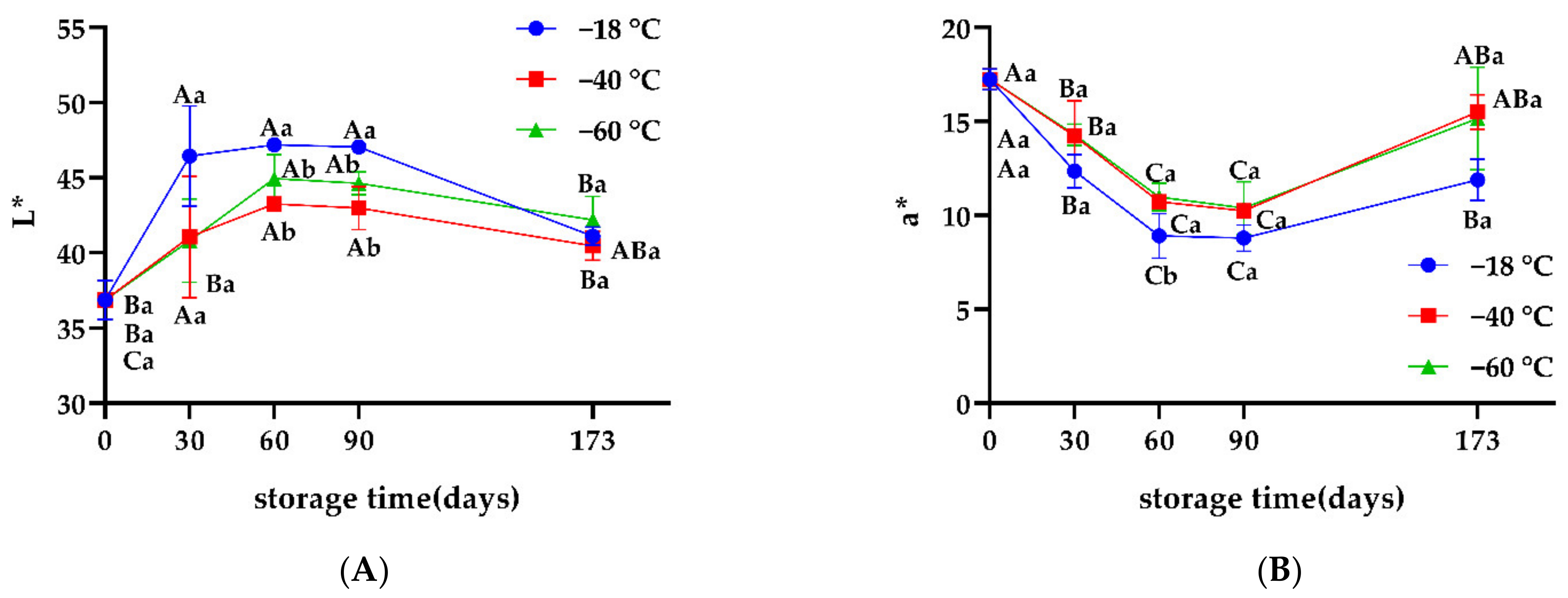
3.3. Color
3.4. Shear Force
3.5. TBARS and Carbonyl Content
3.6. Volatile Organic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omarov, R.; Gorlov, I.; Zakotin, V.; Shlykov, S. Development of Marble Beef Technology. In Proceedings of the 16th International Scientific Conference on Engineering for Rural Development, Jelgava, Latvia, 24–26 May 2017. [Google Scholar]
- Greene, J.L. U.S. Beef: It’s What’s for China. Available online: https://digital.library.unt.edu/ark:/67531/metadc1043365/ (accessed on 27 April 2022).
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, R.; Kong, B.H.; Zhang, Y.G. Effect of Superchilling Storage on Quality Characterizes of Beef as Compared with Chilled and Frozen Preservation. Adv. Mater. Res. 2012, 554–556, 1195–1201. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Hsiao, H.-I. Food quality and safety risk diagnosis in the food cold chain through failure mode and effect analysis. Food Control 2021, 120, 107501. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Coombs, C.E.O.; Morris, S.; Kerr, M.J.; Hopkins, D.L. Effect of long term chilled (up to 5 weeks) then frozen (up to 12 months) storage at two different sub-zero holding temperatures on beef: 1. Meat quality and microbial loads. Meat Sci. 2017, 133, 133–142. [Google Scholar] [CrossRef]
- Turgut, S.S.; Işıkçı, F.; Soyer, A. Antioxidant activity of pomegranate peel extract on lipid and protein oxidation in beef meatballs during frozen storage. Meat Sci. 2017, 129, 111–119. [Google Scholar] [CrossRef]
- Choi, M.-J.; Abduzukhurov, T.; Park, D.H.; Kim, E.J.; Hong, G.-P. Effects of Deep Freezing Temperature for Long-term Storage on Quality Characteristics and Freshness of Lamb Meat. Food Sci. Anim. Resour. 2018, 38, 959–969. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Lee, S.; Park, D.H.; Kim, H.; Choi, M.-J. Physicochemical Properties of Pork Neck and Chicken Leg Meat under Various Freezing Temperatures in a Deep Freezer. Food Sci. Anim. Resour. 2020, 40, 444–460. [Google Scholar] [CrossRef]
- Utrera, M.; Morcuende, D.; Estévez, M. Temperature of frozen storage affects the nature and consequences of protein oxidation in beef patties. Meat Sci. 2014, 96, 1250–1257. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Mehmood, W.; Qian, S.; Sun, Z.; Zhang, C.; Blecker, C. Effect of Storage Temperatures on the Moisture Migration and Microstructure of Beef. J. Food Qual. 2018, 2018, e3873179. [Google Scholar] [CrossRef]
- Lee, H.-S.; Park, Y.I.; Kang, S. Effects of fat meat and storage temperature on the qualities of frozen minced beef products. Qual. Assur. Saf. Crops Foods 2021, 13, 93–104. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Erkan, N.; Özden, Ö. Quality assessment of whole and gutted sardines (Sardina pilchardus) stored in ice. Int. J. Food Sci. Technol. 2008, 43, 1549–1559. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; del Mar Dobao-Prieto, M.; Arce, L.; Valcárcel, M. Determination of volatile compounds by GC–IMS to assign the quality of virgin olive oil. Food Chem. 2015, 187, 572–579. [Google Scholar] [CrossRef]
- Kaale, L.D.; Eikevik, T.M. The development of ice crystals in food products during the superchilling process and following storage, a review. Trends Food Sci. Technol. 2014, 39, 91–103. [Google Scholar] [CrossRef]
- Grujić, R.; Petrović, L.; Pikula, B.; Amidžić, L. Definition of the optimum freezing rate—1. Investigation of structure and ultrastructure of beef M. longissimus dorsi frozen at different freezing rates. Meat Sci. 1993, 33, 301–318. [Google Scholar] [CrossRef]
- Martino, M.N.; Otero, L.; Sanz, P.D.; Zaritzky, N.E. Size and location of ice crystals in pork frozen by high-pressure-assisted freezing as compared to classical methods. Meat Sci. 1998, 50, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, E.J.; Park, D.H.; Ji, Y.R.; Kang, G.; Choi, M.-J. Deep freezing to maintain the freshness of pork loin during long-term storage. Food Sci. Biotechnol. 2021, 30, 701–710. [Google Scholar] [CrossRef]
- Zhang, Y.; Ertbjerg, P. On the origin of thaw loss: Relationship between freezing rate and protein denaturation. Food Chem. 2019, 299, 125104. [Google Scholar] [CrossRef]
- Wang, F.; Liang, R.; Zhang, Y.; Gao, S.; Zhu, L.; Niu, L.; Luo, X.; Mao, Y.; Hopkins, D.L. Effects of packaging methods combined with frozen temperature on the color of frozen beef rolls. Meat Sci. 2021, 171, 108292. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, S.H.; Choi, J.H.; Choi, Y.S.; Kim, H.Y.; Hwang, K.E.; Park, J.H.; Song, D.H.; Kim, C.J. Effects of rigor state, thawing temperature, and processing on the physicochemical properties of frozen duck breast muscle. Poult. Sci. 2012, 91, 2662–2667. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Kikugawa, K.; Kato, T.; Hayasaka, A. Formation of dityrosine and other fluorescent amino acids by reaction of amino acids with lipid hydroperoxides. Lipids 1991, 26, 922–929. [Google Scholar] [CrossRef]
- Lan, Y.; Shang, Y.; Song, Y.; Dong, Q. Changes in the quality of superchilled rabbit meat stored at different temperatures. Meat Sci. 2016, 117, 173–181. [Google Scholar] [CrossRef]
- Sasaki, K.; Mitsumoto, M.; Kawabata, K. Relationship between lipid peroxidation and fat content in Japanese Black beef Longissimus muscle during storage. Meat Sci. 2001, 59, 407–410. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Jiang, S.-T.; Hwang, D.-C.; Chen, C.-S. Effect of Storage Temperatures on the Formation of Disulfides and Denaturation of Milkfish Actomyosin. J. Food Sci. 1988, 53, 1333–1335. [Google Scholar] [CrossRef]
- Saraiva, C.; Oliveira, I.; Silva, J.A.; Martins, C.; Ventanas, J.; García, C. Implementation of multivariate techniques for the selection of volatile compounds as indicators of sensory quality of raw beef. J. Food Sci. Technol. 2015, 52, 3887–3898. [Google Scholar] [CrossRef] [Green Version]



| Time | 0 Days | 30 Days | 60 Days | 90 Days | 173 Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile Organic Compounds | −18 °C | −40 °C | −60 °C | −18 °C | −40 °C | −60 °C | −18 °C | −40 °C | −60 °C | −18 °C | −40 °C | −60 °C | |
| Alcohols | |||||||||||||
| 1-Propanol | 3150 ± 96 | 697 ± 77 a | 695 ± 17 a | 807 ± 107 a | 889 ± 57 b | 844 ± 40 b | 1695 ± 57 a | 2872 ± 347 a | 3096 ± 153 a | 2223 ± 156 b | 285 ± 93 b | 368 ± 33 b | 590 ± 121 a |
| 1-Butanol | 526 ± 43 | 670 ± 114 a | 763 ± 54 a | 756 ± 33 a | - | - | - | - | - | - | 876 ± 12 a | 563 ± 69 b | 426 ± 98 c |
| 1-Pentanol | 829 ± 20 | 765 ± 68 a | 777 ± 17 a | 676 ± 128 a | 702 ± 20 a | 625 ± 17 b | 447 ± 56 c | 360 ± 74 a | 427 ± 67 a | 419 ± 68 a | 981 ± 7 a | 954 ± 15 b | 955 ± 10 b |
| n-Hexanol | 581 ± 181 | 812 ± 3 a | 815 ± 5 a | 701 ± 110 a | 732 ± 111 a | 772 ± 7 a | 578 ± 132 a | 434 ± 60 b | 521 ± 74 ab | 550 ± 37 a | 930 ± 18 a | 426 ± 135 c | 711 ± 110 b |
| Heptanol | - | - | - | - | - | - | - | 30 ± 5 a | 43 ± 18 a | 36 ± 10 a | - | - | - |
| 2-Hexen-1-ol | 180 ± 37 | 173 ± 46 a | 190 ± 13 a | 137 ± 64 a | 63 ± 37 a | 62 ± 26 a | 31 ± 16 a | - | - | - | 124 ± 13 a | 100 ± 15 a | 94 ± 26 a |
| 1-Octen-3-ol | 529 ± 110 | 835 ± 28 a | 965 ± 57 a | 836 ± 146 a | 208 ± 23 a | 222 ± 32 a | 127 ± 34 b | 343 ± 51 b | 588 ± 189 a | 265 ± 62 b | 635 ± 98 a | 409 ± 67 b | 519 ± 38 ab |
| 2ethyl-1-hexanol | - | 63 ± 5 a | 62 ± 4 a | 55 ± 2 b | 158 ± 51 a | 194 ± 29 a | 119 ± 36 a | - | - | - | - | - | - |
| Aldehydes | |||||||||||||
| Butanal | - | - | - | - | - | - | - | 1596 ± 338 a | 1384 ± 175 a | 1720 ± 76 a | - | - | - |
| Pentanal | 769 ± 33 | 735 ± 37 a | 748 ± 11 a | 623 ± 109 b | 725 ± 19 a | 681 ± 24 a | 243 ± 84 b | - | - | - | - | - | - |
| Hexanal | 664 ± 24 | 652 ± 28 a | 632 ± 17 a | 640 ± 54 a | 630 ± 30 a | 594 ± 23 a | 474 ± 75 b | 431 ± 11 b | 492 ± 38 a | 449 ± 43 ab | 859 ± 5 a | 897 ± 36 a | 881 ± 15 a |
| Heptanal | 553 ± 42 | 472 ± 63 a | 452 ± 57 a | 400 ± 85 a | 245 ± 63 a | 257 ± 78 a | 180 ± 65 a | 199 ± 69 a | 226 ± 19 a | 207 ± 18 a | - | - | - |
| Octanal | 245 ± 6 | 223 ± 14 a | 217 ± 12 a | 183 ± 22 b | 290 ± 39 a | 299 ± 21 a | 204 ± 77 b | 111 ± 29 b | 162 ± 16 a | 122 ± 12 b | 916 ± 93 a | 696 ± 116 b | 814 ± 18 ab |
| n-Nonanal | 324 ± 9 | 301 ± 14 a | 295 ± 16 a | 252 ± 23 b | 60 ± 5 ab | 67 ± 6 a | 46 ± 18 b | 193 ± 36 b | 273 ± 19 a | 254 ± 24 a | 237 ± 10 a | 209 ± 27 a | 229 ± 16 a |
| Benzaldehyde | - | 53 ± 15 a | 57 ± 5 a | 39 ± 10 a | 260 ± 43 a | 248 ± 56 a | 47 ± 35 b | - | - | - | 568 ± 14 a | 572 ± 3 a | 568 ± 20 a |
| Ketones | |||||||||||||
| 2-Propanone | - | 3147 ± 299 b | 3342 ± 105 ab | 3655 ± 172 a | 3113 ± 52 a | 3166 ± 45 a | 3187 ± 407 a | - | - | - | - | - | - |
| 2-Butanone | - | 332 ± 88 a | 406 ± 11 a | 441 ± 78 a | 589 ± 50 a | 628 ± 32 a | 404 ± 83 b | 2130 ± 488 a | 1969 ± 522 a | 2404 ± 222 a | - | - | - |
| 2-Pentanone | - | 282 ± 59 a | 270 ± 35 a | 257 ± 57 a | 421 ± 48 a | 376 ± 69 a | 373 ± 71 a | - | - | - | - | - | - |
| 2-Hexanone | - | 130 ± 4 a | 141 ± 10 a | 119 ± 36 a | 155 ± 59 a | 140 ± 55 a | 78 ± 26 a | - | - | - | - | - | - |
| 2-Heptanone | 144 ± 19 | 201 ± 23 a | 238 ± 41 a | 219 ± 33 a | 44 ± 34 a | 25 ± 2 a | 28 ± 16 a | 166 ± 30 b | 152 ± 36 b | 354 ± 153 a | 166 ± 16 a | 133 ± 60 a | 145 ± 45 a |
| 2-Octanone | - | 49 ± 23 a | 66 ± 19 a | 46 ± 31 a | 71 ± 33 a | 65 ± 4 ab | 26 ± 2 b | - | - | - | - | - | - |
| Cyclohexanone | - | 62 ± 5 a | 67 ± 5 a | 61 ± 6 a | - | - | - | - | - | - | - | - | - |
| 2,3-Pentanedione | 286 ± 42 | - | - | - | - | - | - | - | - | - | - | - | - |
| 2,3-Butanedione | 402 ± 25 | 332 ± 120 b | 402 ± 60 b | 665 ± 112 a | 630 ± 71 b | 802 ± 68 a | 205 ± 93 c | 257 ± 64 a | 267 ± 70 a | 224 ± 26 a | 274 ± 20 b | 193 ± 94 b | 432 ± 36 a |
| Esters | |||||||||||||
| Ethylacetate | 873 ± 89 | 794 ± 27 a | 732 ± 76 a | 742 ± 29 a | 437 ± 59 a | 513 ± 99 a | 431 ± 47 a | 2655 ± 302 a | 1402 ± 99 b | 3081 ± 337 a | 383 ± 32 b | 536 ± 33 a | 538 ± 48 a |
| Isopropyl acetate | - | 373 ± 99 b | 588 ± 102 a | 499 ± 85 ab | - | - | - | 319 ± 52 b | 551 ± 97 a | 365 ± 22 b | - | - | - |
| Isoamyl acetate | - | - | - | - | - | - | - | 31 ± 5 a | 34 ± 5 a | 60 ± 34 a | - | - | - |
| Others | |||||||||||||
| Styrene | - | - | - | - | - | - | - | 117 ± 48 a | 128 ± 15 a | 94 ± 7 a | 77 ± 3 a | 45 ± 10 b | 45 ± 6 b |
| Methylpyrazine | - | 101 ± 55 | 123 ± 24 a | 119 ± 42 a | - | - | - | - | - | - | - | - | - |
| Acetophenone | - | - | - | - | - | - | - | - | - | - | 205 ± 1 a | 205 ± 3 a | 209 ± 7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Liu, Y.; Bai, Y.; Teng, S.; Guo, Y.; Dou, H.; Ye, K. Effect of Different Degrees of Deep Freezing on the Quality of Snowflake Beef during Storage. Foods 2022, 11, 2175. https://doi.org/10.3390/foods11152175
Chang Y, Liu Y, Bai Y, Teng S, Guo Y, Dou H, Ye K. Effect of Different Degrees of Deep Freezing on the Quality of Snowflake Beef during Storage. Foods. 2022; 11(15):2175. https://doi.org/10.3390/foods11152175
Chicago/Turabian StyleChang, Yawei, Yan Liu, Yun Bai, Shuang Teng, Yiping Guo, Han Dou, and Keping Ye. 2022. "Effect of Different Degrees of Deep Freezing on the Quality of Snowflake Beef during Storage" Foods 11, no. 15: 2175. https://doi.org/10.3390/foods11152175
APA StyleChang, Y., Liu, Y., Bai, Y., Teng, S., Guo, Y., Dou, H., & Ye, K. (2022). Effect of Different Degrees of Deep Freezing on the Quality of Snowflake Beef during Storage. Foods, 11(15), 2175. https://doi.org/10.3390/foods11152175




