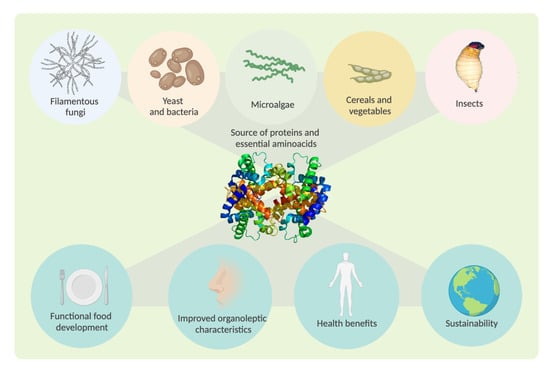
Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation
Abstract
1. Introduction
2. Single-Cell Protein as a Source of Dietary Proteins
2.1. General Characteristics of SCP
2.2. Use of SCP from Yeasts and Bacteria in Food Production
3. Filamentous Fungi Are Invaluable Sources of Dietary Proteins
3.1. Fermented Foods Obtained Using Filamentous Fungi
3.2. Mycoproteins as Food Ingredients
3.3. Barriers Limiting Consumption of Filamentous Fungi-Based Foods
4. Chances and Issues Related to Microalgae as Sources of Proteins and Other Nutrients
4.1. Use of Microalgae as Additional Food Ingredients
4.2. Sensory and Nutritional Issues Related to the Dietary Intake of Microalgae
4.3. Legislation and Additional Issues for Future Research
5. Pros and Cons of Vegetables as Sources of Dietary Proteins
Tradition-Based Innovation in Fermented Foods of Vegetable Origin
6. Advantages from “Hybridization”: The Case of Vegetable/Milk Mixed Foods
6.1. Traditional MFPs
6.2. Novel MFPs
7. Insects as Sources of Dietary Proteins
7.1. Application of Insects to Food Products
7.2. Issues and Solutions Related to the Use of Insects as Protein-Rich Foods
8. Implications and Limitations
9. Challenges in the Field of Meat-Alternative Protein Sources
10. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worldometers from 1950 to Current Year: Elaboration of Data by United Nations, Department of Economic and Social Affairs, Population Division. Available online: https://www.worldometers.info/world-population/ (accessed on 28 June 2022).
- FAO/WHO. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017; p. 136. [Google Scholar]
- Nadathur, S.; Wanasundara, J.; Scanlin, L. Proteins in the diet: Challenges in feeding the global population. In Sustainable Protein Sources; Nadathur, S., Wanasundara, J., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–19. [Google Scholar] [CrossRef]
- Bonnet, C.; Bouamra-Mechemache, Z.; Réquillart, V.; Treich, N. Viewpoint: Regulating meat consumption to improve health, the environment and animal welfare. Food Pol. 2020, 97, 101847. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 28 June 2022).
- FAO. World Livestock 2011—Livestock in Food Security; FAO: Rome, Italy, 2011. [Google Scholar]
- Hu, F.B.; Otis, B.O.; McCarthy, G. Can plant-based meat alternatives be part of a healthy and sustainable diet? JAMA 2019, 322, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; Corpet, D.E. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat intake and risk of colorectal adenomas: A systematic review and meta-analysis of epidemiological studies. Cancer Causes Control. 2013, 24, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr 2017, 105, 1462–1473. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Dissabandara, L.; Gopalan, V. A critical overview on the biological and molecular features of red and processed meat in colorectal carcinogenesis. J. Gastroenterol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Grundy, A.; Poirier, A.E.; Khandwala, F.; McFadden, A.; Friedenreich, C.M.; Brenner, D.R. Cancer incidence attributable to red and processed meat consumption in Alberta in 2012. Can. Med. Assoc. J. 2016, 4, E768–E775. [Google Scholar] [CrossRef][Green Version]
- World Economic Forum. Available online: http://www3.weforum.org/docs/WEF_White_Paper_Alternative_Proteins.pdf (accessed on 28 June 2022).
- Van der Weele, C.; Feindt, P.; van der Goot, A.J.; van Mierlo, B.; van Boekel, M. Meat alternatives: An integrative comparison. Trends Food Sci. Technol. 2019, 88, 505–512. [Google Scholar] [CrossRef]
- Thavamani, A.; Sferra, T.J.; Sankararaman, S. Meet the meat alternatives: The value of alternative protein sources. Curr. Nutr. Rep. 2020, 9, 346–355. [Google Scholar] [CrossRef]
- SYSTEMIC. Available online: https://systemic-hub.eu/coordination/ (accessed on 28 June 2022).
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.; Ramos, O.L.; Vicente, A.A. Emergent food proteins—Towards sustainability, health and innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Kulus, M.; Jankowski, M.; Dompe, C.; Bryl, R.; Petitte, J.N.; Kempisty, B.; Mozdziak, P. COVID-19 pandemic is a call to search for alternative protein sources as food and feed: A review of possibilities. Nutrients 2021, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Rosell, C.M.; Rosene, S.; Bover-Cid, S.; Castellari, M. Non-animal proteins as cutting-edge ingredients to reformulate animal-free foodstuffs: Present status and future perspectives. Crit. Rev. Food Sci. Nutr. 2021, 27, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Dominik, S.; Tobin, A.B.; Stockmann, R.; Simon, C.; Howitt, C.A.; Belobrajdic, D.P.; Paull, C.; Vanhercke, T. Perspectives on future protein production. J. Agr. Food Chem. 2021, 69, 15076–15083. [Google Scholar] [CrossRef]
- Anupama; Ravindra, P. Value-added food: Single cell protein. Biotechnol. Adv. 2000, 18, 459–479. [Google Scholar] [CrossRef]
- Food out of Thin Air. Available online: https://ifst.onlinelibrary.wiley.com/doi/full/10.1002/fsat.3402_12.x (accessed on 28 June 2022).
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single cell protein production: A review. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 251–262. [Google Scholar]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single cell protein—State-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single Cell Protein: A potential substitute in human and animal nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Leger, D.; Matassa, S.; Noor, E.; Shepon, A.; Milo, R.; Bar-Even, A. Photovoltaic-driven microbial protein production can use land and sunlight more efficiently than conventional crops. Proc. Natl. Acad. Sci. USA 2021, 118, e2015025118. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef]
- Linder, T. Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Sec. 2019, 11, 265–278. [Google Scholar] [CrossRef]
- Erdman, M.D.; Bergen, W.G.; Reddy, C.A. Amino acid profiles and presumptive nutritional assessment of single-cell protein from certain lactobacilli. Appl. Environ. Microbiol. 1977, 33, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, D.; Liu, Y. Production of single cell protein from soy molasses using Candida tropicalis. Ann. Microbiol. 2012, 62, 1165–1172. [Google Scholar] [CrossRef]
- Nasseri, A.; Rasoul-Amini, S.; Morowvat, M.; Ghasemi, Y. Single cell protein: Production and process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Ciani, M.; Lippolis, A.; Fava, F.; Rodolfi, L.; Niccolai, A.; Tredici, M.R. Microbes: Food for the future. Foods 2021, 10, 971. [Google Scholar] [CrossRef]
- Sillman, J.; Nygren, L.; Kahiluoto, H.; Ruuskanen, V.; Tamminen, A.; Bajamundi, C.; Nappa, M.; Wuokko, M.; Lindh, T.; Vainikka, P.; et al. Bacterial protein for food and feed generated via renewable energy and direct air capture of CO2: Can it reduce land and water use? Glob. Food Secur. Agr. 2019, 22, 25–32. [Google Scholar] [CrossRef]
- Somda, M.K.; Nikiema, M.; Keita, I.; Mogmenga, I.; Kouhounde, S.H.; Dabire, Y.; Coulibaly, W.H.; Taale, E.; Traore, A.S. Production of single cell protein (SCP) and essentials amino acids from Candida utilis FMJ12 by solid state fermentation using mango waste supplemented with nitrogen sources. Afr. J. Biotechnol. 2018, 17, 716–723. [Google Scholar] [CrossRef]
- Albers, E.; Larsson, C.; Lidn, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [CrossRef]
- Abou-Zeid, A.-Z.A.; Khan, J.A.; Abulnaja, K.O. On methods for reduction of nucleic acids content in a single-cell protein from gas oil. Biores. Technol. 1995, 52, 21–24. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Asif, M.; Razzaq, Z.U.; Nazir, A.; Maan, A.A. Sustainable food industrial waste management through single cell protein production and characterization of protein enriched bread. Food Biosci. 2022, 46, 101406. [Google Scholar] [CrossRef]
- Martinez, M.; Sanchez-Montero, J.; Sinisterra, J.; Ballesteros, A. New insolubilized derivatives of ribonuclease and endonuclease for elimination of nucleic acids in single cell protein concentrates. Biotech. Appl. Biochem. 1990, 12, 643–652. [Google Scholar] [CrossRef]
- Razzaq, Z.U.; Khan, M.K.I.; Maan, A.A.; Rahman, S.U. Characterization of single cell protein from Saccharomyces cerevisiae for nutritional, functional and antioxidant properties. J. Food Meas. Charact. 2020, 14, 2520–2528. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Athanasiadis, I.; Kanellaki, M.; Bekatorou, A.; Blekas, G.; Kiosseoglou, V. Functional properties of single cell protein produced by kefir microflora. Food Res. Int. 2003, 36, 431–438. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for future food systems: Precision fermentation can complement the scope and applications of traditional fermentation. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef] [PubMed]
- El Youssef, C.; Bonnarme, P.; Fraud, S.; Péron, A.-C.; Helinck, S.; Landaud, S. Sensory improvement of a pea protein-based product using microbial co-cultures of lactic acid bacteria and yeasts. Foods 2020, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Ben-Harb, S.; Irlinger, F.; Saint-Eve, A.; Panouillé, M.; Souchon, I.; Bonnarme, P. Versatility of microbial consortia and sensory properties induced by the composition of different milk and pea protein-based gels. LWT 2020, 118, 108720. [Google Scholar] [CrossRef]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008, 77, 1191–1205. [Google Scholar] [CrossRef]
- Kirk, P.C.P.; Minter, D.; Stalpers, J. Dictionary of the Fungi, 10th ed.; CBS: The Hague, The Netherlands, 2011; p. 784. [Google Scholar]
- Jia, L.; Dong, J.; Wang, R.; Mao, S.; Lu, F.; Singh, S.; Wang, Z.; Liu, X. Identification and characterization of the steroid 15α-hydroxylase gene from Penicillium raistrickii. Appl. Microbiol. Biotechnol. 2017, 101, 6409–6418. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Pandey, A.K. Fungal-derived natural product: Synthesis, function, and applications. In Recent Advancement in White Biotechnology through Fungi: Volume 2: Perspective for Value-Added Products and Environments; Yadav, A.N., Singh, S., Mishra, S., Gupta, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 229–248. [Google Scholar] [CrossRef]
- Jiang, Z.-D.; An, Z. Bioactive fungal natural products through classic and biocombinatorial approaches. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 245–272. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1443–1476. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi—Potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef]
- Agarwal, S.; Kushwaha, A.; Verma, V.; Singh, M. Nutritional attributes of Pleurotus mushroom. In Incredible World of Biotechnology; Singh, V.V., Singh, A.K., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 13–24. [Google Scholar]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, C.W.; Cook, D.J. Food, Fermentation, and Micro-Organisms; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for future foods. J. Fut. Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Khosravi-Darani, K.; Hosseini, H.; Farshi, P.; Reihani, S.F.S. Mycoproteins as safe meat substitutes. J. Clean. Product. 2020, 253, 119958. [Google Scholar] [CrossRef]
- Derbyshire, D.E. Fungal protein—What is it and what is the health evidence? A systematic review focusing on mycoprotein. Front. Sust. Food Syst. 2021, 5, 18. [Google Scholar] [CrossRef]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Pamela, M.; Altero, A.; Laura, P. Nutritional value of mushrooms widely consumed in Italy. Food Chem. 2001, 73, 321–325. [Google Scholar] [CrossRef]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.-M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- Cheung, P. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Aydoğdu, M.; Gölükçü, M. Nutritional value of huitlacoche, maize mushroom caused by Ustilago maydis. Food Sci. Technol. 2017, 37, 531–535. [Google Scholar] [CrossRef][Green Version]
- Moore, D.; Robson, G.D.; Trinci, A.P. 21st Century Guidebook to Fungi; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Souza Filho, P.F.; Andersson, D.; Ferreira, J.A.; Taherzadeh, M.J. Mycoprotein: Environmental impact and health aspects. World J. Microbiol. Biotechnol. 2019, 35, 147. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, C.; Mahboubi, A.; Atmowidjojo, A.; Ramadhani, A.; Wainaina, S.; Millati, R.; Wikandari, R.; Niklasson, C.; Taherzadeh, M.J. Cultivation of edible filamentous fungus Aspergillus oryzae on volatile fatty acids derived from anaerobic digestion of food waste and cow manure. Biores. Technol. 2021, 337, 125410. [Google Scholar] [CrossRef]
- Wiebe, M.G. QuornTM myco-protein—Overview of a successful fungal product. Mycologist 2004, 18, 17–20. [Google Scholar] [CrossRef]
- Finnigan, T.; Needham, L.; Abbott, C. Mycoprotein: A healthy new protein with a low environmental impact. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 305–325. [Google Scholar] [CrossRef]
- Bottin, J.H.; Swann, J.R.; Cropp, E.; Chambers, E.S.; Ford, H.E.; Ghatei, M.A.; Frost, G.S. Mycoprotein reduces energy intake and postprandial insulin release without altering glucagon-like peptide-1 and peptide tyrosine-tyrosine concentrations in healthy overweight and obese adults: A randomised-controlled trial. Brit. J. Nutr. 2016, 116, 360–374. [Google Scholar] [CrossRef]
- Nout, M.J.R.; Aidoo, K.E. Asian fungal fermented food. In Industrial Applications; Hofrichter, M., Ed.; Springer: Berlin/Heidelberg, Berlin, 2011; pp. 29–58. [Google Scholar] [CrossRef]
- Moore, D.; Chiu, S.W. Fungal products as food. In Bio-Exploitation of Filamentous Fungi. Fungal Diversity Research Series; Pointing, S.B., Hyde, K.D., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 223–251. [Google Scholar]
- Sastraatmadja, D.D.; Tomita, F.; Kasai, T. Production of high-quality oncom, a traditional Indonesian fermented food, by the inoculation with selected mold strains in the form of pure culture and solid inoculum. J. Grad. Sch. Agric. Hokkaido Univ. 2002, 70, 111–127. [Google Scholar]
- Stodolak, B.; Starzyńska-Janiszewska, A.; Bączkowicz, M. Aspergillus oryzae (koji mold) and Neurospora intermedia (oncom mold) application for flaxseed oil cake processing. LWT 2020, 131, 109651. [Google Scholar] [CrossRef]
- Romulo, A.; Surya, R. Tempe: A traditional fermented food of Indonesia and its health benefits. Int. J. Gastron. Food Sci. 2021, 26, 100413. [Google Scholar] [CrossRef]
- Tsukahara, M.; Shinzato, N.; Tamaki, Y.; Namihira, T.; Matsui, T. Red yeast rice fermentation by selected Monascus sp. with deep-red color, lovastatin production but no citrinin, and effect of temperature-shift cultivation on lovastatin production. Appl. Biochem. Biotechnol. 2009, 158, 476–482. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Nair, R.B.; Andersson, D.; Lennartsson, P.R.; Taherzadeh, M.J. Vegan-mycoprotein concentrate from pea-processing industry byproduct using edible filamentous fungi. Fungal Biol. Biotechnol. 2018, 5, 5. [Google Scholar] [CrossRef]
- Valverde, M.E.; Paredes-López, O.; Pataky, J.K.; Guevara-Lara, F.; Pineda, T. Huitlacoche (Ustilago maydis) as a food source—Biology, composition, and production. Crit. Rev. Food Sci. Nutr. 1995, 35, 191–229. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.A.; Geiselman, P.J.; Lovejoy, J.; Greenway, F.; Volaufova, J.; Martin, C.K.; Arnett, C.; Ortego, L. Effects of consuming mycoprotein, tofu or chicken upon subsequent eating behaviour, hunger and safety. Appetite 2006, 46, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, F.; de Oliveira Santana, W.; Fontana, R.C.; Camassola, M. Use of Pleurotus albidus mycoprotein flour to produce cookies: Evaluation of nutritional enrichment and biological activity. Innov. Food Sci. Emerg. Technol. 2021, 68, 102642. [Google Scholar] [CrossRef]
- Wiebe, M. Myco-protein from Fusarium venenatum: A well-established product for human consumption. Appl. Microbiol. Biotechnol. 2002, 58, 421–427. [Google Scholar] [CrossRef]
- Hellwig, C.; Gmoser, R.; Lundin, M.; Taherzadeh, M.J.; Rousta, K. Fungi burger from stale bread? a case study on perceptions of a novel protein-rich food product made from an edible fungus. Foods 2020, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Gultom, S.O.; Hu, B. Review of microalgae harvesting via co-pelletization with filamentous fungus. Energies 2013, 6, 5921–5939. [Google Scholar] [CrossRef]
- Jareonsin, S.; Pumas, C. Advantages of heterotrophic microalgae as a host for phytochemicals production. Front. Bioeng. Biotechnol. 2021, 9, 628597. [Google Scholar] [CrossRef]
- Schüler, L.; de Morais, E.G.; Trovão, M.; Machado, A.; Carvalho, B.; Carneiro, M.; Maia, I.; Soares, M.; Duarte, P.; Barros, A.; et al. Isolation and characterization of novel Chlorella vulgaris mutants with low chlorophyll and improved protein contents for food applications. Front. Bioeng. Biotechnol. 2020, 8, 469. [Google Scholar] [CrossRef]
- Xie, T.; Xia, Y.; Zeng, Y.; Li, X.; Zhang, Y. Nitrate concentration-shift cultivation to enhance protein content of heterotrophic microalga Chlorella vulgaris: Over-compensation strategy. Biores. Technol. 2017, 233, 247–255. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Masui, H.; Tanaka, H. Sequential heterotrophic/autotrophic cultivation—An efficient method of producing Chlorella biomass for health food and animal feed. J. Appl. Phycol. 1997, 9, 359–366. [Google Scholar] [CrossRef]
- Da Silva, S.P.; Ferreira do Valle, A.; Perrone, D. Microencapsulated Spirulina maxima biomass as an ingredient for the production of nutritionally enriched and sensorially well-accepted vegan biscuits. LWT 2021, 142, 110997. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Menegol, T.; Diprat, A.B.; Rodrigues, E.; Rech, R. Effect of temperature and nitrogen concentration on biomass composition of Heterochlorella luteoviridis. Food Sci. Technol. 2017, 37, 28–37. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Fu, J.; Xiong, J.; Zhang, C. Production of fucoxanthin, chrysolaminarin, and eicosapentaenoic acid by Odontella aurita under different nitrogen supply regimes. J. Biosci. Bioeng. 2018, 126, 723–729. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Pereira, H.; Silva, J.; Santos, T.; Gangadhar, K.N.; Raposo, A.; Nunes, C.; Coimbra, M.A.; Gouveia, L.; Barreira, L.; Varela, J. Nutritional potential and toxicological evaluation of Tetraselmis sp. CTP4 microalgal biomass produced in industrial photobioreactors. Molecules 2019, 24, 3192. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Z.; Wei, D.; Chen, W.; Xie, J. Mixotrophic Chlorella pyrenoidosa as cell factory for ultrahigh-efficient removal of ammonium from catalyzer wastewater with valuable algal biomass coproduction through short-time acclimation. Bioresour. Technol. 2021, 333, 125151. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Joint FAO/WHO. Protein quality evaluation. FAO Food Nutr. Pap. 1991, 51, 1–66. [Google Scholar]
- Caporgno, M.P.; Böcker, L.; Müssner, C.; Stirnemann, E.; Haberkorn, I.; Adelmann, H.; Handschin, S.; Windhab, E.J.; Mathys, A. Extruded meat analogues based on yellow, heterotrophically cultivated Auxenochlorella protothecoides microalgae. Innov. Food Sci. Emerg. Technol. 2020, 59, 102275. [Google Scholar] [CrossRef]
- Ścieszka, S.; Gorzkiewicz, M.; Klewicka, E. Innovative fermented soya drink with the microalgae Chlorella vulgaris and the probiotic strain Levilactobacillus brevis ŁOCK 0944. LWT 2021, 151, 112131. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae biomass as an additional ingredient of gluten-free bread: Dough rheology, texture quality and nutritional properties. Algal Res. 2020, 50, 101998. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Soleimanian-Zad, S.; Alavi, N.; Nazari, E.; Eskandari, M.H. Effect of Spirulina (Arthrospira platensis) powder on probiotic bacteriologically acidified feta-type cheese. J. Appl. Phycol. 2019, 31, 1085–1094. [Google Scholar] [CrossRef]
- Thirumdas, R.; Brnčić, M.; Brnčić, S.R.; Barba, F.J.; Gálvez, F.; Zamuz, S.; Lacomba, R.; Lorenzo, J.M. Evaluating the impact of vegetal and microalgae protein sources on proximate composition, amino acid profile, and physicochemical properties of fermented Spanish “chorizo” sausages. J. Food Process. Preserv. 2018, 42, e13817. [Google Scholar] [CrossRef]
- Grahl, S.; Strack, M.; Mensching, A.; Mörlein, D. Alternative protein sources in Western diets: Food product development and consumer acceptance of spirulina-filled pasta. Food Qual. Prefer. 2020, 84, 103933. [Google Scholar] [CrossRef]
- Janczyk, P.; Wolf, C.; Souffrant, W.B. Evaluation of nutritional value and safety of the green microalgae Chlorella vulgaris treated with novel processing methods. Arch. Zootech. 2005, 8, 132–147. [Google Scholar]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Becher, W. 18 microalgae in human and animal nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley Online Library: Hoboken, NJ, USA, 2004. [Google Scholar]
- Barbosa, M.J.; Zijffers, J.W.; Nisworo, A.; Vaes, W.; van Schoonhoven, J.; Wijffels, R.H. Optimization of biomass, vitamins, and carotenoid yield on light energy in a flat-panel reactor using the A-stat technique. Biotechnol. Bioeng. 2005, 89, 233–242. [Google Scholar] [CrossRef]
- Barka, A.; Blecker, C. Microalgae as a potential source of single-cell proteins. A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 427–436. [Google Scholar] [CrossRef]
- Ljubic, A.; Thulesen, E.T.; Jacobsen, C.; Jakobsen, J. UVB exposure stimulates production of vitamin D3 in selected microalgae. Algal Res. 2021, 59, 102472. [Google Scholar] [CrossRef]
- Edelmann, M.; Aalto, S.; Chamlagain, B.; Kariluoto, S.; Piironen, V. Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J. Food Comp. Anal. 2019, 82, 103226. [Google Scholar] [CrossRef]
- Santhakumaran, P.; Ayyappan, S.M.; Ray, J.G. Nutraceutical applications of twenty-five species of rapid-growing green-microalgae as indicated by their antibacterial, antioxidant and mineral content. Algal Res. 2020, 47, 101878. [Google Scholar] [CrossRef]
- Batista, S.; Pintado, M.; Marques, A.; Abreu, H.; Silva, J.L.; Jessen, F.; Tulli, F.; Valente, L.M. Use of technological processing of seaweed and microalgae as strategy to improve their apparent digestibility coefficients in European seabass (Dicentrarchus labrax) juveniles. J. Appl. Phycol. 2020, 32, 3429–3446. [Google Scholar] [CrossRef]
- Yim, H.E.; Yoo, K.H.; Seo, W.H.; Won, N.H.; Hong, Y.S.; Lee, J.W. Acute tubulointerstitial nephritis following ingestion of Chlorella tablets. Pediatr. Nephrol. 2017, 22, 887–888. [Google Scholar] [CrossRef]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R. A new paradigm to search for allergenic proteins in novel foods by integrating proteomics analysis and in silico sequence homology prediction: Focus on spirulina and chlorella microalgae. Talanta 2022, 240, 123188. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Bühler, J.M.; Schlangen, M.; Möller, A.C.; Bruins, M.E.; van der Goot, A.J. Starch in plant-based meat replacers: A new approach to using endogenous starch from cereals and legumes. Starch/Staerke 2022, 74, 2100157. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Lijiao, K.; Shaoping, N.; Jielun, H.; Sunan, W.; Zhouya, B.; Junqiao, W.; Yaomin, Z.; Jun, J.; Qin, Z.; Ke, S. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Patil, G.; Mian, R.; Vuong, T.; Pantalone, V.; Song, Q.; Chen, P.; Shannon, G.J.; Carter, T.C.; Nguyen, H.T. Molecular mapping and genomics of soybean seed protein: A review and perspective for the future. Theor. Appl. Genet. 2017, 130, 1975–1991. [Google Scholar] [CrossRef] [PubMed]
- Hajos, G.; Gelencsér, E.; Grant, G.; Bardocz, S.; Sakhri, M.; Duguid, T.J.; Newman, A.M.; Pusztai, A. Effect of proteolytic modification and methionine enrichment on the nutritional value of soya albumins for rats. J. Nutr. Biochem. 1996, 7, 481–487. [Google Scholar] [CrossRef]
- Ajani, E.K.; Orisasona, O.; Omitoyin, B.O.; Osho, E.F. Total replacement of fishmeal by soybean meal with or without methionine fortification in the diets of Nile tilapia, Oreochromis niloticus. J. Fish. Aquat. Sci. 2016, 11, 238–243. [Google Scholar] [CrossRef][Green Version]
- De Oliveira, J.E.D.; De Souza, N.; Jordão, A.A., Jr.; Marchin, J.S. Methionine supplementation of soya products: Effects on nitrogen balance parameters. Arch. Latinoam. Nutr. 1998, 48, 35–40. [Google Scholar]
- Mota, C.; Santos, M.; Mauro, R.; Samman, N.; Matos, A.S.; Torres, D.; Castanheira, I. Protein content and amino acids profile of pseudocereals. Food Chem. 2016, 193, 55–61. [Google Scholar] [CrossRef]
- De Almeida Costa, G.E.; da Silva Queiroz-Monici, K.; Reis, S.M.P.M.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Cavazos, A.; Gonzalez de Mejia, E. Identification of bioactive peptides from cereal storage proteins and their potential role in prevention of chronic diseases. Compr. Rev. Food Sci. 2013, 12, 364–380. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef]
- Brückner-Gühmann, M.; Banovic, M.; Drusch, S. Towards an increased plant protein intake: Rheological properties, sensory perception and consumer acceptability of lactic acid fermented, oat-based gels. Food Hydrocol. 2019, 96, 201–208. [Google Scholar] [CrossRef]
- Ames, N.; Storsley, J.; Thandapilly, S.J. Functionality of beta-glucan from oat and barley and its relation with human health. In Cereal Grain-Based Functional Foods: Carbohydrate and Phytochemical Components; The Royal Society of Chemistry: London, UK, 2019; pp. 147–166. [Google Scholar] [CrossRef]
- Sterna, V.; Zute, S.; Brunava, L. Oat grain composition and its nutrition benefice. Agric. Agric. Sci. Procedia 2016, 8, 252–256. [Google Scholar] [CrossRef]
- Torbica, A.; Belović, M.; Popović, L.; Čakarević, J.; Jovičić, M.; Pavličević, J. Comparative study of nutritional and technological quality aspects of minor cereals. J. Food Sci. Technol. 2021, 58, 311–322. [Google Scholar] [CrossRef]
- Mensa-Wilmot, Y.; Phillips, R.; Lee, J.; Eitenmiller, R. Formulation and evaluation of cereal/legume-based weaning food supplements. Plant Foods Hum. Nutr. 2003, 58, 1–14. [Google Scholar] [CrossRef]
- Patel, H.; Chandra, S.; Alexander, S.; Soble, J.; Williams, K.A. Plant-based nutrition: An essential component of cardiovascular disease prevention and management. Cur. Cardiol. Rep. 2017, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Andrade, C.; Olías, R.; Jiménez-López, J.C.; Clemente, A. Aspectos de las legumbres nutricionales y beneficiosos para la salud humana. Arbor 2016, 192, a313. [Google Scholar]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Chardigny, J.-M.; Walrand, S. Plant protein for food: Opportunities and bottlenecks. OCL Oilseeds Fats Crops Lipids 2016, 23, 6. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Rothenberg, M.E.; Brandt, E.B. Molecular mechanisms in allergy and clinical immunology. J. Allergy Clin. Immunol. 2005, 34, 411–420. [Google Scholar]
- Jnawali, P.; Kumar, V.; Tanwar, B. Celiac disease: Overview and considerations for development of gluten-free foods. Food Sci. Hum. Wellness 2016, 5, 169–176. [Google Scholar] [CrossRef]
- Parca, F.; Koca, Y.O.; Aydın, U. Nutritional and antinutritional factors of some pulses seed and their effects on human health. Int. J. Sec. Metabol. 2018, 5, 331–342. [Google Scholar] [CrossRef]
- Robinson, G.; Balk, J.; Domoney, C. Improving pulse crops as a source of protein, starch and micronutrients. Nutr. Bull. 2019, 44, 202–215. [Google Scholar] [CrossRef] [PubMed]
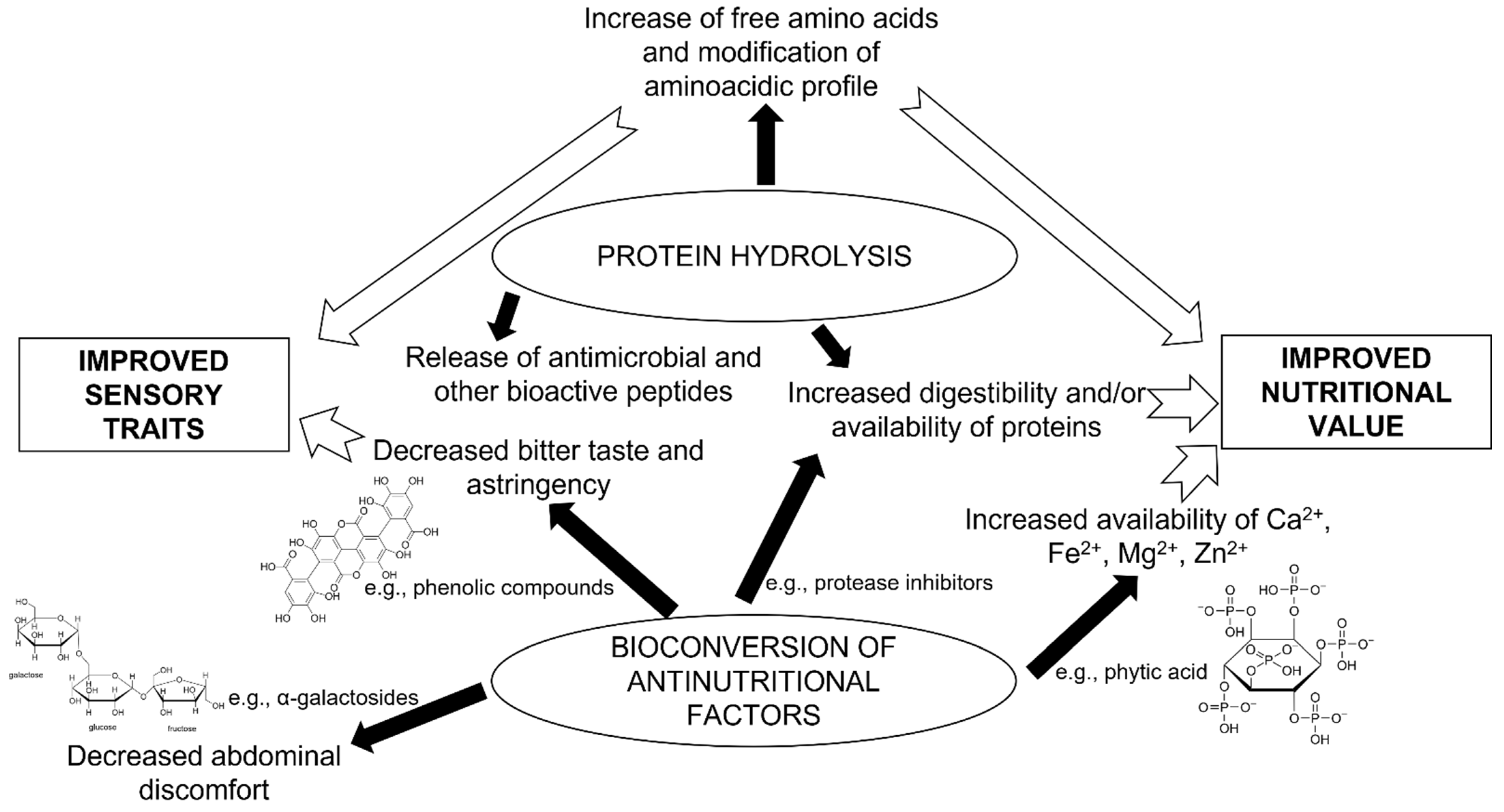
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological factors affecting biogenic amine content in foods: A review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef]
- Licandro, H.; Ho, P.H.; Nguyen, T.K.C.; Petchkongkaew, A.; Van Nguyen, H.; Chu-Ky, S.; Nguyen, T.V.A.; Lorn, D.; Waché, Y. How fermentation by lactic acid bacteria can address safety issues in legumes food products? Food Control 2020, 110, 106957. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Jambunathan, R.; Hall, S.; Sudhir, P.; Rajan, V.; Sadhana, V. Uses of Tropical Grain Legumes. In Proceedings of the Consultants Meeting, Patancheru, India, 27–30 March 1989. [Google Scholar]
- González-Montemayor, A.M.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C.; López-Badillo, C.M.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R. Green bean, pea and mesquite whole pod flours nutritional and functional properties and their effect on sourdough bread. Foods 2021, 10, 2227. [Google Scholar] [CrossRef]
- Wu, H.; Rui, X.; Li, W.; Xiao, Y.; Zhou, J.; Dong, M. Whole-grain oats (Avena sativa L.) as a carrier of lactic acid bacteria and a supplement rich in angiotensin I-converting enzyme inhibitory peptides through solid-state fermentation. Food Funct. 2018, 9, 2270–2281. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.; Surasani, V.K.R. Quinoa protein isolate supplemented pasta: Nutritional, physical, textural and morphological characterization. LWT 2021, 135, 110045. [Google Scholar] [CrossRef]
- Molfetta, M.; Celano, G.; Minervini, F. Functional, nutritional, and sensory quality of mixed flours-based breads as compared to durum wheat semolina-based breads. Foods 2021, 10, 1613. [Google Scholar] [CrossRef]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT 2017, 82, 296–302. [Google Scholar] [CrossRef]
- Bravo-Núñez, Á.; Gómez, M. Enrichment of cakes and cookies with pulse flours. A review. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Coda, R.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G. Sourdough lactic acid bacteria: Exploration of non-wheat cereal-based fermentation. Food Microbiol. 2014, 37, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2018, 302, 47–58. [Google Scholar] [CrossRef]
- Hou, J.W.; Yu, R.C.; Chou, C.C. Changes in some components of soymilk during fermentation with bifidobacteria. Food Res. Int. 2000, 33, 393–397. [Google Scholar] [CrossRef]
- Song, Y.S.; Frias, J.; Martinez-Villaluenga, C.; Vidal-Valdeverde, C.; Gonzalez de Mejia, E. Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem. 2008, 108, 571–581. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Wang, N.; Shi, Y.; Sun, J.; Le, G. Evaluation of peanut flour fermented with lactic acid bacteria as a probiotic food. Food Sci. Technol. Int. 2007, 13, 469–475. [Google Scholar] [CrossRef]
- Pontonio, E.; Raho, S.; Dingeo, C.; Centrone, D.; Carofiglio, V.E.; Rizzello, C.G. Nutritional, functional, and technological characterization of a novel gluten-and lactose-free yogurt-style snack produced with selected lactic acid bacteria and Leguminosae flours. Front. Microbiol. 2020, 11, 1664. [Google Scholar] [CrossRef]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.; Magalhães Júnior, A.I.; Soccol, C.R. A review of selection criteria for starter culture development in the food fermentation industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Pontonio, E.; Dingeo, C.; Di Cagno, R.; Blandino, M.; Gobbetti, M.; Rizzello, C.G. Brans from hull-less barley, emmer and pigmented wheat varieties: From by-products to bread nutritional improvers using selected lactic acid bacteria and xylanase. Int. J. Food Microbiol. 2020, 313, 108384. [Google Scholar] [CrossRef] [PubMed]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant-versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino acid availability of a dairy and vegetable protein blend compared to single casein, whey, soy, and pea proteins: A double-blind, cross-over trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Tamime, A.; O’connor, T. Kishk—A dried fermented milk/cereal mixture. Int. Dairy J. 1995, 5, 109–128. [Google Scholar] [CrossRef]
- Mazzoli, R.; Riedel, K.; Pessione, E. Bioactive compounds from microbes. Front. Microbiol. 2017, 8, 392. [Google Scholar] [CrossRef]
- Tamime, A.; Muir, D.; Barclay, M.; Khaskheli, M.; McNulty, D. Laboratory-made Kishk from wheat, oat and barley: 2. Compositional quality and sensory properties. Food Res. Int. 1997, 30, 319–326. [Google Scholar] [CrossRef]
- Tamime, A.; Muir, D.; Khaskheli, M.; Barclay, M. Effect of processing conditions and raw materials on the properties of Kishk 1. Compositional and microbiological qualities. LWT 2000, 33, 444–451. [Google Scholar] [CrossRef]
- Daglioǧlu, O. Tarhana as a traditional Turkish fermented cereal food. Its recipe, production and composition. Food/Nahrung 2000, 44, 85–88. [Google Scholar] [CrossRef]
- Kivanc, M.; Funda, E.G. A functional food: A traditional Tarhana fermentation. Food Sci. Technol. 2017, 37, 269–274. [Google Scholar] [CrossRef]
- Lazos, E.S.; Aggelousis, G.; Bratakos, M. The fermentation of trahanas: A milk-wheat flour combination. Plant Foods Hum. Nut. 1993, 44, 45–62. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Nielsen, D.S.; Karapinar, M.; Jakobsen, M. Identification of lactic acid bacteria isolated from Tarhana, a traditional Turkish fermented food. Int. J. Food Microbiol. 2009, 135, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, Ö.; Özel, S.; Çon, A.H. Comparison of lactic acid bacteria diversity during the fermentation of Tarhana produced at home and on a commercial scale. Food Sci. Biotechnol. 2017, 26, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Soyuçok, A.; Zafer Yurt, M.N.; Altunbas, O.; Ozalp, V.C.; Sudagidan, M. Metagenomic and chemical analysis of Tarhana during traditional fermentation process. Food Biosci. 2021, 39, 100824. [Google Scholar] [CrossRef]
- Özel, S.; Sabanoğlu, S.; Çon, A.H.; Şimşek, Ö. Diversity and stability of yeast species during the fermentation of Tarhana. Food Biotechnol. 2015, 29, 117–129. [Google Scholar] [CrossRef]
- Bellici, A.E.; Karasu-Yalcin, S.; Eryasar-Orer, K.; Yalcin, E. MALDI-TOF/TOF mass spectrometry for determination of yeast diversity in traditional cornelian cherry tarhana produced with different cereal/pseudocereal flours. Ann. Microbiol. 2019, 69, 613–625. [Google Scholar] [CrossRef]
- Bilgiçli, N. Effect of buckwheat flour on chemical and functional properties of tarhana. LWT 2009, 42, 514–518. [Google Scholar] [CrossRef]
- Toufeili, I.; Melki, C.; Shadarevian, S.; Robinson, R.K. Some nutritional and sensory properties of bulgur and whole wheatmeal kishk (a fermented milk-wheat mixture). Food Qual. Pref. 1998, 10, 9–15. [Google Scholar] [CrossRef]
- Aktaş, K.; Akın, N. Influence of rice bran and corn bran addition on the selected properties of tarhana, a fermented cereal based food product. LWT 2020, 129, 109574. [Google Scholar] [CrossRef]
- Bilgiçli, N.; Elgün, A.; Türker, S. Effects of various phytase sources on phytic acid content, mineral extractability and protein digestibility of tarhana. Food Chem. 2006, 98, 329–337. [Google Scholar] [CrossRef]
- Chen, X.; Singh, M.; Bhargava, K.; Ramanathan, R. Yogurt fortification with chickpea (Cicer arietinum) flour: Physicochemical and sensory effects. J. Am. Oil Chem. Soc. 2018, 95, 1041–1048. [Google Scholar] [CrossRef]
- Dabija, A.; Codină, G.G.; Gâtlan, A.-M.; Sănduleac, E.T.; Rusu, L. Effects of some vegetable proteins addition on yogurt quality. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 181–192. [Google Scholar]
- Yousseef, M.; Lafarge, C.; Valentin, D.; Lubbers, S.; Husson, F. Fermentation of cow milk and/or pea milk mixtures by different starter cultures: Physico-chemical and sensorial properties. LWT 2016, 69, 430–437. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Saint-Eve, A.; Panouillé, M.; Souchon, I.; Bonnarme, P.; Dugat-Bony, E.; Irlinger, F. Design of microbial consortia for the fermentation of pea-protein-enriched emulsions. Int. J. Food Microbiol. 2019, 293, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, N.; Yazıcı, G.; Şimşek, Ö.; Özkal, S.G.; Con, A.H. The effect of lactic acid bacteria and yeast usage on aroma development during tarhana fermentation. Food Biosci. 2018, 26, 30–37. [Google Scholar] [CrossRef]
- Canon, F.; Mariadassou, M.; Maillard, M.-B.; Falentin, H.; Parayre, S.; Madec, M.-N.; Valence-Bertel, F.; Henry, G.; Laroute, V.; Daveran-Mingot, M.-L. Function-driven design of lactic acid bacteria co-cultures to produce new fermented food associating milk and lupin. Front. Microbiol. 2020, 11, 2850. [Google Scholar] [CrossRef]
- Akin, Z.; Ozcan, T. Functional properties of fermented milk produced with plant proteins. LWT 2017, 86, 25–30. [Google Scholar] [CrossRef]
- Goencue, A.; Celik, I. Investigation of some properties of gluten-free tarhanas produced by red, green and yellow lentil whole flour. Food Sci. Technol. 2020, 40, 574–581. [Google Scholar] [CrossRef]
- Atasoy, R.; Hendek Ertop, M. Assessment of nutritional and bioactive properties for gluten-free tarhana containing various legumes and cereals. J. Food Process Preserv. 2021, 45, e15606. [Google Scholar] [CrossRef]
- Bodenheimer, F.S. Insects as human food. In Insects as Human Food; Springer: Berlin/Heidelberg, Germany, 1951; pp. 7–38. [Google Scholar]
- Lanfranchi, G.B. Minilivestock consumption in the Ancient Near East: The case of locusts. In Ecological Implications of Minilivestock: Potential of Insects, Rodents, Frogs And Snails; Paoletti, M., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 163–174. ISBN 978-1-57808-339-8. [Google Scholar]
- Pechal, J.L.; Benbow, M.E.; Kamng’ona, A.W.; Safalaoh, A.; Masamba, K.; Kang’ombe, J. The need for alternative insect protein in Africa. Ann. Entomol. Soc. Am. 2019, 112, 566–575. [Google Scholar] [CrossRef]
- Patel, S.; Suleria, H.A.R.; Rauf, A. Edible insects as innovative foods: Nutritional and functional assessments. Trends Food Sci. Technol. 2019, 86, 352–359. [Google Scholar] [CrossRef]
- Vantomme, P. Farming insects as a viable and global source of animal proteins. Atti Accademia Nazionale Italiana Entomologia 2015, 63, 57–63. [Google Scholar]
- Van Huis, A. Edible insects contributing to food security? Agric. Food Secur. 2015, 4, 20. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; Van Loon, J.J. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Köhler, R.; Irias-Mata, A.; Ramandey, E.; Purwestri, R.; Biesalski, H.K. Nutrient composition of the Indonesian sago grub (Rhynchophorus bilineatus). Int. J. Trop. Insect Sci. 2020, 40, 677–686. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Musundire, R.; Zvidzai, C.; Chidewe, C.; Ngadze, R.; Macheka, L.; Manditsera, F.; Mubaiwa, J.; Masheka, A. Nutritional and bioactive compounds composition of Eulepida mashona, an edible beetle in Zimbabwe. J. Insects Food Feed 2016, 2, 179–187. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Mai, C. Forest Insects as Food: Humans Bite Back. In Proceedings of the Workshop on Asia-Pacific Resources and Their Potential for Development, Chiang Mai, Thailand, 19–21 February 2008; FAO, Ed.; RAP Publication Food and Agriculture Organization of the United Nations, Regional Office for Asia and the Pacific: Bangkok, Thailand, 2010. [Google Scholar]
- Aochen, C.; Krishnappa, R.; Firake, D.; Pyngrope, S.; Aochen, S.; Ningombam, A.; Behere, G.; Ngachan, S. Loungu (Carpenter worm): Indigenous delicious insects with immense dietary potential in Nagaland state, India. Indian J. Tradit. Knowl. 2020, 19, 145–151. [Google Scholar]
- Tomotake, H.; Katagiri, M.; Yamato, M. Silkworm pupae (Bombyx mori) are new sources of high quality protein and lipid. J. Nutr. Sci. Vitaminol. 2010, 56, 446–448. [Google Scholar] [CrossRef]
- Paul, D.; Dey, S. Essential amino acids, lipid profile and fat-soluble vitamins of the edible silkworm Bombyx mori (Lepidoptera: Bombycidae). Int. J. Trop. Insect Sci. 2014, 34, 239–247. [Google Scholar] [CrossRef]
- Omotoso, O.T. An evaluation of the nutrients and some anti-nutrients in silkworm, Bombyx mori L. (Bombycidae: Lepidoptera). Jordan J. Biol. Sci. 2015, 8, 45–50. [Google Scholar] [CrossRef]
- Finke, M.D. Nutrient composition of bee brood and its potential as human food. Ecol. Food Nutr. 2005, 44, 257–270. [Google Scholar] [CrossRef]
- Borremans, A.; Bußler, S.; Sagu, S.T.; Rawel, H.; Schlüter, O.K.; Leen, V.C. Effect of blanching plus fermentation on selected functional properties of mealworm (Tenebrio molitor) powders. Foods 2020, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- Homann, A.; Ayieko, M.A.; Konyole, S.; Roos, N. Acceptability of biscuits containing 10% cricket (Acheta domesticus) compared to milk biscuits among 5-10-year-old Kenyan schoolchildren. J. Insects Food Feed 2017, 3, 95–103. [Google Scholar] [CrossRef]
- Akande, A.O.; Jolayemi, O.S.; Adelugba, V.A.; Akande, S.T. Silkworm pupae (Bombyx mori) and locusts as alternative protein sources for high-energy biscuits. J. Asia-Pac. Entomol. 2020, 23, 234–241. [Google Scholar] [CrossRef]
- Biró, B.; Sipos, M.A.; Kovács, A.; Badak-Kerti, K.; Pásztor-Huszár, K.; Gere, A. Cricket-enriched oat biscuit: Technological analysis and sensory evaluation. Foods 2020, 9, 1561. [Google Scholar] [CrossRef]
- Ramírez-Rivera, E.J.; Hernández-Santos, B.; Juárez-Barrientos, J.M.; Torruco-Uco, J.G.; Ramírez-Figueroa, E.; Rodríguez-Miranda, J. Effects of formulation and process conditions on chemical composition, color parameters, and acceptability of extruded insect-rich snack. J. Food Proc. Preserv. 2021, 45, e15499. [Google Scholar] [CrossRef]
- Angaman, D.M.; Ehouman, A.G.; Boko, A.C.E. Propriétés physico-chimiques, fonctionnelles et microbiologiques de la farine de maïs germé enrichie de larves d’insectes comestibles Rhynchophorus phoenicis et Oryctes owariensis. J. Appl. Biosci. 2021, 158, 16310–16320. [Google Scholar] [CrossRef]
- Da Rosa Machado, C.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Nutritional, sensory, and texture quality of bread and cookie enriched with house cricket (Acheta domesticus) powder. J. Food Process. Preserv. 2020, 44, e14601. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Kinyuru, J.; Kenji, G.; Njoroge, M. Process development, nutrition and sensory qualities of wheat buns enriched with edible termites (Macrotermes subhylanus) from Lake Victoria region, Kenya. Afr. J. Food Agric. Nutr. Dev. 2009, 9, 1739–1750. [Google Scholar] [CrossRef]
- Idolo, I. Nutritional and quality attributes of wheat buns enriched with the larvae of Rhynchophorus phoenicis F. Pak. J. Nutr. 2010, 9, 1043–1046. [Google Scholar] [CrossRef]
- Park, Y.-S.; Choi, Y.-S.; Hwang, K.-E.; Kim, T.-K.; Lee, C.-W.; Shin, D.-M.; Han, S.G. Physicochemical properties of meat batter added with edible silkworm pupae (Bombyx mori) and transglutaminase. Korean J. Food Sci. Anim. Resour. 2017, 37, 351. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Duda, A.; Adamczak, J.; Chełmińska, P.; Juszkiewicz, J.; Kowalczewski, P. Quality and nutritional/textural properties of durum wheat pasta enriched with cricket powder. Foods 2019, 8, 46. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jedras, M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 2013, 4, 287–291. [Google Scholar] [CrossRef]
- Ndiritu, A.K.; Kinyuru, J.N.; Kenji, G.M.; Gichuhi, P.N. Extraction technique influences the physico-chemical characteristics and functional properties of edible crickets (Acheta domesticus) protein concentrate. J. Food Meas. Charact. 2017, 11, 2013–2021. [Google Scholar] [CrossRef]
- Purschke, B.; Tanzmeister, H.; Meinlschmidt, P.; Baumgartner, S.; Lauter, K.; Jäger, H. Recovery of soluble proteins from migratory locust (Locusta migratoria) and characterisation of their compositional and techno-functional properties. Food Res. Int. 2018, 106, 271–279. [Google Scholar] [CrossRef]
- Stull, V.J.; Kersten, M.; Bergmans, R.S.; Patz, J.A.; Paskewitz, S. Crude protein, amino acid, and iron content of Tenebrio molitor (Coleoptera, Tenebrionidae) reared on an agricultural byproduct from maize production: An exploratory study. Ann. Entomol. Soc. Am. 2019, 112, 533–543. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effect of diet on the growth performance, feed conversion, and nutrient content of the house cricket. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Mlček, J.; Adámková, A.; Adámek, M.; Borkovcová, M.; Bednářová, M.; Kouřimská, L. Selected nutritional values of field cricket (Gryllus assimilis) and its possible use as a human food. Indian J. Tradit. Knowl. 2018, 17, 518–524. [Google Scholar]
- Lucchese-Cheung, T.; de Aguiar, L.; Spers, E.; De Lima, L. The Brazilians’ sensorial perceptions for novel food–cookies with insect protein. J. Insects Food Feed 2021, 7, 287–299. [Google Scholar] [CrossRef]
- Luna, G.C.; Martin-Gonzalez, F.S.; Mauer, L.; Liceaga, A. Cricket (Acheta domesticus) protein hydrolysates’ impact on the physicochemical, structural and sensory properties of tortillas and tortilla chips. J. Insects Food Feed 2021, 7, 109–120. [Google Scholar] [CrossRef]
- Akande, O.A.; Falade, O.O.; Badejo, A.A.; Adekoya, I. Assessment of mulberry silkworm pupae and African palm weevil larvae as alternative protein sources in snack fillings. Heliyon 2020, 6, e03754. [Google Scholar] [CrossRef] [PubMed]
- Awobusuyi, T.D.; Pillay, K.; Siwela, M. Consumer acceptance of biscuits supplemented with a sorghum–insect meal. Nutrients 2020, 12, 895. [Google Scholar] [CrossRef]
- Adámek, M.; Adámková, A.; Mlček, J.; Borkovcová, M.; Bednářová, M. Acceptability and sensory evaluation of energy bars and protein bars enriched with edible insect. Potravin. Slovak J. Food Sci. 2018, 12, 431–437. [Google Scholar] [CrossRef]
- Tao, J.; Davidov-Pardo, G.; Burns-Whitmore, B.; Cullen, E.; Li, Y. Effects of edible insect ingredients on the physicochemical and sensory properties of extruded rice products. J. Insects Food Feed 2017, 3, 263–278. [Google Scholar] [CrossRef]
- Akullo, J.; Agea, J.G.; Obaa, B.B.; Acai, J.O.; Nakimbugwe, D. Process development, sensory and nutritional evaluation of honey spread enriched with edible insects flour. Afr. J. Food Sci. 2017, 11, 30–39. [Google Scholar] [CrossRef]
- Ebenebe, C.; Okpoko, V. Preliminary studies on alternative substrate for multiplication of African palm weevil under captive management. J. Insects Food Feed 2016, 2, 171–177. [Google Scholar] [CrossRef]
- Raksasat, R.; Lim, J.W.; Kiatkittipong, W.; Kiatkittipong, K.; Ho, Y.C.; Lam, M.K.; Font-Palma, C.; Zaid, H.F.M.; Cheng, C.K. A review of organic waste enrichment for inducing palatability of black soldier fly larvae: Wastes to valuable resources. Environ. Pol. 2020, 267, 115488. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Bonacci, T.; Reguzzoni, M.; Casartelli, M.; Grimaldi, A.; Tettamanti, G.; Brandmayr, P. An in-depth description of head morphology and mouthparts in larvae of the black soldier fly Hermetia illucens. Arthropod Struct. Dev. 2020, 58, 100969. [Google Scholar] [CrossRef] [PubMed]
- Castro-López, C.; Santiago-López, L.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Liceaga, A.M.; García, H.S.; Hernández-Mendoza, A. An insight to fermented edible insects: A global perspective and prospective. Food Res. Int. 2020, 137, 109750. [Google Scholar] [CrossRef]
- Kuttiyatveetil, J.R.; Mitra, P.; Goldin, D.; Nickerson, M.T.; Tanaka, T. Recovery of residual nutrients from agri-food byproducts using a combination of solid-state fermentation and insect rearing. Int. J. Food Sci. Technol. 2019, 54, 1130–1140. [Google Scholar] [CrossRef]
- Liu, H.; Tan, B.; Kong, X.; Li, J.; Li, G.; He, L.; Bai, M.; Yin, Y. Dietary insect powder protein sources improve protein utilization by regulation on intestinal amino acid-chemosensing system. Animals 2020, 10, 1590. [Google Scholar] [CrossRef]
- Young, W.; Arojju, S.K.; McNeill, M.R.; Rettedal, E.; Gathercole, J.; Bell, N.; Payne, P. Feeding bugs to bugs: Edible insects modify the human gut microbiome in an in vitro fermentation model. Front. Microbiol. 2020, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Pini, N.; Granchi, L. Technological feature assessment of lactic acid bacteria isolated from cricket powder’s spontaneous fermentation as potential starters for cricket-wheat bread production. Foods 2020, 9, 1322. [Google Scholar] [CrossRef]
- De Smet, J.; Lenaerts, S.; Borremans, A.; Scholliers, J.; Van Der Borght, M.; Van Campenhout, L. Stability assessment and laboratory scale fermentation of pastes produced on a pilot scale from mealworms (Tenebrio molitor). LWT 2019, 102, 113–121. [Google Scholar] [CrossRef]
- Klunder, H.; Wolkers-Rooijackers, J.; Korpela, J.M.; Nout, M.R. Microbiological aspects of processing and storage of edible insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Losses, inefficiencies and waste in the global food system. Agric. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat alternatives: Life cycle assessment of most known meat substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Onwezen, M.C.; Bouwman, E.P.; Reinders, M.J.; Dagevos, H. A systematic review on consumer acceptance of alternative proteins: Pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite 2021, 159, 105058. [Google Scholar] [CrossRef] [PubMed]

| Origin | Food | Energy | Proteins a | Carbohydrates a | Lipids a | Saturated Fatty acid a | Fiber a | Vitamins (μg/100 g) | Mineral (mg/100 g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B6 | B9 | B12 | Ca | P | Fe | Mg | Zn | K | ||||||||
| Fungal | Mycoprotein * | 85 | 11 | 3 | 2.9 | 0.7 | 6 | 100 | 114 | 0.72 | 48 | 290 | 0.4 | 49 | 7.6 | 71 |
| Shiitake (cooked) | 55 | 1.6 | 12.3 | 0.2 | 0.1 | N | N | N | N | 3 | 29 | 0.4 | 14 | N | 12 | |
| Vegetable | Tofu, soybean (steamed) | 73 | 8.1 | 0.7 | 4.2 | N | N | 70 | 15 | N | N | 95 | 1.2 | 23 | 0.7 | 63 |
| Chickpea (re-heated) | 129 | 8.4 | 18.3 | 3 | 0.29 | 7.1 | 380 | 35 | N | 48 | 141 | 1.9 | 44 | 1.1 | 281 | |
| Animal | Chicken breast (casseroled) | 160 | 28.4 | N b | 5.2 | 29.6 | 0.9 | 360 | 6 | N | 9 | 210 | 0.5 | 25 | 1.1 | 270 |
| Beef mince (stewed) | 209 | 21.8 | N | 13.5 | 47.5 | N | 170 | 5 | 0.8 | 11 | 93 | 0.83 | 11 | 2.1 | 163 | |
| Species | Substrate | Protein Content |
|---|---|---|
| Aspergillus flavus | Rice bran | 10 |
| Aspergillus niger | Apple pomace | 17–20 |
| Banana wastes | 18 | |
| Rice bran | 11 | |
| Stick water | 49 | |
| Potato starch processing waste | 38 | |
| Waste liquor | 50 | |
| Aspergillus ochraceus | Rice bran | 10 |
| Aspergillus oryzae | Rice bran (deoiled) | 24 |
| Neurospora (Chrysonilia) sitophila | Lignin | 39 |
| Cladosporium cladosporioides | Rice bran | 10 |
| Fusarium semitectum | Rice bran | 10 |
| Monascus ruber | Rice bran | 10 |
| Penicillium citrinum | Rice bran | 10 |
| Pleurotus floridanus | Wheat straw | 63 |
| Trichoderma harzianum | Cheese whey filtrate | 34 |
| Trichoderma viride | Citrus pulp | 32 |
| Species | Proteins | Carbohydrates | Lipids | Ashes | Ref. |
|---|---|---|---|---|---|
| Arthrospira platensis * | 70.9 | 18.8 | 9.6 | – | [83] |
| Spirulina maxima | 80 | 0.6 | 7.6 | 11.6 | [88] |
| Spirulina sp. LEB 18 * | 53.6–62.9 | 5.7–10.2 | 12–11 | 10.2–23.7 | [83] |
| Aphanizomenon flosaquae * | 62 | 23 | 3 | – | [89] |
| Heterochlorella luteoviridis * | 13.8 | 63.1 | 9.9 | – | [90] |
| Chlorella pyrenoidosa * | 31.5 | 12.9 | 30.5 | – | [91] |
| Chlorella vulgaris * | 51.0–58.0 | 12–17 | 14–22 | – | [89] |
| Chlamydomonas reinhardtii * | 48 | 17 | 21 | [89] | |
| Odontella aurita * | 25.0 | 66.1 | 14.5 | – | [92] |
| Tetraselmis chuii * | 35–40 | 30–32 | 5–8 | 14–16 | [93] |
| Tetraselmis CTP4 | 40.5–42.7 | 46.5–41.2 | 4.9–5.6 | 7.5–8.2 | [94] |
| Dunaliella salina | 15.6–23.5 | 6.0–4.8 | 60.8–68.3 | – | [95] |
| Nannochloropisis oculata | 57 | 8 | 32 | – | [89] |
| Tisochrysis lutea | 42.9 | 8.6 | 27.9 | 9.7 | [89] |
| Haematococcus pluvialis | 48 | 27 | 15 | – | [89] |
| Scenedesmus obliquus | 43.1 | 16.4 | 10.7 | 20 | [89] |
| Species | Ile § | Leu | Val | Lys | Phe | Tyr | Met | Cys | Trp | Thr | Ala | Arg | Asp | Glu | Gly | His | Pro | Ser | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arthrospira platensis * | 6.7 | 9.8 | 7.1 | 4.8 | 5.3 | 5.3 | 2.5 | 0.9 | 0.3 | 6.2 | 9.5 | 7.3 | 11.8 | 10.3 | 5.7 | 2.2 | 4.2 | 5.1 | [83] |
| Spirulina maxima | 6.0 | 8.0 | 6.5 | 4.6 | 4.9 | 3.9 | 1.4 | 0.4 | 1.4 | 4.6 | 6.8 | 6.5 | 8.6 | 12.6 | 4.8 | 1.8 | 3.9 | 4.2 | [88] |
| Spirulina sp. LEB 18 * | 4.4 | 8.0 | 4.6 | 2.9 | 5.7 | 3.2 | 1.6 | 0.47 | 2.5 | 4.9 | 6.5 | 4.9 | 9.2 | 10.7 | 5.2 | 2.7 | 4.0 | 4.3 | [83] |
| Aphanizomenon sp | 2.9 | 5.2 | 3.2 | 3.5 | 2.5 | - | 0.7 | 0.2 | 0.7 | 3.3 | 4.7 | 3.8 | 4.7 | 7.8 | 2.9 | 0.9 | 2.9 | 2.9 | [89] |
| Heterochlorella luteoviridis * | 1.8 | 8.1 | 2.9 | 8.7 | 5.4 | 2.7 | 1.8 | 0.4 | 0.6 | 5.2 | 11.1 | 5.6 | 0.3 | 1.3 | 9.6 | 1.8 | 5.5 | 6.8 | [90] |
| Chlorella pyrenoidosa * | 6.2 | 3.4 | 5.2 | 8.1 | 3.8 | 1.2 | 3.3 | 2.8 | n.d. | 3.4 | 5.1 | 5.9 | 8.1 | 7.8 | 9.8 | 1.6 | n.d. | 2.8 | [91] |
| Chlorella vulgaris * | 3.8 | 8.8 | 5.5 | 8.4 | 5.0 | 3.4 | 2.2 | 1.4 | 2.1 | 4.8 | 7.9 | 6.4 | 9.0 | 11.6 | 5.8 | 2.0 | 4.8 | 4.1 | [89] |
| Chlamydomonas reinhardtii * | 1.7 | 6.9 | 3.0 | 8.1 | 5.0 | 3.1 | 2.0 | 0.3 | 0.3 | 4.2 | 10.5 | 9.2 | 0.4 | 0.7 | 8.0 | 2.1 | 4.6 | 6.2 | [89] |
| Tetraselmis chuii * | 3.5 | 7.5 | 4.9 | 5.7 | 4.8 | 3.1 | 2.5 | 2.9 | 2.4 | 4.1 | 6.1 | 9.6 | 14.4 | 12.3 | 6.7 | 1.6 | 3.7 | 4.3 | [93] |
| Tetraselmis CTP4 | 1.1 | 2.2 | 1.5 | 1.7 | 1.4 | 0.8 | 0.6 | 0.3 | 0.4 | 1.3 | 2.0 | 1.7 | 2.9 | 3.6 | 1.6 | 0.1 | 1.3 | 1.2 | [94] |
| Dunaliella salina | 4.0 | 9.6 | 7.2 | 6.0 | 6.9 | 4.9 | 2.8 | 1.6 | 0.2 | 5.2 | 11.0 | 8.2 | 9.6 | 12.4 | 8.7 | 1.7 | 5.2 | 4.8 | [95] |
| Haematococcus pluvialis | 0.5 | 1.2 | 0.6 | 0.7 | 0.6 | 0.4 | 0.1 | – | n.d. | 0.6 | 1.3 | 0.7 | 1.4 | 1.9 | 0.9 | – | – | 0.9 | [89] |
| Scenedesmus obliquus | 3.6 | 7.3 | 6.0 | 5.6 | 4.8 | 3.2 | 1.5 | 0.6 | 0.3 | 5.1 | 9.0 | 7.1 | 8.4 | 10.7 | 7.1 | 2.1 | 3.9 | 3.8 | [89] |
| Soybean | 5.3 | 7.7 | 5.3 | 6.4 | 5.0 | 3.7 | 1.3 | 1.9 | 1.4 | 4.0 | 5.0 | 7.4 | 1.3 | 19.0 | 4.5 | 2.6 | 5.3 | 5.8 | [96] |
| FAO/WHO | 2.8 | 6.6 | 3.5 | 5.8 | – | – | – | – | 1.1 | 3.4 | – | – | – | – | – | – | 1.9 | – | [97] |
| Species | Proteins | Limiting EAA * | Carbohydrates | Lipids | Fiber | Ashes | Ref. |
|---|---|---|---|---|---|---|---|
| Barley | 9.9–11.60 | Met | 77.7 | 1.2–1.9 | 15.2–15.6 | 1.6–2.6 | [116,125,126] |
| Rye | 8.8–11.4 | Cys, Met | 60.7 | 1.7–2.5 | 12.9–13.2 | 2.02 | [125,126,127] |
| Triticale | 12.3 | Met | nr ¥ | 1.74 | 18.1 | 2.33 | [125] |
| Spelt | 14.6 | Lys | 53.9 | 2.4 | 10.7 | nr | [126,128] |
| Maize | 9.4–10.60 | Cys | 74 | 4.7 | 7.3 | nr | [116,126,127] |
| Rice | 7.1–15 | Trp | 80.0 | 0.7–20 | 1.3–11 | 1.35–9.9 | [127] |
| Millet | 9.5–11.7 | Lys | 73 | 4.2 | 1.8–8.5 | 1.17 | [125,126] |
| Sorghum | 10.5–12.6 | Cys, Met | 75 | 2.2–3.3 | 6.3–12.1 | 2.15 | [116,125,127] |
| Oat | 8.8–17 | Trp, Cys | 66.3 | 4.9–6.9 | 11.25–11.6 | nr | [116,125,127] |
| Buckwheat | 12.5–14.8 | Trp | 58.9 | 2.1–3.6 | 8.3–29.5 | 2.1 | [116,127] |
| Amaranth | 14.5–16.5 | Trp | 61.4 | 5.7–10.2 | 8.8–20.6 | 2.5 | [116,126] |
| Quinoa | 13–14.5 | Trp | 64.2 | 5.2–7.2 | 7.2–14.2 | 2.9 | [116,126] |
| Pea | 15.3–21.9 | Trp | 52.5 | 2.34–7.3 | 10.4–30.7 | 2.39–3 | [119] |
| Fava bean | 21.87–31.2 | Met, Cys | nr | 2.1–12.45 | 24.7–31.74 | 3.13–3.4 | [119] |
| Chickpea | 18.5–24.7 | Met, Cys | 54.0 | 1.5–6.7 | 9.88–18.8 | 3.15–3.7 | [116] |
| Lentil | 20.06–25.25 | Trp | 56.4 | 2.15–3.27 | 6.8–33.6 | 2.0–2.8 | [119] |
| Soybean | 34.05–44.53 | Met | nr | 14.13–22.44 | 4.2–32.2 | 3.9–5.05 | [116,119] |
| Lupin | 29.5–48.2 | Lys, Trp, Met | nr | 4.5–10.4 | 11.6–47.5 | 3.5–4.9 | [116,120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Dugat-Bony, E.; Bonnarme, P.; Minervini, F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods 2022, 11, 2065. https://doi.org/10.3390/foods11142065
Molfetta M, Morais EG, Barreira L, Bruno GL, Porcelli F, Dugat-Bony E, Bonnarme P, Minervini F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods. 2022; 11(14):2065. https://doi.org/10.3390/foods11142065
Chicago/Turabian StyleMolfetta, Mariagrazia, Etiele G. Morais, Luisa Barreira, Giovanni Luigi Bruno, Francesco Porcelli, Eric Dugat-Bony, Pascal Bonnarme, and Fabio Minervini. 2022. "Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation" Foods 11, no. 14: 2065. https://doi.org/10.3390/foods11142065
APA StyleMolfetta, M., Morais, E. G., Barreira, L., Bruno, G. L., Porcelli, F., Dugat-Bony, E., Bonnarme, P., & Minervini, F. (2022). Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods, 11(14), 2065. https://doi.org/10.3390/foods11142065