Protein Hydrolysates Derived from Animals and Plants—A Review of Production Methods and Antioxidant Activity
Abstract
1. Introduction
2. The Animal Kingdom
2.1. Methods of Obtaining Protein Hydrolysates of Animal Origin
2.2. Antioxidant Activity of Protein Hydrolysates Derived from Fish
2.3. Antioxidant Potency of Protein Hydrolysates Prepared from Insects
2.4. Antioxidant Capacity of Protein Hydrolysates Obtained from Domestic Fowl
2.5. Antioxidant Potential of Protein Hydrolysates Produced from Zoonotic Products
3. The Plants Kingdom
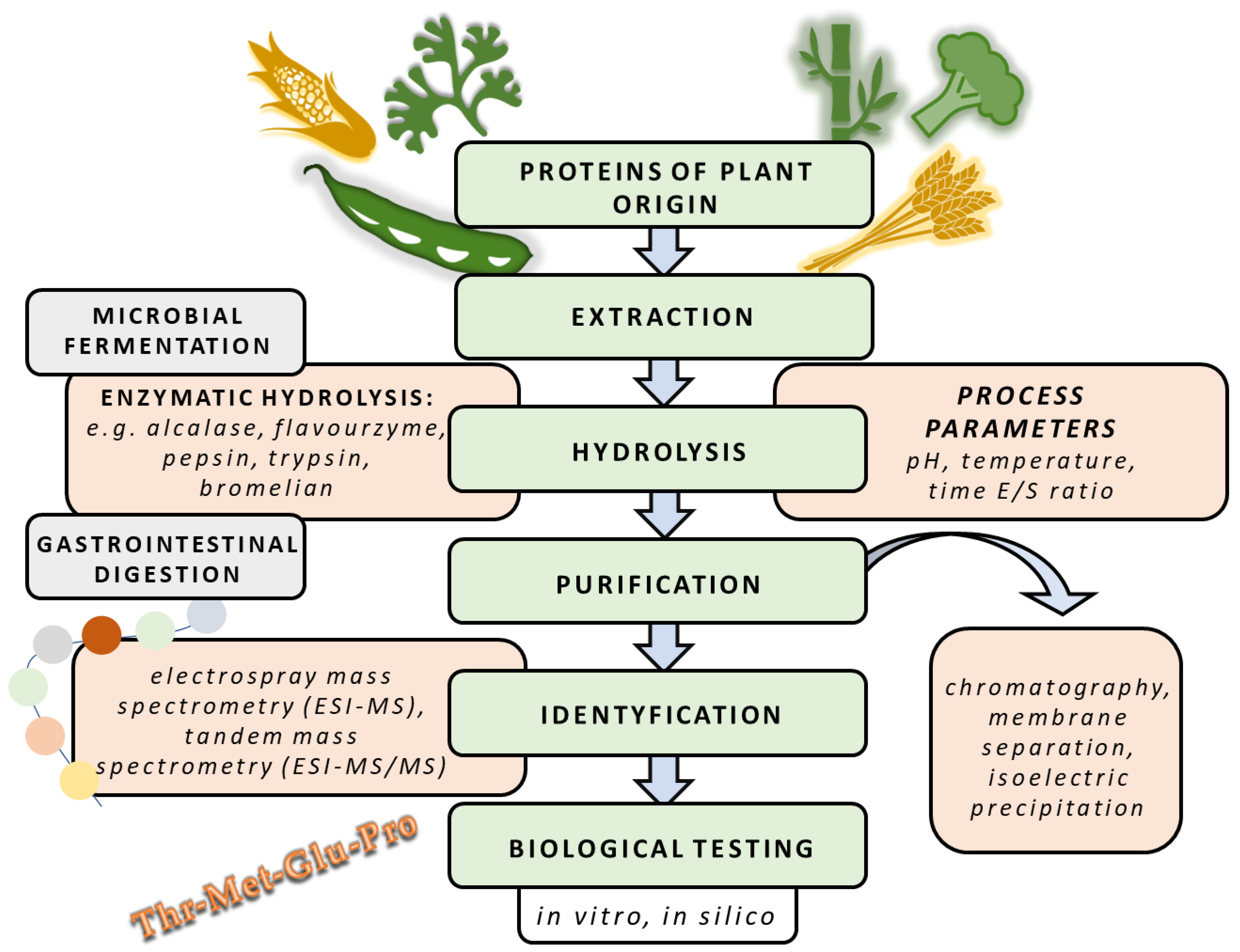
3.1. Methods of Obtaining Protein Hydrolysates of Plant Origin
3.2. Antioxidant Activity of Protein Hydrolysates Derived from Marine Plants
3.3. Antioxidant Capacity of Protein Hydrolysates Obtained from Cucurbitaceae
3.4. Antioxidant Potential of Protein Hydrolysates Produced from Vigna radiata
3.5. Antioxidant Sctivity of Protein Hydrolysates Generated from Zingiberaceae
3.6. Antioxidant Potency of Protein Hydrolysates Formed from Moringa oleifera
4. The Fungus Kingdom
5. Structure—Activity Relationship
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, H.; Tong, T.; Sun, J.; Xu, Y.; Zhao, Z.; Liao, D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014, 154, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.T.; He, R.; Hasan, F.M.; Udenigwe, C.C.; Gill, T.A.; Aluko, R.E. Evaluation of the in vitro antioxidant properties of a cod (Gadus morhua) protein hydrolysate and peptide fractions. Food Chem. 2015, 173, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Kimatu, B.M.; Zhao, L.; Biao, Y.; Ma, G.; Yang, W.; Pei, F.; Hu, Q. Antioxidant potential of edible mushroom (Agaricus bisporus) protein hydrolysates and their ultrafiltration fractions. Food Chem. 2017, 230, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Homayouni-Tabrizi, M.; Asoodeh, A.; Soltani, M. Cytotoxic and antioxidant capacity of camel milk peptides: Effects of isolated peptide on superoxide dismutase and catalase gene expression. J. Food Drug Anal. 2017, 25, 567–575. [Google Scholar] [CrossRef]
- Ghelichi, S.; Shabanpour, B.; Pourashouri, P.; Hajfathalian, M.; Jacobsen, C. Extraction of unsaturated fatty acid-rich oil from common carp (Cyprinus carpio) roe and production of defatted roe hydrolysates with functional, antioxidant, and antibacterial properties. J. Sci. Food Agric. 2018, 98, 1407–1415. [Google Scholar] [CrossRef]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.H.; Zhang, W.G. A review of antioxidant peptides derived from meat muscle and byproducts. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef]
- Ozuna, C.; León-Galván, M.F. Cucurbitaceae seed protein hydrolysates as a potential source of bioactive peptides with functional properties. BioMed Res. Int. 2017, 2017, 2121878. [Google Scholar] [CrossRef]
- López-Barrios, L.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bioactive peptides and hydrolysates from pulses and their potential use as functional ingredients. J. Food Sci. 2014, 79, R273–R283. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Onuh, J.O.; Girgih, A.T.; Aluko, R.E.; Aliani, M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem. 2014, 150, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Roslan, J.; Yunos, K.F.M.; Abdullah, N.; Kamal, S.M.M. Characterization of fish protein hydrolysate from Tilapia (Oreochromis niloticus) by product. Agric. Agric. Sci. Procedia 2014, 2, 312–319. [Google Scholar] [CrossRef]
- Jamil, N.H.; Halim, N.R.; Sarbon, N.M. Optimization of enzymatic hydrolysis condition and functional properties of eel (Monopterus sp.) protein using response surface methodology (RSM). Int. Food Res. J. 2016, 23, 1–9. [Google Scholar]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Kumar, B.D. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J. Food Sci. Technol. 2015, 52, 5817–5825. [Google Scholar] [CrossRef]
- Latorres, J.M.; Rios, D.G.; Saggiomo, G.; Wasielesky, W., Jr. Functional and antioxidant properties of protein hydrolysates obtained from white shrimp (Litopenaeus vannamei). J Food Sci. Technol. 2017, 55, 721–729. [Google Scholar] [CrossRef]
- Al-Shamsi, K.A.; Mudgil, P.; Hassan, H.M.; Maqsood, S. Camel milk protein hydrolysates with improved technofunctional properties and enhanced antioxidant potential in vitro and in food model systems. J. Dairy Sci. 2018, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Chiozzi, R.Z.; Laganà, A. Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in Tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef]
- Cai, X.; Yan, A.; Fu, N.; Wang, S. In vitro antioxidant activities of enzymatic hydrolysate from Schizochytrium sp. and its hepatoprotective effects on acute alcohol-induced liver injury in vivo. Mar. Drugs 2017, 15, 115. [Google Scholar] [CrossRef]
- Gupta, N.; Srivastava, N.; Bhagyawant, S.S. Vicilin—A major storage protein of mungbean exhibits antioxidative potential, antiproliferative effects and ACE inhibitory activity. PLoS ONE 2018, 13, e0191265. [Google Scholar] [CrossRef]
- Mune, M.A.M.; Minka, S.R.; Henle, T. Investigation on antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activity of Bambara bean protein hydrolysates. Food Chem. 2018, 250, 162–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Zhao, M.; Ning, Z.; Sun-Waterhouse, D.; Sun, B. Soy peptide aggregates formed during hydrolysis reduced protein extraction without decreasing their nutritional value. Food Funct. 2017, 8, 4384–4395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhanga, M.; Lina, S.; Cheng, S. Contribution of specific amino acid and secondary structure to the antioxidant property of corn gluten proteins. Food Res. Int. 2018, 105, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Pinho, O.; Ferreira, I.M. Bio-functional properties of sardine protein hydrolysates obtained by brewer´s spent yeast and commercial proteases. J. Sci. Food Agric. 2017, 97, 5414–5422. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.-B.; Kim, J.-G.; Je, J.-Y. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem. 2014, 147, 78–83. [Google Scholar] [CrossRef]
- Sheriff, S.A.; Balasubramanian, S.; Baranitharan, R.; Ponmurugan, P. Synthesis and in vitro antioxidant functions of protein hydrolysate from backbones of Rastrelliger kanagurta by proteolytic enzymes. Saudi J. Biol. Sci. 2014, 21, 19–26. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Wang, Y.-M.; Zhang, B.; Deng, S.-G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Tamboli, E.; Acharya, A.; Su, C.-H.; Gopinath, S.C.B.; Chen, Y.; Velusamy, P. Separation and identification of bioactive peptides from stem of Tinospora cordifolia (Willd.) Miers. PLoS ONE 2018, 13, e0193717. [Google Scholar] [CrossRef]
- Heo, S.-Y.; Ko, S.-C.; Kim, C.S.; Oh, G.W.; Ryu, B.; Qian, Z.J.; Kim, G.; Park, W.S.; Choi, I.W.; Phan, T.T.; et al. A heptameric peptide purifed from Spirulina sp. Gastrointestinal hydrolysate inhibits angiotensin I-converting enzymeand angiotensin II-induced vascular dysfunction in human endothelial cells. Int. J. Mol. Med. 2017, 39, 1072–1082. [Google Scholar] [CrossRef]
- Inthuwanarud, K.; Sangvanich, P.; Puthong, S.; Karnchanatat, A. Antioxidant and antiproliferative activities of protein hydrolysate from the rhizomes of Zingiberaceae plants. Pak. J. Pharm. Sci. 2016, 29, 1893–1900. [Google Scholar]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Materska, M.; Zielińska, E. Antioxidant activity of protein hydrolysates from raw and heat-treated yellow string beans (Phaseolus vulgaris L.). Acta Sci. Pol. Technol. Aliment. 2014, 13, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Phongthai, S.; D’Amico, S.; Schoenlechner, R.; Homthawornchoo, W.; Rawdkuen, S. Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chem. 2018, 240, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G.; Lammi, C.; Boschin, G.; Zanoni, C.; Arnoldi, A. Eploration of potentially bioactive peptides generated from the enzymatic hydrolysis of hempseed proteins. J. Agric. Food Chem. 2017, 65, 10174–10184. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Arya, S.S. Bioactive L. acidissima protein hydrolysates using Box–Behnken design. Biotech 2017, 7, 218. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of fish skin collagen: An opportunity for valorizing fish industry byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Tejpal, C.S.; Vijayagopal, P.; Elavarasan, K.; Prabu, D.L.; Lekshmi, R.G.K.; Asha, K.K.; Anandan, R.; Chatterjee, N.S.; Mathew, S. Antioxidant, functional properties and amino acid composition of pepsin-derived protein hydrolysates from whole tilapia waste as influenced by pre-processing ice storage. J. Food Sci. Technol. 2017, 54, 4257–4267. [Google Scholar] [CrossRef]
- Slizyte, R.; Rommi, K.; Mozuraityte, R.; Eck, P.; Five, K.; Rustad, T. Bioactivities of fish protein hydrolysates from defatted salmon backbones. Biotechnol. Rep. 2016, 11, 99–109. [Google Scholar] [CrossRef]
- Shazly, A.B.; He, Z.; El-Aziz, M.A.; Zeng, M.; Zhang, S.; Qin, F.; Chen, J. Fractionation and identification of novel antioxidant peptides from buffalo and bovine casein hydrolysates. Food Chem. 2017, 232, 753–762. [Google Scholar] [CrossRef]
- Dash, P.; Ghosh, G. Amino acid composition, antioxidant and functional properties of protein hydrolysates from Cucurbitaceae seeds. J. Food Sci. Technol. 2017, 54, 4162–4172. [Google Scholar] [CrossRef]
- Garza, N.G.G.; Koyoc, J.A.C.; Castillo, J.A.T.; Zambrano, E.A.G.; Ancona, D.B.; Guerrero, L.C.; Garcıa, S.R.S. Biofunctional properties of bioactive peptide fractions from protein isolates of moringa seed (Moringa oleifera). J. Food Sci. Technol. 2017, 54, 4268–4276. [Google Scholar] [CrossRef]
- Li, W.; Zhao, T.; Zhang, J.; Xu, J.; Sun-Waterhouse, D.; Zhao, M.; Su, G. Effect of walnut protein hydrolysate on scopolamine-induced learning and memory deficits in mice. J. Food Sci. Technol. 2017, 54, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Su, G.; Wang, S.; Zhang, Q.; Zhang, J.; Zheng, L.; Sun, B.-G.; Zhao, M. Neuroprotective effects of acetylcholinesterase inhibitory peptides from anchovy (Coilia mystus) against glutamate-induced toxicity in PC12 cells. J. Agric. Food Chem. 2017, 65, 11192–11201. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Zhao, T.; Zhao, Y.; Sun-Waterhouse, D.; Qiu, C.; Huang, P.; Zhao, M. Effect of anchovy (Coilia mystus) protein hydrolysate and its Maillard reaction product on combating memory-impairment in mice. Food Res. Int. 2016, 82, 112–120. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibitory activity, antioxidant properties, phenolic content and amino acid profiles of Fucus spiralis L. protein hydrolysate fractions. Mar. Drugs 2017, 15, 311. [Google Scholar] [CrossRef]
- Wang, D.; Shahidi, F. Protein hydrolysate from turkey meat and optimization of its antioxidant potential by response surface methodology. Poult. Sci. 2018, 97, 1824–1831. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef]
- Guan, H.; Diao, X.; Jiang, F.; Han, J.; Kong, B. The enzymatic hydrolysis of soy protein isolate by Corolase PP under high hydrostatic pressure and its effect on bioactivity and characteristics of hydrolysates. Food Chem. 2018, 245, 89–96. [Google Scholar] [CrossRef]
- Choi, J.-S.; Kim, J.-W.; Park, J.B.; Pyo, S.E.; Hong, Y.-K.; Ku, S.K.; Kim, M.-R. Blood glycemia-modulating effects of melanian snail protein hydrolysates in mice with type II diabetes. Int. J. Mol. Med. 2017, 39, 1437–1451. [Google Scholar] [CrossRef][Green Version]
- Sonklin, C.; Laohakunjit, N.; Kerdchoechuen, O.; Ratanakhanokchai, K. Volatile flavour compounds, sensory characteristics and antioxidant activities of mungbean meal protein hydrolysed by bromelain. J. Food Sci. Technol. 2018, 55, 265–277. [Google Scholar] [CrossRef]
- Jemil, I.; Abdelhedi, O.; Nasri, R.; Mora, L.; Jridi, M.; Aristoy, M.-C.; Toldrá, F.; Nasri, M. Novel bioactive peptides from enzymatic hydrolysate of Sardinelle (Sardinella aurita) muscle proteins hydrolysed by Bacillus subtilis A26 proteases. Food Res. Int. 2017, 100, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Pokora, M.; Zambrowicz, A.; Zabłocka, A.; Dąbrowska, A.; Szołtysik, M.; Babij, K.; Eckert, E.; Trziszka, T.; Chrzanowska, J. The use of serine protease from Yarrowia lipolytica yeast in the production of biopeptides from denatured egg white proteins. Acta Biochem. Pol. 2017, 64, 245–253. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, F.; Wu, D.; Yu, C.; Wang, Z.; Du, M. Antioxidant and ACE inhibitory activity of enzymatic hydrolysates from Ruditapes philippinarum. Molecules 2018, 23, 1189. [Google Scholar] [CrossRef] [PubMed]
- Ktari, N.; Bkhairia, I.; Nasri, R.; Kolsi, R.B.A.; Salem, R.B.S.-B.; Amara, I.B.; Zeghal, N.; Salah, B.B.; Salah, R.B.; Nasri, M. Zebra blenny protein hydrolysates as a source of bioactive peptides with prevention effect against oxidative dysfunctions and DNA damage in heart tissues of rats fed a cholesterol-rich diet. Food Res. Int. 2017, 100, 423–432. [Google Scholar] [CrossRef]
- Ktari, N.; Nasri, R.; Mnafgui, K.; Hamden, K.; Belguith, O.; Boudaouara, T.; Feki, A.; Nasri, M. Antioxidative and ACE inhibitory activities of protein hydrolysates from zebra blenny (Salaria basilisca) in alloxan-induced diabetic rats. Process Biochem. 2014, 49, 890–897. [Google Scholar] [CrossRef]
- Kim, M.-R.; Kim, K.-W.; Park, J.B.; Hong, Y.-K.; Ku, S.K.; Choi, J.-S. Anti-obesity effects of yellow catfsh protein hydrolysate on mice fed a 45% kcal high-fat diet. Int. J. Mol. Med. 2017, 40, 784–800. [Google Scholar] [CrossRef]
- Salem, R.B.S.-B.; Bkhairia, I.; Abdelhedi, O.; Nasri, M. Octopus vulgaris protein hydrolysates: Characterization, antioxidant and functional properties. J. Food Sci. Technol. 2017, 54, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, A.; Eckert, E.; Pokora, M.; Bobak, Ł.; Dąbrowska, A.; Szołtysik, M.; Trziszka, T.; Chrzanowska, J. Antioxidant and antidiabetic activities of peptides isolated from a hydrolysate of an egg-yolk protein by-product prepared with a proteinase from Asian pumpkin (Cucurbita ficifolia). RSC Adv. 2015, 5, 10460–10467. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolyste of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Theodore, A.E.; Raghavan, S.; Kristinsson, H.G. Antioxidative activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. J. Agric. Food Chem. 2008, 56, 7459–7466. [Google Scholar] [CrossRef]
- Quian, Z.-J.; Jung, W.K.; Kim, S.K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Qian, Z.J.; Ryu, B.; Ngo, D.H.; Kim, S.K. Purification and identification of antihypertensive peptides from seaweed pipefish (Syngnathus schlegeli) muscle protein hydrolysate. Food Res. Int. 2011, 44, 703–707. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Elavarasan, K.; Naveen Kumar, V.; Shamasundar, B.A. Antioxidant and functional properties of fish protein hydrolysates from fresh water carp (Catla catla) as influenced by the nature of enzyme. J. Food Process. Preserv. 2014, 38, 1207–1214. [Google Scholar] [CrossRef]
- Taheri, A.; Bakhshizadeh, G.A. Antioxidant and ace inhibitory activities of kawakawa (Euthynnus affinis) protein hydrolysate produced by skipjack tuna pepsin. J. Aquat. Food Prod. Technol. 2019, 29, 148–166. [Google Scholar] [CrossRef]
- Romani, V.P.; Martins, V.G.; Goddard, J.M. Radical scavenging polyethylene films as antioxidant active packaging materials. Food Control 2020, 109, 106946. [Google Scholar] [CrossRef]
- Yaghoubzadeh, Z.; Ghadikolaii, F.P.; Kaboosi, H.; Safari, R.; Fattahi, E. Antioxidant activity and anticancer effect of bioactive peptides from rainbow trout (Oncorhynchus mykiss) skin hydrolysate. Int. J. Pept. Res. Ther. 2020, 26, 625–632. [Google Scholar] [CrossRef]
- Sousa, P.; Borges, S.; Pintado, M. Enzymatic hydrolysis of insect Alphitobius diaperinus towards the development of bioactivepeptide hydrolysates. Food Funct. 2020, 11, 3539–3548. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhu, Z.; Li, X.; Sun, S.; Wang, W.; Sadiq, F.A. Identification and characterization of two novel antioxidant peptides from silkworm pupae protein hydrolysates. Eur. Food Res. Technol. 2021, 247, 343–352. [Google Scholar] [CrossRef]
- Li, B.; Chen, F.; Wang, X.; Ji, B.; Wu, Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization–mass spectrometry. Food Chem. 2007, 102, 1135–1143. [Google Scholar] [CrossRef]
- Arise, R.O.; Yekeen, A.A.; Ekun, O.E. In vitro antioxidant and α-amylase inhibitory properties of watermelon seed protein hydrolysates. Environ. Exp. Biol. 2016, 14, 163–172. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Jiang, N.; Tu, J.; Yang, J.; Zhou, Y. Antioxidant and anti-aging activities of Silybum Marianum protein hydrolysate in mice treated with D-galactose. Biomed. Environ. Sci. 2017, 30, 623–631. [Google Scholar] [PubMed]
- Evangelho, J.A.D.; Vanier, N.L.; Pinto, V.Z.; Berrios, J.J.; Dias, A.R.G.; Zavareze, E.D.R. Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef]
- Jara, A.M.R.; Liggieri, C.S.; Bruno, M.A. Preparation of soy protein hydrolysates with antioxidant activityby using peptidases from latex of Maclura pomifera fruits. Food Chem. 2018, 264, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Arise, A.K.; Alashi, A.M.; Nwachukwu, I.D.; Malomo, S.A.; Aluko, R.E.; Amonsou, E.O. Inhibitory properties of bambara groundnut protein hydrolysate and peptide fractions against angiotensin-converting enzymes, renin and free radicals. J. Sci. Food. Agric. 2017, 97, 2834–2841. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef]
- Sabbione, A.C.; Ibañez, S.M.; Martínez, E.N.; Añón, M.C.; Scilingo, A.A. Antithrombotic and antioxidant activity of amaranth hydrolysate obtained by activation of an endogenous protease. Plant. Foods Hum. Nutr. 2016, 71, 174–182. [Google Scholar] [CrossRef]
- Xie, J.; Du, M.; Shen, M.; Wu, T.; Lin, L. Physico-chemical properties, antioxidant activities and angiotensin-I converting enzyme inhibitory of protein hydrolysates from Mung bean (Vigna radiate). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Zhong, H.; Wang, S.; Zhou, Q. Enzymatic hydrolysis of rice dreg protein: Effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012, 134, 1360–1367. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
| Source | Part of Source | Enzymatic Hydrolysis | Enzymatic Deactivation | Dehydration | MW of PH/ Peptides Sequence | Antioxidant Activity PH/Peptide | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Enzyme | E/S Ratio | pH | Temp (°C) | Time (h) | DH (%) | Temp (°C) | Time (min) | pH | Reagent | ||||||
| Ruditapes philippinarum | whole | trypsin | 3:100 | 9 | 45 | 4 | 17.54 | 100 | 10 | - | - | lyophilization | Highest content fraction of MW 1–3 kDa | DPPH 38.77% | Yu et al., 2018 |
| whole | neutrase | 3:100 | 6.5 | 45 | 4 | 18.3 | 100 | 10 | - | - | lyophilization | Highest content fraction of MW <1 kDa | DPPH 68.55% | ||
| whole | pepsin | 3:100 | 4 | 45 | 4 | 8.34 | 100 | 10 | - | - | lyophilization | highest content of MW fraction >5 kDa. | DPPH 63.98% | ||
| Prionace glauca | skin | pepsin | 1:20 | 8 | 55 | 3 | 16.52 | 90 | 5 | - | - | lyophilization | MW: 3 kDa | DPPH 422.97; 416.03 (mg BHT Eq/mL)—retentates (R) and permeates (P) fraction | Blanco et al., 2017 |
| Scyliorhinus canicula | skin | pepsin | 1:20 | 8 | 55 | 3 | 15.80 | 90 | 5 | - | - | lyophilization | MW: 3 kDa | DPPH 603.40; 601.70 (mg BHT Eq/mL)—retentates (R) and permeates (P) fraction | |
| Xiphias gladius | skin | pepsin | 1:20 | 8 | 55 | 3 | 12.56 | 90 | 5 | - | - | lyophilization | MW: 3 kDa | DPPH 465.63; 448.0 (mg BHT Eq/mL)—retentates (R) and permeates (P) fraction | |
| Thunnus albacores | skin | pepsin | 1:20 | 8 | 55 | 3 | 11.49 | 90 | 5 | - | - | lyophilization | MW: 3 kDa | DPPH 435.97; 457.67 (mg BHT Eq/mL)—retentates (R) and permeates (P) fraction | |
| Decapterus maruadsi | muscle | alcalase | 1:100 | 9.5 | 50 | 5 | - | 100 | 15 | - | - | lyophilization | MW III: <5 kDa MW II: 5–10 kDa MW I: >10 kDa HEKVC HDHPVC | DPPH 39.36% —for PH I: 47.23% II: 36.27 III: 50.54 EC50 0.0677 (mM) EC50 0.0310 (mM) | Jiang et al., 2014 |
| muscle | neutral protease | 1:100 | 7 | 50 | 5 | - | 100 | 15 | - | - | lyophilization | n. d. | 32.33% —for PH | ||
| muscle | papain | 1:100 | 7 | 55 | 5 | - | 100 | 15 | - | - | lyophilization | n. d. | 40.21% —for PH | ||
| muscle | pepsin | 1:100 | 2 | 37.5 | 5 | - | 100 | 15 | - | - | lyophilization | n. d. | 32.63% —for pH | ||
| muscle | trypsin | 1:100 | 7.8 | 37.5 | 5 | - | 100 | 15 | - | - | lyophilization | n. d. | 39.37% —for PH | ||
| Oreochromis niloticus | head, skin, trimmings, fins, frames and viscera | pepsin | 1:100 | 2.5 | 37 | 3 | - | 100 | 15 | - | - | spray drying | MW < 18 kDa | 2.5 mg/mL DPPH scavenning 73.62% | Tejpal et al., 2017 |
| Sardinella aurita | muscle | proteases from B. subtilis A26 | 3:1 | 8 | 45 | 5 | 10 | - | - | - | 0.1 N HCl | lyophilization | LVHYAGTVDYN; FRPQQPYPQP LEGDLKLSQE; NVLSGGTTMYPG; VIVEGELERT | highest reducing power and ORAC value | Jemil et al., 2017 |
| Rastrelliger kanagurta | backbones | pepsin | 1:100 | 2 | 37 | 6 | 24.7 | 100 | 10 | - | - | lyophilization | n. d. | DPPH 46% ABTS 58.5% | Sheriff et al., 2017 |
| backbones | papain | 1:100 | 6 | 37 | 3 | 18.1 | 100 | 10 | - | - | lyophilization | n. d. | DPPH 36% ABTS 37.54% | ||
| Navodon septentrionalis | head | papain | 3:20 | 7.0 | 50 | 5 | - | 90 | 10 | - | - | lyophilization | WEGPLK; GPP; GVPLT | DPPH IC50 4.43 mg/mL IC50 1.92 mg/mL IC50 4.54 mg/mL | Chi et al., 2015 |
| Salmon | backbones | Corolase® PP | 1:10 | - | 50 | 2 | 22.1 | ˃90 | 5 | - | - | lyophilization | MW > 2.5 kDa | DPPH 32% * | Slizyte et al., 2016 |
| backbones | Corolase® 7089 | 1:10 | - | 50 | 2 | 18.3 | ˃90 | 5 | - | - | lyophilization | MW 2.5–6.5 kDa | DPPH 25% | ||
| backbones | Protamex® | 1:10 | - | 50 | 2 | 20.9 | ˃90 | 5 | - | - | lyophilization | MW 0.14 kDa—free amino acids | DPPH 38% * | ||
| backbones | Bromelain 400 GDU/g/Papain 100 TU/mg | 1:10 | - | 50 | 2 | 16.8 | ˃90 | 5 | - | - | lyophilization | MW 5.5–6.5 kDa | DPPH about 27% * | ||
| backbones | Protex 6L | 1:10 | - | 50 | 2 | 18.2 | ˃90 | 5 | - | - | lyophilization | MW 2.5–6.5 kDa | DPPH 25–26% * | ||
| backbones | Seabzyme L 200 | 1:10 | - | 50 | 2 | 17.1 | ˃90 | 5 | - | - | lyophilization | MW 5.5–6.5 Da | DPPH 25–26% * | ||
| backbones | trypsin | 1:10 | - | 50 | 2 | 18.1 | ˃90 | 5 | - | - | lyophilization | MW > 2.5 kDa | DPPH about 21% * | ||
| Salmon | pectoral fin | alcalase | 1:100 | 7 | 50 | 8 | around 10 | 100 | 15 | - | - | lyophilization | n. d. | DPPH IC50 4.76 mg/mL | Ahn et al., 2014 |
| pectoral fin | flavourzyme | 1:100 | 7 | 50 | 8 | around 10 | 100 | 15 | - | - | lyophilization | n. d. | DPPH IC50 3.62 mg/mL | ||
| pectoral fin | neutrase | 1:100 | 7 | 50 | 8 | around 10 | 100 | 15 | - | - | lyophilization | n. d. | DPPH IC50 4.95 mg/mL | ||
| pectoral fin | pepsin | 1:100 | 2 | 37 | 8 | around 10 | 100 | 15 | - | - | lyophilization | Phe-Leu-ASN-Glu-Phe-Leu-His-Val (MW 1018.48 Da) | DPPH IC50 1.63 mg/Ml—for preoteolytic hydrolysate DPPH IC50 486 uM—for octapeptide | ||
| pectoral fin | Protamex | 1:100 | 77 | 50 | 8 | around 10 | 100 | 15 | - | - | lyophilization | n.d. | DPPH IC50 4.08 mg/mL | ||
| pectoral fin | trypsin | 1:100 | 37 | 8 | around 10 | 100 | 15 | - | - | lyophilization | n.d. | DPPH IC50 4.53 mg/mL | |||
| Cyprinus Carpio | roe | alcalase | 1.5:100 | 8 | 55 | 3 | 12.7 | 85-95 | 20 | - | - | vacuum drying | n. d. | DPPH IC50 1.151 mg/mL | Chalamaiah et al., 2015 |
| roe | pepsin | 1.5:100 | 2 | 37 | 3 | 30 | 85-95 | 20 | - | - | vacuum drying | n. d. | DPPH IC50 2.25 mg/mL | ||
| roe | trypsin | 1.5:100 | 8 | 37 | 3 | 19.5 | 85-95 | 20 | - | - | vacuum drying | n. d. | DPPH IC50 1.158 mg/mL | ||
| Octopus vulgaris | muscle | protease from Salaria basilica | 1:3 | 8 | 45 | 8 | 17.6 | 80 | 20 | - | - | lyophilization | high MW peptides. | DPPH 53.29% (at 6 mg/mL) | Salem et al., 2017 [57] |
| muscle | protease from Bacillus subtilis A26 | 1:3 | 8 | 45 | 8 | 21 | 80 | 20 | - | - | lyophilization | highest level of peptides, with MW < 0.3 kDa | DPPH 75.1% (at 6 mg/mL) | ||
| Litopenaeus vannamei | whole | Protamex | 1:100 | 7 | 50 | - | 10 and 20 | 90 | 20 | - | - | lyophilization | n. d. | ABTS about 48% (at 5.0 mg/mL)—values for DH 10% about 66% (at 5.0 mg/mL)–DH 20% | Latorres et al., 2017 |
| whole | alcalase | 1:100 | 8 | 50 | - | 10 and 20 | 90 | 20 | - | - | lyophilization | n. d. | ABTS about 68% (at 5.0 mg/mL)–values for DH 10% about 56% (at 5.0 mg/mL)–DH 20% | ||
| Gryllodes sigillatus | whole | α-amylase, pepsin, pancreatin, and bile extract solution | 4:100 | - | 37 | 1 | - | 100 | 5 | - | - | lyophilization | MW ≤ 3.5 kDa | ABTS IC50 15.24 µg/mL DPPH IC50 17.97 µg/mL | Zielińska et al., 2017 |
| Tenebrio molitor | whole | α-amylase, pepsin, pancreatin, and bile extract solution | 4:100 | - | 37 | 1 | - | 100 | 5 | - | - | lyophilization | MW ≤ 3.5 kDa | ABTS IC50 24.31 µg/mL DPPH IC50 85.55 µg/mL | |
| Schistocerca gregaria | whole | α-amylase, pepsin, pancreatin, and bile extract solution | 4:100 | - | 37 | 1 | - | 100 | 5 | - | - | lyophilization | MW ≤ 3.5 kDa | ABTS IC50 12.1 µg/mL DPPH IC50 88.81 µg/mL | |
| Meleagris gallopavo | muscle | flavourzyme | 3:100 | 5.42 | 50.09 | 1.08 | 12.11 | 100 | 12 | - | - | lyophilization | n. d. | ABTS 79.15% DPPH 88.33% | Wang et al., 2018 |
| Gallus gallus domesticus | muscle | flavourzyme | 3:100 | 5.42 | 50.09 | 1.08 | 12.86 | 100 | 12 | - | - | lyophilization | n. d. | ABTS 78.23% DPPH 86.52% | |
| Bovi | muscle | flavourzyme | 3:100 | 5.42 | 50.09 | 1.08 | 10.52 | 100 | 12 | - | - | lyophilization | n.d. | ABTS 70.57% DPPH 80.15% | |
| Camelus dromedarius | milk | mixture of pepsin and pancreatin | - | 5.3 then 7.5 | 37 | 4 | - | 100 | 10 | - | - | lyophilization | LEEQQQTEDEQQDQL (MW: 1860.85 Da, LL-15); YLEELHRLNAGY (1477.63 Da, YY-11); RGLHPVPQ (903.04 Da, RQ-8) | ABTS IC50 < 0.01 mg/mL DPPH IC50 0.01 mg/mL —for YY-11 ABTS IC50 0.03 mg/mL DPPH IC50 0.03 mg/mL —for LL-15 ABTS IC50 0.07 mg/mL DPPH IC50 0.06 mg/mL —for RQ-8 | Homayouni-Tabrizi et al., 2017 |
| Camelus dromedarius | milk | alcalase | 1:100 | 8 | 50 | 6 | 15.5 | 100 | 10 | - | - | lyophilization | n. d | DPPH about 19 µmol TE/g | Al-Shamsi et al., 2018 |
| milk | bromelain | 1:100 | 7 | 50 | 6 | 23.8 | 100 | 10 | - | - | lyophilization | n. d. | DPPH 23 µmol TE/g), | ||
| milk | papain | 1:100 | 7 | 50 | 6 | 39.6 | 100 | 10 | - | - | lyophilization | n. d | DPPH: 21 µmol TE/g | ||
| Buffalo | milk | trypsin | 1:100 | 7-9 | 37 | 3 | - | 90 | 15 | - | - | lyophilization | RELEE, MEDNKQ, RELEEL, LQS, GNACF, | n.d. | Shazly et al., 2017 |
| milk | alcalase | 1:100 | 5-7 | 55 | 3 | - | 90 | 15 | - | - | lyophilization | PRG, TVA, TAAG, HCL, LLSLS | n.d. | ||
| Gallus gallus domesticus | egg white | protease from Y. lipolytica | 1:30 | 8 | 37 | 24 | 38 | 100 | 15 | - | - | lyophilization | MW < 3 kDa | DPPH 0.21 μM Troloxeq/mg | Pokora et al., 2017 |
| Gallus gallus domesticus | egg yolk | proteinase from C. ficifolia | 1:7.52 | 8 | 37 | 4 | - | 100 | 15 | - | - | lyophilization | LAPSLPGKPKPD | DPPH 6.03 μM Troloxeq/mg FRAP 296.07 μg Fe2+/mg | Zambrowicz et al., 2015 [58] |
| Source | Part of Source | Enzymatic Hydrolysis | Enzymatic Deactivation | Dehydration | MW of PH/ Peptides Sequence | Antioxidant Activity PH/Peptide | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Enzyme | E/S Ratio | pH | Temp (°C) | Time (h) | DH (%) | Temp (°C) | Time (min) | pH | Reagent | ||||||
| Tetradesmus obliquus | whole | alcalase | 1:10 | 8 | 60 | 4 | - | - | - | 2 | TFA | lyophilization | WPRGYFL, GPDRPKFLGPF, WYGPDRPKFL, SDWDRF | EC50 = 4.70 EC50 = 13.97 EC50 = 0.82 EC50 = 5.73 | Montone et al., 2018 |
| Palmaria palmata | whole | Corolase® PP | 1:100 | 7 | 50 | 4 | - | 90 | 20 | - | - | lyophilization | SDITRPGGQM | ORAC: 152.43 [TE/μmol] FRAP: 21.23 [TE/μmol] | Harnedy et al., 2017 |
| Fucus spirali | whole | cellulase then bromelain | 1:100 | 4.5 then 7 | 50 then 37 | 20 | - | 100 | 10 | - | - | lyophilization | MW F1: <1 kDa F2: 1–3 kDa F3: >3 kDa | FRAP: F1: 86.03 F2: 32.73 F3: 80.50 | Paiva et al., 2017 |
| Spirulina sp. | whole | pepsin then trypsin and α-chymotrypsin | 1:100 | 2.2 then 6.5 | 37 | 2 then 2.5 | - | 100 | 10 | - | - | lyophilization | TMEPGKP | IC50 0.1 mg/ml | Heo et al., 2017 |
| Schizochytrium sp. | whole | alcalase then flavourzyme | 10:100 then 12.5:100 | 9 then 6.7 | 50 | 6 then 8 | 8.37 then 21.48 | 100 | 10 | - | - | lyophilization |
MW <3 kDa >3 kDa |
DPPH: IC50 350 μg/mL ABTS: IC50 17.5 μg/mL, for: MW <3 kDa | Cai et al., 2017 |
| Agaricus bisporus | fruiting bodies | alcalase | 2.5:100 | 9 | 50 | 4 | about 18 | 100 | 15 | - | - | lyophilization | MW <1 kDa 1–3 k Da 3–5 kDa 5–10 kDa | DPPH (MW < 1; 1–3; 3–5; 5–10 kDa) about EC50 0.64; 0.32; 0.64; 0.61 | Kimatu et al., 2017 |
| fruiting bodies | pancreatin | 2.5:100 | 7.5 | 37 | 4 | about 12.5 | 100 | 15 | - | - | lyophilization, | MW <1 kDa 1–3 kDa 3–5 kDa 5–10 kDa | DPPH (MW < 1; 1–3; 3–5; 5–10 kDa) about EC50 0.64; 0.13; 0.64; 0.21) | ||
| fruiting bodies | flavourzyme, | 2.5:100 | 7 | 50 | 4 | about 3 | 100 | 15 | - | - | lyophilization | MW <1 kDa 1–3 k Da 3–5 kDa 5–10 kDa | DPPH (MW < 1; 1–3; 3–5; 5–10 kDa) about EC50 0.64; 0.25; 0.64; 0.30) | ||
| fruiting bodies | alcalase-pancreatin | 2.5:100 | 9.0 then 7.5 | 50 then 37 | 2 then 2 | about 18.5 | 100 | 15 | - | - | lyophilization | MW <1 kDa 1–3 kDa 3–5 kDa 5–10 kDa | DPPH (MW < 1; 1–3; 3–5; 5–10 kDa) about EC50 0.64; 0.42; 0.64; 0.24) | ||
| fruiting bodies | alcalase-flavourzyme | 2.5:100 | 9.0 then 7.0 | 50 | 2 then 2 | about 16 | 100 | 15 | - | - | lyophilization | MW <1 kDa 1–3 kDa 3–5 kDa 5–10 kDa | DPPH (MW < 1; 1–3; 3–5; 5–10 kDa) about EC50 0.64; 0.38; 0.64; 0.16) | ||
| Zingiberaceae | rhizome | pepsin then pancreatin | 1:20 | 2.5 then 7.5 | 37 | 3 then 3 | - | 80 | 20 | - | - | lyophilization | MW 12.4–12.8 kDa | IC50 41. 78 µg/mL | Inthuwanarud et al., 2016 |
| Cucurbit moschata | seed | trypsin | 1:50 | 7.5 | 37 | 4 | - | heated in water bath | 20 | - | - | lyophilization | n. d. | DPPH: IC50 49.3 µg/mL ABTS: IC50 142.3 µg/mL | Dash et al., 2017 |
| Citrullus lanatus | seed | trypsin | 1:50 | 7.5 | 37 | 4 | - | heated in water bath | 20 | - | - | lyophilization | n. d. | DPPH: IC50=80.3 µg/Ml ABTS: IC50=179 µg/mL | |
| Lagenaria siceraria | seed | trypsin | 1:50 | 7.5 | 37 | 4 | - | heated in water bath | 20 | - | - | lyophilization | n. d. | DPPH: IC50 46 µg/mL ABTS: IC50 108 µg/mL | |
| Citrullus lanatus | seed | pepsin | 1:100 | 2.2 | 37 | 5 | 19.38 | 95–100 | 15 | - | - | lyophilization | n. d. | IC50 2.41 µg/mL | Arise et al., 2016 [72] |
| seed | trypsin | 1:100 | 8 | 37 | 5 | 26.26 | 95–100 | 15 | - | - | lyophilization | n. d. | IC50 2.82 µg/mL | ||
| seed | alcalase | 1:100 | 8 | 60 | 5 | 13.16 | 95–100 | 15 | - | - | lyophilization | n. d. | IC50 3.20 µg/mL | ||
| Silybum marianum | seed | neutrase | 1:60 | 7 | 55 | 2 | - | 100 | 10 | - | - | lyophilization | MW <1 kDa 1–3 kDa 3–10 kDa ≥10 kDa | TAOC 0.89 U/mg (at 800 mg/kg) | Zhu et al., 2017 [73] |
| Mungfaba | defatted mungbean meal | bromelain | 5, 10, 15, 20:100 | 6 | 50 | 6, 12, 18, 24 | 50.4 | 95 | 15 | - | - | evaporation | MW <10 kDa | DPPH and ABTS 80; 90% | Sonklin et al., 2018 |
| Vigna radiata | seed | alcalase | 20 × 104 U g−1 | 9.5 | 60 | 2.5 | 61.5 | 100 | 10 | - | - | lyophilization | n.d. | DPPH and ABTS: IC50 = 0.77 and 0.78 µg/mL, | Gupta et al., 2018 |
| seed | trypsin | 25 × 104 U g−1 | 8 | 37 | 3.5 | 46.4 | 100 | 10 | - | - | lyophilization | n.d. | IC50 about 1.3 µg/mL | ||
| Phaseolus vulgaris | pod | pepsin | 1:20 | 2 | 37 | 2 | - | 100 | 5 | - | - | lyophilization | n. d. | DPPH and ABTS 46.12%; 92.32%—hydrolysates obtained from heat treated beans | Karaś et al., 2014 |
| Phaseolus vulgaris | seed | alcalase | 1:20 | 2 | 50 | 7 | 11.5 | 90 | 10 | - | - | lyophilization | n. d. | higher ABTS scavening activity for alcalase treated protein | Evangelho et al., 2017 [74] |
| seed | pepsin | 1:20 | 2 | 37 | 2 | 27.09 | 90 | 10 | - | - | lyophilization | n. d. | |||
| Vigna subterranea | seed | alcalase | 4:100 | 7 | 55 | 24 | 38 | 95 | 5 | - | - | lyophilization | hydrolysates produced using trypsin contained higher large-size peptides (>3.5 kDa) compared to the other hydrolysates | DPPH 0.781 µg trolox eq./mg | Mune et al., 2018 |
| seed | trypsin | 1:100 | 7 | 55 | 24 | 22 | 95 | 5 | - | - | lyophilization | DPPH 5.52 µg trolox eq./mg | |||
| seed | thermolysin | 1:100 | 8 | 70 | 24 | 27.5 | 95 | 5 | - | - | lyophilization | DPPH 1.323 µg trolox eq./mg) | |||
| Glycine max | defatted soy flour | peptidases from latex of Maclura pomifera fruits | 1:10 | 8.0 | 45 | 3 | 36.2 | 100 | 7 | - | - | - | Theoretical sequences of peptides (D)LDIFLSSVDINEGAL(L) (I)PAAYPFVVNATSNLNFLA(F) R)FQTLFKNQYGHVRVLQRFN(K) (Y)NLQSGDALRVPAGTTFYV(V) | IC50 31.6 µg/mL, ABTS 157.6 µg trolox eq./mg ORAC 176.9 µm TE/g | Jara et al., 2018 [75] |
| Glycine max | defatted soy flakes | Corolase PP | 3:100 | 7.5 | 50 | 5 | about 26 for 80 MPa, about 27 for 100 MPa, about 28 for 120 MPa, about 29 for 200 MPa, about 31 for 300 MPa | 100 | 10 | - | - | lyophilization | MW < 3 kDa | ABTS 30.6% | Guan et al., 2018 |
| Voandzeia subterranea | seed | alcalase | 1:100 | 8 | 50 | 4 | - | 90 | 15 | 4 | 2M HCl | lyophilization | n.d. | EC50 about 25 μg/mL | Arise et al., 2017 [76] |
| seed | pepsin | 1:100 | 2 | 37 | 4 | - | 90 | 15 | 4 | 2M NaOH | lyophilization | EC50 about 22 μg/mL | |||
| seed | trypsin | 1:100 | 8 | 37 | 4 | - | 90 | 15 | 4 | 2M HCl | lyophilization | EC50 22 μg/mL | |||
| Frumentum | corn gluten meal | alcalase | 9.13:100 | 8.6 | 50 | 2.5 | - | 100 | 10 | - | - | lyophilization | AGIPM, AGLPM, HALGA, and HAIGA H1: MW < 1 kDa) H2: 10 kDa < MW < 30 kDa | DPPH H1: 66.89% H2: 71.49% | Jiang et al., 2018 |
| Rice furfures | defatted rice bran | pepsin then trypsin | 1:100 | 1.5 then 7 | 37 | 2 then 2 | - | 95 | 10 | - | - | lyophilization | F1: MW < 3 kDa, F2: MW 3–5 kDa, and F3: MW 5–10 kDa) | F1;F2;F3 DPPH 66.25; 58.57; 43.98 µmoL Trolox equivalent/g ABTS 425.81; 430.12; 403.28 µmoL Trolox equivalent/g | Phongthai et al., 2018 |
| Pennisetum glaucum | seed | trypsin | 1:100 | 6.5 | 37 | 3 | - | 80 | 20 | - | - | lyophilization | SDRDLLGPNNQYLPK | DPPH 67.66%, ABTS 78.81%, Fe2+ chelating ability 51.20%, | Agrawal et al., 2016 [77] |
| Amaranthus hypochondriacus | seed | endogenous aspartic protease | - | 2 | 40 | 16 | 5.3 | 85 | 10 | - | - | lyophilization | n.d. | hydrolysate ORAC IC50 0.058 ABTS IC50 2.1 mg/mL | Sabbione et al., 2016 [78] |
| Cannabis sativa | seed | pepsin | 1:50 | 2 | 37 | 16 | 19.7 | 95 | 10 | - | - | - | Pepsin hydrolysis—high number of peptides (1000–1500; 2000–2500 Da) | n.d. | Aiello et al., 2017 |
| seed | trypsin | 1:50 | 8 | 37 | 16 | 46.6 | 95 | 10 | - | - | - | n.d. | |||
| seed | pancreatin | 1:50 | 8 | 37 | 16 | 47.5 | 95 | 10 | - | - | - | n.d. | |||
| seed | pepsin then the mixture of trypsin and pancreatin | 1:20 then 1:25 | 2 then 8.5 | 37 | 2 then 4 | 34 | 95 | 10 | - | - | - | n.d. | |||
| Tinospora cordifolia | stem | papain | 1:100 | 6.8 | 37 | 2 | - | 100 | 0.1 | - | - | lyophilization | VLYSTPVKMWEPGR; VITVVATAGSETMR; HIGININSR | SRCA 64.15% at 0.0125 mg/mL | Pachaiappan et al., 2018 |
| stem | pepsin | 1:3.33 | 2.2 | 37 | 2 | - | - | - | 8 | Na2CO3 | lyophilization | n.d. | DPPH about 52%—for trypsin hydrolysate | ||
| stem | trypsin and α-chymotrypsin | 1:5 | 7.8 then 8 | 37 | 3 | - | 100 | 0.1 | - | - | lyophilization | n.d. | DPPH 79.04%—for trypsin hydrolysate after 30 min digestion | ||
| Moringa oleifera | seed | trypsin | 1:5 | 7.8 | 37 | 2.5 and 5 | - | 80 | 10 | - | - | lyophilization | Peptide fraction > 10 kDa | ABTS 24.74; 32.81% | Garza et al., 2017 |
| seed | chymotrypsin | 1:5 | 7.8 | 37 | 2.5 and 5 | - | 80 | 10 | - | - | lyophilization | Peptide fraction > 10 kDa | ABTS 35.32; 37.87% | ||
| seed | pepsin–trypsin | 1:5 | 2 then 7.8 | 37 | 2.5 and 5 | - | 80 | 10 | - | - | lyophilization | Peptide fraction > 10 kDa | ABTS 29.15; 29.30% | ||
| Limonia acidissima | seed | pepsin | 2.5:100 | 2 | 37 | 42.41 | 39.82 | 100 | 10 | - | - | air-drying | n.d | DPPH 32.94% ABTS 88.18% | Sonawane et al., 2017 |
| Juglans regia L. | defatted walnut meal | pancreatin and viscozyme L | 0.8:100 | 7 | 55 | 16 | 6.6 | 95 | 15 | - | - | lyophilization | n.d |
ORAC 1752.98 μmol TE/g ABTS 237.94 μmol TE/g) at 0.4 mg/mL | Li et al., 2017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czelej, M.; Garbacz, K.; Czernecki, T.; Wawrzykowski, J.; Waśko, A. Protein Hydrolysates Derived from Animals and Plants—A Review of Production Methods and Antioxidant Activity. Foods 2022, 11, 1953. https://doi.org/10.3390/foods11131953
Czelej M, Garbacz K, Czernecki T, Wawrzykowski J, Waśko A. Protein Hydrolysates Derived from Animals and Plants—A Review of Production Methods and Antioxidant Activity. Foods. 2022; 11(13):1953. https://doi.org/10.3390/foods11131953
Chicago/Turabian StyleCzelej, Michał, Katarzyna Garbacz, Tomasz Czernecki, Jacek Wawrzykowski, and Adam Waśko. 2022. "Protein Hydrolysates Derived from Animals and Plants—A Review of Production Methods and Antioxidant Activity" Foods 11, no. 13: 1953. https://doi.org/10.3390/foods11131953
APA StyleCzelej, M., Garbacz, K., Czernecki, T., Wawrzykowski, J., & Waśko, A. (2022). Protein Hydrolysates Derived from Animals and Plants—A Review of Production Methods and Antioxidant Activity. Foods, 11(13), 1953. https://doi.org/10.3390/foods11131953







