Black Bean Pasta Meals with Varying Protein Concentrations Reduce Postprandial Glycemia and Insulinemia Similarly Compared to White Bread Control in Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Recruitment and Selection Criteria
2.2. Study Design
2.3. Testing Protocol
2.3.1. Test Day Procedures
2.3.2. Subjective Appetite, Sensory, and Gastrointestinal Surveys
2.3.3. Test Meals
Pasta preparation
Particle size distribution
2.4. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Study Power
3.3. Food Frequency Questionnaires (FFQs) and 24 h Food Logs
3.4. Subjective Appetite Measures
3.5. Sensory
3.6. Gastrointestinal Symptoms
3.7. Postprandial Glucose Response
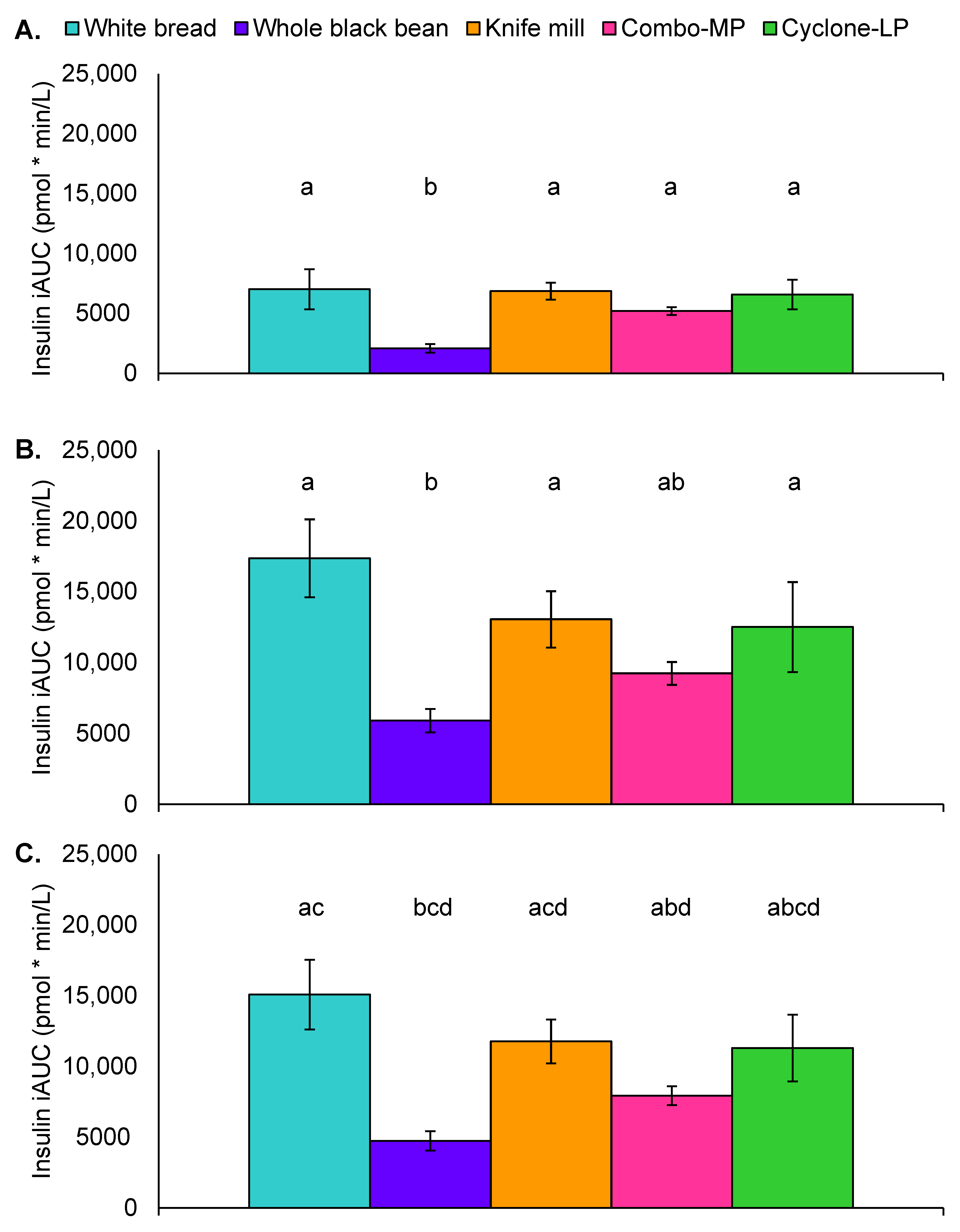
3.8. Postprandial Insulin Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levenstein, H. American response to Italian food, 1880–1930. Food Foodways 1985, 1, 1–23. [Google Scholar] [CrossRef]
- National Pasta Association. Pasta 101. Pasta Facts. Available online: https://sharethepasta.org/pasta-101/pasta-iq/pasta-facts/ (accessed on 19 January 2022).
- Papanikolaou, Y. Pasta consumption is linked to greater nutrient intakes and improved diet quality in american children and adults, and beneficial weight-related outcomes only in adult females. Front. Nutr. 2020, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; Debruyn, J.; Ronksley, P.E.; Shaheen, A.A.; et al. Incidence of celiac disease is increasing over time: A systematic review and meta-analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef]
- Johnston, C.S.; Snyder, D.; Smith, C. Commercially available gluten-free pastas elevate postprandial glycemia in comparison to conventional wheat pasta in healthy adults: A double-blind randomized crossover trial. Food Funct. 2017, 8, 3139–3144. [Google Scholar] [CrossRef]
- Hooper, S.D.; Glahn, R.P.; Cichy, K.A. Single varietal dry bean (Phaseolus vulgaris L.) pastas: Nutritional profile and consumer acceptability. Plant Foods Hum. Nutr. 2019, 74, 342–349. [Google Scholar] [CrossRef]
- Davitt, E.D.; Winham, D.M.; Heer, M.M.; Shelley, M.C.; Knoblauch, S.T. Predictors of Plant-Based Alternatives to Meat Consumption in Midwest University Students. J. Nutr. Educ. Behav. 2021, 53, 564–572. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Havemeier, S.; Erickson, J.; Slavin, J. Dietary guidance for pulses: The challenge and opportunity to be part of both the vegetable and protein food groups. Ann. N. Y. Acad. Sci. 2017, 1392, 58–66. [Google Scholar] [CrossRef]
- Mollard, R.C.; Zykus, A.; Luhovyy, B.L.; Nunez, M.F.; Wong, C.L.; Anderson, G.H. The acute effects of a pulse-containing meal on glycaemic responses and measures of satiety and satiation within and at a later meal. Br. J. Nutr. 2012, 108, 509–517. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Thompson, A.M.; Tees, M.T.; Nguyen, C.H.; Winham, D.M. Non-soy legume consumption lowers cholesterol levels: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Lam, H.-M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- Ha, V.; Sievenpiper, J.L.; de Souza, R.J.; Jayalath, V.H.; Mirrahimi, A.; Agarwal, A.; Chiavaroli, L.; Mejia, S.B.; Sacks, F.M.; Di Buono, M.; et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: A systematic review and meta-analysis of randomized controlled trials. Can. Med. Assoc. J. 2014, 186, E252–E262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; De Souza, R.J.; Choo, V.L.; Ha, V.; Cozma, A.I.; Chiavaroli, L.; Mirrahimi, A.; Mejia, S.B.; Di Buono, M.; Bernstein, A.M.; et al. Effects of dietary pulse consumption on body weight: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 1213–1223. [Google Scholar] [CrossRef] [Green Version]
- Hartman, T.J.; Albert, P.S.; Zhang, Z.; Bagshaw, D.; Kris-Etherton, P.M.; Ulbrecht, J.; Miller, C.K.; Bobe, G.; Colburn, N.H.; Lanza, E. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J. Nutr. 2010, 140, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, A.M.; Winham, D.M.; Thompson, S. V Phaseolus beans: Impact on glycaemic response and chronic disease risk in human subjects. Br. J. Nutr. 2012, 108 (Suppl. S1), S52–S65. [Google Scholar] [CrossRef] [Green Version]
- McCrory, M.A.; Hamaker, B.R.; Lovejoy, J.C.; Eichelsdoerfer, P.E. Pulse consumption, satiety, and weight management. Adv. Nutr. 2010, 1, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Darmadi-Blackberry, I.; Wahlqvist, M.L.; Kouris-Blazos, A.; Steen, B.; Lukito, W.; Horie, Y.; Horie, K. Legumes: The most important dietary predictor of survival in older people of different ethnicities. Asia Pac. J. Clin. Nutr. 2004, 13, 217–220. [Google Scholar]
- Dietary Guidelines for Americans, 2020–2025 and Online Materials|Dietary Guidelines for Americans. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials (accessed on 23 November 2021).
- Mitchell, D.C.; Marinangeli, C.P.F.; Pigat, S.; Bompola, F.; Campbell, J.; Pan, Y.; Curran, J.M.; Cai, D.J.; Jaconis, S.Y.; Rumney, J. Pulse intake improves nutrient density among us adult consumers. Nutrients 2021, 13, 2668. [Google Scholar] [CrossRef]
- Turco, I.; Bacchetti, T.; Morresi, C.; Padalino, L.; Ferretti, G. Polyphenols and the glycaemic index of legume pasta. Food Funct. 2019, 10, 5931–5938. [Google Scholar] [CrossRef]
- Trevisan, S.; Pasini, G.; Simonato, B. An overview of expected glycaemic response of one ingredient commercial gluten free pasta. LWT 2019, 109, 13–16. [Google Scholar] [CrossRef]
- Giuberti, G.; Gallo, A.; Cerioli, C.; Fortunati, P.; Masoero, F. Cooking quality and starch digestibility of gluten free pasta using new bean flour. Food Chem. 2015, 175, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Flores-Silva, P.C.; Rodriguez-Ambriz, S.L.; Bello-Pérez, L.A. Gluten-free snacks using plantain-chickpea and maize blend: Chemical composition, starch digestibility, and predicted glycemic index. J. Food Sci. 2015, 80, C961–C966. [Google Scholar] [CrossRef] [PubMed]
- Tazrart, K.; Zaidi, F.; Lamacchia, C.; Haros, M. Effect of durum wheat semolina substitution with broad bean flour (Vicia faba) on the Maccheronccini pasta quality. Eur. Food Res. Technol. 2016, 242, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Winham, D.M.; Hutchins, A.M.; Melde, C.L. Pinto bean, navy bean, and black-eyed pea consumption do not significantly lower the glycemic response to a high glycemic index treatment in normoglycemic adults. Nutr. Res. 2007, 27, 535–541. [Google Scholar] [CrossRef]
- Thompson, S.V.; Winham, D.M.; Hutchins, A.M. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: A cross-over study. Nutr. J. 2012, 11, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winham, D.M.; Hutchins, A.M.; Thompson, S.V. Glycemic response to black beans and chickpeas as part of a rice meal: A randomized cross-over trial. Nutrients 2017, 9, 1095. [Google Scholar] [CrossRef] [Green Version]
- Noah, L.; Guillon, F.; Bouchet, B.; Buléon, A.; Molis, C.; Gratas, M.; Champ, M. Digestion of Carbohydrate from White Beans (Phaseolus vulgaris L.) in Healthy Humans. J. Nutr. 1998, 128, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Wursch, P.; Del Vedovo, S.; Koellreutter, B. Cell structure and starch nature as key determinants of the digestion rate of starch in legume. Am. J. Clin. Nutr. 1986, 43, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Golay, A.; Coulston, A.M.; Hollenbeck, C.B.; Kaiser, L.L.; Würsch, P.; Reaven, G.M. Comparison of metabolic effects of white beans processed into two different physical forms. Diabetes Care 1986, 9, 260–266. [Google Scholar] [CrossRef]
- Tovar, J.; Granfeldt, Y.; Björck, I.M. Effect of processing on blood glucose and insulin responses to starch in legumes. J. Agric. Food Chem. 1992, 40, 1846–1851. [Google Scholar] [CrossRef]
- Courcelles, J. Pulse Flours—The fastest growing flour in the industry | Miller Magazine. Available online: https://millermagazine.com/blog/pulse-floursthe-fastest-growing-flour-in-the-industry-4096 (accessed on 18 January 2022).
- Thakur, S.; Scanlon, M.G.; Tyler, R.T.; Milani, A.; Paliwal, J. Pulse flour characteristics from a wheat flour miller’s perspective: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 775–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanlon, M.G.; Thakur, S.; Tyler, R.T.; Milani, A.; Der, T.; Paliwal, J. The critical role of milling in pulse ingredient functionality. Cereal Foods World 2018, 63, 201–206. [Google Scholar] [CrossRef] [Green Version]
- De Lauzon, B.; Romon, M.; Deschamps, V.; Lafay, L.; Borys, J.M.; Karlsson, J.; Ducimetière, P.; Charles, M.A. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J. Nutr. 2004, 134, 2372–2380. [Google Scholar] [CrossRef]
- Block, G.; Gillespie, C.; Rosenbaum, E.H.; Jenson, C. A rapid food screener to assess fat and fruit and vegetable intake. Am. J. Prev. Med. 2000, 18, 284–288. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Mattes, R.D. Effects of a combination fiber system on appetite and energy intake in overweight humans. Physiol. Behav. 2007, 90, 705–711. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781482216912. [Google Scholar]
- Winham, D.M.; Hutchins, A.M. Perceptions of flatulence from bean consumption among adults in 3 feeding studies. Nutr. J. 2011, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, A.; Winham, D.M. Pinto beans and green beans result in comparable glycemic control in adults with type 2 diabetes: A randomized pilot trial pinto beans and green beans result in comparable glycemic control in adults. Food Sci. Nutr. Technol. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- De La Hera, E.; Gomez, M.; Rosell, C.M. Particle size distribution of rice flour affecting the starch enzymatic hydrolysis and hydration properties. Carbohydr. Polym. 2013, 98, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AACC. Approved Method 66-50.01 Pasta and Noodle Cooking Quality—Firmness, 10th ed.; Cereals & Grains Association: St. Paul, MN, USA, 2000. [Google Scholar]
- USDA Agricultural Research Service. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 12 July 2020).
- NutritionQuest Web-Based Wellness Solutions. Free Assessment Tools For Individuals—Wellness—NutritionQuest. Available online: https://www.nutritionquest.com/wellness/free-assessment-tools-for-individuals/ (accessed on 11 May 2020).
- Duh, M.S.; Lefebvre, P.; Fastenau, J.; Piech, C.T.; Waltzman, R.J. Assessing the clinical benefits of erythropoietic agents using area under the hemoglobin change curve. Oncologist 2005, 10, 438–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverri, E.J.; Randolph, J.M.; Kappagoda, C.T.; Park, E.; Edirisinghe, I.; Burton-Freeman, B.M. Assessing beans as a source of intrinsic fiber on satiety in men and women with metabolic syndrome. Appetite 2017, 118, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Novotny, J.A.; Bornhorst, G.M.; Baer, D.J. Food processing and structure impact the metabolizable energy of almonds. Food Funct. 2016, 7, 4231–4238. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Walnuts consumed by healthy adults provide less available energy than predicted by the atwater factors. J. Nutr. 2016, 146, 9–13. [Google Scholar] [CrossRef]
- Granfeldt, Y.; Björck, I. Glycemic response to starch in pasta: A study of mechanisms of limited enzyme availability. J. Cereal Sci. 1991, 14, 47–61. [Google Scholar] [CrossRef]
- Korczak, R.; Timm, D.; Ahnen, R.; Thomas, W.; Slavin, J.L. High protein pasta is not more satiating than high fiber pasta at a lunch meal, nor does it decrease mid-afternoon snacking in healthy men and women. J. Food Sci. 2016, 81, S2240–S2245. [Google Scholar] [CrossRef]
- Kristensen, M.; Jensen, M.G.; Riboldi, G.; Petronio, M.; Bügel, S.; Toubro, S.; Tetens, I.; Astrup, A. Wholegrain vs. refined wheat bread and pasta. effect on postprandial glycemia, appetite, and subsequent ad libitum energy intake in young healthy adults. Appetite 2010, 54, 163–169. [Google Scholar] [CrossRef]
- Winham, D.M.; Davitt, E.D.; Heer, M.M.; Shelley, M.C. Pulse knowledge, attitudes, practices, and cooking experience of midwestern us university students. Nutrients 2020, 12, 3499. [Google Scholar] [CrossRef]
- Fulgoni, V.L.; Bailey, R. Association of pasta consumption with diet quality and nutrients of public health concern in adults: National health and nutrition examination survey 2009–2012. Curr. Dev. Nutr. 2017, 1, e001271. [Google Scholar] [CrossRef] [Green Version]





| Mill Type | d (0.1) µm | d (0.5) µm | d (0.9) µm | MV µm |
|---|---|---|---|---|
| Knife mill | 11.3 a | 62.4 a | 251.3 a | 104.2 a |
| Compression/Decompression | ||||
| Combo-MP | 2.4 c | 16.7 c | 33.4 b | 17.2 c |
| Cyclone-LP | 2.8 b | 20.4 b | 52.3 b | 26.2 b |
| Characteristics | White Bread | Whole Black Beans | Knife Mill | Combo-MP | Cyclone-LP |
|---|---|---|---|---|---|
| Total weight (g) | 111.5 | 409.6 | 451.5 | 450.5 | 417.4 |
| Pasta Sauce (g) | - | 213.0 | 213.0 | 213.0 | 213.0 |
| Bread (g) | 111.5 | - | - | - | - |
| Pulses (g) | - | 196.6 | 238.5 | 237.5 | 204.4 |
| Energy (kcal) | 289.4 | 445.8 | 426.0 | 440.3 | 388.2 |
| Total Carbohydrate (g) | 52.6 | 78.2 | 72.1 | 72.4 | 69.5 |
| Fiber (g) | 2.6 a | 28.2 b | 22.1 b | 22.4 b | 19.5 b |
| Available CHO (g) | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Pasta Sauce (g) | - | 20.0 | 20.0 | 20.0 | 20.0 |
| Bread (g) | 50.0 | - | - | - | - |
| Pulses (g) | - | 30.0 | 30.0 | 30.0 | 30.0 |
| Protein (g) | 10.2 a,c | 20.9 b,d | 19.5 b,d | 20.5 b,d | 13.7 b,c |
| Fat (g) | 4.3 | 5.4 | 6.5 | 7.5 | 6.1 |
| Total Starch (g) | 32.6 | 21.5 | 22.0 | 21.1 | 20.8 |
| Meal | Appearance | Aroma | Flavor | Texture | Meal Size |
|---|---|---|---|---|---|
| White bread control | 5.5 ± 1.5 a | 6.1 ± 1.1 | 6.4 ± 1.3 | 6.6 ± 1.3 a | 4.8 ± 1.6 |
| Whole black beans 2 | 3.9 ± 1.2 b | 5.6 ± 1.0 | 5.6 ± 1.2 | 4.6 ± 1.4 b | 5.5 ± 1.4 |
| Knife mill | 5.3 ± 2.0 a | 6.1 ± 1.0 | 6.3 ± 2.2 | 5.9 ± 1.9 a,c | 5.9 ± 1.3 |
| Combo-MP 3 | 5.4 ± 2.0 a | 6.3 ± 1.5 | 6.1 ± 1.8 | 5.5 ± 2.0 a,b | 5.7 ± 1.0 |
| Cyclone-LP | 5.4 ± 1.7 a | 6.5 ± 1.2 | 6.3 ± 1.6 | 5.8 ± 1.6 a,c | 5.5 ± 1.0 |
| Pasta sauce alone | --- | --- | 6.6 ± 1.7 | 6.6 ± 1.4 | --- |
| Pre-Test | White Bread | Black Beans | Knife Mill | Combo-MP | Cyclone-LP | |
|---|---|---|---|---|---|---|
| Flatulence | ||||||
| No Change | 83.8 (62) | 86.7 (13) | 46.7 (7) | 46.7 (7) | 44.4 (8) | 70.6 (12) |
| Increased | 12.2 (9) | 13.3 (2) | 46.7 (7) | 46.7 (7) | 50.0 (9) | 29.4 (5) |
| Decreased | 4.1 (3) | 0 | 6.7 (1) | 6.7 (1) | 5.6 (1) | 0 |
| Bloating | ||||||
| No Change | 85.1 (63) | 73.3 (11) | 66.7 (10) | 71.4 (10) | 83.3 (15) | 76.5 (13) |
| Increased | 13.5 (10) | 26.7 (4) | 33.3 (5) | 14.3 (2) | 16.7 (3) | 23.5 (4) |
| Decreased | 1.4 (1) | 0 | 0 | 14.3 (2) | 0 | 0 |
| Stool frequency | ||||||
| No Change | 82.8 (53) | 78.6 (11) | 73.3 (11) | 53.3 (8) | 77.8 (14) | 88.2 (15) |
| Increased | 7.8 (5) | 7.1 (1) | 13.3 (2) | 40.0 (6) | 16.7 (3) | 11.8 (2) |
| Decreased | 9.4 (6) | 14.3 (2) | 13.3 (2) | 6.7 (1) | 5.6 (1) | 0 |
| Stool consistency | ||||||
| No Change | 89.1 (57) | 85.7 (12) | 93.3 (14) | 73.3 (11) | 77.8 (14) | 88.2 (15) |
| More Loose | 9.4 (6) | 0 | 6.7 (1) | 26.7 (4) | 11.1 (2) | 5.9 (1) |
| More Firm | 1.6 (1) | 14.3 (2) | 0 | 0 | 11.1 (2) | 5.9 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winham, D.M.; Thompson, S.V.; Heer, M.M.; Davitt, E.D.; Hooper, S.D.; Cichy, K.A.; Knoblauch, S.T. Black Bean Pasta Meals with Varying Protein Concentrations Reduce Postprandial Glycemia and Insulinemia Similarly Compared to White Bread Control in Adults. Foods 2022, 11, 1652. https://doi.org/10.3390/foods11111652
Winham DM, Thompson SV, Heer MM, Davitt ED, Hooper SD, Cichy KA, Knoblauch ST. Black Bean Pasta Meals with Varying Protein Concentrations Reduce Postprandial Glycemia and Insulinemia Similarly Compared to White Bread Control in Adults. Foods. 2022; 11(11):1652. https://doi.org/10.3390/foods11111652
Chicago/Turabian StyleWinham, Donna M., Sharon V. Thompson, Michelle M. Heer, Elizabeth D. Davitt, Sharon D. Hooper, Karen A. Cichy, and Simon T. Knoblauch. 2022. "Black Bean Pasta Meals with Varying Protein Concentrations Reduce Postprandial Glycemia and Insulinemia Similarly Compared to White Bread Control in Adults" Foods 11, no. 11: 1652. https://doi.org/10.3390/foods11111652
APA StyleWinham, D. M., Thompson, S. V., Heer, M. M., Davitt, E. D., Hooper, S. D., Cichy, K. A., & Knoblauch, S. T. (2022). Black Bean Pasta Meals with Varying Protein Concentrations Reduce Postprandial Glycemia and Insulinemia Similarly Compared to White Bread Control in Adults. Foods, 11(11), 1652. https://doi.org/10.3390/foods11111652








