Effect of Fixation Methods on Biochemical Characteristics of Green Teas and Their Lipid-Lowering Effects in a Zebrafish Larvae Model
Abstract
:1. Introduction
2. Materials and Methods
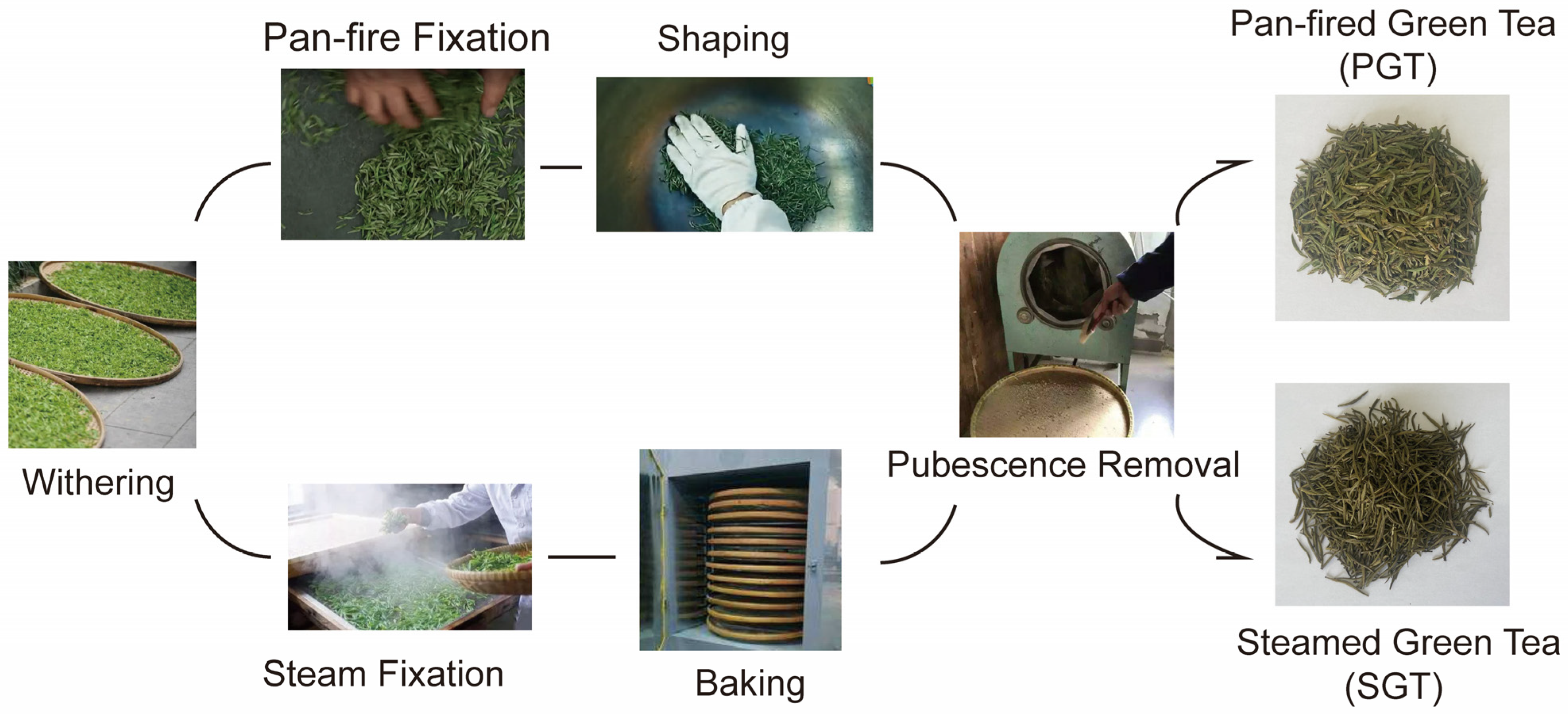
2.1. Materials
2.2. Analysis of Volatile Compounds
2.3. Determination of Amino Acids
2.4. Quantification of Gallic Acid, Catechins and Alkaloids
2.5. Taste Evaluation by Electronic Tongue
2.6. The Lipid-Lowering Effect Based on a Zebrafish Larvae Model
2.7. Statistical Analysis
3. Results and Discussion
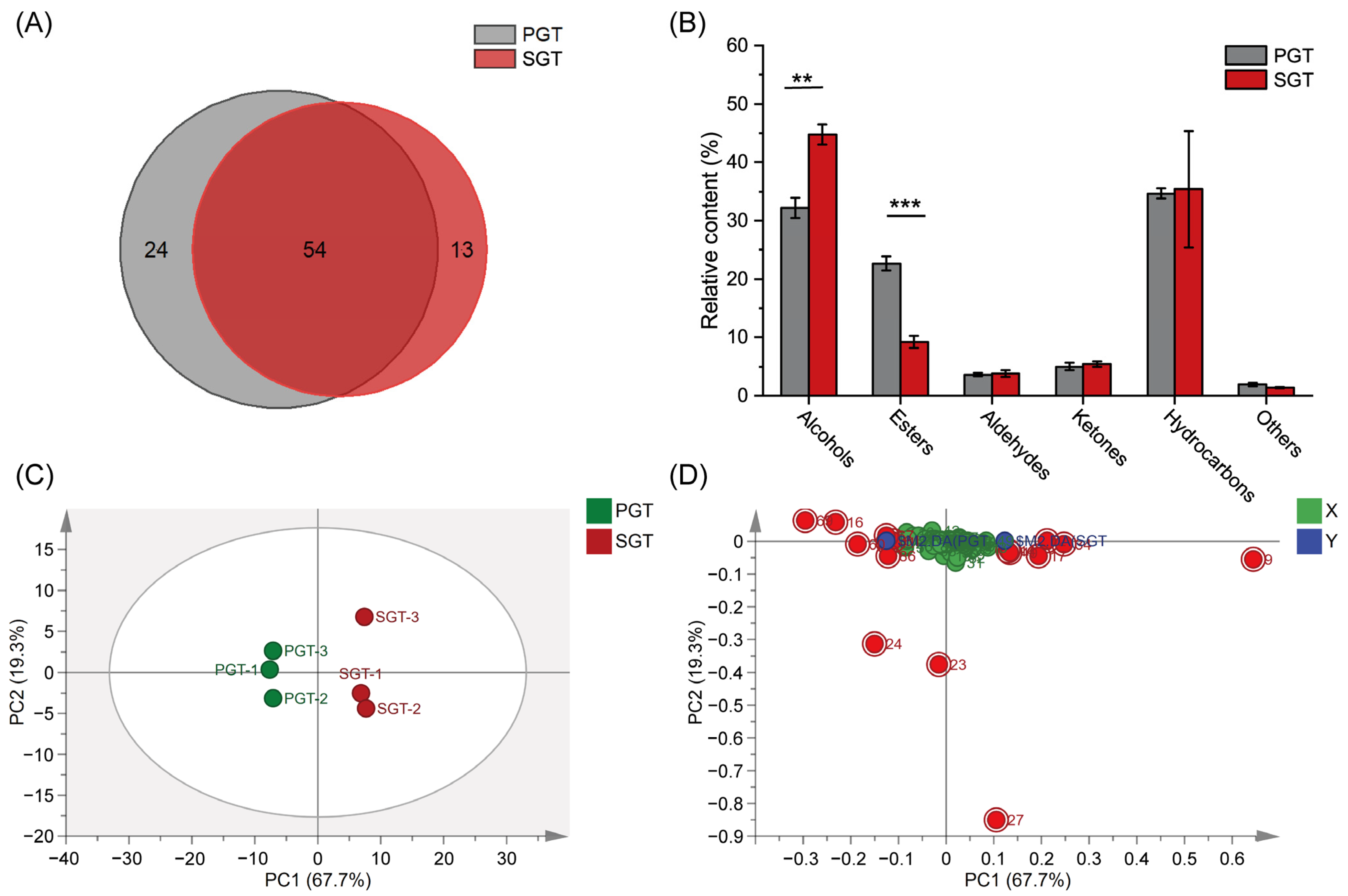
3.1. Effect of Fixation Methods on Volatile Profiles
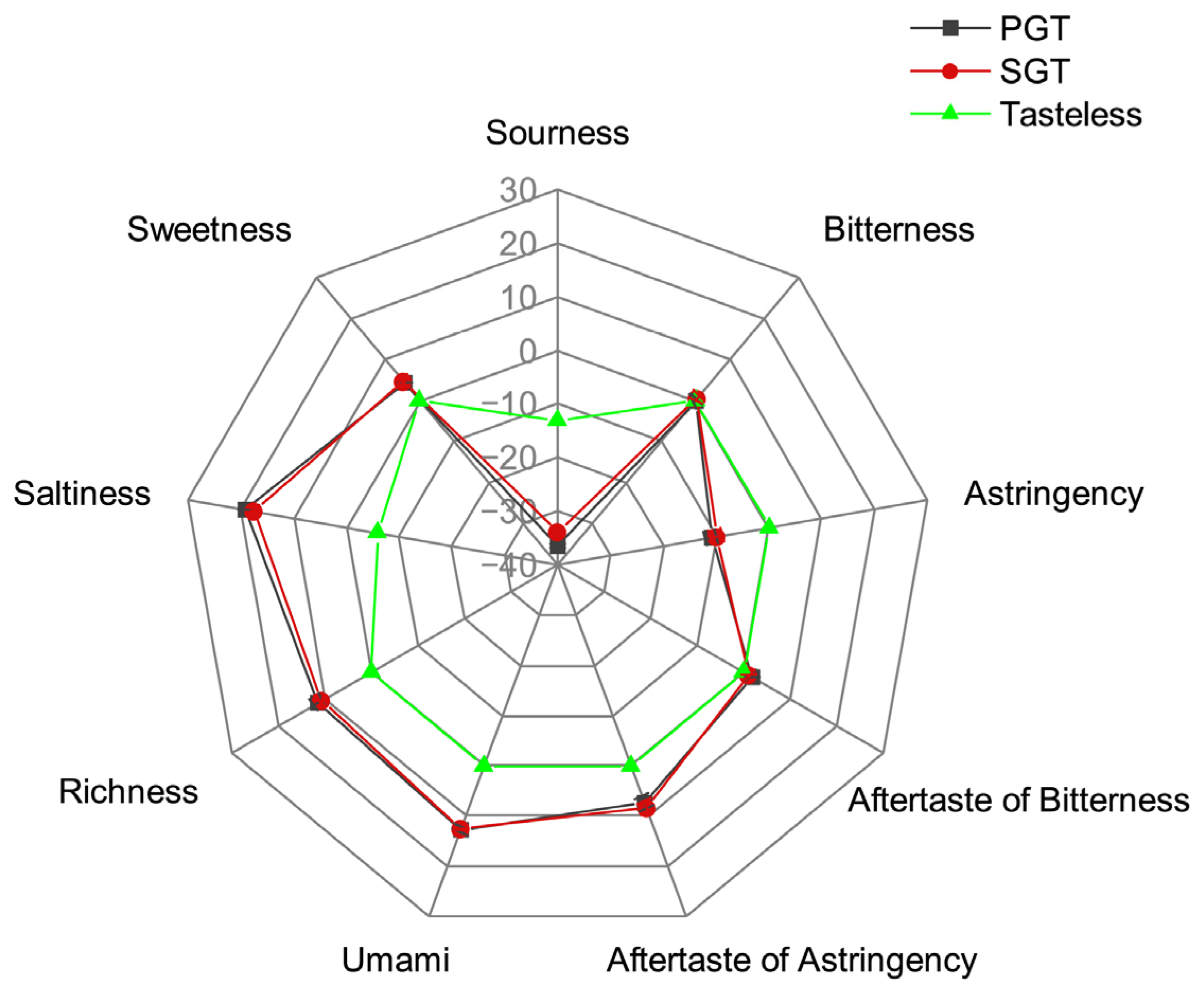
3.2. Effect of Fixation Methods on Taste Characteristics
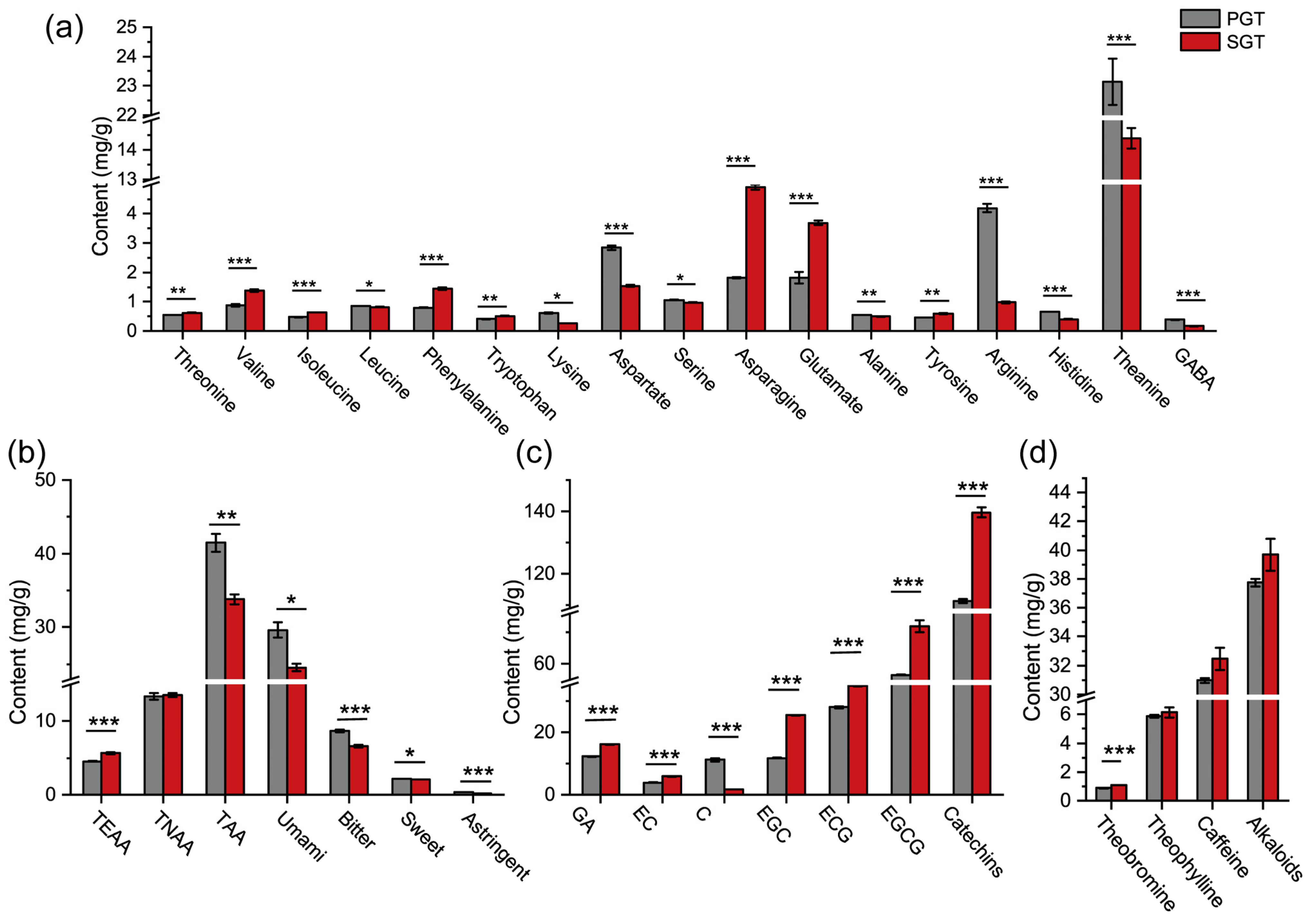
3.3. Effect of Fixation Methods on Chemical Compositions
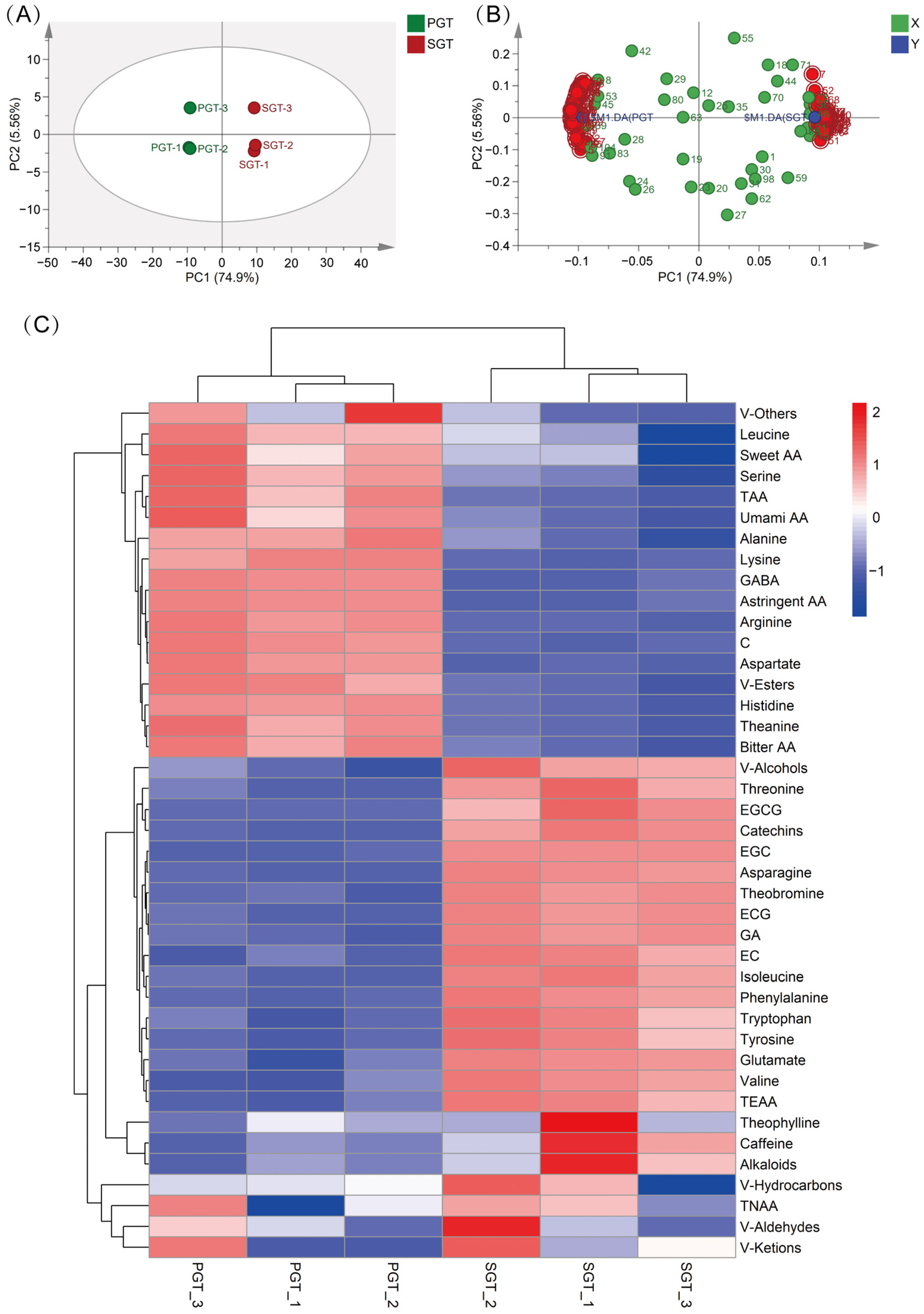
3.4. Multivariate Statistical Analysis of Biochemical Data
3.5. Effect of Fixation Methods on Lipid-Lowering Activity in a Zebrafish Larvae Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reygaert, W.C. An Update on the Health Benefits of Green Tea. Beverages 2017, 3, 6. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutu. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, Y.P.; Zhong, K.; Gao, H. Comprehensive evaluation of the composition of Mingshan Laochuancha green tea and demonstration of hypolipidemic activity in a zebrafish obesity model. RSC Adv. 2019, 9, 41269–41279. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hua, J.; Yuan, H.; Deng, Y.; Zhou, Q.; Yang, Y.; Dong, C.; Zeng, J.; Jiang, Y. Investigation on green tea lipids and their metabolic variations during manufacturing by nontargeted lipidomics. Food Chem. 2021, 339, 128114. [Google Scholar] [CrossRef]
- Yue, P.; Yuan, D. Effects of different fixation methods on the quality of green tea. Trans. Chin. Soc. Agri. Eng. 2009, 25, 275–279. [Google Scholar]
- Wang, S.; Gong, Z.; Chen, X.; Liu, Y.; Lu, S. Research and prospects on fresh tea leaves fixing process in rencent five years. Hubei Agri. Sci. 2017, 56, 2008–2011. [Google Scholar]
- Han, Z.X.; Rana, M.M.; Liu, G.F.; Gao, M.J.; Li, D.X.; Wu, F.G.; Li, X.B.; Wan, X.C.; Wei, S. Green tea flavour determinants and their changes over manufacturing processes. Food Chem. 2016, 212, 739–748. [Google Scholar] [CrossRef]
- Wang, H.; Hua, J.; Jiang, Y.; Yang, Y.; Wang, J.; Yuan, H. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Res. Int. 2020, 136, 109479. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Y.; Chen, J.; Wang, F.; Feng, Z.; Yin, J.; Zeng, L.; Xu, Y. Effects of baking treatment on the sensory quality and physicochemical properties of green tea with different processing methods. Food Chem. 2022, 380, 132217. [Google Scholar] [CrossRef]
- Zaker, B.S.K.; Saghebjoo, M.; Islami, F. Effectiveness of high-intensity interval training and high-protein diet on TNF-α protein level in colon tissue of obese male rats: The importance of diet modifying. Obes. Med. 2022, 31, 100403. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhao, Y.; Zhu, H.; Liang, N.; Ma, K.Y.; Lei, L.; He, W.; et al. Beneficial effects of tea water extracts on the body weight and gut microbiota in C57BL/6J mice fed with a high-fat diet. Food Funct. 2019, 10, 2847–2860. [Google Scholar] [PubMed]
- Zheng, G.D.; Sayama, K.; Okubo, T.; Juneja, L.R.; Oguni, I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo 2004, 18, 55–62. [Google Scholar] [PubMed]
- Xiao, Y.; Zhong, K.; Bai, J.; Wu, Y.; Zhang, J.; Gao, H. The biochemical characteristics of a novel fermented loose tea by Eurotium cristatum (MF800948) and its hypolipidemic activity in a zebrafish model. LWT-Food Sci. Technol. 2020, 117, 108629. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Y.; Zhong, K.; Wu, Y.; Gao, H. Delving into the Biotransformation Characteristics and Mechanism of Steamed Green Tea Fermented by Aspergillus niger PW-2 Based on Metabolomic and Proteomic Approaches. Foods 2022, 11, 865. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Y.; Zhong, K.; Bai, J.; Wu, Y.; Zhang, J.; Gao, H. Characteristics and chemical compositions of Pingwu Fuzhuan brick-tea, a distinctive post-fermentation tea in Sichuan province of China. Int. J. Food Prop. 2019, 22, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Wang, J.; Wei, Y.; Deng, W.; Wan, X.; Bao, G.; Xie, Z.; Ling, T.; Ning, J. Metabolomics based on UHPLC-Orbitrap-MS and global natural product social molecular networking reveals effects of time scale and environment of storage on the metabolites and taste quality of raw Pu-erh tea. J. Agric. Food. Chem. 2019, 67, 12084–12093. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.; Wan, X.; Zhu, H.; Liu, Q.; Wen, Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem. 2021, 341, 128230. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Q.; Xiang, L.; Liang, Y. Recent Advances in Volatiles of Teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef]
- Cui, J.; Zhai, X.; Guo, D.; Du, W.; Gao, T.; Zhou, J.; Schwab, W.G.; Song, C. Characterization of Key Odorants in Xinyang Maojian Green Tea and Their Changes during the Manufacturing Process. J. Agric. Food Chem. 2021, 70, 279–288. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Qin, Z.; Pang, X.; Chen, D.; Cheng, H.; Hu, X.; Wu, J. Evaluation of Chinese tea by the electronic nose and gas chromatography–mass spectrometry: Correlation with sensory properties and classification according to grade level. Food Res. Int. 2013, 53, 864–874. [Google Scholar] [CrossRef]
- Onetto, C.A.; Borneman, A.R.; Schmidt, S.A. Investigating the effects of Aureobasidium pullulans on grape juice composition and fermentation. Food Microbiol. 2020, 90, 103451. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Y.; Zhang, S.; Luo, L.; Zeng, L. Effect of brewing conditions on phytochemicals and sensory profiles of black tea infusions: A primary study on the effects of geraniol and beta-ionone on taste perception of black tea infusions. Food Chem. 2021, 354, 129504. [Google Scholar] [CrossRef]
- Horanni, R.; Engelhardt, U.H. Determination of amino acids in white, green, black, oolong, pu-erh teas and tea products. J. Food Compos. Anal. 2013, 31, 94–100. [Google Scholar] [CrossRef]
- Wang, H.; Hua, J.; Yu, Q.; Li, J.; Wang, J.; Deng, Y.; Yuan, H.; Jiang, Y. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chem. 2021, 363, 130131. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Z.Y.; Lu, M.L.; Li, P.L.; Tan, J.F.; Chen, M.; Lv, H.P.; Zhu, Y.; Zhang, Y.; Guo, L.; et al. Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtypes of white tea. Food Res. Int. 2018, 106, 909–919. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.Q.; Granato, D.; Xu, Y.Q.; Ho, C.T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Qi, G.; Liu, Q. Studies on the components of catechin in green tea treated by different de-enzyming process. J. Chin. Insti. Food Sci. Technol. 2001, 02, 1–4. [Google Scholar]
- Zhu, M.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.; Xu, W.; Li, J.; Lin, H.; Zhang, Z.; Xiao, J.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Fan, F.; Shi, M.; Nie, Y.; Zhao, Y.; Ye, J.; Liang, Y. Differential behaviors of tea catechins under thermal processing: Formation of non-enzymatic oligomers. Food Chem. 2016, 196, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, F.; Liu, Z.; Huang, J.; Xu, Z.; Li, Y.; Chen, J.; Gong, Y.; Yang, X. Comparison of catechins and volatile compounds among different types of tea using high performance liquid chromatograph and gas chromatograph mass spectrometer. Int. J. Food Sci. Tech. 2011, 46, 1406–1412. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J. Analysis of chemical components in green tea in relation with perceived quality, a case study with Longjing teas. Int. J. Food Sci. Technol. 2009, 44, 2476–2484. [Google Scholar] [CrossRef]
- Wang, Y.; Kan, Z.; Thompson, H.J.; Ling, T.; Ho, C.; Li, D.; Wan, X. Impact of Six Typical Processing Methods on the Chemical Composition of Tea Leaves Using a Single Camellia sinensis Cultivar, Longjing 43. J. Agric. Food. Chem. 2019, 67, 5423–5436. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Shimada, Y.; Nakayama, H.; Katsuzaki, H.; Kim, Y.; Chu, D.; Juneja, L.R.; Kuroyanagi, J.; Nishimura, N. Preventive Effects of Green Tea Extract against Obesity Development in Zebrafish. Molecules 2021, 26, 2627. [Google Scholar] [CrossRef]
- Koo, S.I.; Noh, S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Zhou, F.; Ouyang, J.; Wang, Q.; Li, Y.; Wu, J.; Huang, J.; Liu, Z. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Guo, L.; Zhu, R.; Yang, D.; Xiao, Y.; Wu, Y.; Zhong, K.; Huang, Y.; Gao, H. Effect of Fixation Methods on Biochemical Characteristics of Green Teas and Their Lipid-Lowering Effects in a Zebrafish Larvae Model. Foods 2022, 11, 1582. https://doi.org/10.3390/foods11111582
Li M, Guo L, Zhu R, Yang D, Xiao Y, Wu Y, Zhong K, Huang Y, Gao H. Effect of Fixation Methods on Biochemical Characteristics of Green Teas and Their Lipid-Lowering Effects in a Zebrafish Larvae Model. Foods. 2022; 11(11):1582. https://doi.org/10.3390/foods11111582
Chicago/Turabian StyleLi, Maoyun, Lulu Guo, Ruixue Zhu, Dongmei Yang, Yue Xiao, Yanping Wu, Kai Zhong, Yina Huang, and Hong Gao. 2022. "Effect of Fixation Methods on Biochemical Characteristics of Green Teas and Their Lipid-Lowering Effects in a Zebrafish Larvae Model" Foods 11, no. 11: 1582. https://doi.org/10.3390/foods11111582
APA StyleLi, M., Guo, L., Zhu, R., Yang, D., Xiao, Y., Wu, Y., Zhong, K., Huang, Y., & Gao, H. (2022). Effect of Fixation Methods on Biochemical Characteristics of Green Teas and Their Lipid-Lowering Effects in a Zebrafish Larvae Model. Foods, 11(11), 1582. https://doi.org/10.3390/foods11111582