Nutrition and Sensory Evaluation of Solid-State Fermented Brown Rice Based on Cluster and Principal Component Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Composition of BR
2.3. Solid-State Fermentation
2.3.1. Pretreatment of BR
2.3.2. Activation of Strains
2.3.3. Inoculation
2.4. Nutrition Characteristics of BR
2.4.1. Soluble (SDF) and Insoluble Dietary Fiber (IDF) Contents
2.4.2. Total Arabinoxylans Content (TAX)
2.4.3. β-Glucan Content
2.4.4. Phytic Acid Content
2.4.5. Total γ-Oryzanol Content (TOC)
2.4.6. Total Polyphenol Content and Total Antioxidant Capacity (TAC)
2.5. Sensory Characteristics of BR
2.5.1. Color Analysis
2.5.2. Sensory Assessment
2.6. Statistical Analysis
3. Results and Discussion
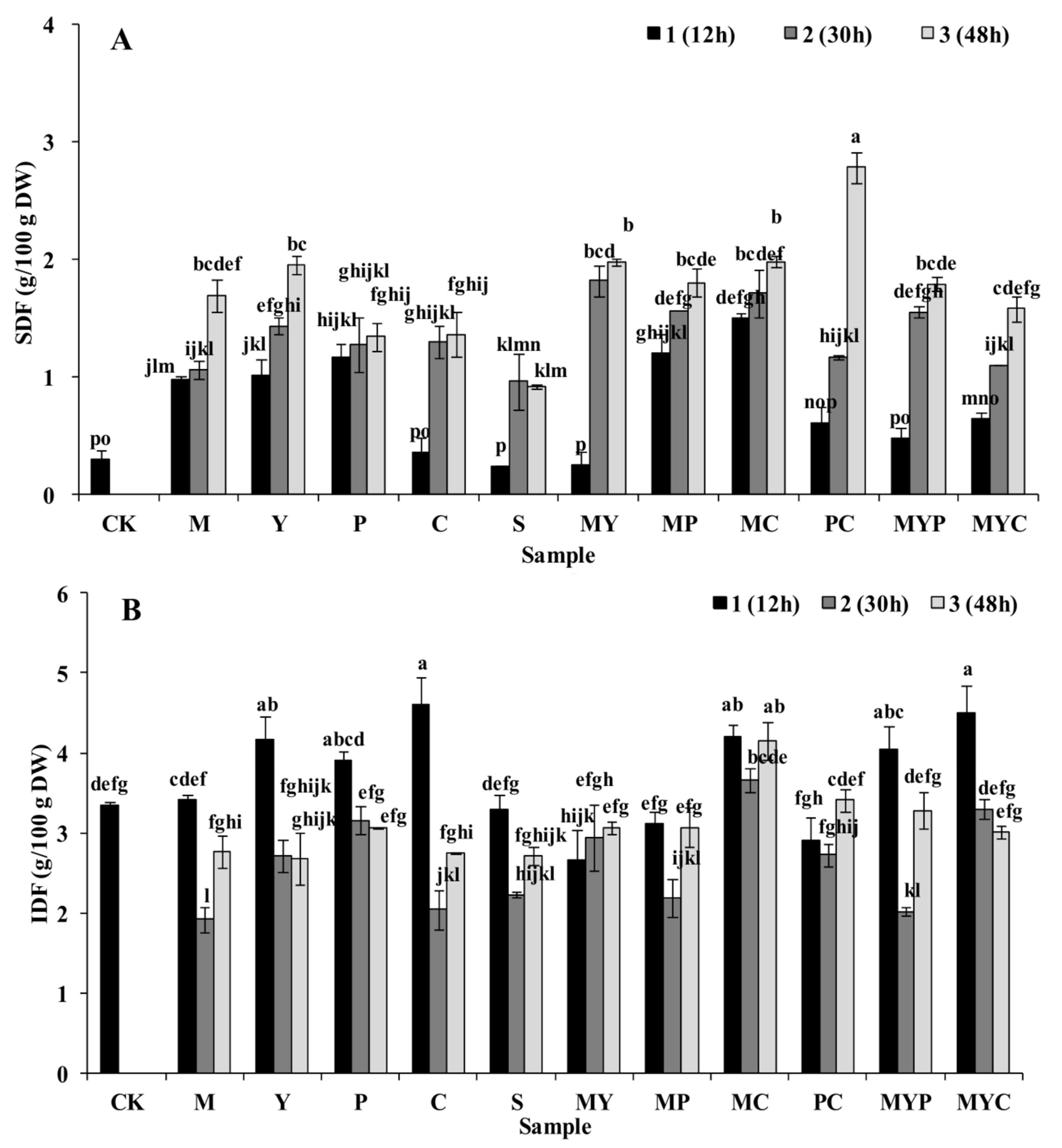
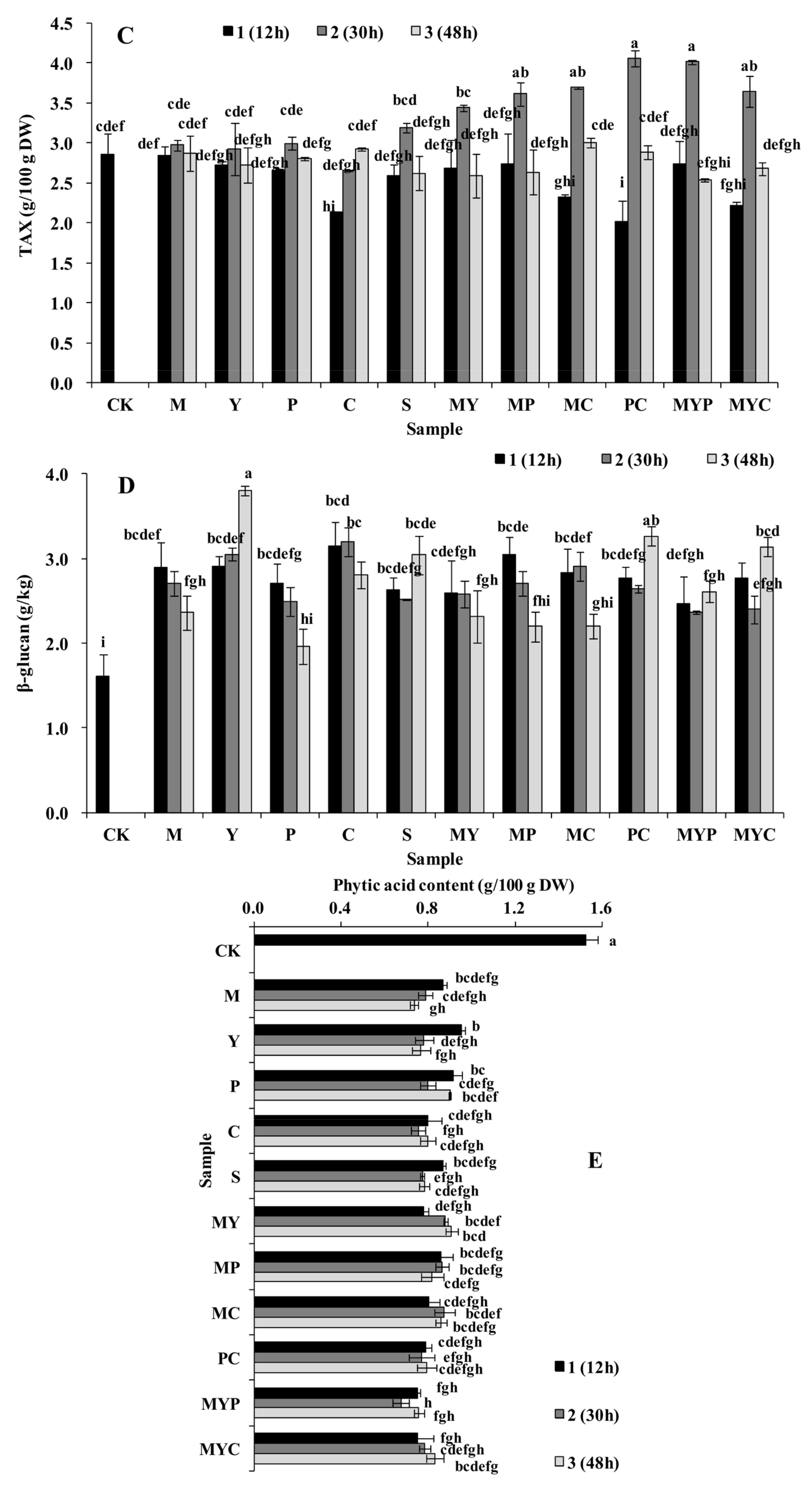
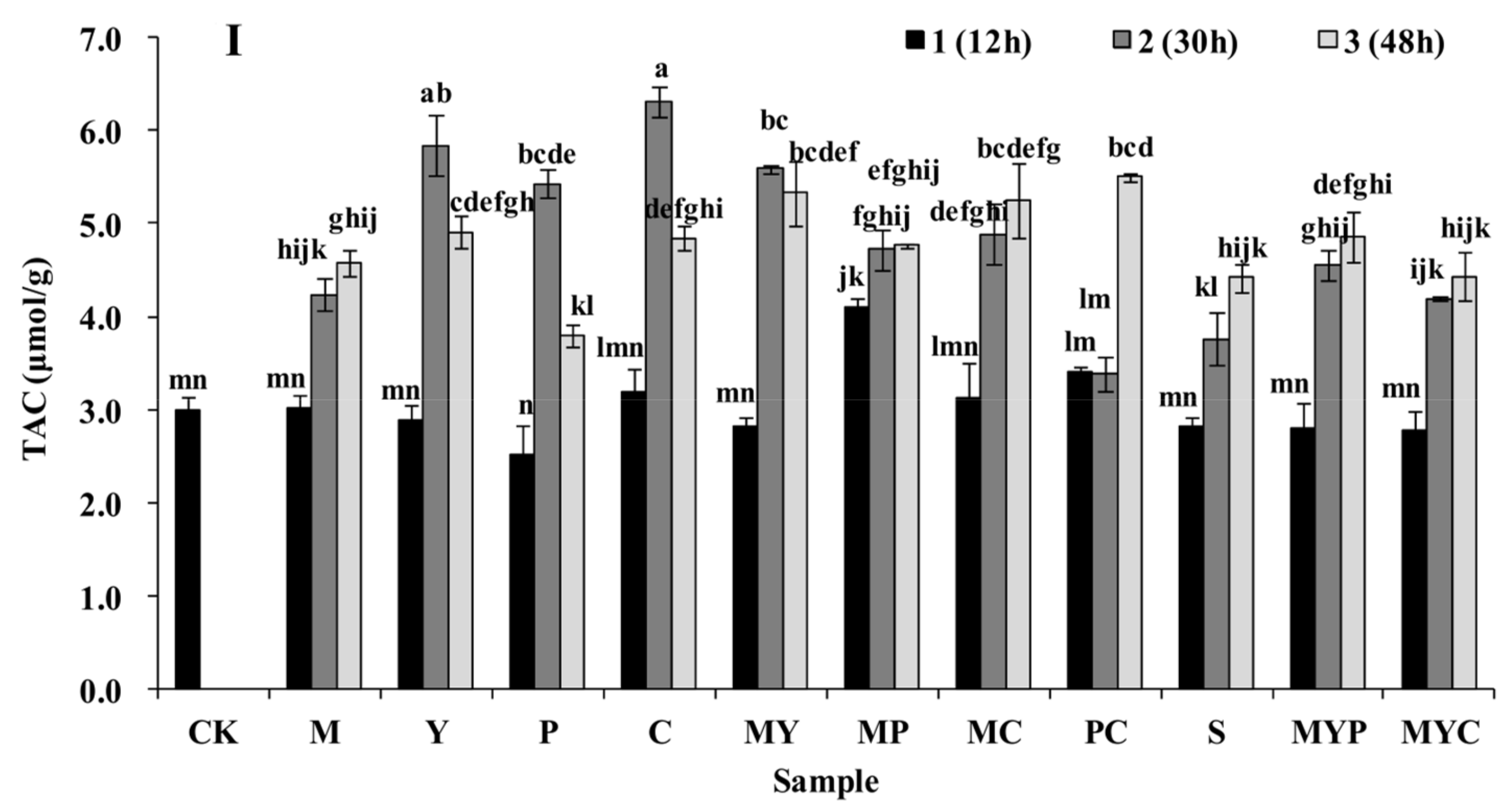
3.1. Nutrition Characteristics of BR
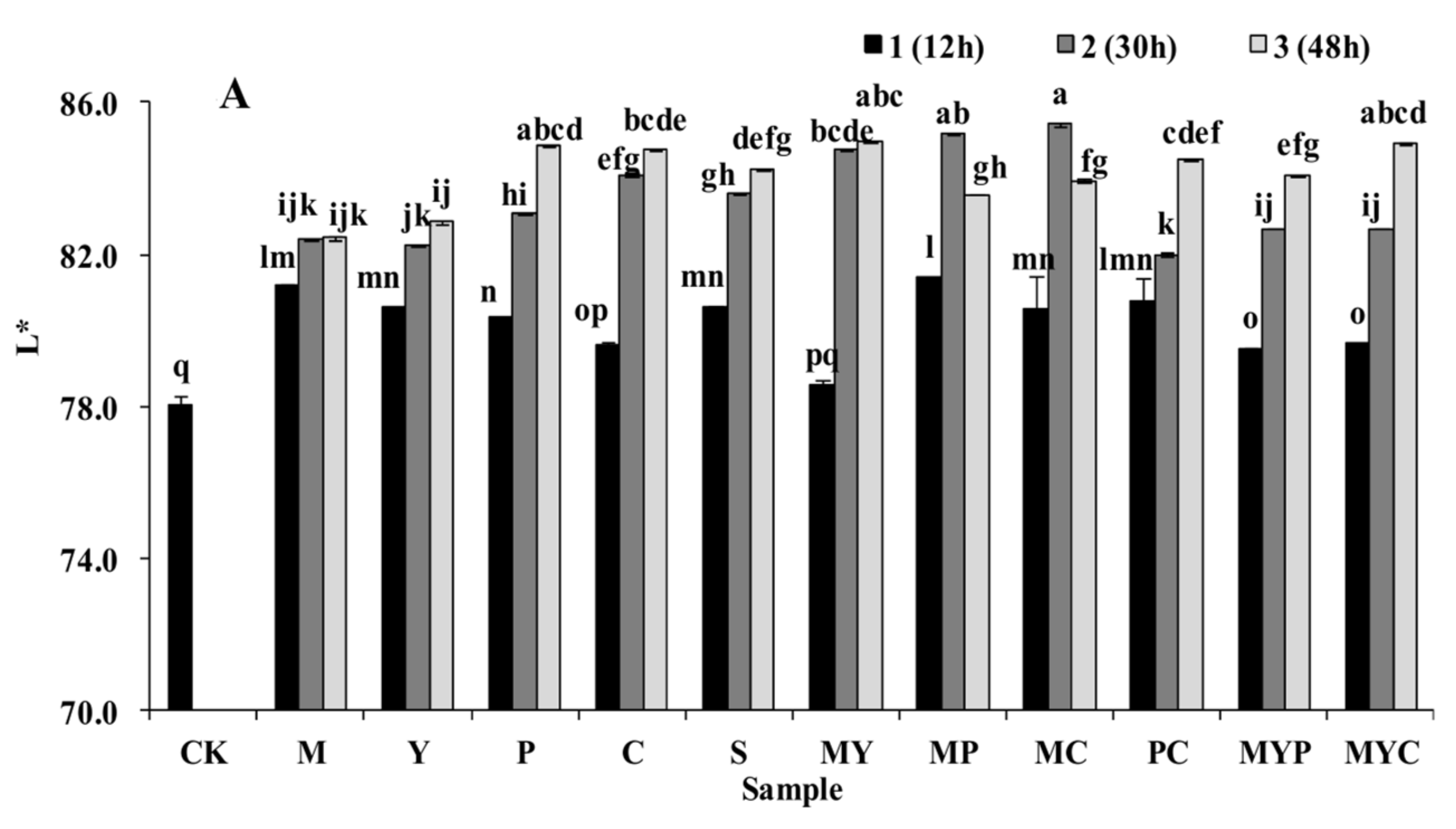
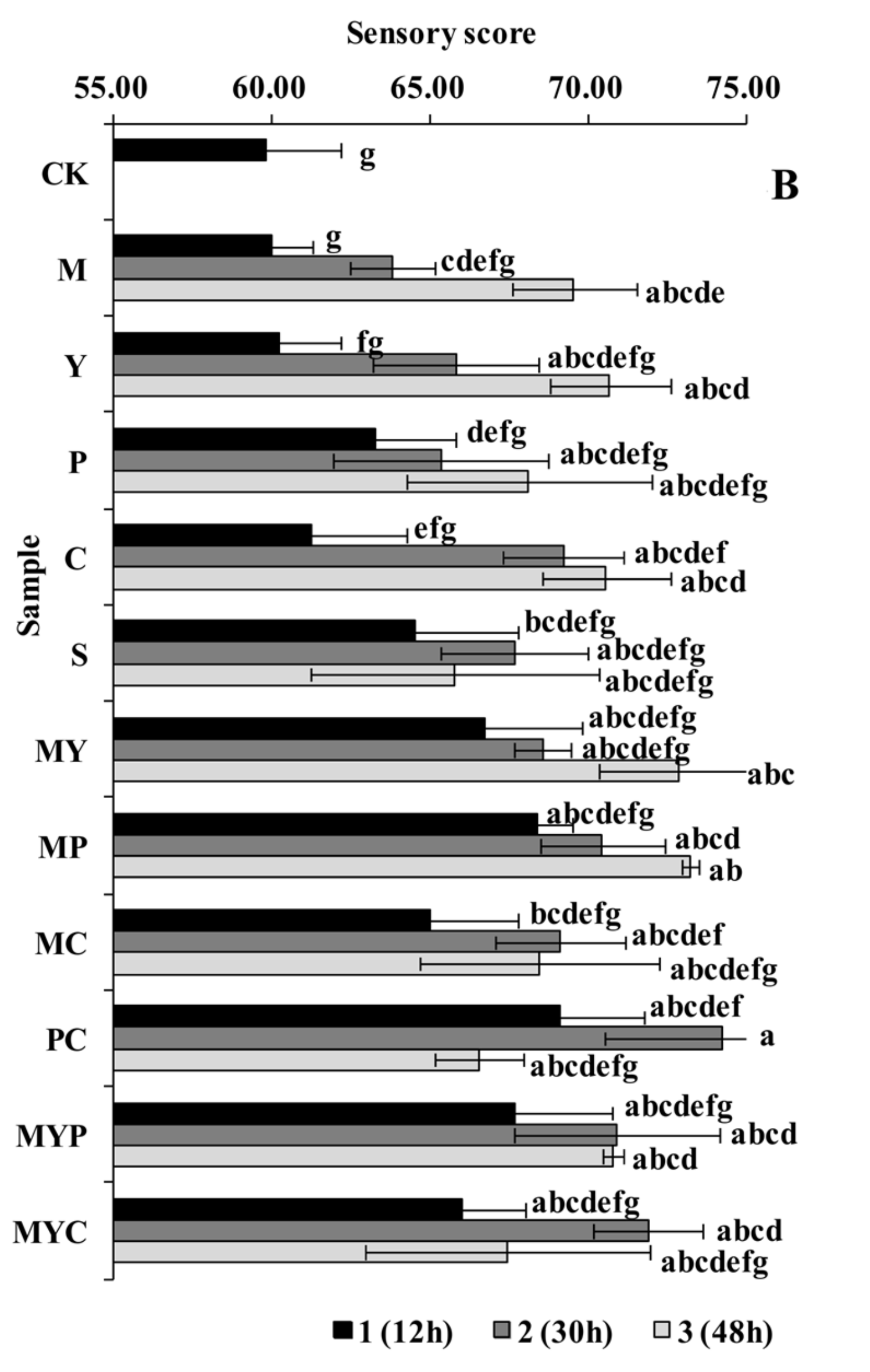
3.2. Sensory Characteristics of BR
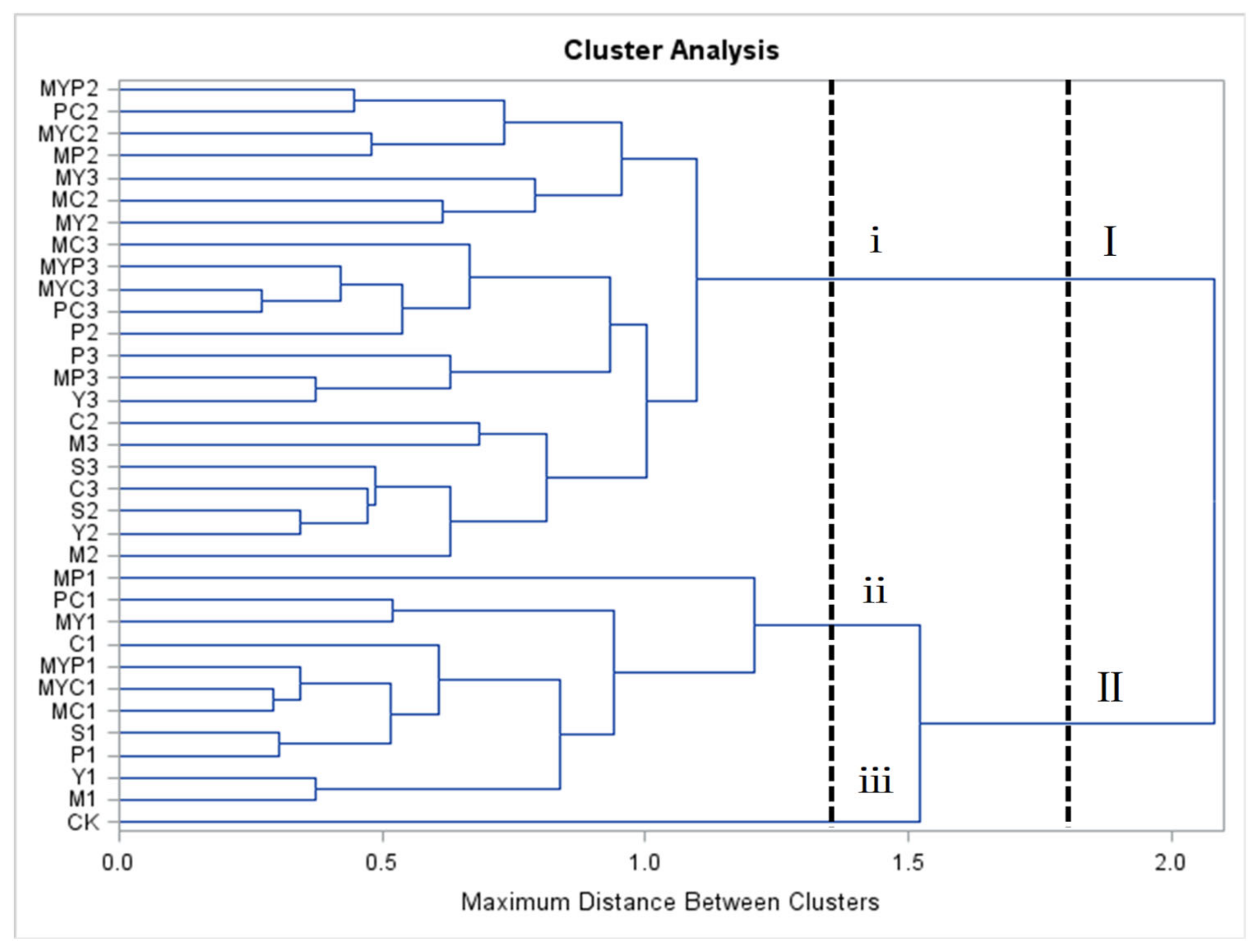
3.3. Cluster Analysis
3.4. Principal Components Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bagchi, T.B.; Sharma, S.G.; Chattopadhyay, K. Development of NIRS models to predict protein and amylose content of brown rice and proximate compositions of rice bran. Food Chem. 2015, 191, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arzuaga, M.; Cho, S.; Billiris, M.A.; Siebenmorgen, T.; Seo, H. Impacts of degree of milling on the appearance and aroma characteristics of raw rice. J. Sci. Food Agric. 2016, 96, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.; Liu, C.; Li, B.; Zhou, W.; Chen, H.; Li, T.; Wu, J.; Zeng, Z.; Wang, Y.; Si, X.; et al. Phytochemical profiles of rice and their cellular antioxidant activity against abap induced oxidative stress in human hepatocellular carcinoma hepg2 cells. Food Chem. 2020, 318, 126484. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Cheng, L.; Li, X.; Zhang, D.; Wu, G.; Zhang, H.; Wang, L.; Qian, H.; Wang, Y. Effect of cooking methods on solubility and nutrition quality of brown rice powder. Food Chem. 2018, 274, 444–451. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Suzuki, K.; Yasui, Y.; Kasumi, T. Bio-functional components in the processed pre-germinated brown rice by a twin-screw extruder. J. Food Compos. Anal. 2005, 18, 303–316. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, F.; Ramaswamy, H.S.; Zhu, S.; Yu, L.; Zhang, Q. Effect of soaking and single/two cycle high pressure treatment on water absorption, color, morphology and cooked texture of brown rice. J. Food Sci. Technol. 2017, 54, 1655–1664. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Su, X.; Shi, F.; Wang, L.; Chen, Z. High-temperature air-fluidization-induced changes in the starch texture, rheological properties, and digestibility of germinated brown rice. Starch-Stärke 2017, 68, 1600328. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, S.K.; Park, J.; Park, H.Y.; Choi, H.S.; Choi, I.; Han, S.I.; Chung, H.J.; Jeong, D.; Oh, S.K. Effect of cell wall degrading enzyme treatment on the phenolic content and antioxidant activity of brown rice. J. Korean Soc. Food Sci. Nutr. 2018, 47, 605–611. [Google Scholar] [CrossRef]
- Lang, G.H.; Kringel, D.H.; Acunha, T.; Ferreira, C.D.; Oliveira, M.D. Cake of brown, black and red rice: Influence of transglutaminase on technological properties, in vitro starch digestibility and phenolic compounds. Food Chem. 2020, 318, 126480. [Google Scholar] [CrossRef]
- Cho, D.H.; Lim, S.T. Germinated brown rice and its bio-functional compounds. Food Chem. 2016, 196, 259–271. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, L.; Xu, C.; Mei, J.; Li, Y. Effects of germination and high hydrostatic pressure processing on mineral elements, amino acids and antioxidants in vitro bioaccessibility, as well as starch digestibility in brown rice (Oryza sativa L.). Food Chem. 2017, 214, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Ukpong, E.S.; Onyeka, E.U.; Omeire, G.C.; Ogueke, C. Farro 57 rice cultivar: A comparative study of the nutritional composition of its parboiled milled rice, brown rice and germinated brown rice. Asian Food Sci. J. 2021, 20, 52–60. [Google Scholar] [CrossRef]
- Devanthi, P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Nevijo, Z. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. Biomed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razak, D.L.A.; Rashid, N.Y.A.; Jamaluddin, A.; Ghani, A.A.; Manan, M.A. Brewer’s rice: A potential substrate for cosmeceutical bio-ingredient production by solid state fermentation using Aspergillus oryzae. Malays. J. Microbiol. 2019, 15, 160–166. [Google Scholar] [CrossRef]
- Liang, C.H.; Syu, J.L.; Lee, Y.L.; Mau, J.L. Nonvolatile taste components of solid-state fermented adlay and rice by Phellinus linteus. LWT-Food Sci. Technol. 2009, 42, 1738–1743. [Google Scholar] [CrossRef]
- Saman, P.; Fuciños, P.; Vázquez, J.A.; Pandiella, S.S. Fermentability of brown rice and rice bran for growth of human Lactobacillus plantarum NCIMB 8826. Food Technol. Biotechnol. 2011, 49, 128–132. [Google Scholar] [CrossRef]
- Kuo, C.H.; Shieh, C.J.; Huang, S.M.; Wang, H.M.D.; Huang, C.Y. The effect of extrusion puffing on the physicochemical properties of brown rice used for saccharification and Chinese rice wine fermentation. Food Hydrocoll. 2019, 94, 363–370. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.; Hao, L.; Du, P.; Liu, L. The bioavailability of soybean polysaccharides and their metabolites on gut microbiota in the simulator of the human intestinal microbial ecosystem (SHIME). Food Chem. 2021, 362, 130233. [Google Scholar] [CrossRef]
- Ilowefah, M.; Chinma, C.; Bakar, J.; Ghazali, H.; Muhammad, K.; Makeri, M. Fermented brown rice flour as functional food ingredient. Foods 2014, 3, 149–159. [Google Scholar] [CrossRef]
- Bressiani, J.; Santetti, G.S.; Oro, T.; Esteres, V.; Gularte, M.A. Hydration properties and arabinoxylans content of whole wheat flour intended for cookie production as affected by particle size and brazilian cultivars. LWT-Food Sci. Technol. 2021, 150, 111918. [Google Scholar] [CrossRef]
- Pascual, C.; Massaretto, I.L.; Kawassaki, F.; Barros, R.; Noldin, J.A.; Marquez, U. Effects of parboiling, storage and cooking on the levels of tocopherols, tocotrienols and γ-oryzanol in brown rice (Oryza sativa L.). Food Res. Int. 2013, 50, 676–681. [Google Scholar] [CrossRef]
- Liu, L.; Guo, J.; Zhang, R.; Wei, Z.; Deng, Y.; Guo, J.; Zhang, M. Effect of degree of milling on phenolic profiles and cellular antioxidant activity of whole brown rice. Food Chem. 2015, 185, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1988, 46, 4113–4117. [Google Scholar] [CrossRef]
- Wu, N.; Li, H.; Tan, B.; Zhang, M.; Xiao, Z.; Tian, X.; Zhai, X.; Liu, M.; Liu, Y.; Wang, L.; et al. Free and bound phenolic profiles of the bran from different rice varieties and their antioxidant activity and inhibitory effects on α-amylose and α-glucosidase. J. Cereal Sci. 2018, 82, 206–212. [Google Scholar] [CrossRef]
- De Souza, C.T.; Soares, S.R.; Queiroz, A.F.S.; Santos, A.M.P.; Ferreira, S.L.C. Determination and evaluation of the mineral composition of breadfruit (Artocarpus altilis) using multivariate analysis technique. Microchem. J. 2016, 128, 84–88. [Google Scholar] [CrossRef]
- Tian, H.; Li, F.; Qin, L.; Yu, H.; Ma, X. Quality evaluation of beef seasonings using gas chromatography-mass spectrometry and electronic nose: Correlation with sensory attributes and classification according to grade level. Food Anal. Methods 2015, 8, 1522–1534. [Google Scholar] [CrossRef]
- Katina, K. Sourdough: A tool for the improved flavour, texture and shelf-life of wheat bread. Vtt Publ. 2005, 569, 3–92. [Google Scholar]
- Rizal, S.; Kustyawati, M.E.; Murhadi; Hasanudin, U. The growth of yeast and fungi, the formation of β-glucan, and the antibacterial activities during soybean fermentation in producing tempeh. Int. J. Food Sci. 2021, 2021, 6676042. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, X.; Zhu, K. Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef]
- Wang, X.; Geng, X.; Egashira, Y.; Sanada, H. Purification and characterization of a feruloyl esterase from the intestinal bacterium lactobacillus acidophilus. Appl. Environ. Microbiol. 2004, 70, 2367–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ijaz, U.; Nadeem, H.U.; Shafique, A.; Rasheed, A.; Azeem, F. Rice bran composition and its emerging potential applications. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Wang, K.; Liu, X. Structural modification of hemicelluloses and lignin based on the biorefinery process with white-rot fungal. Carbohydr. Polym. 2016, 153, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.L.; Wang, L.; Lü, J.; Zhu, Y.Y.; Shen, R.L. Structural, antioxidant and adsorption properties of dietary fiber from foxtail millet (Setaria italica) bran. J. Sci. Food Agric. 2019, 99, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tan, B. Effects of different solid-state fermentation ratios of S. cerevisiae and L. plantarum on physic-chemical properties of wheat bran and the quality of whole wheat bread. J. Sci. Food Agric. 2021, 101, 4551–4560. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Sun, X.; Zhang, Q.; Yang, N. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agric. 2020, 100, 3283–3290. [Google Scholar] [CrossRef]
- Li, Y.; Teng, F.; Shi, F.; Wang, L.; Li, Y.; Chen, Z. Effect of solid-state fermentation by Lactobacillus plantarum on the cooking quality, microstructure, and physicochemical properties of brown rice. Starch-Stärke 2019, 71, 1800160. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, D.; Zhang, P.; Liu, F.; Liang, J. Seedling evaluation of six walnut rootstock species originated in china based on principal component analysis and cluster analysis. Sci. Hortic. 2020, 265, 109212. [Google Scholar] [CrossRef]
- Vichi, M.; Saporta, G. Clustering and disjoint principal component analysis. Comput. Stat. Data Anal. 2009, 53, 3194–3208. [Google Scholar] [CrossRef]
- Mir, J.I.; Ahmed, N.; Singh, D.B.; Padder, B.A.; Shafi, W.; Zaffer, S.; Hamid, A.; Bhat, H.A. Diversity evaluation of fruit quality of apple (Malus × domestica Borkh.) germplasm through cluster and principal component analysis. Indian J. Plant Physiol. 2017, 22, 221–226. [Google Scholar] [CrossRef]
- Šamec, D.; Maretić, M.; Lugarić, I.; Mešić, A.; Salopek-Sondi, B.; Duralija, B. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 2016, 194, 828–834. [Google Scholar] [CrossRef]

| Groups | Label | Inoculation Proportion | BR Weight (g) | Suspension Volume (mL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Lb. plantarum | S. cerevisiae | R. oryzae | A. oryzae | N. sitophila | Sterile Water | ||||
| Control group | CK | - | 300 | 0 | 0 | 0 | 0 | 0 | 90 |
| Study group | M | Lb. plantarum | 300 | 3 | 0 | 0 | 0 | 0 | 87 |
| Y | S. cerevisiae | 0 | 3 | 0 | 0 | 0 | |||
| P | R. oryzae | 0 | 0 | 3 | 0 | 0 | |||
| C | A. oryzae | 0 | 0 | 0 | 3 | 0 | |||
| S | N. sitophila | 0 | 0 | 0 | 0 | 3 | |||
| MY | Lb. plantarum: S. cerevisiae = 1:1 | 1.5 | 1.5 | 0 | 0 | 0 | |||
| MP | Lb. plantarum: R. oryzae = 1:1 | 1.5 | 0 | 1.5 | 0 | 0 | |||
| MC | Lb. plantarum: A. oryzae = 1:1 | 1.5 | 0 | 0 | 1.5 | 0 | |||
| PC | R. oryzae: A. oryzae = 1:1 | 0 | 0 | 1.5 | 1.5 | 0 | |||
| MYP | Lb. plantarum: S. cerevisiae: R. oryzae = 1:1:1 | 1.5 | 1.5 | 1.5 | 0 | 0 | 85.5 | ||
| MYC | Lb. plantarum: S. cerevisiae: A. oryzae = 1:1:1 | 1.5 | 1.5 | 0 | 1.5 | 0 | |||
| Principal Component | Eigenvalue | Difference | Proportion (%) | Cumulative (%) |
|---|---|---|---|---|
| Z1 | 5.2160 | 3.0709 | 52.16 | 52.16 |
| Z2 | 2.1451 | 1.1440 | 21.45 | 73.61 |
| Z3 | 1.0011 | 0.4844 | 10.01 | 83.62 |
| Z4 | 0.5066 | 0.1103 | 5.07 | 88.69 |
| Z5 | 0.4164 | 0.0889 | 4.16 | 92.85 |
| Z6 | 0.3175 | 0.0925 | 3.18 | 96.03 |
| Z7 | 0.2250 | 0.0770 | 2.25 | 98.28 |
| Z8 | 0.1480 | 0.1261 | 1.48 | 99.76 |
| Z9 | 0.0220 | 0.0197 | 0.22 | 99.98 |
| Z10 | 0.0023 | 0.02 | 100.00 |
| Quality Traits | Z1 | Z2 | Z3 |
|---|---|---|---|
| X1 | −0.310 | −0.132 | 0.206 |
| X2 | 0.166 | 0.557 | −0.627 |
| X3 | 0.342 | −0.072 | 0.259 |
| X4 | −0.161 | 0.720 | 0.381 |
| X5 | 0.281 | −0.104 | −0.134 |
| X6 | 0.346 | 0.032 | 0.364 |
| X7 | 0.335 | 0.287 | 0.036 |
| X8 | 0.421 | −0.022 | 0.182 |
| X9 | 0.298 | −0.231 | −0.366 |
| X10 | 0.395 | 0.020 | 0.184 |
| Samples | Fermentation Time | Z1 | Z2 | Z3 | Z | Ranking Order |
|---|---|---|---|---|---|---|
| CK | 12 | 251.79 | −17.66 | 35.35 | 1310.84 | 29 |
| M1 | 289.47 | −17.79 | 45.71 | 1517.49 | 23 | |
| Y1 | 254.17 | −20.05 | 29.64 | 1312.40 | 28 | |
| P1 | 242.35 | −20.31 | 24.77 | 1245.34 | 32 | |
| C1 | 230.99 | −19.55 | 22.54 | 1185.46 | 34 | |
| S1 | 244.20 | −18.73 | 27.08 | 1260.70 | 31 | |
| MY1 | 288.09 | −21.67 | 44.75 | 1500.96 | 24 | |
| MP1 | 269.47 | −16.18 | 31.94 | 1402.80 | 26 | |
| MC1 | 264.83 | −19.03 | 36.60 | 1377.18 | 27 | |
| PC1 | 278.05 | −21.39 | 37.29 | 1441.72 | 25 | |
| MYP1 | 241.60 | −20.25 | 25.50 | 1242.28 | 33 | |
| MYC1 | 246.22 | −17.58 | 28.01 | 1274.59 | 30 | |
| M2 | 30 | 390.90 | −26.45 | 79.90 | 2062.17 | 11 |
| Y2 | 376.20 | −19.84 | 75.48 | 1995.27 | 14 | |
| P2 | 377.91 | −18.45 | 82.68 | 2014.36 | 13 | |
| C2 | 414.07 | −18.28 | 88.73 | 2209.39 | 2 | |
| S2 | 368.38 | −22.33 | 68.80 | 1942.43 | 17 | |
| MY2 | 412.61 | −17.46 | 84.92 | 2199.72 | 3 | |
| MP2 | 401.17 | −17.42 | 86.95 | 2142.18 | 6 | |
| MC2 | 380.67 | −20.18 | 74.08 | 2016.46 | 12 | |
| PC2 | 374.55 | −23.74 | 68.67 | 1971.45 | 15 | |
| MYP2 | 348.73 | −21.88 | 57.60 | 1829.72 | 22 | |
| MYC2 | 354.31 | −21.52 | 68.39 | 1870.35 | 20 | |
| M3 | 48 | 389.85 | −22.70 | 83.99 | 2068.84 | 10 |
| Y3 | 375.21 | −32.47 | 71.59 | 1959.10 | 16 | |
| P3 | 394.92 | −24.05 | 78.97 | 2087.36 | 9 | |
| C3 | 409.19 | −24.08 | 85.73 | 2168.51 | 4 | |
| S3 | 361.32 | −26.44 | 71.06 | 1899.08 | 19 | |
| MY3 | 434.88 | −13.88 | 97.40 | 2336.08 | 1 | |
| MP3 | 368.92 | −29.25 | 70.78 | 1932.41 | 18 | |
| MC3 | 353.03 | −25.24 | 67.17 | 1854.51 | 21 | |
| PC3 | 398.14 | −22.54 | 88.06 | 2116.50 | 8 | |
| MYP3 | 402.29 | −25.99 | 88.69 | 2131.38 | 7 | |
| MYC3 | 406.38 | −25.36 | 93.78 | 2159.13 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Ye, Y.; Wang, L.; Tan, B. Nutrition and Sensory Evaluation of Solid-State Fermented Brown Rice Based on Cluster and Principal Component Analysis. Foods 2022, 11, 1560. https://doi.org/10.3390/foods11111560
Zhang D, Ye Y, Wang L, Tan B. Nutrition and Sensory Evaluation of Solid-State Fermented Brown Rice Based on Cluster and Principal Component Analysis. Foods. 2022; 11(11):1560. https://doi.org/10.3390/foods11111560
Chicago/Turabian StyleZhang, Duqin, Yanjun Ye, Luyao Wang, and Bin Tan. 2022. "Nutrition and Sensory Evaluation of Solid-State Fermented Brown Rice Based on Cluster and Principal Component Analysis" Foods 11, no. 11: 1560. https://doi.org/10.3390/foods11111560
APA StyleZhang, D., Ye, Y., Wang, L., & Tan, B. (2022). Nutrition and Sensory Evaluation of Solid-State Fermented Brown Rice Based on Cluster and Principal Component Analysis. Foods, 11(11), 1560. https://doi.org/10.3390/foods11111560






