Effects of Different Zn2+ Concentrations and High Hydrostatic Pressures (HHP) on Chlorophyll Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. 100 °C Thermal Treatment
2.4. Color Measurement
2.5. Antioxidant Activity Determination
2.5.1. ABTS+• Radical Scavenging Assay
2.5.2. DPPH Radical-Scavenging Assay
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. Zn2+ Replacement Rate Analysis
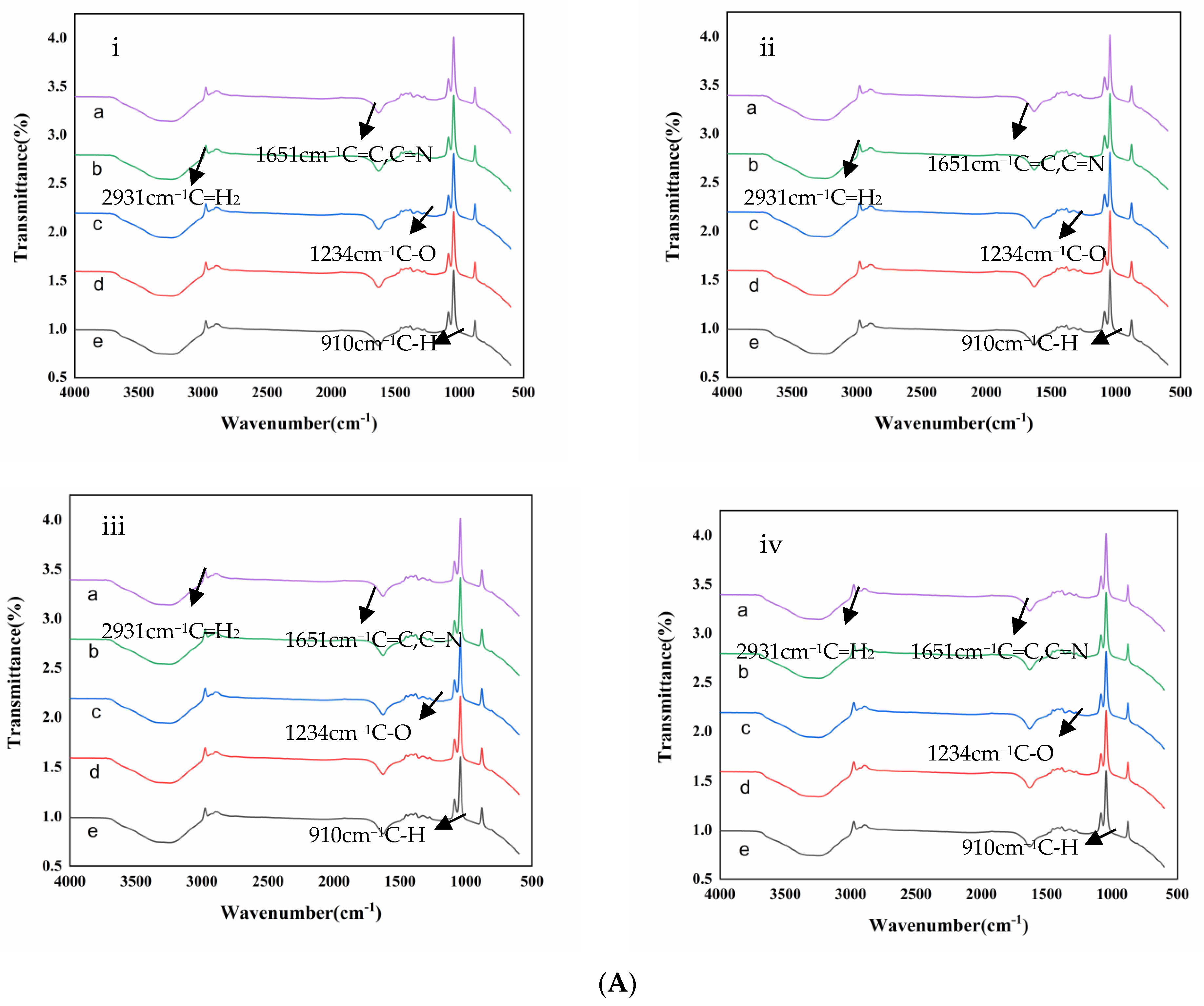
2.7. Structure Characterization Analysis
2.7.1. FTIR Analysis
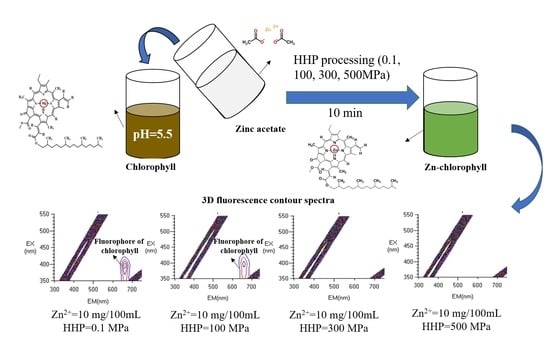
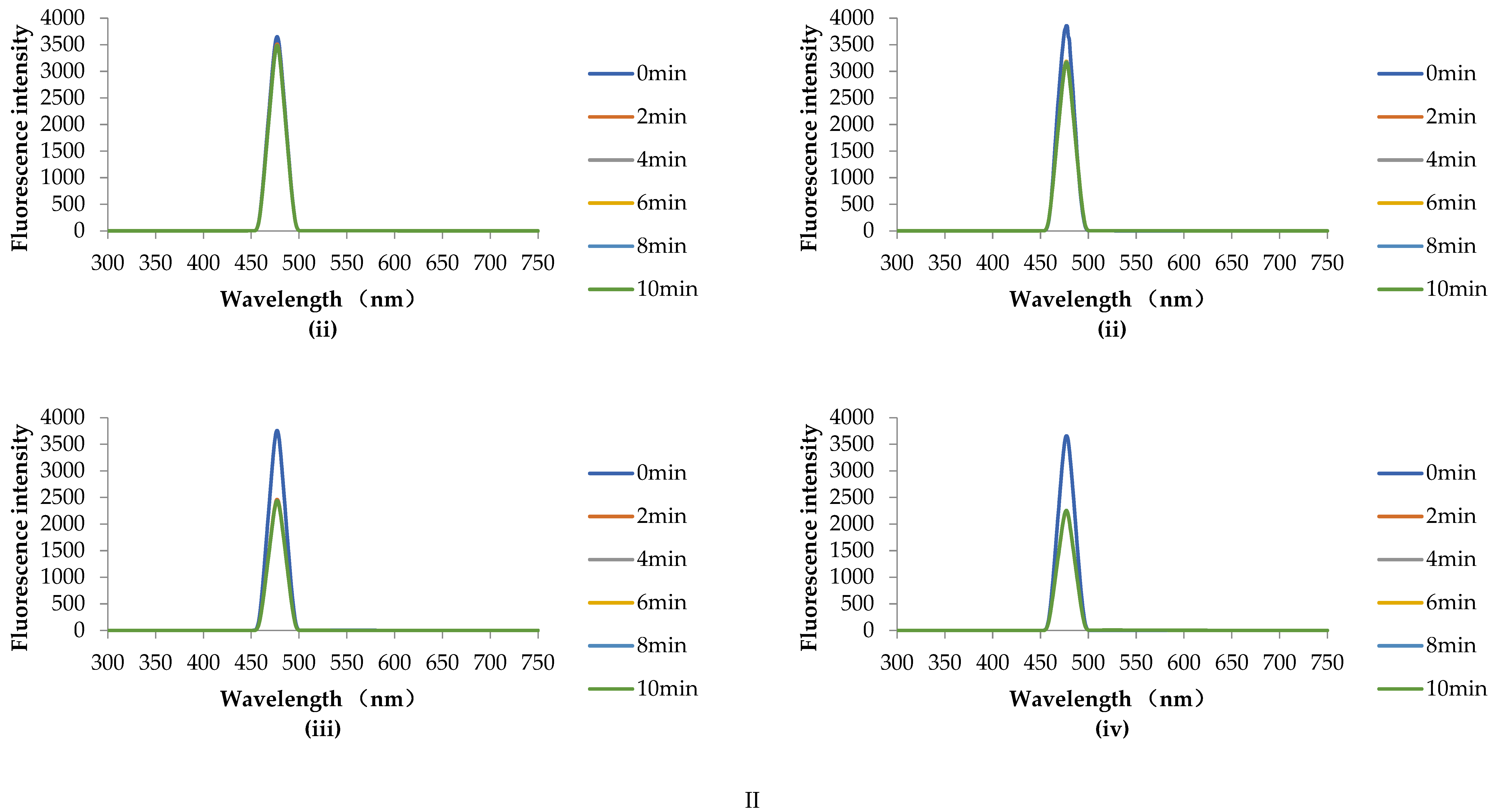
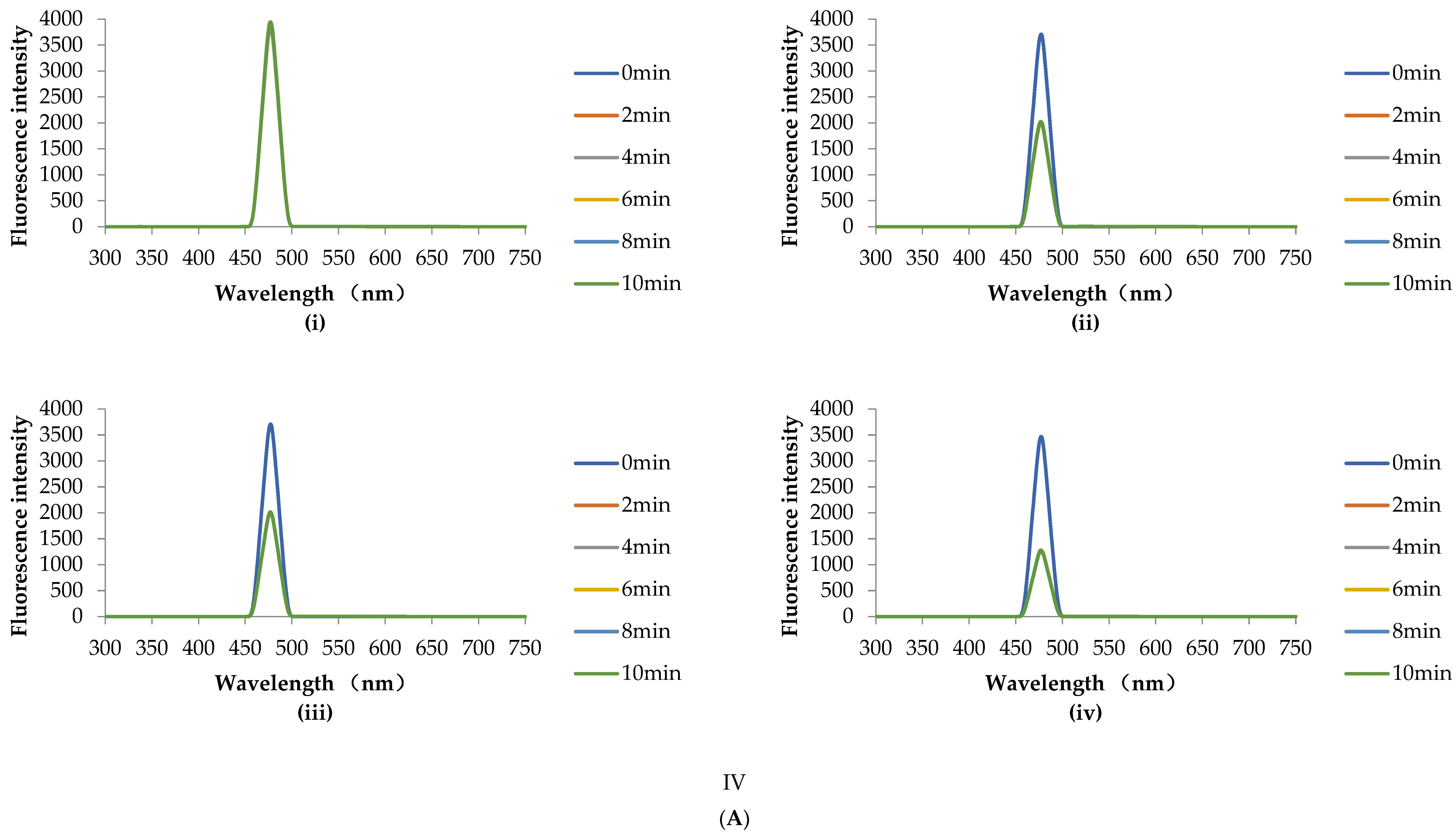
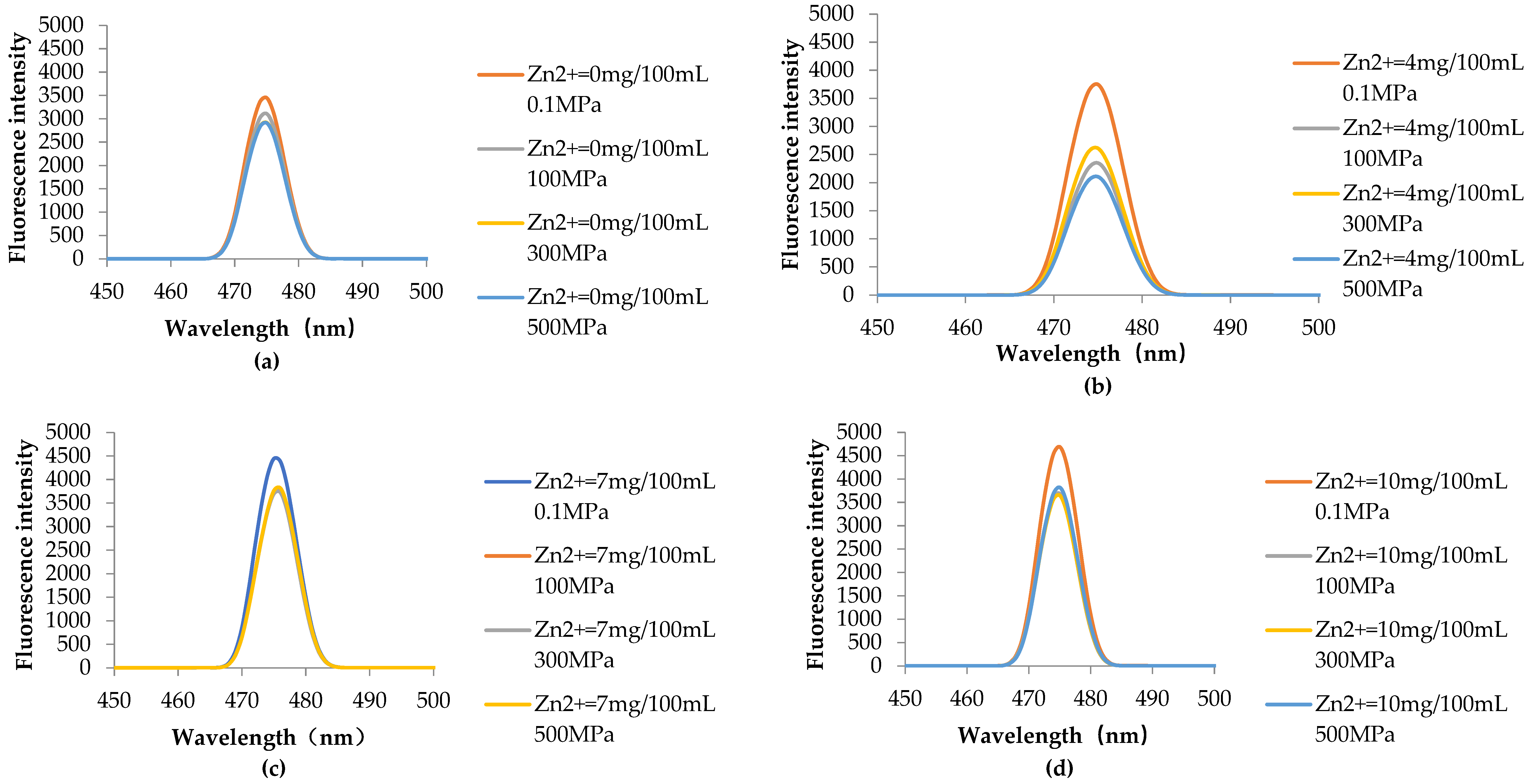
2.7.2. HHP Fluorescence Online Analysis
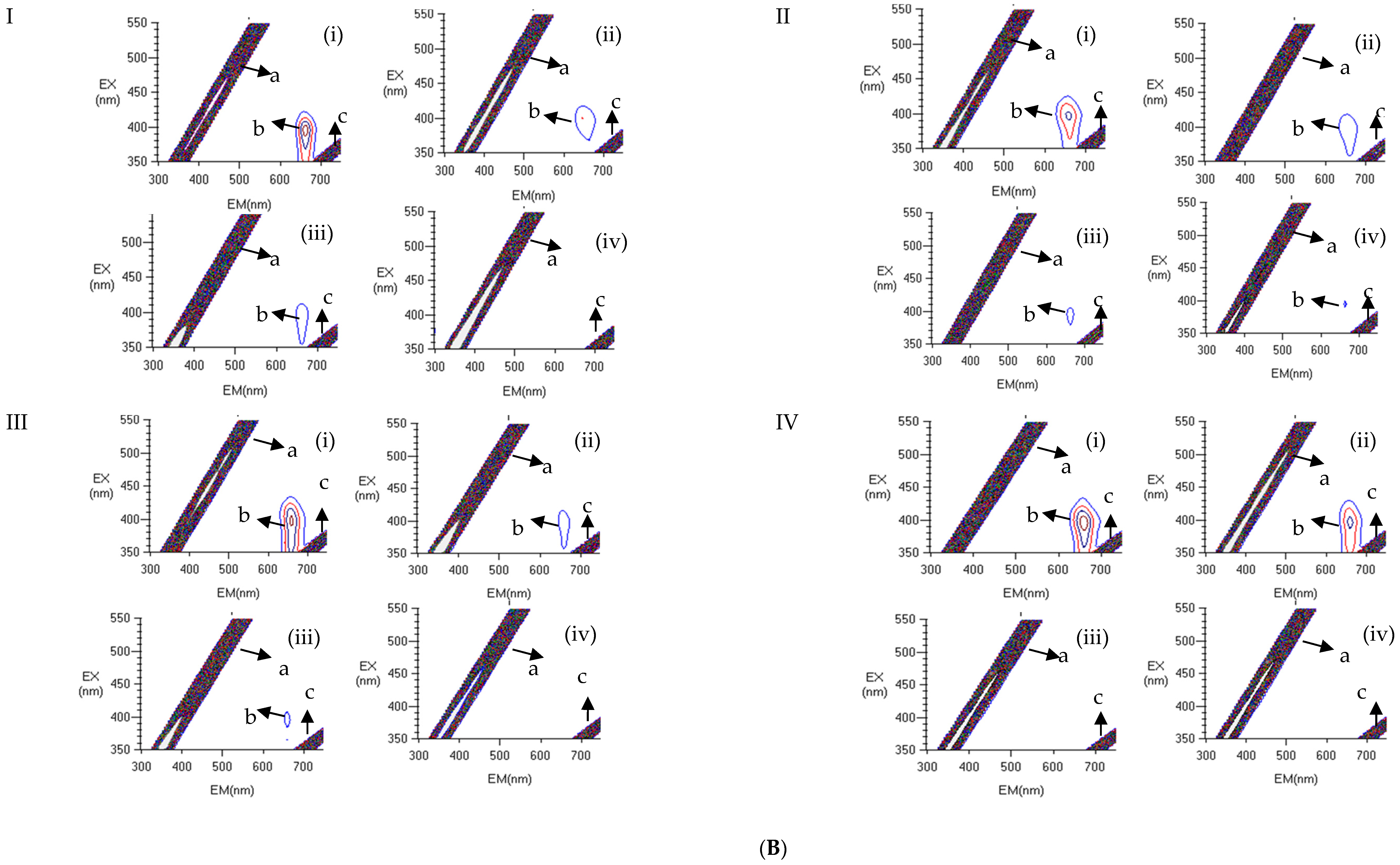
2.7.3. Fluorescence Scanning
2.8. Statistical Analysis
3. Results and Discussion
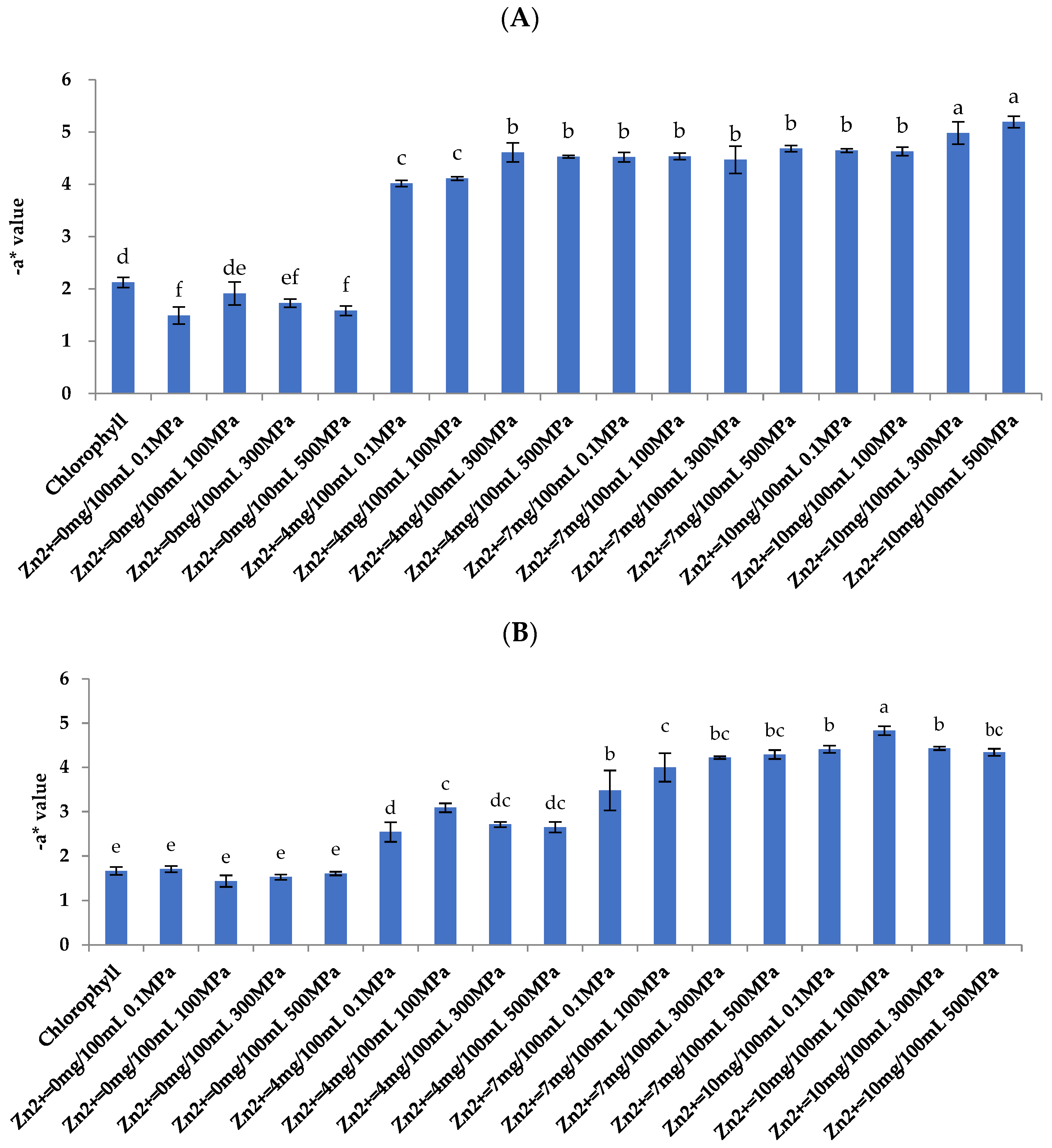
3.1. Color Analysis
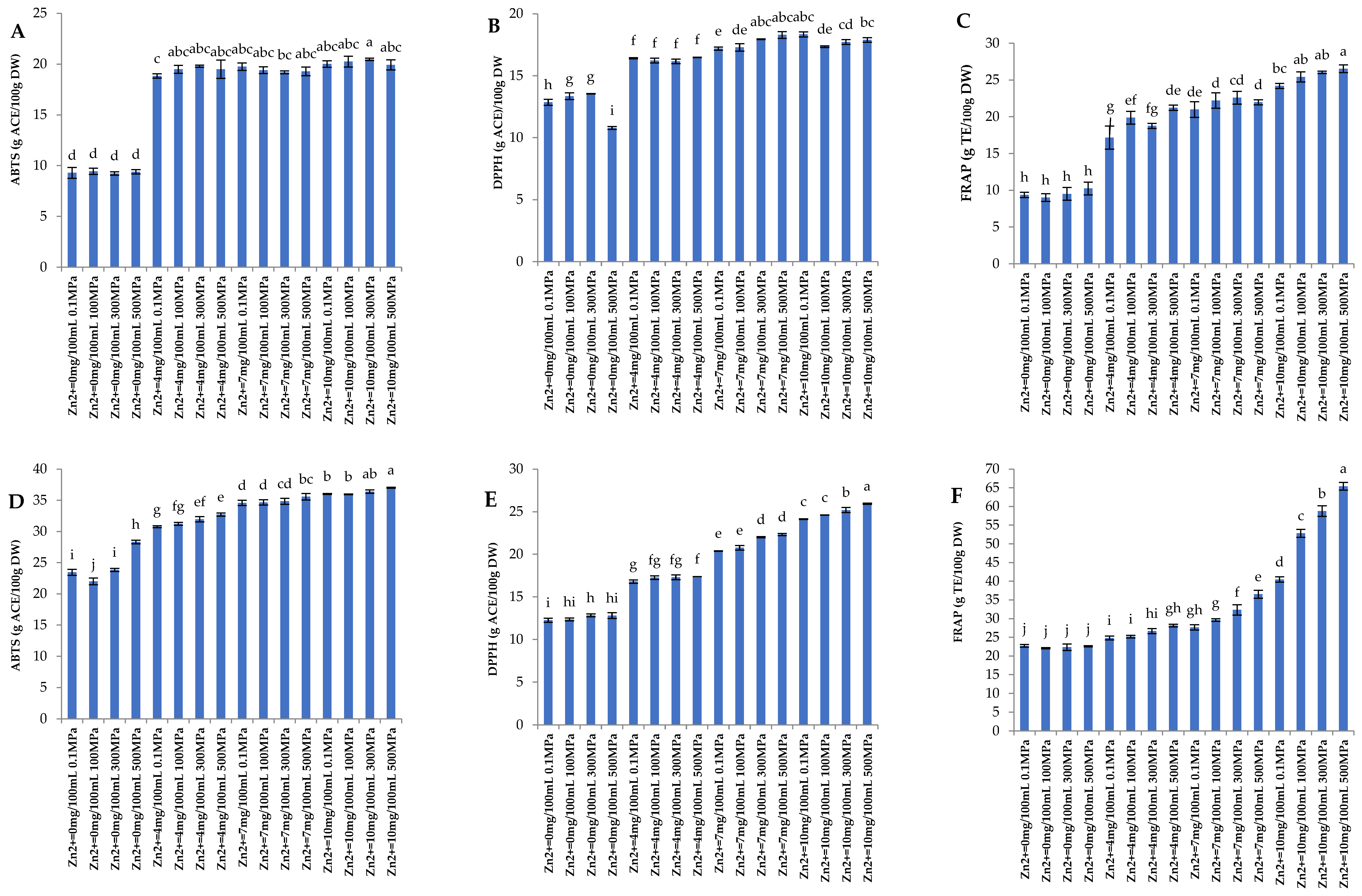
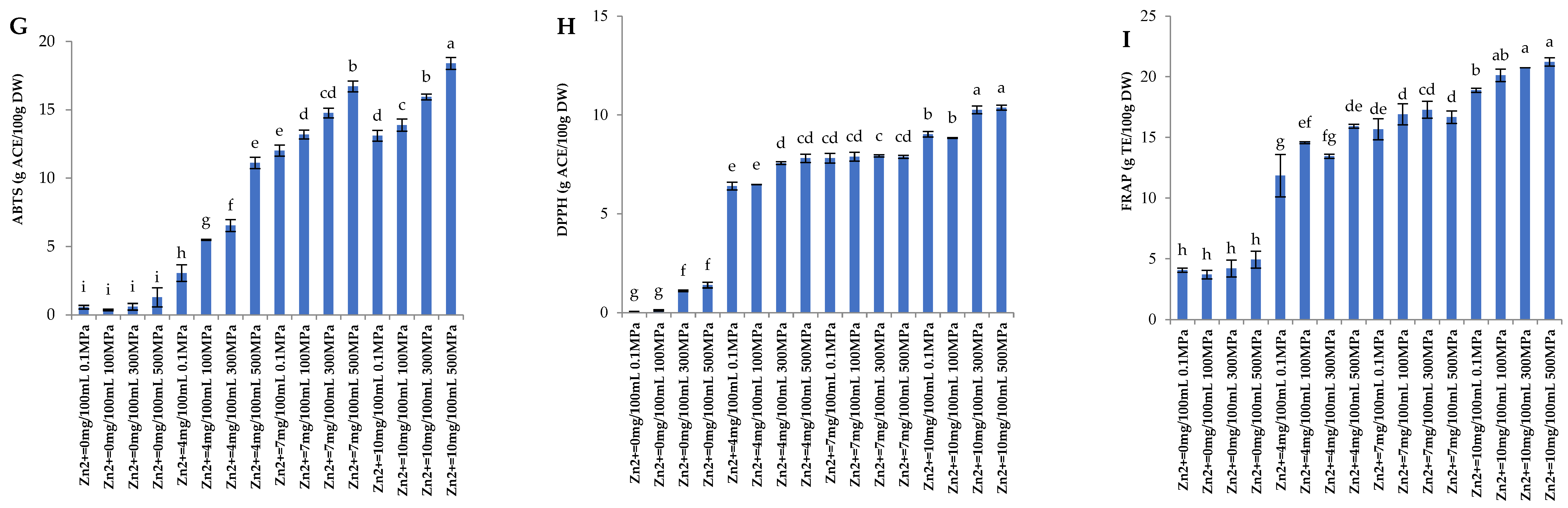
3.2. Antioxidant Activity
3.3. Zn2+ Replacement Rate Analysis
3.4. Structure Characterization Analysis
3.4.1. FTIR Analysis
3.4.2. HHP Fluorescence Online Analysis
3.5. Thermal Stability Analysis
3.5.1. Color Analysis
3.5.2. Antioxidant Activity
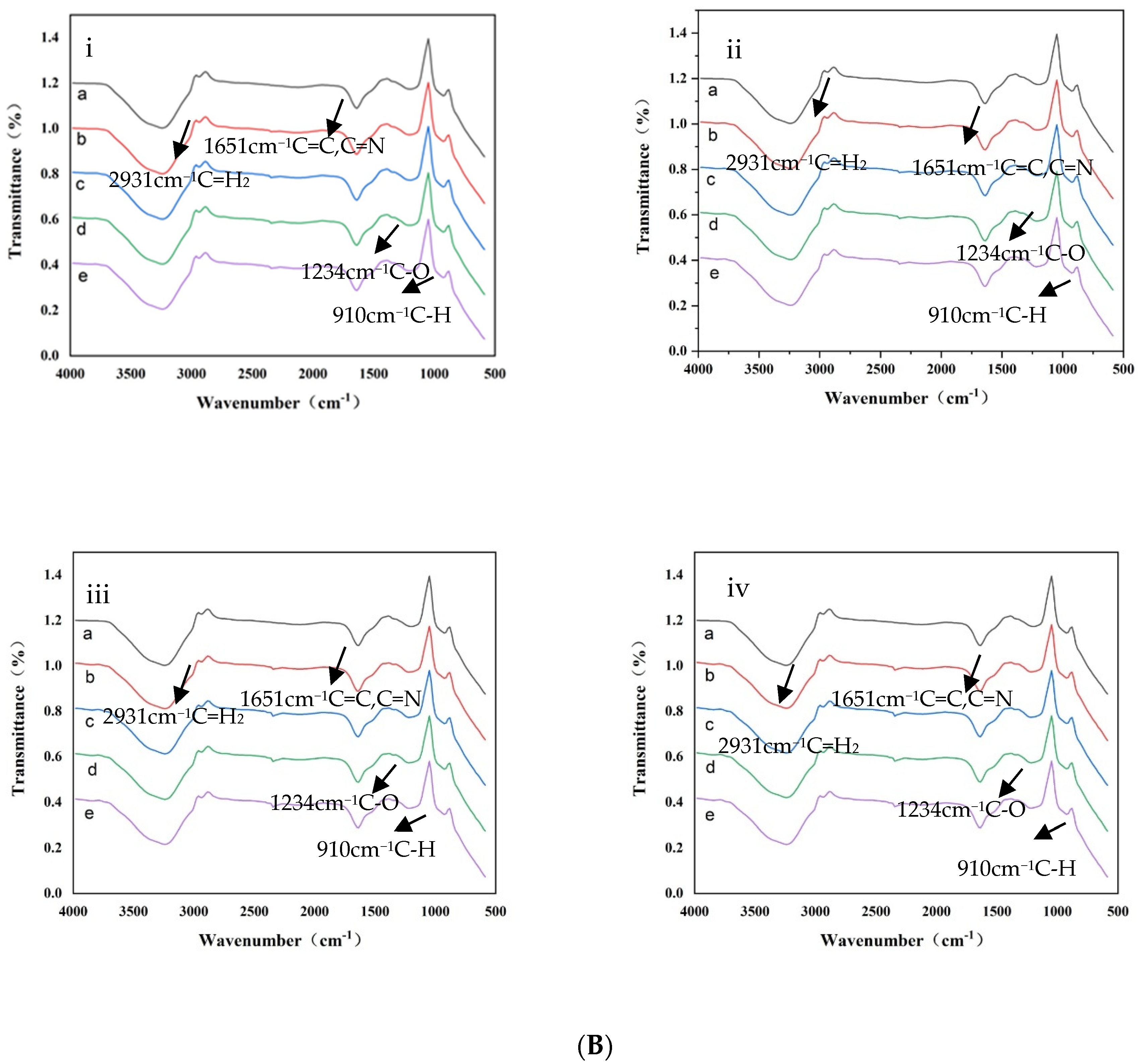
3.5.3. FTIR Analysis
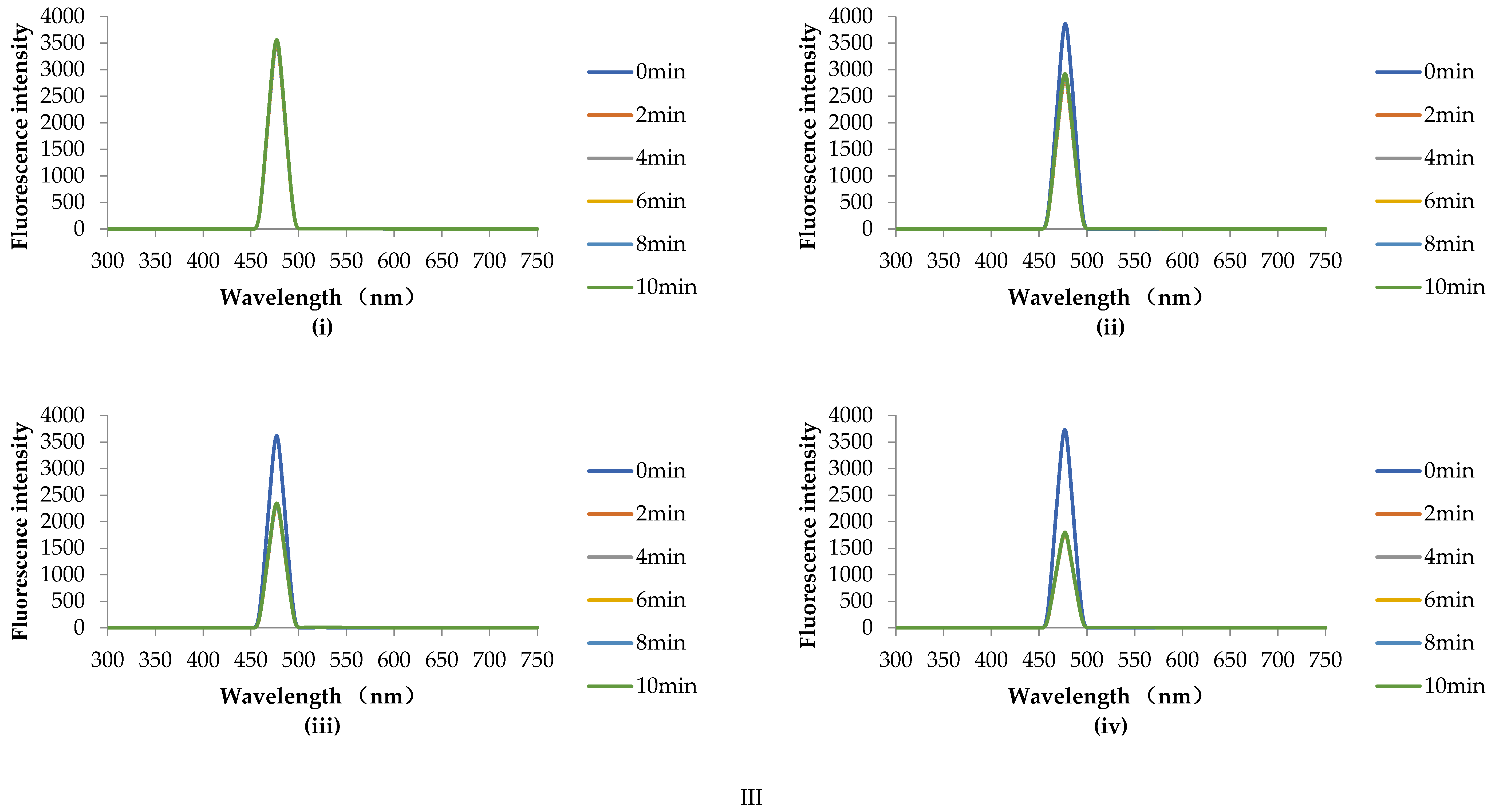
3.5.4. Fluorescence Scanning Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowacka, M.; Dadan, M.; Janowicz, M.; Wiktor, A.; Witrowa-Rajchert, D.; Mandal, R.; Pratap-Singh, A.; Janiszewska-Turak, E. Effect of nonthermal treatments on selected natural food pigments and color changes in plant material. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5097–5144. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 867–901. [Google Scholar] [CrossRef]
- Dadan, M.; Nowacka, M. The assessment of the possibility of using ethanol and ultrasound to design the properties of dried carrot tissue. Appl. Sci. 2021, 11, 689. [Google Scholar] [CrossRef]
- Pratap Singh, A.; Yen, P.P.L.; Ramaswamy, H.S.; Singh, A. Recent advances in agitation thermal processing. Curr. Opin. Food Sci. 2018, 23, 90–96. [Google Scholar] [CrossRef]
- Managa, M.G.; Mpai, S.; Remize, F.; Garcia, C.; Sivakumar, D. Impact of moist cooking methods on colour, anti-nutritive compounds and phenolic metabolites in African nightshade (Solanum retroflexum Dun.). Food Chem. 2020, 325, 126805. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Salman, F.; Almusallam, A.S. Effect of blanching on thermal color degradation kinetics and rheological behavior of rocket (Eruca sativa) puree. J. Food Eng. 2013, 119, 660–667. [Google Scholar] [CrossRef]
- Kwartiningsih, E.; Ramadhani, A.N.; Putri, N.G.A.; Damara, V.C.J. Chlorophyll extraction methods review and chlorophyll stability of katuk leaves (Sauropus androgynous). J. Phys. Conf. Ser. 2021, 1858, 12015. [Google Scholar] [CrossRef]
- Rahayuningsih, E.; Pamungkas, M.S.; Olvianas, M.; Putera, A.D.P. Chlorophyll extraction from suji leaf (Pleomele angustifolia Roxb.) with ZnCl2 stabilizer. J. Food Sci. Technol. 2018, 55, 1028–1036. [Google Scholar] [CrossRef]
- Inasti, D.; Andarwulan, N.; Purnomo, E.H.; Wulandari, N. Stability of chlorophyll as natural colorant: A review for suji (Dracaena Angustifolia Roxb.) leaves’ case. Curr. Res. Nutr. Food Sci. 2018, 6, 609. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Pratap Singh, A.; Ramaswamy, H.S. A controlled agitation process for improving quality of canned green beans during agitation thermal processing. J. Food Sci. 2016, 81, E1399–E1411. [Google Scholar] [CrossRef] [Green Version]
- Chasapis, C.; Ntoupa, P.; Spiliopoulou, C.; Stefanidou, M. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.Y.; Gillen, C.M.; Barnett, T.C.; Walker, M.J.; McEwan, A.G. An antimicrobial role for zinc in innate immune defense against group a streptococcus. J. Infect. 2014, 209, 1500–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rux, G.; Gelewsky, R.; Schlüter, O.; Herppich, W.B. High hydrostatic pressure treatment effects on selected tissue properties of fresh horticultural products. Innov. Food Sci. Emerg. Technol. 2020, 61, 102326. [Google Scholar] [CrossRef]
- Hu, X.M.; Ma, T.; Ao, L.; Kang, H.; Hu, X.S.; Song, Y.; Liao, X.J. Effect of high hydrostatic pressure processing on textural properties and microstructural characterization of fresh-cut pumpkin (Cucurbita pepo). J. Food Process Eng. 2020, 43, e13379. [Google Scholar] [CrossRef]
- Finten, G.; Agüero, M.V.; Jagus, R.J.; Niranjan, K. High hydrostatic pressure blanching of baby spinach (Spinacia oleracea L.). J. Food Sci. Technol. 2006, 73, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.X.; Deng, J.J.; Luo, D.S.; Bao, Y.J.; Liao, X.J.; Gao, H.Y.; Wu, J.H. Comparative study of high hydrostatic pressure and high temperature short time processing on quality of clear and cloudy Se-enriched kiwifruit juices. J. Food Sci. Technol. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Bae, H.H.; Yi, G.; Go, Y.S.; Ha, J.Y.; Choi, Y.; Son, J.H.; Shin, S.; Jung, T.W.; Lee, S. Measuring Antioxidant Activity in Yellow Corn (Zea mays L.) Inbreds from Three Different Geographic Regions. Appl. Biol. Chem. 2021, 64, 56. [Google Scholar] [CrossRef]
- Makori, S.I.; Mu, T.H.; Sun, H.N. Total Polyphenol Content, Antioxidant Activity, and Individual Phenolic Composition of Different Edible Parts of 4 Sweet Potato Cultivars. Nat. Prod. Commun. 2020, 15, 1934578. [Google Scholar] [CrossRef]
- Makori, S.I.; Mu, T.H.; Sun, H.N. Physicochemical Properties, Antioxidant Activities, and Binding Behavior of 3,5-di-O-caffeoylquinic Acid with Beta-lactoglobulin Colloidal Particles. Food Chem. 2021, 347, 129084. [Google Scholar] [CrossRef]
- Das, A.; Guyer, L.; Hörtensteiner, S. Chlorophyll and Chlorophyll Catabolite Analysis by HPLC. Methods Mol. Biol. 2018, 1744, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Mathiyalagan, S.; Mandal, B.K.; Sinha, M.; Ling, Y.C. Synthesis of different metallochlorophyllins and quantification in food samples by reversed phase—High performance liquid chromatography. Nat. Prod. Res. 2019, 33, 3120–3126. [Google Scholar] [CrossRef] [PubMed]
- Li, F.W.; Zhou, L.; Cao, J.R.; Wang, Z.H.; Liao, X.J.; Zhang, Y. Aggregation induced by the synergy of sodium chloride and high-pressure improves chlorophyll stability. Food Chem. 2022, 366, 130577. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.M.; Hou, G.G.; Sun, J.J.; Zhang, Z.Z.; Wan, X.C. Effects of green tea powder on the quality attributes of hard red winter wheat flour and Chinese steamed bread. Int. J. Food Sci. Technol. 2019, 54, 576–582. [Google Scholar] [CrossRef]
- Senklang, P.; Anprung, P. Optimizing enzymatic extraction of Zn–chlorophyll derivatives from pandan leaf using response surface methodology. J. Food Process Pres. 2010, 34, 759–776. [Google Scholar] [CrossRef]
- Bechaieb, R.; Fredj, A.B.; Akacha, A.B.; Gérard, H. Interactions of copper (ii) and zinc (ii) with chlorophyll: Insights from density functional theory studies. New J. Chem. 2016, 40, 4543–4549. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Nguyen, Q.D.; Nguyen, T.V. Kinetic Study on Chlorophyll and Antioxidant Activity from Polyscias fruticosa (L.) Harms Leaves via Microwave-Assisted Extraction. Molecules 2021, 26, 3761. [Google Scholar] [CrossRef] [PubMed]
- Suryani, C.L.; Dwiwahyuningsih, T.; Supriyadi, S.; Santoso, U. Derivatization of chlorophyll from pandan (Pandanus amaryllifolius Roxb.) leaves and their antioxidant activity. Periodico Tche Quimica 2020, 17, 1110–1126. [Google Scholar] [CrossRef]
- Kang, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, Characterization, and Functional Properties of Chlorophylls, Pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Baiano, A.; Del Nobile, M.A. Antioxidant Compounds from Vegetable Matrices: Biosynthesis, Occurrence, and Extraction Systems. Crit. Rev. Food Sci. Nutr. 2016, 56, 2053–2068. [Google Scholar] [CrossRef]
- Konwar, M.; Baruah, G.D.; Konwar, M. On the nature of vibrational bands in the FTIR spectra of medicinal plant leaves. Arch. Appl. Sci. Res. 2011, 3, 214–221. [Google Scholar]
- Petrovic, J.; Nikolic, G.; Markovic, D. In vitro complexes of copper and zinc with chlorophyll. J. Serb. Chem. Soc. 2006, 71, 501–512. [Google Scholar] [CrossRef]
- Tao, D.D.; Li, F.W.; Hu, X.S.; Liao, X.S.; Zhang, Y. Quality comparison of “Laba” garlic processed by high hydrostatic pressure and high pressure carbon dioxide. Sci. Rep. 2020, 10, 3719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreault, V.; Hénaux, L.; Bazinet, L.; Doyen, A. Pretreatment of Flaxseed Protein Isolate by High Hydrostatic Pressure: Impacts on Protein Structure, Enzymatic Hydrolysis and Final Hydrolysate Antioxidant Capacities. Food Chem. 2017, 221, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Zakharenkova, S.A.; Dobrovolskii, A.A.; Garshev, A.V.; Statkus, M.A.; Beklemishev, M.K. Chlorophyll-based self-assembled nanostructures for fluorescent sensing of aminoglycoside antibiotics. ACS Sustain. Chem. Eng. 2021, 9, 3408–3415. [Google Scholar] [CrossRef]
- Qu, F.; Gong, N.; Wang, S.H.; Gao, Y.; Sun, C.L.; Fang, W.H.; Men, Z.W. Effect of pH on fluorescence and absorption of aggregates of chlorophyll a and carotenoids. Dye. Pigment. 2020, 173, 107975. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Molecular structure, stability and cytotoxicity of natural green colorants produced from Centella asiatica L. leaves treated by steaming and metal complexations. Food Chem. 2017, 232, 387–394. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Yahia, E.M.; Ornelas-Paz, J.D.; Victoria-Campos, C.I.; Ibarra-Junquera, V.; Pérez-Martínez, J.D.; Escalante-Minakata, P. Antioxidant Activity and Content of Chlorophylls and Carotenoids in Raw and Heat-processed Jalapeño Peppers at Intermediate Stages of Ripening. Food Chem. 2014, 146, 188–196. [Google Scholar] [CrossRef]
- Dzwolak, W.; Kato, M.; Taniguchi, Y. Fourier transform infrared spectroscopy in high-pressure studies on proteins. BBA-Protein Struct. Mol. Enzymol. 2002, 1595, 131–144. [Google Scholar] [CrossRef]
- Hu, X.; Xu, X.; Jin, Z.; Tian, Y.; Bai, Y.; Xie, Z. Retrogradation properties of rice starch gelatinized by heat and high hydrostatic pressure (HHP). J. Food Eng. 2011, 106, 262–266. [Google Scholar] [CrossRef]

| Sample | Chlorophyll a (mg/100 mL) | Chlorophyll b (mg/100 mL) | Pheophytin a (mg/100 mL) | Pheophytin b (mg/100 mL) | Zn2+ Replacement Rate (%) |
|---|---|---|---|---|---|
| Zn2+ = 0 mg/100 mL 0.1 MPa | 2.13 ± 0.01 | 2.03 ± 0.03 | 0.56 ± 0.07 | 0.74 ± 0.17 | 0.00 ± 0.00 c |
| Zn2+ = 0 mg/100 mL 100 MPa | 3.75 ± 0.27 | 2.04 ± 0.01 | 0.35 ± 0.06 | 0.87 ± 0.09 | 0.00 ± 0.00 c |
| Zn2+ = 0 mg/100 mL 300 MPa | 2.16 ± 0.05 | 1.99 ± 0.02 | 0.45 ± 0.03 | 1.16 ± 0.16 | 0.00 ± 0.00 c |
| Zn2+ = 0 mg/100 mL 500 MPa | 2.15 ± 0.02 | 2.09 ± 0.02 | 0.42 ± 0.02 | 1.00 ± 0.19 | 0.00 ± 0.00 c |
| Zn2+ = 4 mg/100 mL 0.1 MPa | 4.20 ± 0.90 | 2.18 ± 0.02 | 0.70 ± 0.18 | 1.13 ± 0.25 | 16.03 ± 9.43 b |
| Zn2+ = 4 mg/100 mL 100 MPa | 3.35 ± 1.08 | 2.05 ± 0.04 | 0.43 ± 0.01 | 0.89 ± 0.00 | 27.24 ± 8.45 ab |
| Zn2+ = 4 mg/100 mL 300 MPa | 2.99 ± 0.85 | 2.09 ± 0.08 | 0.44 ± 0.07 | 0.67 ± 0.17 | 30.85 ± 7.08 ab |
| Zn2+ = 4 mg/100 mL 500 MPa | 2.13 ± 0.03 | 2.06 ± 0.03 | 0.43 ± 0.06 | 0.74 ± 0.24 | 39.31 ± 1.20 ab |
| Zn2+ = 7 mg/100 mL 0.1 MPa | 2.63 ± 0.50 | 2.04 ± 0.04 | 0.38 ± 0.05 | 0.68 ± 0.20 | 36.94 ± 5.32 ab |
| Zn2+ = 7 mg/100 mL 100 MPa | 2.15 ± 0.01 | 2.03 ± 0.09 | 0.53 ± 0.04 | 0.85 ± 0.21 | 38.15 ± 2.99 ab |
| Zn2+ = 7 mg/100 mL 300 MPa | 2.22 ± 0.04 | 2.03 ± 0.03 | 0.56 ± 0.09 | 1.15 ± 0.19 | 33.73 ± 0.96 ab |
| Zn2+ = 7 mg/100 mL 500 MPa | 2.50 ± 0.27 | 2.03 ± 0.00 | 0.34 ± 0.07 | 0.73 ± 0.12 | 37.82 ± 0.92 ab |
| Zn2+ = 10 mg/100 mL 0.1 MPa | 2.13 ± 0.02 | 2.01 ± 0.11 | 0.64 ± 0.34 | 0.83 ± 0.31 | 37.63 ± 8.72 ab |
| Zn2+ = 10 mg/100 mL 100 MPa | 2.26 ± 0.07 | 2.00 ± 0.01 | 0.44 ± 0.08 | 0.97 ± 0.33 | 36.94 ± 5.32 ab |
| Zn2+ = 10 mg/100 mL 300 MPa | 2.14 ± 0.04 | 2.06 ± 0.01 | 0.47 ± 0.03 | 0.77 ± 0.29 | 39.66 ± 3.07 ab |
| Zn2+ = 10 mg/100 mL 500 MPa | 2.16 ± 0.05 | 2.02 ± 0.02 | 0.46 ± 0.02 | 0.55 ± 0.03 | 42.34 ± 0.80 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Sun, H.; Mu, T. Effects of Different Zn2+ Concentrations and High Hydrostatic Pressures (HHP) on Chlorophyll Stability. Foods 2022, 11, 2129. https://doi.org/10.3390/foods11142129
Hu Y, Sun H, Mu T. Effects of Different Zn2+ Concentrations and High Hydrostatic Pressures (HHP) on Chlorophyll Stability. Foods. 2022; 11(14):2129. https://doi.org/10.3390/foods11142129
Chicago/Turabian StyleHu, Yuwei, Hongnan Sun, and Taihua Mu. 2022. "Effects of Different Zn2+ Concentrations and High Hydrostatic Pressures (HHP) on Chlorophyll Stability" Foods 11, no. 14: 2129. https://doi.org/10.3390/foods11142129
APA StyleHu, Y., Sun, H., & Mu, T. (2022). Effects of Different Zn2+ Concentrations and High Hydrostatic Pressures (HHP) on Chlorophyll Stability. Foods, 11(14), 2129. https://doi.org/10.3390/foods11142129