Development of a Highly Sensitive and Specific ic-ELISA and Lateral Flow Immunoassay for Diacetoxyscirpenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
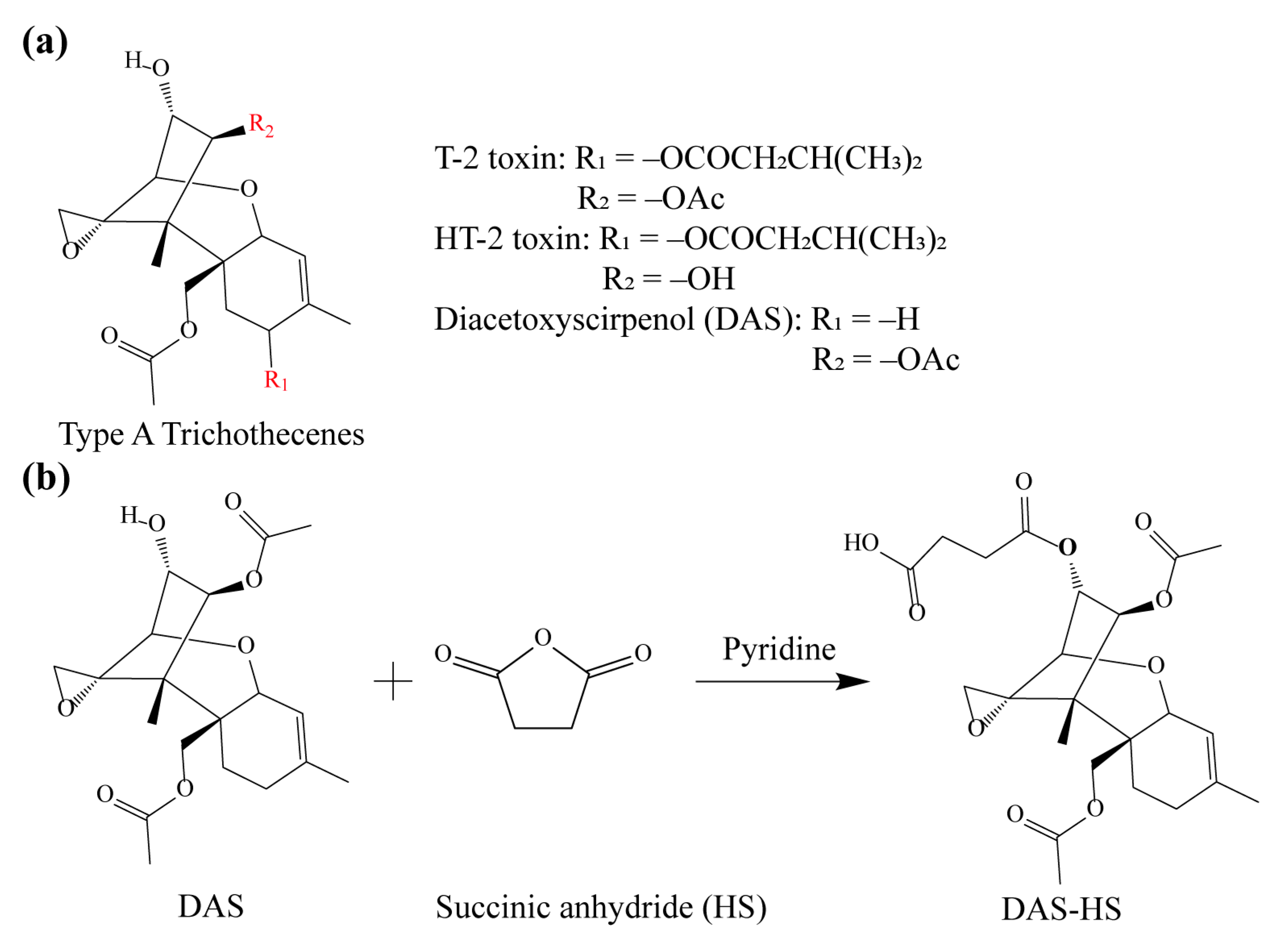
2.2. Synthesis of Hapten and Antigen
2.3. Production of mAb
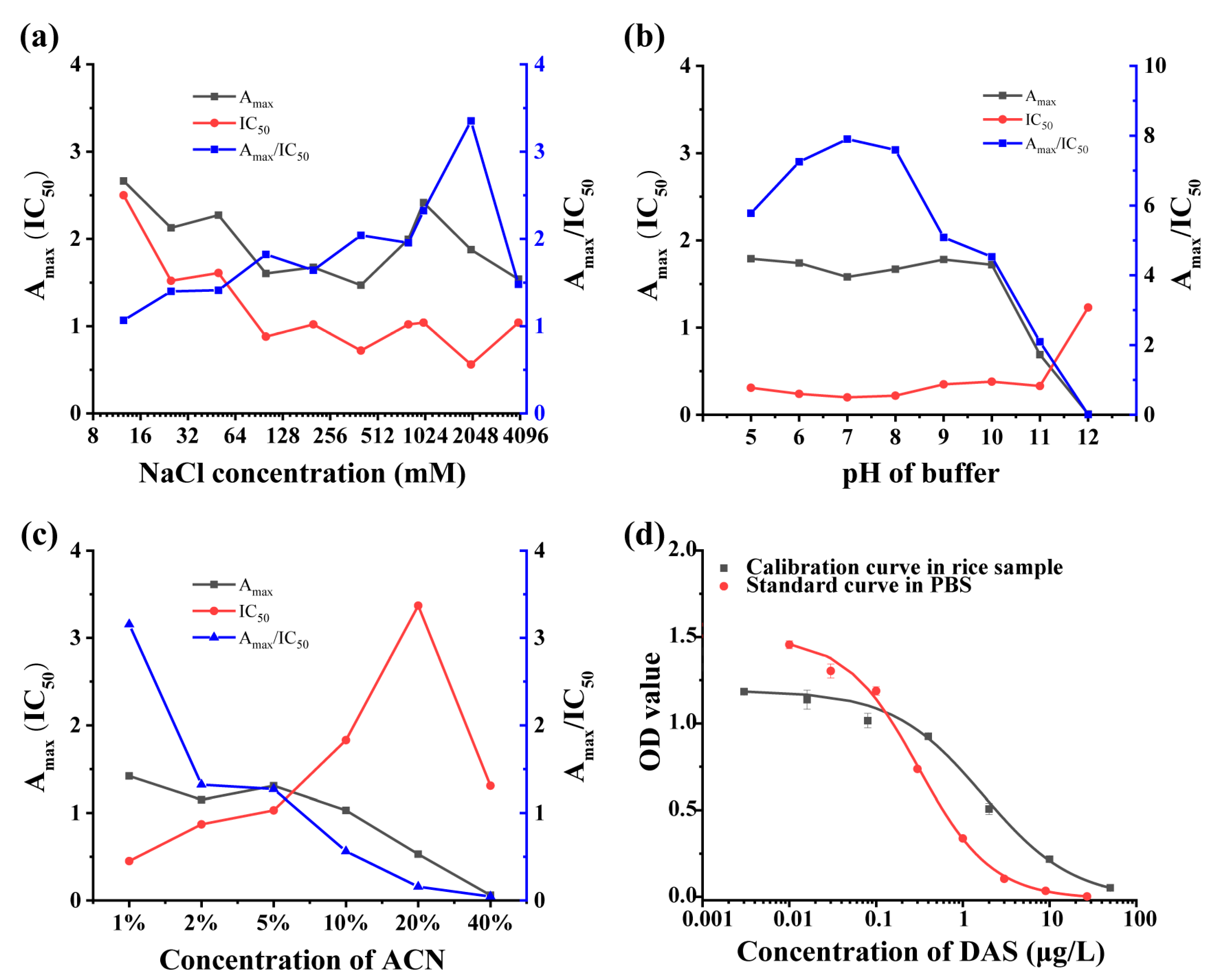
2.4. Development and Optimization of ic-ELISA
2.5. Matrix Effect and Recovery
2.6. Preparation of mAb-Labeled Colloidal Gold Nanoparticles (AuNPs)
2.7. Preparation of Lateral Flow Immunoassay Strip
2.8. Extraction of Antibody Variable Region Sequences, Homology Modeling and Molecular Docking
3. Results and Discussion
3.1. Preparation and Identification of Hapten and Antigen
3.2. Production of mAb to DAS
3.3. Optimization and Development of ic-ELISA
3.4. Matrix Effect and Recovery of ic-ELISA
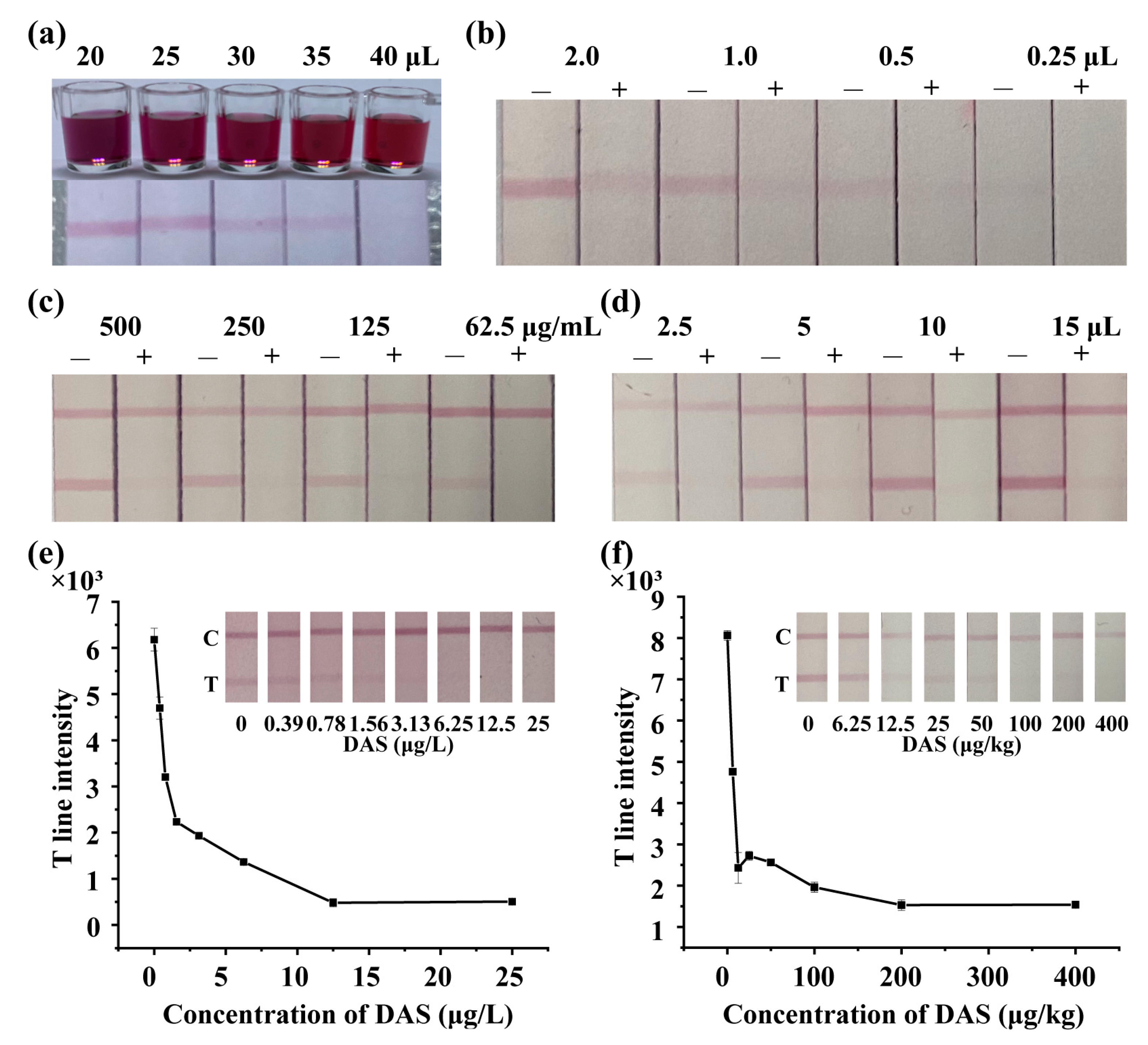
3.5. Development of LFIA
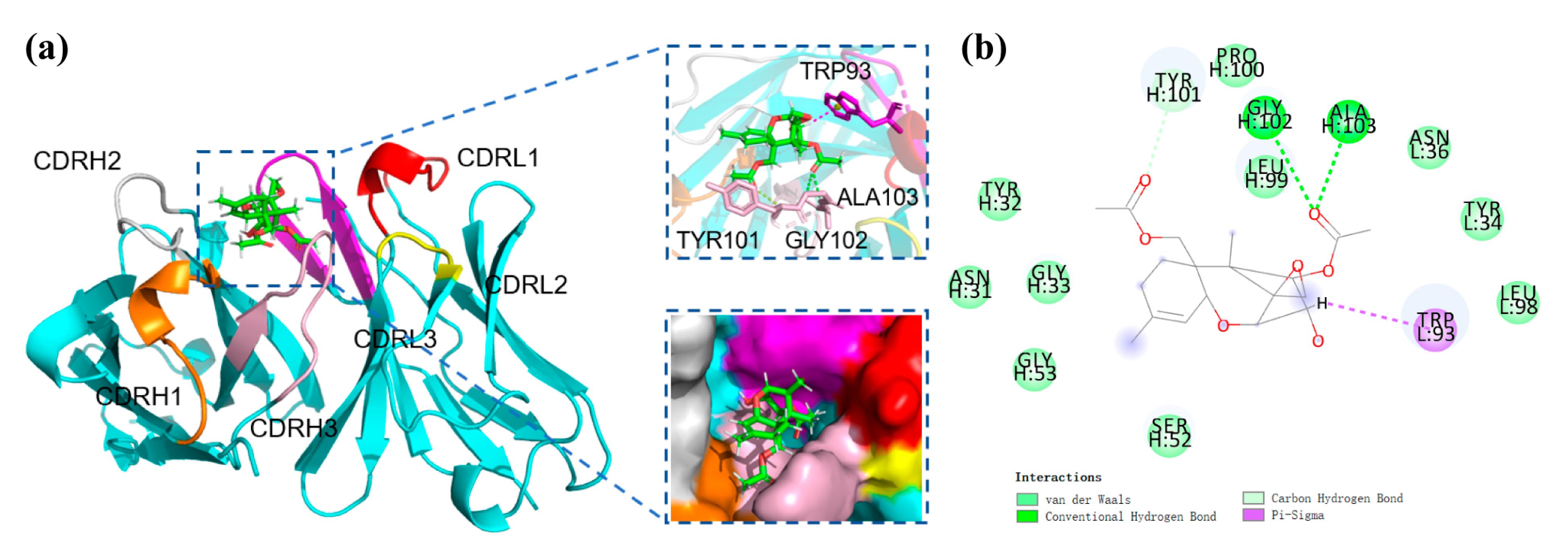
3.6. Recognition Mechanism of mAb to DAS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nesic, K.; Ivanovic, S.; Nesic, V. Fusarial toxins: Secondary metabolites of Fusarium fungi. Rev. Environ. Contam. Toxicol. 2014, 228, 101–120. [Google Scholar] [CrossRef]
- Barros, G.; Zanon, M.A.; Palazzini, J.; Haidukowski, M.; Pascale, M.; Chulze, S. Trichothecenes and zearalenone production by Fusarium equiseti and Fusarium semitectum species isolated from Argentinean soybean. Food Addit. Contam. Part A 2012, 29, 1436–1442. [Google Scholar] [CrossRef]
- Thrane, U.; Adler, A.; Clasen, P.E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef]
- Wu, D.; Li, S.; Li, P.; Jiang, A.; Liu, Z.; Zhang, Y.; Wang, J.; Yang, Z.; Wei, Z. Diacetoxyscirpenol-induced heterophil extracellular traps contribute to the immune toxicity of liver injury in chickens. Food Chem. Toxicol. 2021, 148, 111926. [Google Scholar] [CrossRef]
- Ayral, A.M.; Dubech, N.; Le Bars, J.; Escoula, L. In vitro effect of diacetoxyscirpenol and deoxynivalenol on microbicidal. Mycopathologia 1992, 120, 121–127. [Google Scholar] [CrossRef]
- Thanakarn Nasri, R.R.B.; ten Voorde, S.; Fink-Gremmels, J. Differential induction of apoptosis by type A and B trichothecenes in Jurkat T-lymphocytes. Toxicol. In Vitro 2006, 20, 832–840. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, K.; Wu, W.; Zhang, H. Anorectic responses to T-2 toxin, HT-2 toxin, diacetoxyscirpenol and neosolaniol correspond to plasma elevations of neurotransmitters 5-hydroxytryptamine and substance P. Ecotoxicol. Environ. Saf. 2018, 161, 451–458. [Google Scholar] [CrossRef]
- Bottex, C.; Martin, A.; Fontanges, R. Action of a mycotoxin (diacetoxyscirpenol) on the immune response of the mouse-interaction with an immunomodulator (OM-89). Immunopharmacol. Immunotoxicol. 1990, 12, 311–325. [Google Scholar] [CrossRef]
- Chi, M.; Robison, T.; Mirocha, C.; Reddy, K. Acute toxicity of 12,13-epoxytrichothecenes in one-day-old broiler chicks. Appl. Environ. Microbiol. 1978, 35, 636–640. [Google Scholar] [CrossRef] [Green Version]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [Green Version]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [Green Version]
- Hassanane, M.; Abdalla, E.; El-Fiky, S.; Amer, M.; Hamdy, A. Mutagenicity of the Mycotoxin diacetoxyscirpenol on somatic and germ cells of mice. Mycotoxin Res. 2000, 16, 53–64. [Google Scholar] [CrossRef]
- Rocha, M.E.B.; Freire, F.C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, W.; Kuca, K. From hypoxia and hypoxia-inducible factors (HIF) to oxidative stress: A new understanding of the toxic mechanism of mycotoxins. Food Chem. Toxicol. 2020, 135, 110968. [Google Scholar] [CrossRef]
- Yang, S.; De Boevre, M.; Zhang, H.; De Ruyck, K.; Sun, F.; Wang, Z.; Cao, X.; Shen, J.; De Saeger, S.; Zhang, S. Unraveling the in vitro and in vivo metabolism of diacetoxyscirpenol in various animal species and human using ultrahigh-performance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 8571–8583. [Google Scholar] [CrossRef]
- Schollenberger, M.; Muller, H.M.; Ernst, K.; Sondermann, S.; Liebscher, M.; Schlecker, C.; Wischer, G.; Drochner, W.; Hartung, K.; Piepho, H.P. Occurrence and distribution of 13 trichothecene toxins in naturally contaminated maize plants in Germany. Toxins 2012, 4, 778–787. [Google Scholar] [CrossRef]
- Aniołowska, M.; Steininger, M. Determination of trichothecenes and zearalenone in different corn (Zea mays) cultivars for human consumption in Poland. J. Food Compos. Anal. 2014, 33, 14–19. [Google Scholar] [CrossRef]
- Njumbe Ediage, E.; Van Poucke, C.; De Saeger, S. A multi-analyte LC-MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: The forgotten sample matrix. Food Chem. 2015, 177, 397–404. [Google Scholar] [CrossRef]
- Schollenberger, M.; Müller, H.M.; Rüfle, M.; Drochner, W. Natural occurrence of 16 Fusarium toxins in edible oil marketed in Germany. Food Control 2008, 19, 475–482. [Google Scholar] [CrossRef]
- Yoshinari, T.; Takeda, N.; Watanabe, M.; Sugita-Konishi, Y. Development of an Analytical Method for Simultaneous Determination of the Modified Forms of 4,15-Diacetoxyscirpenol and their Occurrence in Japanese Retail Food. Toxins 2018, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- García-Moraleja, A.; Font, G.; Mañes, J.; Ferrer, E. Simultaneous determination of mycotoxin in commercial coffee. Food Control 2015, 57, 282–292. [Google Scholar] [CrossRef]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type A and type B) in processed cereal-based baby foods by GC-MS. Food Chem. 2015, 182, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carrasco, Y.; Ruiz, M.J.; Font, G.; Berrada, H. Exposure estimates to Fusarium mycotoxins through cereals intake. Chemosphere 2013, 93, 2297–2303. [Google Scholar] [CrossRef]
- Escrivá, L.; Manyes, L.; Font, G.; Berrada, H. Analysis of trichothecenes in laboratory rat feed by gas chromatography-tandem mass spectrometry. Food Addit. Contam. Part A 2016, 33, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Carballo, D.; Font, G.; Ferrer, E.; Berrada, H. Evaluation of Mycotoxin Residues on Ready-to-Eat Food by Chromatographic Methods Coupled to Mass Spectrometry in Tandem. Toxins 2018, 10, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romera, D.; Mateo, E.M.; Mateo-Castro, R.; Gómez, J.V.; Gimeno-Adelantado, J.V.; Jiménez, M. Determination of multiple mycotoxins in feedstuffs by combined use of UPLC-MS/MS and UPLC-QTOF-MS. Food Chem. 2018, 267, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, X.; Wen, K.; Li, Y.; Zhang, X.; Ma, M.; Yu, X.; Yu, W.; Shen, J.; Wang, Z. Class-specific monoclonal antibodies and dihydropteroate synthase in bioassays used for the detection of sulfonamides: Structural insights into recognition diversity. Anal. Chem. 2019, 91, 2392–2400. [Google Scholar] [CrossRef]
- Dong, B.; Li, H.; Sun, J.; Li, Y.; Mari, G.M.; Yu, X.; Yu, W.; Wen, K.; Shen, J.; Wang, Z. Magnetic assisted fluorescence immunoassay for sensitive chloramphenicol detection using carbon dots@CaCO3 nanocomposites. J. Hazard. Mater. 2021, 402, 123942. [Google Scholar] [CrossRef]
- Chu, F.S.; Liang, M.Y.; Zhang, G.S. Production and characterization of antibody against diacetoxyscirpenol. Appl. Environ. Microbiol. 1984, 48, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.S.; Schubring, S.L.; Chu, F.S. Improved method for production of antibodies against T-2 toxin and diacetoxyscirpenol in rabbits. Appl. Environ. Microbiol. 1986, 51, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Schubring, S.L.; Chu, F.S. An indirect enzyme-linked immunosorbent assay for the detection of diacetoxyscirpenol in wheat and corn. Mycotoxin Res. 1987, 3, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Klaffer, U.; Märtlbauer, E.; Terplan, G. Development of a sensitive enzyme-linked immunosorbent assay for the detection of diacetoxyscirpenol. Int. J. Food Microbiol. 1988, 6, 9–17. [Google Scholar] [CrossRef]
- Mills, E.; Johnston, J.; Kemp, H.A.; Morgan, M. An enzyme-linked immunosorbent assay for diacetoxyscirpenol applied to the analysis of wheat. J. Sci. Food Agric. 1988, 42, 225–233. [Google Scholar] [CrossRef]
- Fan, T.S.; Schubring, S.L.; Wei, R.D.; Chu, F.S. Production and characterization of a monoclonal antibody cross-reactive with most group A trichothecenes. Appl. Environ. Microbiol. 1988, 54, 2959–2963. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.; Wu, X.; Cui, G.; Liu, L.; Kuang, H.; Xu, C. Development of an ic-ELISA and immunochromatographic strip assay for the detection of diacetoxyscirpenol in rice. ACS Omega 2020, 5, 17876–17882. [Google Scholar] [CrossRef]
- Tang, X.; Wu, J.; Wu, W.; Zhang, Z.; Zhang, W.; Zhang, Q.; Zhang, W.; Chen, X.; Li, P. Competitive-type pressure-dependent immunosensor for highly sensitive detection of diacetoxyscirpenol in wheat via monoclonal antibody. Anal. Chem. 2020, 92, 3563–3571. [Google Scholar] [CrossRef]
- Li, H.; Ma, S.; Zhang, X.; Li, C.; Dong, B.; Mujtaba, M.; Wei, Y.; Liang, X.; Yu, X.; Wen, K.; et al. Generic hapten synthesis, broad-specificity monoclonal antibodies preparation, and ultrasensitive ELISA for five antibacterial synergists in chicken and milk. J. Agric. Food Chem. 2018, 66, 11170–11179. [Google Scholar] [CrossRef]
- Dou, L.; Zhao, B.; Bu, T.; Huang, Q.; Yan, L.; Huang, L.; Wang, Y.; Wang, J.; Zhang, D. Highly sensitive detection of a small molecule by a paired labels recognition system based lateral flow assay. Anal. Bioanal. Chem. 2018, 410, 3161–3170. [Google Scholar] [CrossRef]
- Reader, R.H.; Workman, R.G.; Maddison, B.C.; Gough, K.C. Advances in the production and batch reformatting of phage antibody libraries. Mol. Biotechnol. 2019, 61, 801–815. [Google Scholar] [CrossRef] [Green Version]
| Antibody Type | IC50 (μg/L) | Immunoassays | Reference |
|---|---|---|---|
| pAb | 15 | RIA | [29] |
| pAb | Not mentioned | ic-ELISA | [30] |
| pAb | 10 | ic-ELISA | [31] |
| pAb | Not mentioned | ic-ELISA | [32] |
| pAb | Not mentioned | ic-ELISA | [33] |
| mAb | Not mentioned | RIA | [34] |
| mAb | 5.97 | ic-ELISA | [35] |
| mAb | 3.08 | competitive-type pressure-dependent immunosensor | [36] |
| mAb | 0.31 | ic-ELISA | This study |
| Analyte | IC50 (μg/L) | Cross-Reactivity (%) |
|---|---|---|
| DAS | 0.31 | 100 |
| T-2 toxin | >5000 | <0.1 |
| HT-2 toxin | >5000 | <0.1 |
| Aflatoxin B1 | >5000 | <0.1 |
| Fumonisin B1 | >5000 | <0.1 |
| Deoxynivalenol | >5000 | <0.1 |
| Citreoviridin | >5000 | <0.1 |
| Ochratoxin A | >5000 | <0.1 |
| Zearalenone | >5000 | <0.1 |
| Spiked Level (μg/kg) | Mean Recovery (%) | Inter-Assay CV (%) | Intra-Assay CV (%) |
|---|---|---|---|
| 50 | 114 | 10 | 4 |
| 106 | 7 | ||
| 109 | 13 | ||
| 100 | 95 | 12 | 14 |
| 120 | 12 | ||
| 124 | 4 | ||
| 200 | 96 | 12 | 5 |
| 106 | 13 | ||
| 104 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, S.; Shang, W.; Bai, Y.; Dou, L.; Zhang, S.; Shen, J.; Wang, Z.; Wen, K. Development of a Highly Sensitive and Specific ic-ELISA and Lateral Flow Immunoassay for Diacetoxyscirpenol. Foods 2022, 11, 1548. https://doi.org/10.3390/foods11111548
Shao S, Shang W, Bai Y, Dou L, Zhang S, Shen J, Wang Z, Wen K. Development of a Highly Sensitive and Specific ic-ELISA and Lateral Flow Immunoassay for Diacetoxyscirpenol. Foods. 2022; 11(11):1548. https://doi.org/10.3390/foods11111548
Chicago/Turabian StyleShao, Shibei, Wenhua Shang, Yuchen Bai, Leina Dou, Suxia Zhang, Jianzhong Shen, Zhanhui Wang, and Kai Wen. 2022. "Development of a Highly Sensitive and Specific ic-ELISA and Lateral Flow Immunoassay for Diacetoxyscirpenol" Foods 11, no. 11: 1548. https://doi.org/10.3390/foods11111548
APA StyleShao, S., Shang, W., Bai, Y., Dou, L., Zhang, S., Shen, J., Wang, Z., & Wen, K. (2022). Development of a Highly Sensitive and Specific ic-ELISA and Lateral Flow Immunoassay for Diacetoxyscirpenol. Foods, 11(11), 1548. https://doi.org/10.3390/foods11111548







