METTL3 Regulated the Meat Quality of Rex Rabbits by Controlling PCK2 Expression via a YTHDF2–N6-Methyladenosine Axis
Abstract
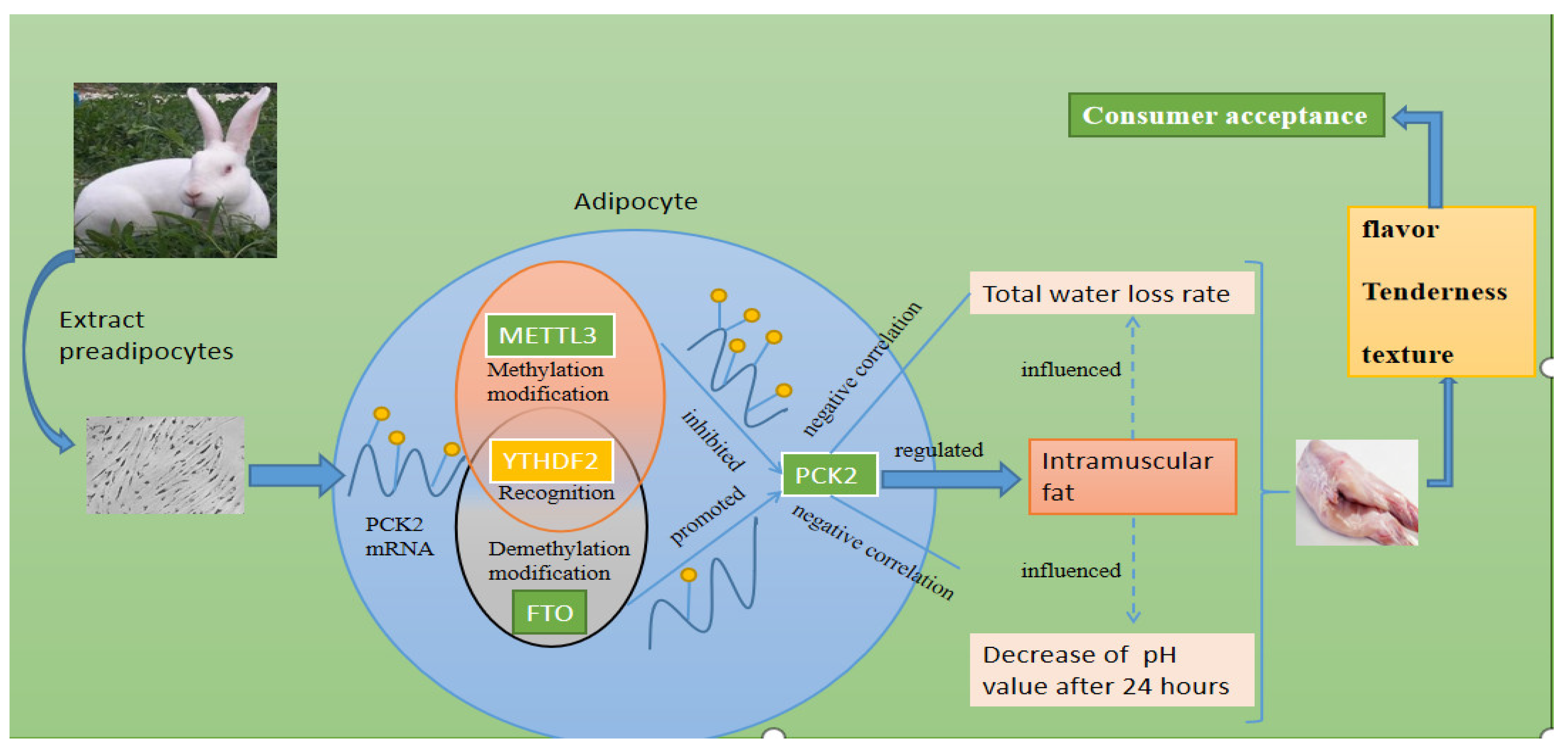
:1. Introduction
2. Material and Methods
2.1. Animals and Tissue Collection
2.2. Sample Collection
2.3. Cell Culture and Transfection
2.4. RT-qPCR
2.5. Measurement of PCK2 in Blood
2.6. Gene-Specific m6A qPCR
2.7. Western Blotting
2.8. Measurement of Triglyceride Content and Oil Red-O Staining
2.9. Determination of Meat Quality Traits
2.10. Statistical Analysis
3. Results
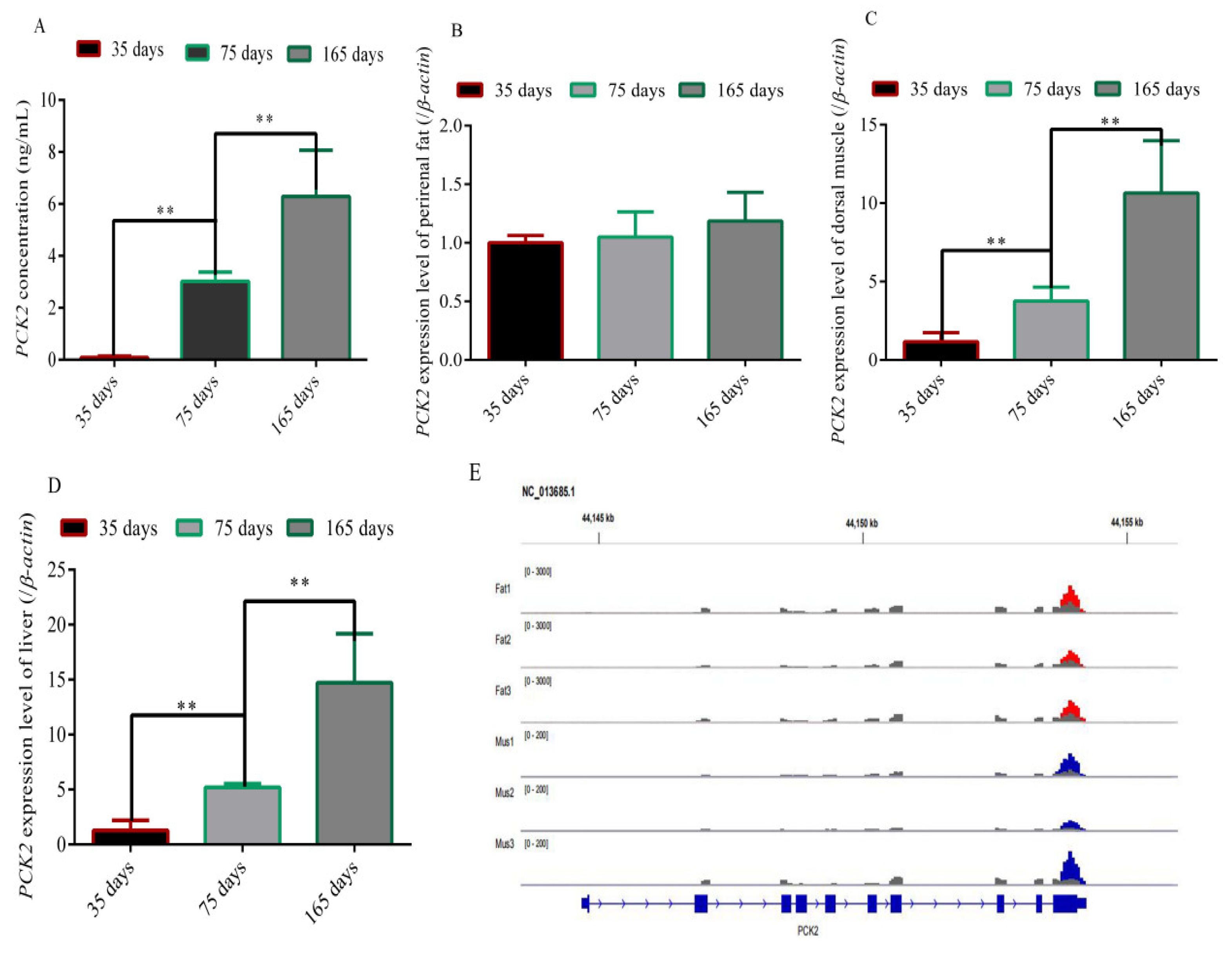
3.1. Analysis of the Content of PCK2 in Blood, the Expression of the PCK2 Gene and m6A Modification of the PCK2 Gene in Different Tissues of Rex Rabbits
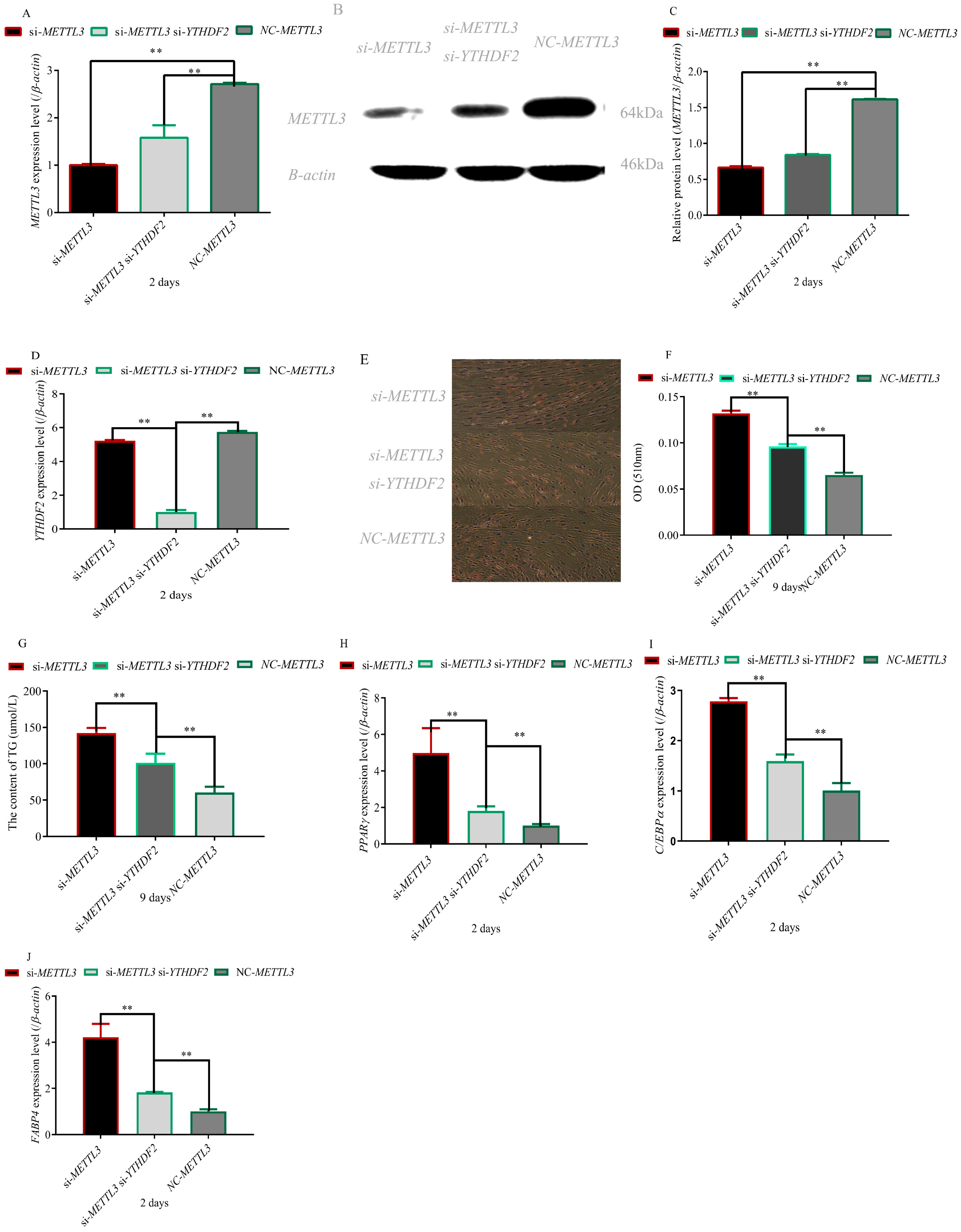
3.2. Knockout of YTHDF2 Gene Partially Restored the Effect of METTL3 Knockout on Adipocyte Differentiation
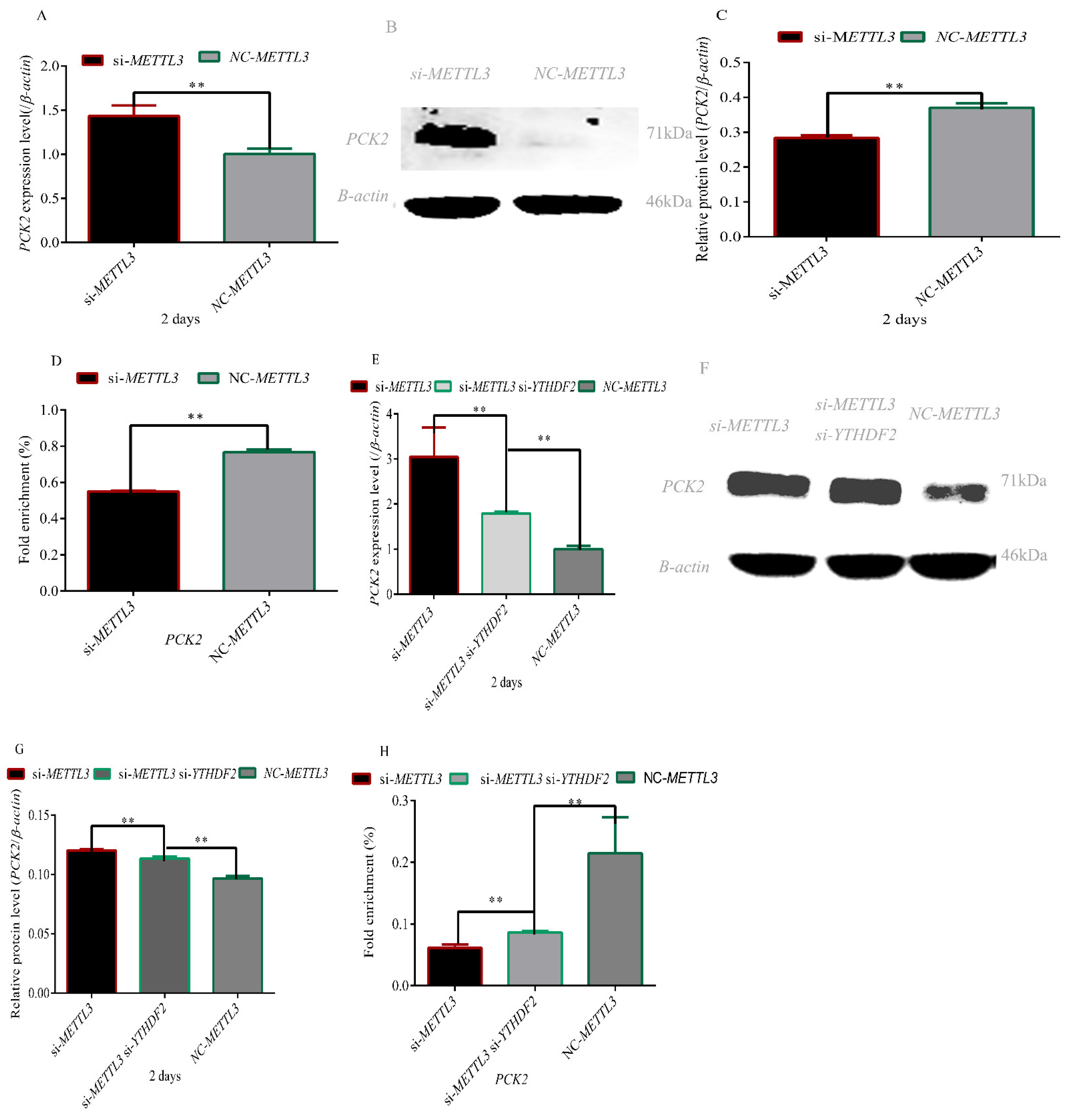
3.3. Effect of METTL3 and YTHDF2 on the Expression Level of the PCK2 Gene
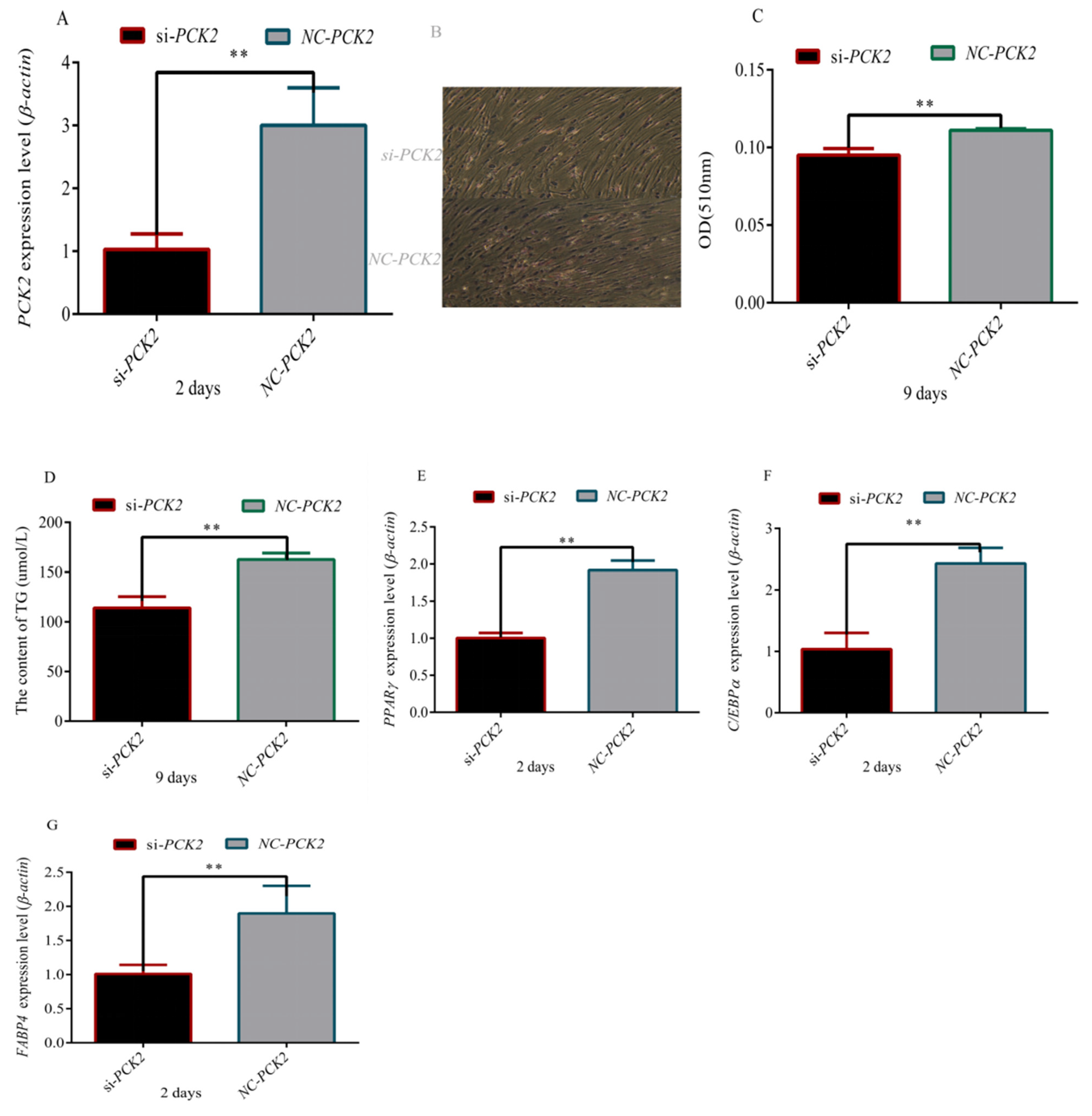
3.4. Effect of PCK2 on the Differentiation of Preadipocytes
3.5. Correlation Analysis between Expression Level of the PCK2 Gene in the Longissimus Lumborum and Meat Quality Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| m6A | N6-methyladenosine |
| NCD | non-communicable disease |
| PCK2 | phosphoenolpyruvate carboxykinase 2 |
| YTHDF2 | YTH domain family 2 |
| METTL3 | methyltransferase-like 3 |
| METTL14 | methyltransferase-like 14 |
| WTAP | Wilms tumor 1–associated protein |
| PPARγ | peroxisome proliferator-activated receptor-γ |
| C/EBPα | CCAAT/enhancer-binding family of proteins |
| FABP4 | fatty-acid-binding protein 4 |
| TG | triglyceride content |
References
- Dalle, Z.A.; Zs, S. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T. The role of intramuscular connective tissue in meat texture. Anim. Sci. J. 2010, 81, 21–27. [Google Scholar] [CrossRef]
- Ge, K.; Ye, P.; Yang, L.; Kuang, J.; Chen, X.; Geng, Z. Comparison of slaughter performance, meat traits, serum lipid parameters and fat tissue between Chaohu ducks with high- and low-intramuscular fat content. Anim. Biotechnol. 2020, 31, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Watkins, P.; Ball, A.; Krishnamurthy, R.; Piyasiri, U.; Sewell, J.; OrtuñO, J.; Stark, J.; Warner, R. Impact of Brassica and Lucerne Finishing Feeds and Intramuscular Fat on Lamb Eating Quality and Flavor. A Cross-Cultural Study Using Chinese and Non-Chinese Australian Consumers. J. Agric. Food Chem. 2016, 64, 6856–6868. [Google Scholar] [CrossRef] [PubMed]
- Starkey, C.P.; Geesink, G.H.; Collins, D.; Oddy, V.H.; Hopkins, D.L. Do sarcomere length, collagen content, pH, intramuscular fat and desmin degradation explain variation in the tenderness of three ovine muscles? Meat Sci. 2016, 113, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Di, S.L.; Brugiapaglia, A.; Galloni, M.; Destefanis, G.; Lisa, C. Effect of the leptin c.73TC mutation on carcass traits in beef cattle. Anim. Genet. 2007, 38, 316–317. [Google Scholar]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure—ScienceDirect. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Mortensen, M.; Andersen, H.J.; Engelsen, S.B.; Bertram, H.C. Effect of freezing temperature, thawing and cooking rate on water distribution in two pork qualities. Meat Sci. 2006, 72, 34–42. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Hsu, P.J.; Chen, Y.S.; Yang, Y.G. Dynamic transcriptomic m6A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef] [Green Version]
- Zhen, B.; Liu, Y.; Zhao, Y.; Yao, Y.; Wang, X. A dynamic reversible RNA N6-methyladenosine modification: Current status and perspectives. J. Cell. Physiol. 2019, 234, 7948–7956. [Google Scholar]
- Wang, Y.; Li, Y.; Toth, J.I.; Petroski, M.; Zhang, Z.; Jing, C.Z. N-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014, 16, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, L.; Chen, J.; Wang, Y. mRNA m6A methylation downregulates adipogenesis in porcine adipocytes. Biochem. Biophys. Res. Commun. 2015, 459, 201–207. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Sun, B.F.; Shi, Y.; Yang, X.; Xiao, W.; Hao, Y.J.; Ping, X.L.; Chen, Y.S.; Wang, W.J.; et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014, 24, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Tymoczko, J.; Stryer, L. Biochemistry; W. H. Freeman and Company: New York, NY, USA, 2002; pp. 93–126. [Google Scholar]
- Franckhauser, S.; Munoz, S.; Pujol, A. Increased Fatty Acid Re-esterification by PEPCK Overexpression in Adipose Tissue Leads to Obesity Without Insulin Resistance. Diabetes 2002, 51, 624–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, K.Y.; Li, M.; Yu, B.; Fan, M.; Yerle, B.L. Assignment porcine PCK1 and PCK2 genes to SSC17 and SSC7, respectively, by radiation hybrid mapping. Anim. Genet. 2005, 36, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, W.; Jin, B.; Zhang, X.; Ma, F.; Xu, X. Candidate gene expression affects intramuscular fat content and fatty acid composition in pigs. J. Appl. Genet. 2013, 54, 113–118. [Google Scholar] [CrossRef]
- Oshel, K.M.; Knight, J.B.; Cao, K.T.; Thai, M.V.; Olson, A.L. Identification of a 30-Base Pair Regulatory Element and Novel DNA Binding Protein That Regulates the Human GLUT4 Promoter in Transgenic Mice. J. Biol. Chem. 2000, 275, 23666–23673. [Google Scholar] [CrossRef] [Green Version]
- Hamam, D.; Ali, D.; Kassem, M.; Aldahmash, A.; Alajez, N.M. MicroRNAs as Regulators of Adipogenic Differentiation of Mesenchymal Stem Cells. Stem Cells Dev. 2014, 24, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Prayitno, D.S.; Phillips, C.J.C.; Omed, H. The Effects of Color of Lighting on the Behavior and Production of Meat Chickens. Poult. Sci. 1997, 76, 452–457. [Google Scholar] [CrossRef]
- Wang, W.; Xue, W.; Xu, X.; Jin, B.; Zhang, X. Correlations of genes expression in PPAR signalling pathway with porcine meat quality traits. Czech J. Anim. Sci. 2016, 61, 333–339. [Google Scholar]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, Z.; Yu, L.; Li, Y.; Liang, M.; Zhou, L. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. J. Cell. Biochem. 2018, 119, 5676–5685. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Hu, S.; Lai, T.; Wang, J.; Wang, L.; Lai, S. MiR-9-5p promotes rabbit preadipocyte differentiation by suppressing leptin gene expression. Lipids Health Dis. 2020, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Wang, L.; Hu, S.; Du, K.; Lai, S. Association of leptin mRNA expression with meat quality trait in Tianfu black rabbits. Anim. Biotechnol. 2020, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J. Some differences between avian and mammaeian biochemistry. Int. J. Biochem. 1977, 8, 269–275. [Google Scholar] [CrossRef]
- Pilo, B.; George, J.C. Diurnal and seasonal variation in liver glycogen and fat in relation to metabolic status of liver and m. pectoralis in the migratory starling, Sturnus roseus, wintering in India. Comp. Biochem. Physiol. A Comp. Physiol. 1983, 74, 601–604. [Google Scholar] [CrossRef]
- Yasuhara, M.; Ohama, T.; Matsuki, N.; Saito, H.; Teramoto, T. Induction of fatty liver by fasting in suncus. J. Lipid Res. 1991, 32, 887–891. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- Wu, R.; Jiang, D.; Wang, Y.; Wang, X. N (6)-Methyladenosine (m(6)A) Methylation in mRNA with A Dynamic and Reversible Epigenetic Modification. Mol. Biotechnol. 2016, 58, 450–459. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, L.; Wang, M.; Xiong, Q.; Guo, Y.; Liang, Y.; Li, J.; Sheng, R.; Deng, P.; Wang, Y.; et al. Mettl3-mediated m6A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masatoshi, K.; Mitsuru, O.; Takayoshi, S.; Motoharu, A.; Toshihiro, U.; Aya, I.; Naoki, K.; Yukiko, O.; Naoto, K.; Ryo, S. The RNA methyltransferase complex of WTAP, METTL3, and METTL14 regulates mitotic clonal expansion in adipogenesis. Mol. Cell. Biol. 2018, 38, 16–18. [Google Scholar]
- Xie, W.; Ma, L.L.; Xu, Y.Q.; Wang, B.H.; Li, S.M. METTL3 inhibits hepatic insulin sensitivity via N6-methyladenosine modification of Fasn mRNA and promoting fatty acid metabolism—ScienceDirect. Biochem. Biophys. Res. Commun. 2019, 518, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Byrne, C.D.; Zhang, J.L.; Petry, C.J.; Hales, C.N. Programming of hepatic insulin-sensitive enzymes in offspring of rat dams fed a protein-restricted diet. Am. J. Physiol. 1997, 272, 1083–1090. [Google Scholar] [CrossRef]
- Rozance, P.J.; Limesand, S.W.; Barry, J.S.; Brown, L.D.; Hay, W.W. Chronic late-gestation hypoglycemia upregulates hepatic PEPCK associated with increased PGC1α mRNA and phosphorylated CREB in fetal sheep. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E365–E370. [Google Scholar] [CrossRef] [Green Version]
- Berger, J. Peroxisome proliferator-activated receptor-gamma ligands inhibit adipocyte 11beta -hydroxysteroid dehydrogenase type 1 expression and activity. J. Biol. Chem. 2001, 276, 12629–12635. [Google Scholar] [CrossRef] [Green Version]
- Kotelevtsev, Y.; Holmes, M.C.; Burchell, A.; Houston, P.M.; Schmoll, D.; Jamieson, P.; Best, R.; Brown, R.; Edwards, C.; Seckl, J.R. 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity orstress. Proc. Natl. Acad. Sci. USA 1997, 94, 14924–14929. [Google Scholar] [CrossRef] [Green Version]
- Forhead, A.J.; Poore, K.R.; Mapstone, J.; Fowden, A.L. Developmental regulation of hepatic and renal gluconeogenic enzymes by thyroid hormones in fetal sheep during late gestation. J. Physiol. 2003, 548, 941–947. [Google Scholar] [CrossRef]
- Franko, K.L.; Giussani, D.A.; Forhead, A.J.; Fowden, A.L. Effects of dexamethasone on the glucogenic capacity of fetal, pregnant, and non-pregnant adult sheep. J. Endocrinol. 2007, 192, 67–73. [Google Scholar] [CrossRef]
- Chen, H.; Gao, S.; Liu, W.; Wong, C.C.; Wu, J.; Wu, J.; Liu, D.; Gou, H.; Kang, W.; Zhai, J. RNA N6-Methyladenosine Methyltransferase METTL3 Facilitates Colorectal Cancer by Activating the m6A-GLUT1-mTORC1 Axis and Is a Therapeutic Target—ScienceDirect. Gastroenterology 2020, 160, 1284–1300. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Deng, K.; Pan, M.; Liu, S.; Zuo, J. Down-regulation of PCK2 inhibits the invasion and metastasis of laryngeal carcinoma cells. Am. J. Transl. Res. 2020, 12, 3842–3857. [Google Scholar]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef]
- Xiang, Y.; Laurent, B.; Hsu, C.H.; Nachtergaele, S.; Lu, Z.; Sheng, W.; Xu, C.; Hen, H.C.; Jian, O.; Wang, S. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature 2017, 552, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Patil, D.; Zhou, J.; Zinoviev, A.; Jaffrey, S. 5’ UTR m6A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Li, Y.; Tian, W.; Xiang, Z.; Gabriele, M. Modification of N6-methyladenosine RNA methylation on heat shock protein expression. PLoS ONE 2018, 13, e0198604. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhang, Q.; Liu, X.; Zhou, H.; Cao, J.L. Mettl3 contributes to inflammatory pain by targeting TET1 in YTHDF2-dependent manner. Pain 2021, 7. Publish ahead of printing. [Google Scholar]
- Zhou, D.; Tang, W.; Xu, Y.; Xu, Y.; Wang, G. METTL3/YTHDF2 m6A axis accelerates colorectal carcinogenesis through epigenetically suppressing YPEL5. Mol. Oncol. 2021, 15, 2172–2184. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.W.; Reshef, L. Glyceroneogenesis revisited. Biochimie 2003, 85, 1199–1205. [Google Scholar] [CrossRef]
- Stark, R.; Pasquel, F.; Turcu, A.; Pongratz, R.L.; Roden, M.; Cline, G.W.; Shulman, G.I.; Kibbey, R.G. Phosphoenolpyruvate Cycling via Mitochondrial Phosphoenolpyruvate Carboxykinase Links Anaplerosis and Mitochondrial GTP with Insulin Secretion. J. Biol. Chem. 2009, 284, 26578–26590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polonsky, K.S.; Given, B.; Hirsch, L.; Shapiro, E.T.; Cauter, E.V. Quantitative study of insulin secretion and clearance in normal and obese subjects. J. Clin. Investig. 1988, 81, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Zavaroni, I.; Bruschi, F.; Alpi, O.; Pezzarossa, A.; Guerra, L.; Dall’Aglio, E.; Coscelli, C.; Butturini, U. Peripheral Hyperinsulinemia of Simple Obesity: Pancreatic Hypersecretion or Impaired Insulin Metabolism? J. Clin. Endocrinol. Metab. 1984, 59, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Meistas, M.T.; Margolis, S.; Kowarski, A.A. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am. J. Physiol. 1983, 245, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Lucas, A.; Duarte, J.; Sunny, N.E.; Satapati, S.; He, T.T.; Fu, X.; Bermúdez, J.; Burgess, S.C.; Perales, J.C. PEPCK-M expression in mouse liver potentiates, not replaces, PEPCK-C mediated gluconeogenesis. J. Hepatol. 2013, 59, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Kumanova, M.; Hart, L.S.; Sloane, K.; Zhang, H.; Panis, D.D.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Ron, D.; Koumenis, C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. Embo J. 2014, 29, 2082–2096. [Google Scholar] [CrossRef] [Green Version]
- Ding, B.; Parmigiani, A.; Divakaruni, A.S.; Archer, K.; Murphy, A.N.; Budanov, A.V. Sestrin2 is induced by glucose starvation via the unfolded protein response and protects cells from non-canonical necroptotic cell death. Sci. Rep. 2016, 6, 22538. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.; Fortuno, E.S.; Suh, J.M.; Stenesen, D.; Tang, W.; Parks, E.J.; Adams, C.M.; Townes, T.; Graff, J.M. Atf4 Regulates Obesity, Glucose Homeostasis, and Energy Expenditure. Diabetes 2009, 58, 2565–2573. [Google Scholar] [CrossRef] [Green Version]
- Okeudo, N.J.; Moss, B.W. Interrelationships amongst carcass and meat quality characteristics of sheep. Meat Sci. 2005, 69, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.H.; Byrne, K.A.; Reverter, A.; Harper, G.S.; Taniguchi, M.; Mcwilliam, S.M.; Mannen, H.; Oyama, K.; Lehnert, S.A. Transcriptional profiling of skeletal muscle tissue from two breeds of cattle. Mamm. Genome 2005, 16, 201–210. [Google Scholar] [CrossRef]
- Schäfer, A.; Rosenvold, K.; Purslow, P.P.; Andersen, H.J.; Henckel, P. Physiological and structural events post mortem of importance for drip loss in pork. Meat Sci. 2002, 61, 355–366. [Google Scholar] [CrossRef]
- Zotte, A.D. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livest. Prod. Sci. 2002, 75, 11–32. [Google Scholar] [CrossRef]
- Anne, L.; Bénédicte, L.; Isabelle, L.; Thierry, A.; Muriel, B.; Louis, L.; Brigitte, P.; JérMe, B. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 2016, 1–14. [Google Scholar]
| Gene Name | Primer Sequence (5′-3′) | Tm (°C) | Product Size (bp) |
|---|---|---|---|
| YTHDF2 | CAGACACAGCCATTGCCTCCAC | 60 | 122 |
| CCGTTATGACCGAACCCACTGC | |||
| METTL3 | CCCACCTCAGTGGATCTGTT | 60 | 189 |
| ACCCAGAGGAAGAGAAAGCC | |||
| PCK2 | AACAGGAGGTGCGTGACATT | 60 | 250 |
| GGGACAGGGAGTGTGAGAAG | |||
| PPARγ | GAGGACATCCAGGACAACC | 61 | 168 |
| GTCCGTCTCCGTCTTCTTT | |||
| β-actin | GGAGATCGTGCGGGACAT | 61.4 | 318 |
| GTTGAAGGTGGTCTCGTGGAT | |||
| C/EBPα | GCGGGAACGAACAACAT | 64 | 172 |
| GGCGGTCATTGTCACTGGTC | |||
| FABP4 | GGCCAGGAATTTGATGAAGTC | 61.4 | 140 |
| AGTTTATCGCCCTCCCGTT | |||
| si-YTHDF2 | CAUGAAUACUAUAGACCAATT | ||
| UUGGUCUAUAGUAUUCAUGTT | |||
| si-METTL3 | UCAAGGAACAACAGAGCAATT | ||
| UUGCUCUGUUGUUCCUUAGTT | |||
| si-PCK2 | GGGAACAGGAGGUGCGUGATT | ||
| UCACGCACCUCCUGUUCCCTT | |||
| Negative Control | UUCUCCGAACGUGUCACGUTT | ||
| ACGUGACACGUUCGGAGAATT |
| Expression Level/Meat Quality Traits | IntramuscuLAR Fat Content | Cooked Meat Rate | Drip Loss | Reduced pH Value | Total Water Loss Rate | |
|---|---|---|---|---|---|---|
| Expression level of PCK2 | Age of 35 days | 0.94 ** | 0.45 | 0.58 | 0.39 | −0.87 * |
| Age of 75 days | 0.85 * | −0.46 | 0.21 | −0.85 * | −0.79 * | |
| Age of 165 days | 0.82 * | −0.04 | −0.28 | −0.88 * | −0.74 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Zhu, T.; Ren, Z. METTL3 Regulated the Meat Quality of Rex Rabbits by Controlling PCK2 Expression via a YTHDF2–N6-Methyladenosine Axis. Foods 2022, 11, 1549. https://doi.org/10.3390/foods11111549
Luo G, Zhu T, Ren Z. METTL3 Regulated the Meat Quality of Rex Rabbits by Controlling PCK2 Expression via a YTHDF2–N6-Methyladenosine Axis. Foods. 2022; 11(11):1549. https://doi.org/10.3390/foods11111549
Chicago/Turabian StyleLuo, Gang, Tongyan Zhu, and Zhanjun Ren. 2022. "METTL3 Regulated the Meat Quality of Rex Rabbits by Controlling PCK2 Expression via a YTHDF2–N6-Methyladenosine Axis" Foods 11, no. 11: 1549. https://doi.org/10.3390/foods11111549
APA StyleLuo, G., Zhu, T., & Ren, Z. (2022). METTL3 Regulated the Meat Quality of Rex Rabbits by Controlling PCK2 Expression via a YTHDF2–N6-Methyladenosine Axis. Foods, 11(11), 1549. https://doi.org/10.3390/foods11111549





