Glochidion wallichianum Leaf Extract as a Natural Antioxidant in Sausage Model System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of MPE
2.3. Determination of Total Extractable Phenolic Content, Free Radical Scavenging Activities, and Reducing Power
2.4. Preparation of SMS
2.5. Determination of Lipid Oxidation
2.6. Determination of Protein Oxidation
2.7. Determination of Redness Index and Discoloration
2.8. Determination of pH and Expressible Drip
2.9. Statistical Analysis
3. Results and Discussion
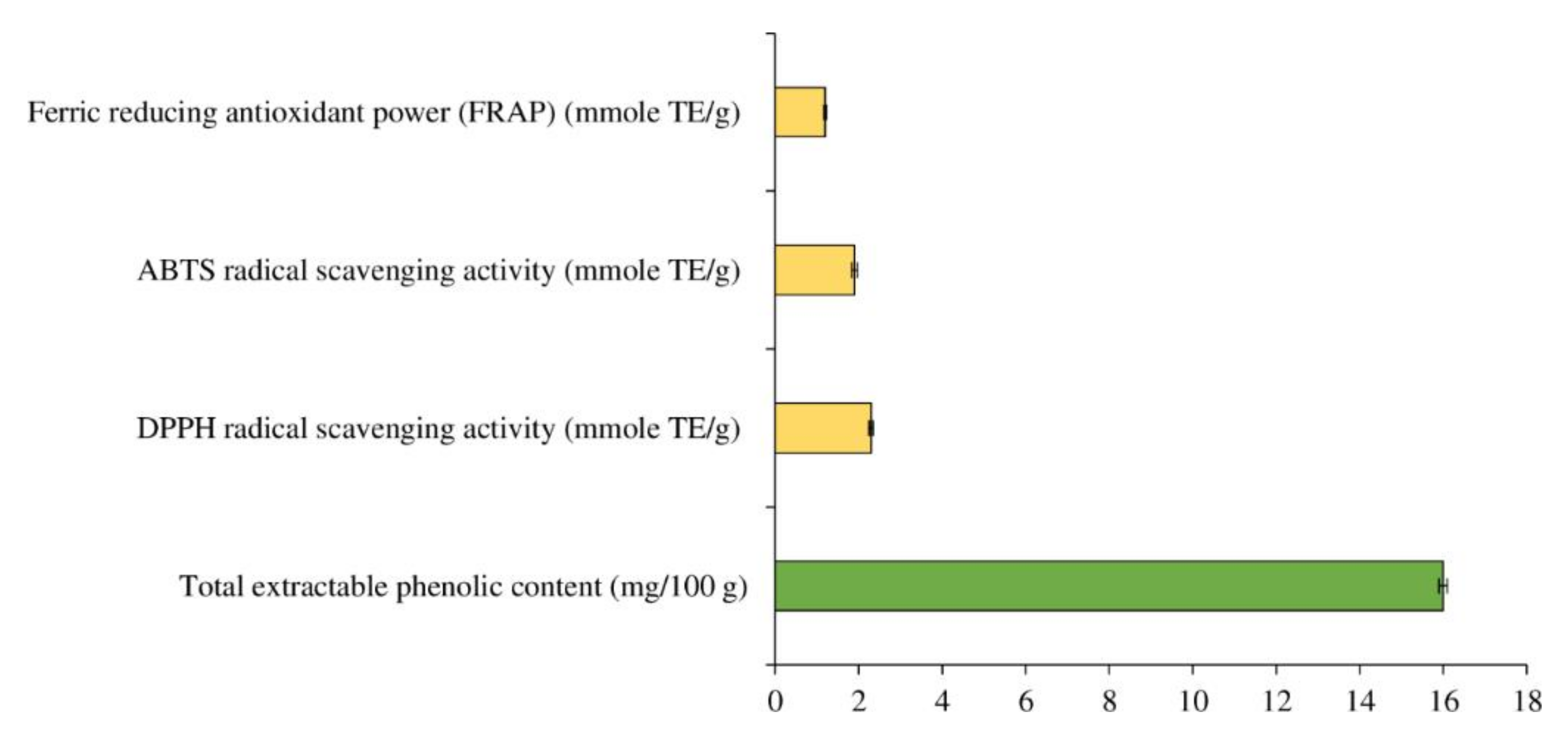
3.1. Total Extractable Phenolic Content and In Vitro Antioxidant Activity of MPE
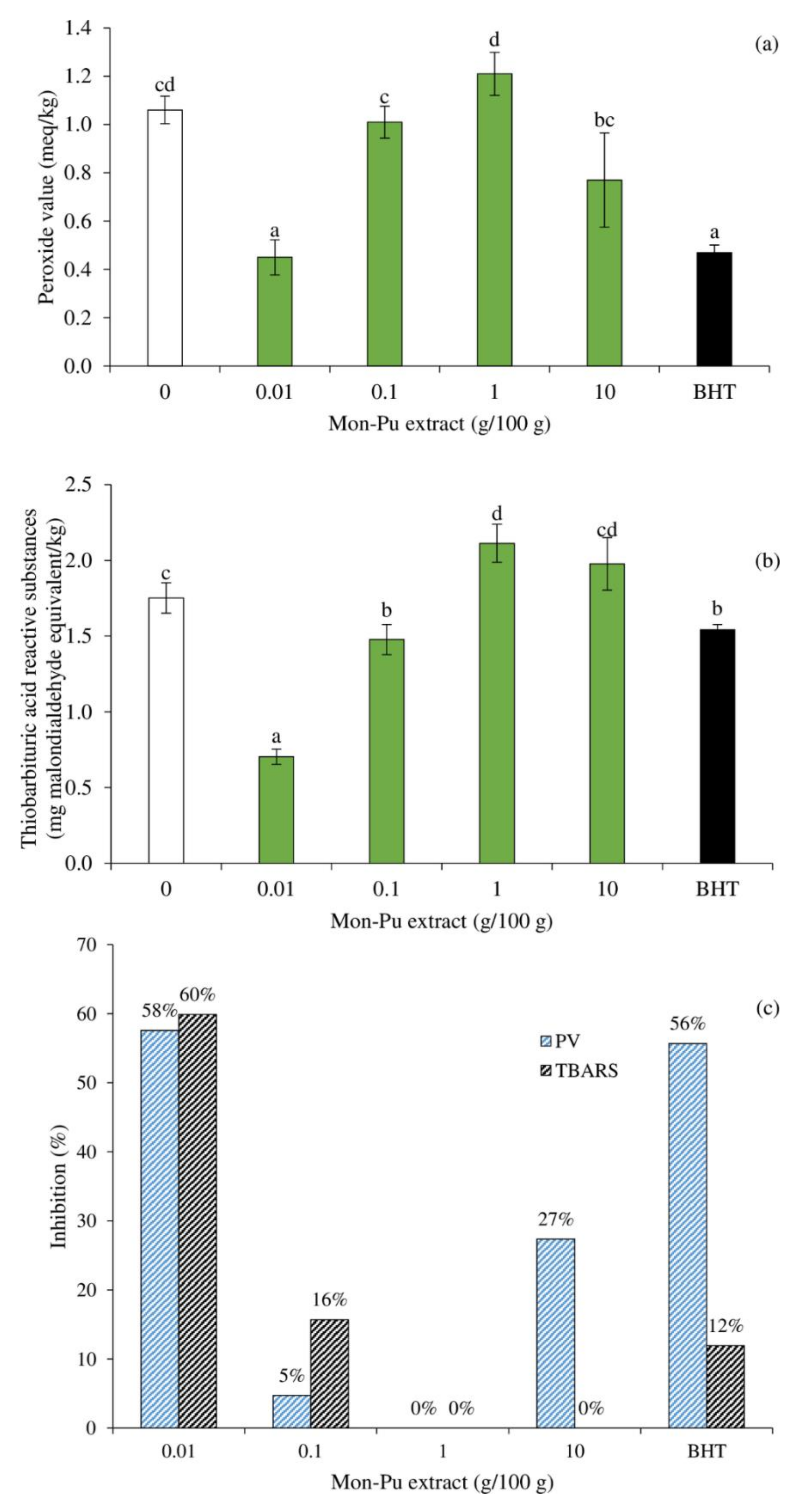
3.2. Effect of MPE on Lipid Oxidation of Cooked SMS
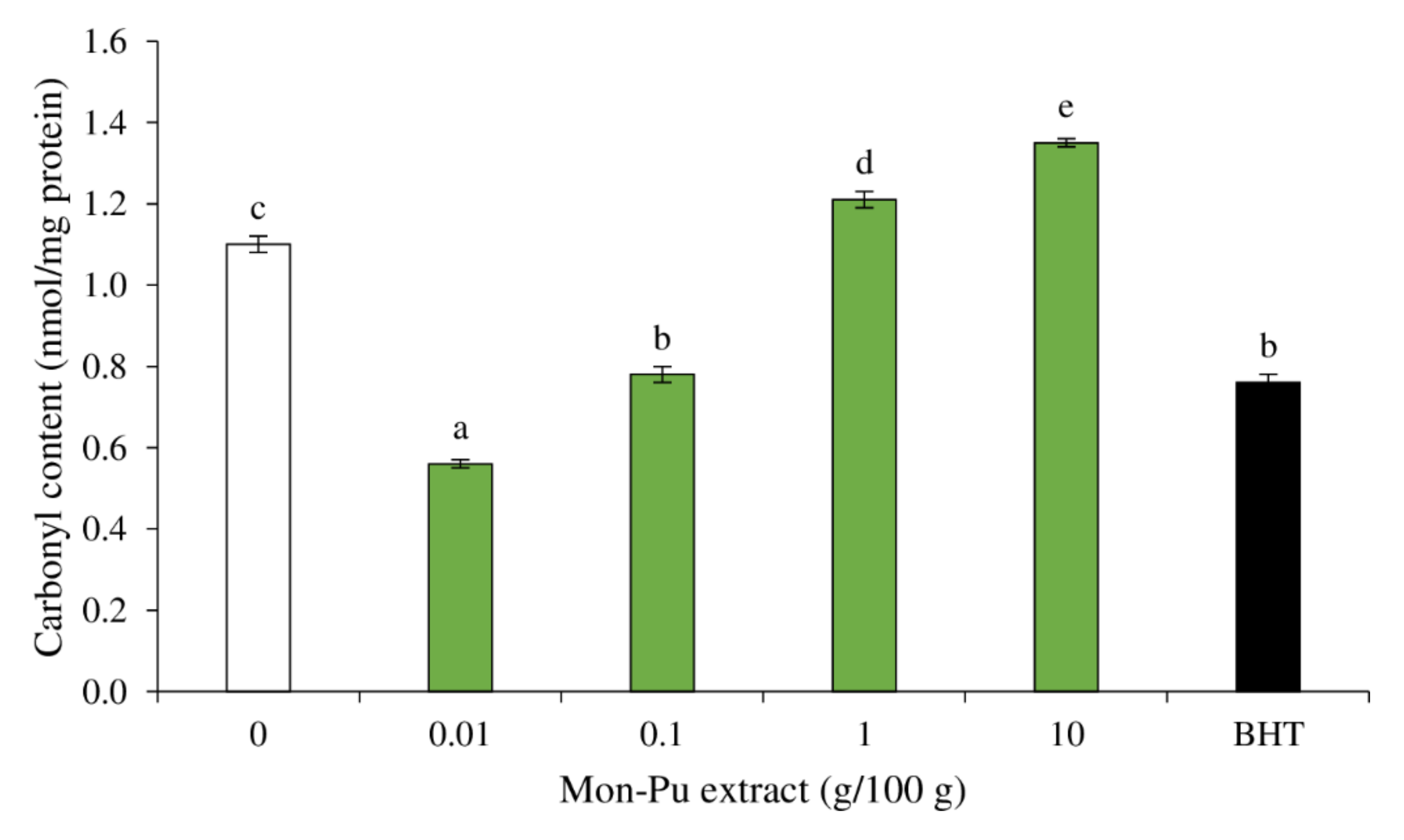
3.3. Effect of MPE on Protein Oxidation of Cooked SMS
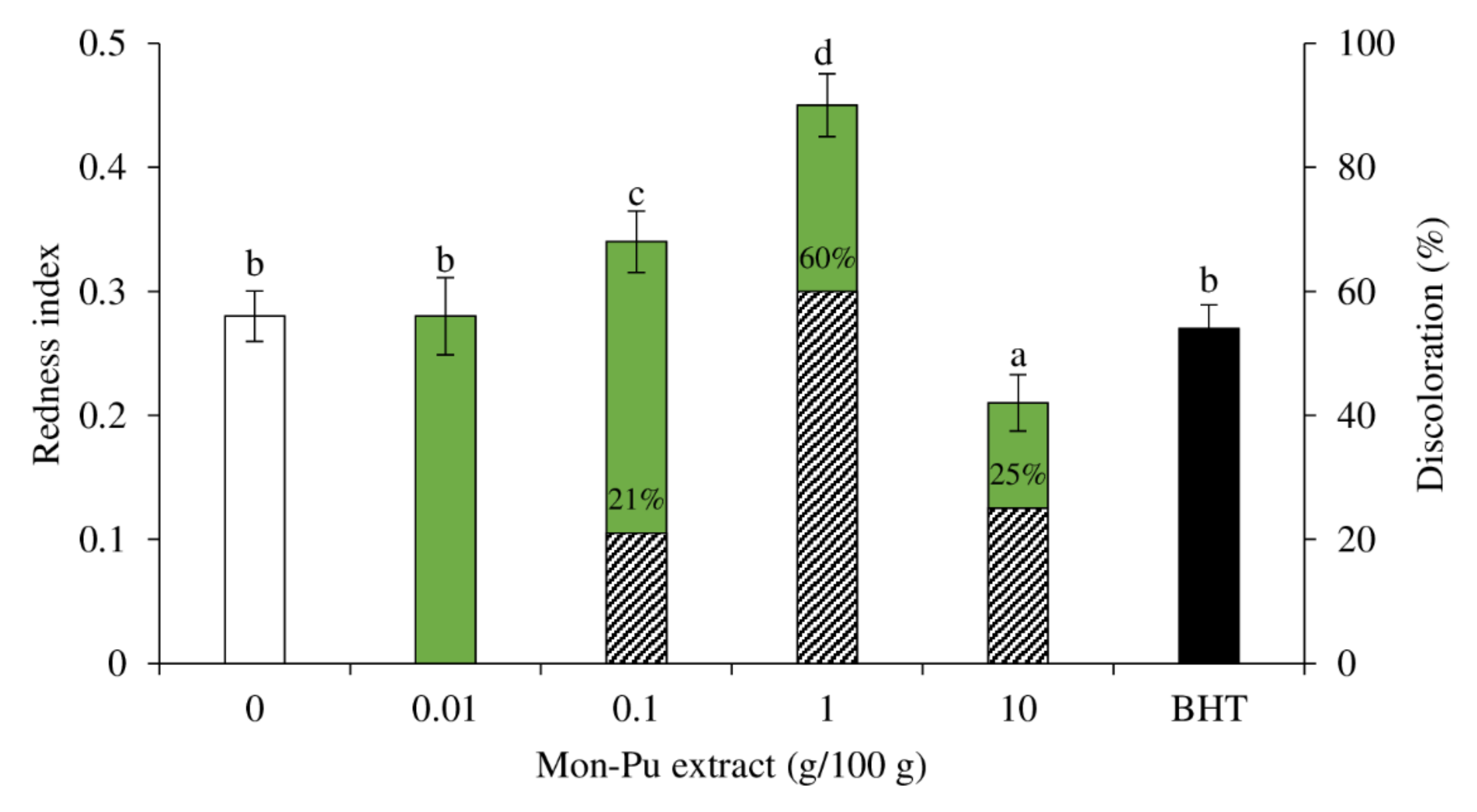
3.4. Effect of MPE on Redness Index and Discoloration of Cooked SMS
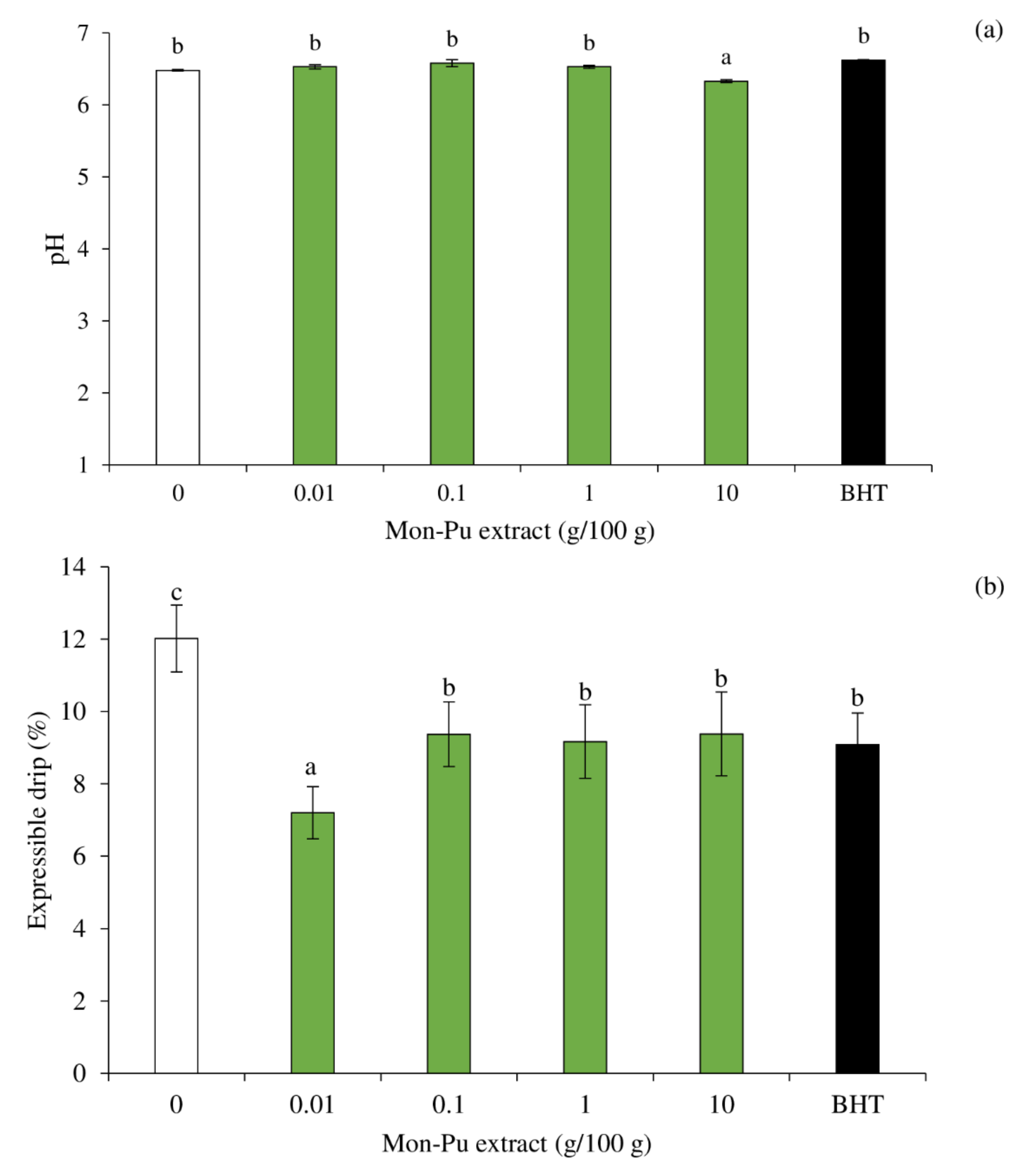
3.5. Effect of MPE on pH and Expressible Drip of Cooked SMS
3.6. Effect of MPE on Lipid Oxidation of Cooked SMS during Refrigerated Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Yin, J.; Zhang, J.; Richards, M. Factors affecting lipid oxidation due to pig and turkey hemolysate. J. Agric. Food Chem. 2017, 65, 8011–8017. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, A.B.; da Silva, M.V.; da Silva Lannes, S.C. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Chaijan, M.; Panpipat, W. Mechanism of oxidation in foods of animal origin. In Natural Antioxidants, 1st ed.; Banerjee, R., Verma, A.K., Siddiqui, M.W., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 1–37. [Google Scholar]
- Sebranek, J.G.; Sewalt, V.J.H.; Robbins, K.; Houser, T.A. Comparison of a natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2005, 69, 289–296. [Google Scholar] [CrossRef]
- Panpipat, W.; Suttirak, W.; Chaijan, M. Free radical scavenging activity and reducing capacity of five southern Thai indigenous vegetable extracts. Walailak J. Sci. Technol. 2010, 7, 51–60. [Google Scholar]
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef]
- Tikk, K.; Haugen, J.-E.; Andersen, H.J.; Aaslyng, M.D. Monitoring of warmed-over flavor in pork using the electronic nose–correlation to sensory attributes and secondary lipid oxidation products. Meat Sci. 2008, 80, 1254–1263. [Google Scholar] [CrossRef]
- EU Commission. Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union Law 2011, 295, 1–177. [Google Scholar]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–174. [Google Scholar] [CrossRef]
- Sungpud, C.; Panpipat, W.; Sae Yoon, A.; Chaijan, M. Tuning of virgin coconut oil and propylene glycol ratios for maximizing the polyphenol recovery and in vitro bioactivities of mangosteen (Garcinia mangostana L.) pericarp. Process Biochem. 2019, 87, 179–186. [Google Scholar] [CrossRef]
- Karpińska, M.; Borowski, J.; Danowska-Oziewicz, M. The use of natural antioxidants in ready-to-serve food. Food Chem. 2001, 72, 5–9. [Google Scholar] [CrossRef]
- Cando, D.; Morcuende, D.; Utrera, M.; Estévez, M. Phenolic-rich extracts from Willowherb (Epilobium hirsutum L.) inhibit lipid oxidation but accelerate protein carbonylation and discoloration of beef patties. Eur. Food Res. Technol. 2014, 238, 741–751. [Google Scholar] [CrossRef]
- Oktay, M.; Gülçin, İ.; Küfrevioğlu, Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Van Der Sluis, A.A.; Dekker, M.; Skrede, G.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple juice. 1. Effect of existing production methods. J. Agric. Food Chem. 2002, 50, 7211–7219. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef] [Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Chanudom, L.; Bhoopong, P.; Khwanchuea, R.; Tangpong, J. Antioxidant and antimicrobial activities of aqueous & ethanol crude extracts of 13 Thai traditional plants. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 549–558. [Google Scholar]
- Rajan, V.K.; Muraleedharan, K. A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, Gallic acid. Food Chem. 2017, 220, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mendiratta, S.K.; Agarwal, R.K.; Kumar, S.; Soni, A. Evaluation of anti-oxidant and anti-microbial activity of various essential oils in fresh chicken sausages. J. Food Sci. Technol. 2017, 54, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qin, C.; Zhang, P.; Ge, Q.; Wu, M.; Wu, J.; Wang, M.; Wang, Z. Antioxidant effect of apple phenolic on lipid peroxidation in Chinese-style sausage. J. Food Sci. Technol. 2015, 52, 1032–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anantachoke, N.; Kitphati, W.; Mangmool, S.; Bunyapraphatsara, N. Polyphenolic compounds and antioxidant activities of the leaves of Glochidion hypoleucum. Nat. Prod. Commun. 2015, 10, 479–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kongkachuichai, R.; Charoensiri, R.; Yakoh, K.; Kringkasemsee, A.; Insung, P. Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand. Food Chem. 2015, 173, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Alzoreky, N.; Nakahara, K. Antioxidant activity of some edible Yemeni plants evaluated by ferrylmyoglobin/ABTS assay. Food Sci. Technol. Res. 2001, 7, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Tangkanakul, P.; Trakoontivakorn, G.; Jariyavattanavijit, C. Extracts of Thai indigenous vegetables as rancid inhibitor in a model system. Kasetsart J. 2005, 39, 274–283. [Google Scholar]
- Sae-Leaw, T.; Benjakul, S. Prevention of quality loss and melanosis of Pacific white shrimp by cashew leaf extracts. Food Control 2019, 95, 257–266. [Google Scholar] [CrossRef]
- Sae Yoon, A.; Sakdiset, P. Development of microemulsions containing Glochidion wallichianum leaf extract and potential for transdermal and topical skin delivery of gallic acid. Sci. Pharm. 2020, 88, 53. [Google Scholar] [CrossRef]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Rietjens, I.M.; Boersma, M.G.; de Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Sungpud, C.; Panpipat, W.; Sae Yoon, A.; Chaijan, M. Polyphenol extraction from mangosteen (Garcinia mangostana Linn) pericarp by bio-based solvents. Int. Food Res. J. 2020, 27, 111–120. [Google Scholar]
- Sungpud, C.; Panpipat, W.; Chaijan, M.; Sae Yoon, A. Techno-biofunctionality of mangostin extract-loaded virgin coconut oil nanoemulsion and nanoemulgel. PLoS ONE 2020, 15, e0227979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agbor, G.A.; Oben, J.E.; Ngogang, J.Y.; Xinxing, C.; Vinson, J.A. Antioxidant capacity of some herbs/spices from Cameroon: A comparative study of two methods. J. Agric. Food Chem. 2005, 53, 6819–6824. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Panpipat, W.; Cheong, L.Z.; Chaijan, M. Impact of lecithin incorporation on gel properties of bigeye snapper (Priacanthus tayenus) surimi. Int. J. Food Sci. Technol. 2021, 56, 2481–2491. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W.; Nisoa, M. Chemical deterioration and discoloration of semi-dried tilapia processed by sun drying and microwave drying. Dry Technol. 2017, 35, 642–649. [Google Scholar] [CrossRef]
- Chen, H.H.; Chiu, E.M.; Huang, J.R. Color and gel-forming properties of horse mackerel (Trachurus japonucus) as related to washing conditions. J. Food Sci. 1997, 62, 985–991. [Google Scholar] [CrossRef]
- Somjid, P.; Panpipat, W.; Cheong, L.Z.; Chaijan, M. Reduced washing cycle for sustainable mackerel (Rastrelliger kanagurta) surimi production: Evaluation of bio-physico-chemical, rheological, and gel-forming properties. Foods 2021, 10, 2717. [Google Scholar] [CrossRef]
- Botsaris, G.; Orphanides, A.; Yiannakou, E.; Gekas, V.; Goulas, V. Antioxidant and antimicrobial effects of Pistacia lentiscus L. extracts in pork sausages. Food Technol. Biotechnol. 2015, 53, 472–478. [Google Scholar] [CrossRef]
- Gallo, M.; Ferracane, R.; Naviglio, D. Antioxidant addition to prevent lipid and protein oxidation in chicken meat mixed with supercritical extracts of Echinacea angistifolia. J. Supercrit. Fluids 2012, 72, 198–204. [Google Scholar] [CrossRef]
- Vaquero, M.J.R.; Serravalle, L.R.T.; de Nadra, M.C.M.; de Saad Strasser, A.M. Antioxidant capacity and antibacterial activity of phenolic compounds from Argentinean herbs infusions. Food Control 2010, 21, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Capitani, C.D.; Hatano, M.K.; Marques, M.F.; Castro, I.A. Effects of optimized mixtures containing phenolic compounds on the oxidative stability of sausages. Food Sci. Technol. Int. 2013, 19, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Tang, D.; Yang, H.; Wang, X.; Zhu, M.; Liu, X. The dose-dependent effects of polyphenols and malondialdehyde on the emulsifying and gel properties of myofibrillar protein-mulberry polyphenol complex. Food Chem. 2021, 360, 130005. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, L.; Xiang, R.; Liu, X.; Zhu, M. Effects of mulberry polyphenols on oxidation stability of sarcoplasmic and myofibrillar proteins in dried minced pork slices during processing and storage. Meat Sci. 2020, 160, 107973. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Estévez, M.; Heinonen, M. Effect of phenolic compounds on the formation of α-aminoadipic and γ-glutamic semialdehydes from myofibrillar proteins oxidized by copper, iron, and myoglobin. J. Agric. Food Chem. 2010, 58, 4448–4455. [Google Scholar] [CrossRef]
- Chobot, V.; Hadacek, F. Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep. 2011, 16, 242–247. [Google Scholar] [CrossRef]
- Akagawa, M.; Ishii, Y.; Ishii, T.; Shibata, T.; Yotsu-Yamashita, M.; Suyama, K.; Uchida, K. Metal-catalyzed oxidation of protein-bound dopamine. Biochemistry 2006, 45, 15120–15128. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.E.; Hathaway, G.M.; Havlin, R. Oxidation and reduction of iron porphyrins and hemoproteins by quinones and hydroquinones. J. Am. Chem. Soc. 1977, 99, 8032–8039. [Google Scholar] [CrossRef]
- McBride, N.T.; Hogan, S.A.; Kerry, J.P. Comparative addition of rosemary extract and additives on sensory and antioxidant properties of retail packaged beef. Int. J. Food Sci. Technol. 2007, 42, 1201–1207. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of heat treatment on the phenolic com-pounds and antioxidant capacity of citrus peel extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Jiang, J.; True, A.D.; Xiong, Y.L. Myofibrillar protein cross-linking and gelling behavior modified by structurally relevant phenolic compounds. J. Agric. Food Chem. 2021, 69, 1308–1317. [Google Scholar] [CrossRef]
- Balange, A.; Benjakul, S. Enhancement of gel strength of bigeye snapper (Priacanthus tayenus) surimi using oxidised phenolic compounds. Food Chem. 2009, 113, 61–70. [Google Scholar] [CrossRef]
- Cao, Y.; Ai, N.; True, A.D.; Xiong, Y.L. Effects of (−)-epigallocatechin-3-gallate incorporation on the physicochemical and oxidative stability of myofibrillar protein–soybean oil emulsions. Food Chem. 2018, 245, 439–445. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Zhou, F.; Sun, W.; Zhao, M. Controlled formation of emulsion gels stabilized by salted myofibrillar protein under malondialdehyde (MDA)-induced oxidative stress. J. Agric. Food Chem. 2015, 63, 3766–3777. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Gao, Z. Effects of malondialdehyde-induced protein modification on water functionality and physicochemical state of fish myofibrillar protein gel. Food Res. Int. 2016, 86, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Nanditha, B.R.; Jena, B.S.; Prabhasankar, P. Influence of natural antioxidants and their carry-through property in biscuit processing. J. Sci. Food Agric. 2009, 89, 288–298. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongnen, C.; Ruzzama, N.; Chaijan, M.; Cheong, L.-Z.; Panpipat, W. Glochidion wallichianum Leaf Extract as a Natural Antioxidant in Sausage Model System. Foods 2022, 11, 1547. https://doi.org/10.3390/foods11111547
Wongnen C, Ruzzama N, Chaijan M, Cheong L-Z, Panpipat W. Glochidion wallichianum Leaf Extract as a Natural Antioxidant in Sausage Model System. Foods. 2022; 11(11):1547. https://doi.org/10.3390/foods11111547
Chicago/Turabian StyleWongnen, Chantira, Naiya Ruzzama, Manat Chaijan, Ling-Zhi Cheong, and Worawan Panpipat. 2022. "Glochidion wallichianum Leaf Extract as a Natural Antioxidant in Sausage Model System" Foods 11, no. 11: 1547. https://doi.org/10.3390/foods11111547
APA StyleWongnen, C., Ruzzama, N., Chaijan, M., Cheong, L.-Z., & Panpipat, W. (2022). Glochidion wallichianum Leaf Extract as a Natural Antioxidant in Sausage Model System. Foods, 11(11), 1547. https://doi.org/10.3390/foods11111547






