Arabinoxylans Release from Brewers’ Spent Grain Using Extrusion and Solid-State Fermentation with Fusarium oxysporum and the Antioxidant Capacity of the Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Milling Process
2.3. Extrusion Process
2.4. Chemical Characterization
2.5. Inoculum
2.6. Solid-State Fermentation
2.7. Extraction and Purification of Water-Extractable Arabinoxylans
2.8. Determination of Arabinoxylan Content
2.9. Extraction of Free Phenolic Compounds
2.10. Extraction of Bound Phenolic Compounds
2.11. Determination of Total Phenolic Content (TPC)
2.12. Determination of Phenolic Acids Using HPLC
2.13. Determination of DPPH Radical Scavenging Activity
2.14. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization
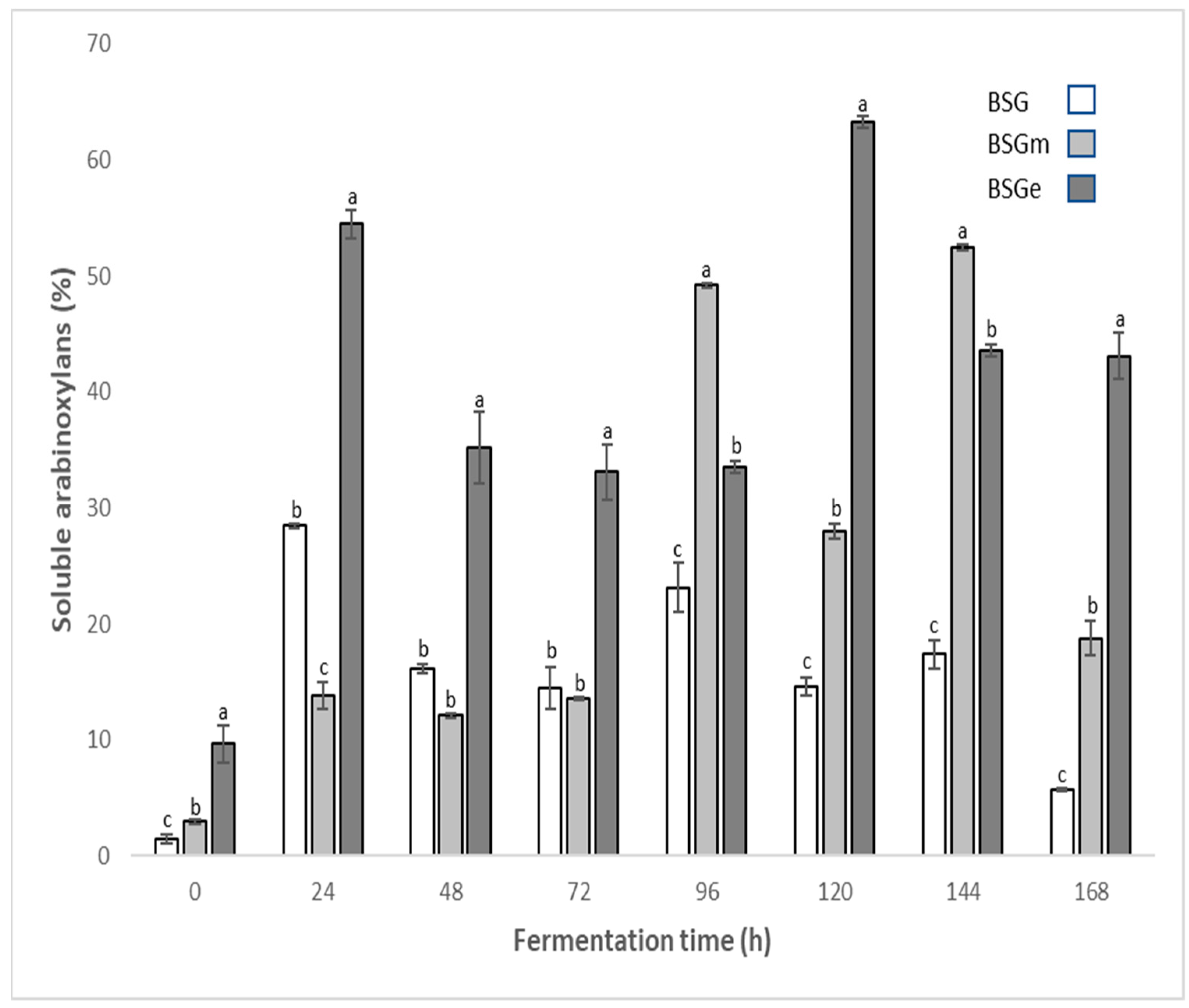
3.2. Soluble Arabinoxylan Content in BSG Extracts
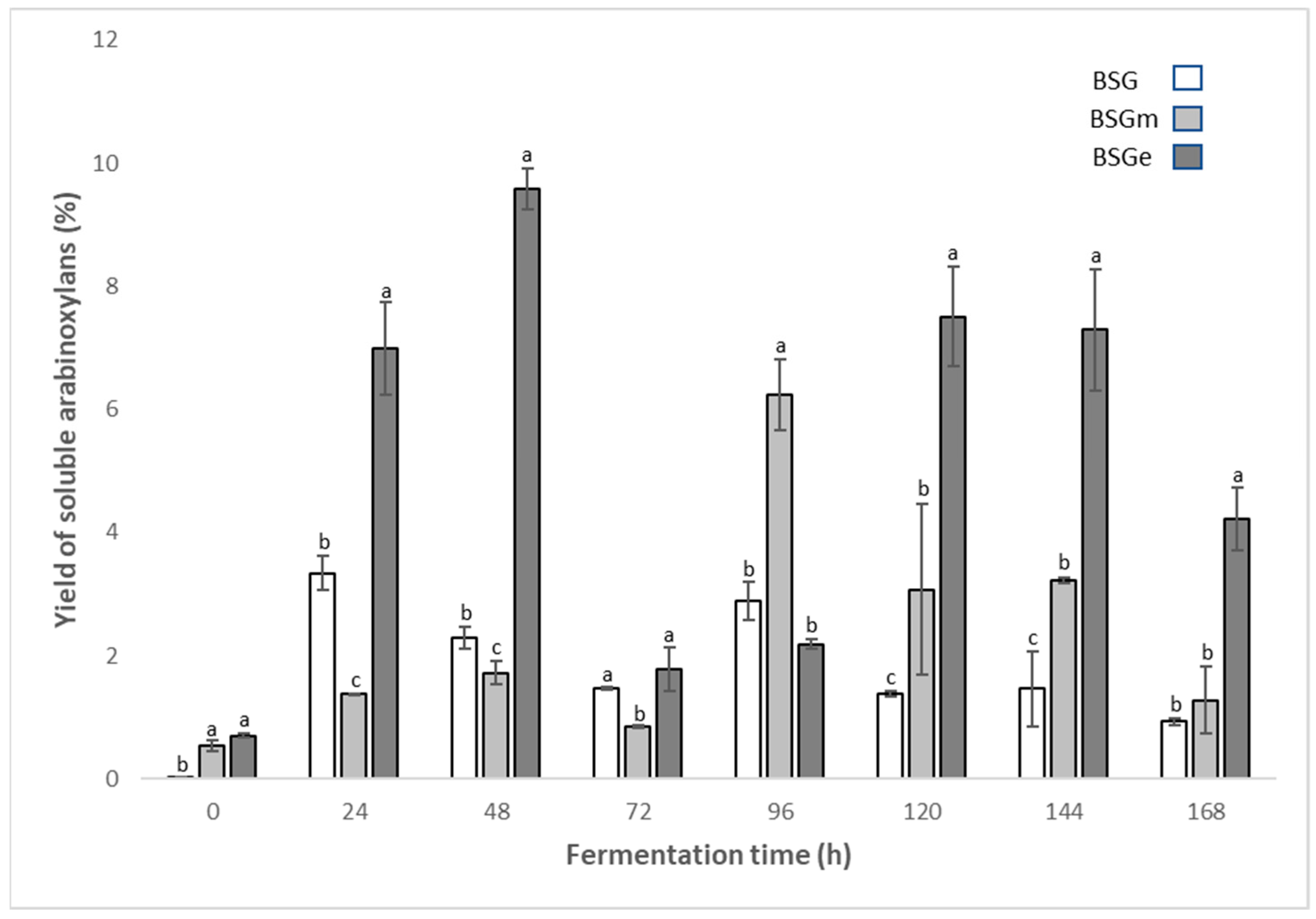
3.3. Yield of Soluble Arabinoxylans from BSG
3.4. Total Phenolic Compounds
3.5. Phenolic Acids
3.6. Antioxidant Capacity by DPPH
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Charlton, P.; Vriesekoop, F. Brewery by-products. In Handbook of Brewing, 3rd ed.; Steward, G.G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 567–589. [Google Scholar]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Eaton, B. An overwiew of brewing. In Handbook of Brewing, 3rd ed.; Steward, G.G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 53–56. [Google Scholar]
- Mussatto, S.I. Brewers’ spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifácio-Lopes, T.; Teixeira, J.A.; Pintado, M. Current extraction techniques towards bioactive compounds from brewer´s spent grain A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.F.; Abu-Ghannam, N. Antioxidant capacity, arabinoxylans content and in vitro glycaemic index of cereal-based snacks incorporated with brewer’s spent grain. LWT-Food Sci. Technol. 2014, 55, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Izydorczyk, M.S. Arabinoxylans. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science and Nutrition; Woodhead Publishing: Sawston, UK, 2021; pp. 339–461. [Google Scholar]
- Steiner, J.; Procopio, S.; Becker, T. Brewer’s spent grain: Source of value-added polysaccharides for the food industry in reference to the health claims. Eur. Food Res. Technol. 2015, 241, 303–315. [Google Scholar] [CrossRef]
- Fadel, A.; Plunkett, A.; Ashworth, J.; Mahmou, A.M.; Ranneh, Y.; El Mohtadi, M.; Li, W. The effect of extrusion screw-speed on the water extractability and molecular weight distribution of arabinoxylans from defatted rice bran. J. Food Sci. Technol. 2018, 55, 1201–1206. [Google Scholar] [CrossRef]
- Panagiotou, G.; Granouillet, P.; Olsson, L. Production and partial characterization of arabinoxylan-degrading enzymes by Penicillium brasilianum under solid-state fermentation. Appl. Microbiol. Biotechnol. 2006, 72, 1117–1124. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, X.; Guo, Y.; Wang, Q.; Peng, D.; Cao, L. Comparison of the immunological activities of arabinoxylans from wheat bran with alkali and xylanase-aided extraction. Carbohydr. Polym. 2010, 81, 784–789. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, C.; Li, W. Extraction and modification technology of arabinoxylans from cereal by-products: A critical review. Food Res. Int. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Reis, S.F.; Coelho, E.; Coimbra, M.A.; Abu-Ghannam, N. Improved efficiency of brewer’s spent grain arabinoxylans by ultrasound-assisted extraction. Ultrason. Sonochemistry 2015, 24, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Heredia-Olea, E.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Effect of extrusion conditions and hydrolysis with fiber-degrading enzymes on the production of C5 and C6 sugars from brewers spent grain for bioethanol production. Biofuel Res. J. 2015, 2, 203–208. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Liu, W.; Wan, J.; Wang, W.; Wu, L.; Yin, Z. Preparation, physicochemical and texture properties of texturized rice produce by improved extrusion cooking technology. J. Cereal Sci. 2011, 54, 473–480. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Xiros, C.; Christakopoulos, P. Enhanced ethanol production from brewer’s spent grain by a Fusarium oxysporum consolidated system. Biotechnol. Biofuels 2009, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, S.T. Solid state fermentation and its applications. In Bioprocessing for Value-Added Products from Renewable Resources: New Technologies and Applications; Yang, S.-T., Ed.; Elsevier: Amsterdam, NL, USA, 2011; pp. 465–489. ISBN 978-0-444-321149. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; John Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Armenta-López, S.E.; Valenzuela-Solano, C.; Hernández-Martínez, R. Identification and molecular analysis of races of Fusarium oxysporum f. sp. lycopersici isolated from tomato in Baja California, México. Mex. J. Phytopathol. 2021, 39, 266–288. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of extractives in biomass. Lab. Anal. Proced. 2008, 1617, 1–9. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- AACC. Approved Methods of Analysis, 11th ed.; The American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Troncoso-Rojas, R.; Sánchez-Estrada, A.; Carvallo, T.; González-León, A.; Aguilar-Valenzuela, A.; Ojeda-Contreras, J.; Tiznado-Hernández, M.E. A fungal elicitor enhances the resistance of tomato fruit to Fusarium oxysporum infection by activating the phenylpropanoid metabolic pathway. Phytoparasitica 2013, 41, 133–142. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Evaluation of Fusarium oxysporum as an enzyme factory for the hydrolysis of brewer’s spent grain with improved biodegradability for ethanol production. Ind. Crops Prod. 2008, 28, 213–224. [Google Scholar] [CrossRef]
- Douglas, S.G. A rapid method for the determination of pentosans in wheat flour. J. Food Chem. 1981, 7, 139–145. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Wang, X.; Gu, Z.; Beta, T. Changes of phenolic profiles and antioxidant activity in canaryseed (Phalaris canariensis L.) during germination. Food Chem. 2016, 194, 608–618. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s spent grain: A review of its potentials and applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar] [CrossRef]
- Bianco, A.; Budroni, M.; Zara, S.; Mannazzu, I.; Fancello, F.; Zara, G. The role of microorganisms on biotransformation of brewers spent grain. Appl. Microbiol. Biotechnol. 2020, 104, 8661–8678. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Johansen, A.Z.; Mussatto, S.I. Evaluation of different pretreatment strategies for protein extraction from brewer’s spent grains. Ind. Crops Prod. 2018, 125, 443–453. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, C.; Li, W.; Ashworth, J. Characterization of nitric oxide modulatory activities of alkaline-extracted and enzymatic-modified arabinoxylans from corn bran in cultured human monocytes. J. Agric. Food Chem. 2016, 64, 8128–8137. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [Green Version]
- Vasanthan, T.; Gaosong, J.; Yeung, J.; Li, J. Dietary fiber profile of barley flour as affected by extrusion cooking. Food Chem. 2002, 77, 35–40. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Rodríguez, A.; De la Rosa-Millán, J.; Heredia-Olea, E.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Shear-induced enhancement of technofunctional properties of whole grain flours through extrusion. Food Hydrocoll. 2021, 111, 106400. [Google Scholar] [CrossRef]
- García-Amezquita, L.E.; Tejada-Ortigoza, V.; Pérez-Carrillo, E.; Serna-Saldívar, S.O.; Campanella, O.H.; Welti-Chanes, J. Functional and compositional changes of orange peel fiber thermally-treated in a twin extruder. Lebensm. Wiss. Technol. 2019, 111, 673–681. [Google Scholar] [CrossRef]
- Srivastava, N.; Mishra, P.K.; Upadhyay, S.N. Industrial Enzymes for Biofuels Production: Recent Updates and Future Trends, 1st ed.; Elsevier: Amsterdam, NL, USA, 2020; Chapter 6. [Google Scholar]
- Alconada, T.M.; Martínez, M.J. Purification and characterization of an extracellular endo-1, 4-β-xylanase from Fusarium oxysporum f. sp. melonis. FEMS Microbiol. Lett. 1994, 118, 305–310. [Google Scholar] [CrossRef]
- Christakopoulos, P.; Nerinckx, W.; Kekos, D.; Macris, B.; Claeyssens, M. The alkaline xylanase III from Fusarium oxysporum F3 belongs to family F/10. Carbohydr. Res. 1997, 302, 191–195. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Di Pietro, A.; Roncero, M.I.G. Purification and characterization of an acidic endo-β-1, 4-xylanase from the tomato vascular pathogen Fusarium oxysporum f. sp. lycopersici. FEMS Microbiol. Lett. 1997, 148, 75–82. [Google Scholar] [CrossRef]
- Christakopoulos, P.; Nerinckx, W.; Kekos, D.; Macris, B.; Claeyssens, M. Purification and characterization of two low molecular mass alkaline xylanases from Fusarium oxysporum F3. J. Biotechnol. 1996, 51, 181–189. [Google Scholar] [CrossRef]
- Gómez-Gómez, E.; Roncero, I.M.; Di Pietro, A.; Hera, C. Molecular characterization of a novel endo-β-1, 4-xylanase gene from the vascular wilt fungus Fusarium oxysporum. Curr. Genet. 2001, 40, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Seiboth, B.; Metz, B. Fungal arabinan and L-arabinose metabolism. Appl. Micrpbiol Biotecnol. 2011, 89, 1665–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemi, P.; Martins, D.; Buchert, J.; Faulds, C.B. Pre-hydrolisis with carbohydrases facilitates the release of protein from brewer’s spent grain. Bioresour Technol. 2013, 136, 529–534. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, M.A.M.; Moreira, A.S.; Domingues, M.R.M.; Coimbra, M.A. Revisiting the structural features of arabinoxylans from brewers’ spent grain. Carbohydr. Polym. 2016, 139, 167–176. [Google Scholar] [CrossRef]
- Severini, C.; Azzollini, D.; Jouppila, K.; Jussi, L.; Derossi, A.; De Pilli, T. Effect of enzymatic and technological treatments on solubilisation of arabinoxylans from brewer’s spent grain. J. Cereal Sci. 2015, 65, 162–166. [Google Scholar] [CrossRef]
- Pandey, A.; Larroche, C.; Ricke, S.C.; Dussap, C.G.; Gnansou, E. Biofuels: Alternative Feedstocks and Conversion Processes, 1st ed.; Academic Press: Cambridge, MA, USA, 2011; Chapter 7. [Google Scholar]
- Ti, H.; Zhang, R.; Zhang, M.; Wei, Z.; Chi, J.; Deng, Y.; Zhang, Y. Effect of extrusion on phytochemical profiles in milled fractions of black rice. Food Chem. 2015, 178, 186–194. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Alvarez-Jubete, L.; Smyth, T.J.; Kilcawley, K.; Rai, D.K.; Gallagher, E. Application of bioprocessing techniques (sourdough fermentation and technological aids) for brewer’s spent grain breads. Food Res. Int. 2015, 73, 107–116. [Google Scholar] [CrossRef]
- Meneses, N.G.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.; Cheng, Z.; Hao, J.; Guo, G.; Liu, J.G.; Lin, C.; Yu, L. Effects of solid-state yeast treatment on the antioxidant properties and protein and fiber compositions of common hard wheat bran. J. Agric. Food Chem. 2007, 55, 10173–10182. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Villela-Castrejón, J.; Pérez-Carrillo, E.; Gómez-Sánchez, C.E.; Gutiérrez-Uribe, J.A. Effects of solid-state fungi fermentation on phenolic content, antioxidant properties and fiber composition of lime cooked maize by-product (nejayote). J. Cereal Sci. 2019, 90, 102837. [Google Scholar] [CrossRef]
- da Costa-Maia, I.; dos Santos-D’Almeida, C.T.; Freire, D.M.G.; Cavalcanti, E.D.A.C.; Cameron, L.C.; Dias, J.F.; Ferreira, M.S.L. Effect of solid-state fermentation over the release of phenolic compounds from brewer’s spent grain revealed by UPLC-MSE. Lebensm. Wiss. Technol. 2020, 133, 110136. [Google Scholar] [CrossRef]
- Mäkelä, M.R.; Marinović, M.; Nousiainen, P.; Liwanag, A.J.; Benoit, I.; Sipilä, J.; Hilden, K.S. Aromatic metabolism of filamentous fungi in relation to the presence of aromatic compounds in plant biomass. Adv. Appl. Microbiol. 2015, 91, 63–137. [Google Scholar] [CrossRef] [PubMed]
- Shary, S.; Kapich, A.N.; Panisko, E.A.; Magnuson, J.K.; Cullen, D.; Hammel, K.E. Differential expression in Phanerochaete chrysosporium of membrane-associated proteins relevant to lignin degradation. Appl. Environ. Microbiol. 2008, 74, 7252–7257. [Google Scholar] [CrossRef] [Green Version]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and nutrient value proposition of brewers spent grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef] [Green Version]
- Szwajgier, D.; Waśko, A.; Targoński, Z.; Niedźwiadek, M.; Bancarzewska, M. The use of a novel ferulic acid esterase from Lactobacillus acidophilus K1 for the release of phenolic acids from brewer’s spent grain. J. Inst. Brew. 2010, 116, 293–303. [Google Scholar] [CrossRef]
- Niemi, P.; Tamminen, T.; Smeds, A.; Viljanen, K.; Ohra-aho, T.; Holopainen-Mantila, U.; Buchert, J. Characterization of lipids and lignans in brewer’s spent grain and its enzymatically extracted fraction. J. Agric. Food Chem. 2012, 60, 9910–9917. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Rizzello, C.G. Bioprocessing of brewers’ spent grain enhances its antioxidant activity: Characterization of phenolic compounds and bioactive peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Factor A Extrusion Process | Factor B Solid-State Fermentation |
|---|---|
| BSG non-extruded | BSG non-fermented |
| BSG extruded | BSG fermented |
| Compound | g/100 g BSG Dry Basis 1 |
|---|---|
| Cellulose | 13.8 ± 0.1 |
| Hemicellulose | 26.4 ± 0.5 |
| Lignin | 18.9 ± 0.2 |
| Water-soluble extract | 10.9 ± 0.9 |
| Ethanol-soluble extract | 13.2 ± 0.3 |
| Compound | BSG Untreated (g/100 g Dry Basis) | BSG Milled (g/100 g Dry Basis) | BSG Extruded (g/100 g Dry Basis) |
|---|---|---|---|
| Moisture | 2.2 ± 0.2 c | 4.2 ± 0.1 b | 8.4 ± 0.1 a |
| Protein | 21.6 ± 0.5 a | 22.2 ± 0.1 a | 21.8 ±0.3 a |
| Lipids | 11.9 ± 0.2 a | 11.7 ± 0.3 a | 12.0 ± 0.1 a |
| Ash | 4.6 ± 0.1 a | 4.7 ± 0.1 a | 4.0 ± 0.0 a |
| Total carbohydrates | 59.7 | 57.2 | 53.8 |
| Total dietary fiber | 61.8 ± 0.6 | 63.8 ± 0.5 | 62.9 ± 1.8 |
| Insoluble fiber | 61.6 ± 0.5 a | 61.8 ± 0.5 a | 60.7 ± 1.1 b |
| Soluble fiber | 0.2 ± 0.1 b | 2.0 ± 0.0 a | 2.2 ± 0.7 a |
| Sample | Antioxidant Capacity of Free Phenolic Extracts Determined by the DPPH Assay | |
|---|---|---|
| % Inhibition | DPPH Values (µmol TE/kg) | |
| BSG | 57.14 c | 1761.58 ± 55.67 c |
| BSGm | 67.74 a | 2207.00 ± 57.53 a |
| BSGe | 60.26 b | 1892.63 ± 51.63 b |
| BSGf 48 h | 93.17 a | 3276.05 ± 35.97 a |
| BSGmf 48 h | 92.9 a | 3265.28 ± 1.96 a |
| BSGef 48 h | 91.76 b | 3216.95 ± 31.53 b |
| BSGf 96 h | 92.56 a | 3250.76 ± 21.54 a |
| BSGmf 96 h | 92.96 a | 3267.26 ± 22.34 a |
| BSGef 96 h | 90.76 b | 3174.85 ± 23.94 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes-Ramirez, J.G.; Vasquez-Lara, F.; Sanchez-Estrada, A.; Troncoso-Rojas, R.; Heredia-Olea, E.; Islas-Rubio, A.R. Arabinoxylans Release from Brewers’ Spent Grain Using Extrusion and Solid-State Fermentation with Fusarium oxysporum and the Antioxidant Capacity of the Extracts. Foods 2022, 11, 1415. https://doi.org/10.3390/foods11101415
Cervantes-Ramirez JG, Vasquez-Lara F, Sanchez-Estrada A, Troncoso-Rojas R, Heredia-Olea E, Islas-Rubio AR. Arabinoxylans Release from Brewers’ Spent Grain Using Extrusion and Solid-State Fermentation with Fusarium oxysporum and the Antioxidant Capacity of the Extracts. Foods. 2022; 11(10):1415. https://doi.org/10.3390/foods11101415
Chicago/Turabian StyleCervantes-Ramirez, Joel G., Francisco Vasquez-Lara, Alberto Sanchez-Estrada, Rosalba Troncoso-Rojas, Erick Heredia-Olea, and Alma R. Islas-Rubio. 2022. "Arabinoxylans Release from Brewers’ Spent Grain Using Extrusion and Solid-State Fermentation with Fusarium oxysporum and the Antioxidant Capacity of the Extracts" Foods 11, no. 10: 1415. https://doi.org/10.3390/foods11101415
APA StyleCervantes-Ramirez, J. G., Vasquez-Lara, F., Sanchez-Estrada, A., Troncoso-Rojas, R., Heredia-Olea, E., & Islas-Rubio, A. R. (2022). Arabinoxylans Release from Brewers’ Spent Grain Using Extrusion and Solid-State Fermentation with Fusarium oxysporum and the Antioxidant Capacity of the Extracts. Foods, 11(10), 1415. https://doi.org/10.3390/foods11101415







