Structure, Antioxidant Activity and In Vitro Hypoglycemic Activity of a Polysaccharide Purified from Tricholoma matsutake
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction and Purification of Crude Polysaccharides
2.3. Chemical Composition Analysis
2.4. Structural Characteristics Analysis
2.5. In Vitro Antioxidant Activity Evaluation
2.6. Hypoglycemic Activity Analysis
2.7. In Vitro Hypoglycemic Assays
2.8. Statistical Analysis
3. Results and Discussion
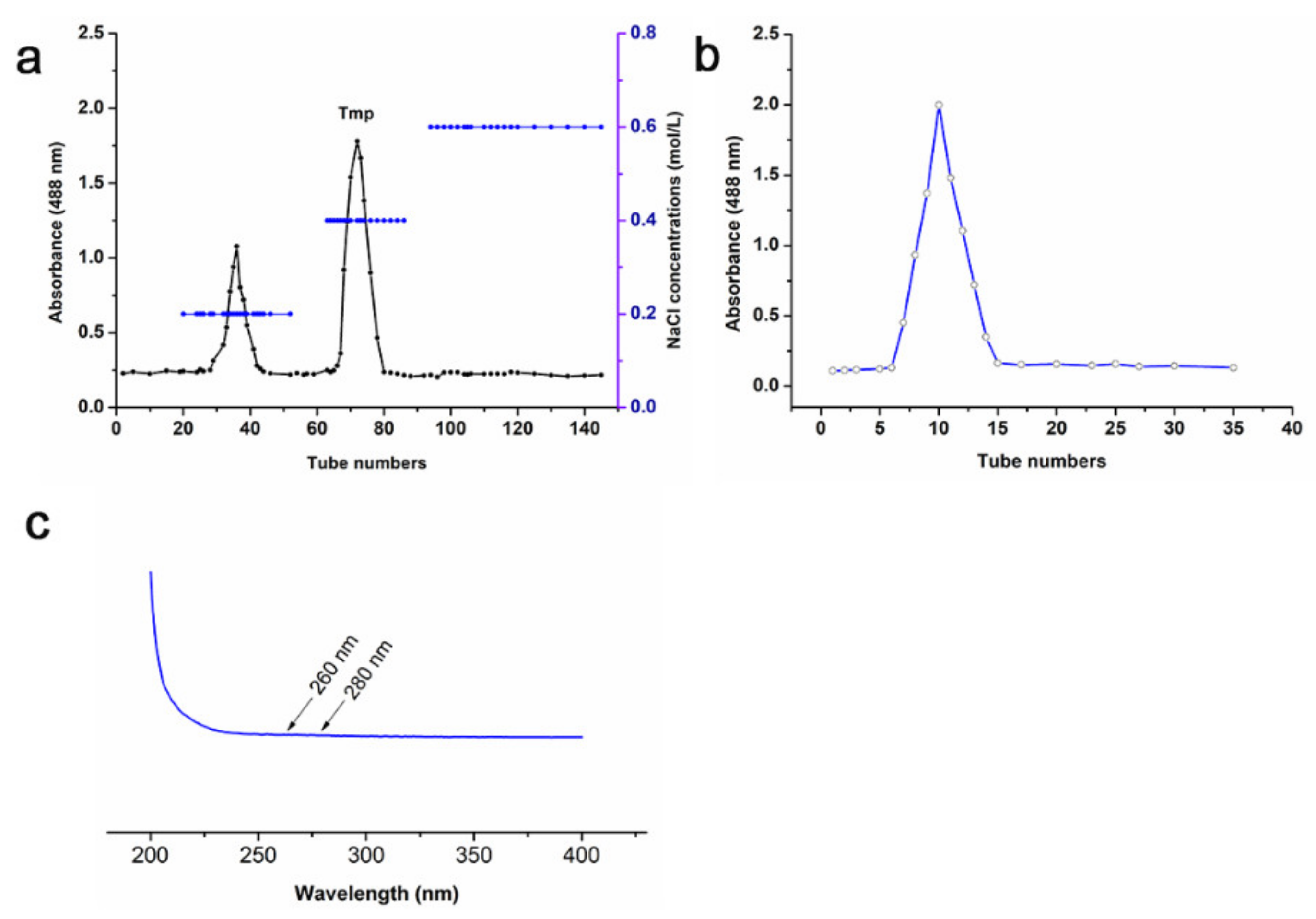
3.1. Physicochemical Property of Tmp
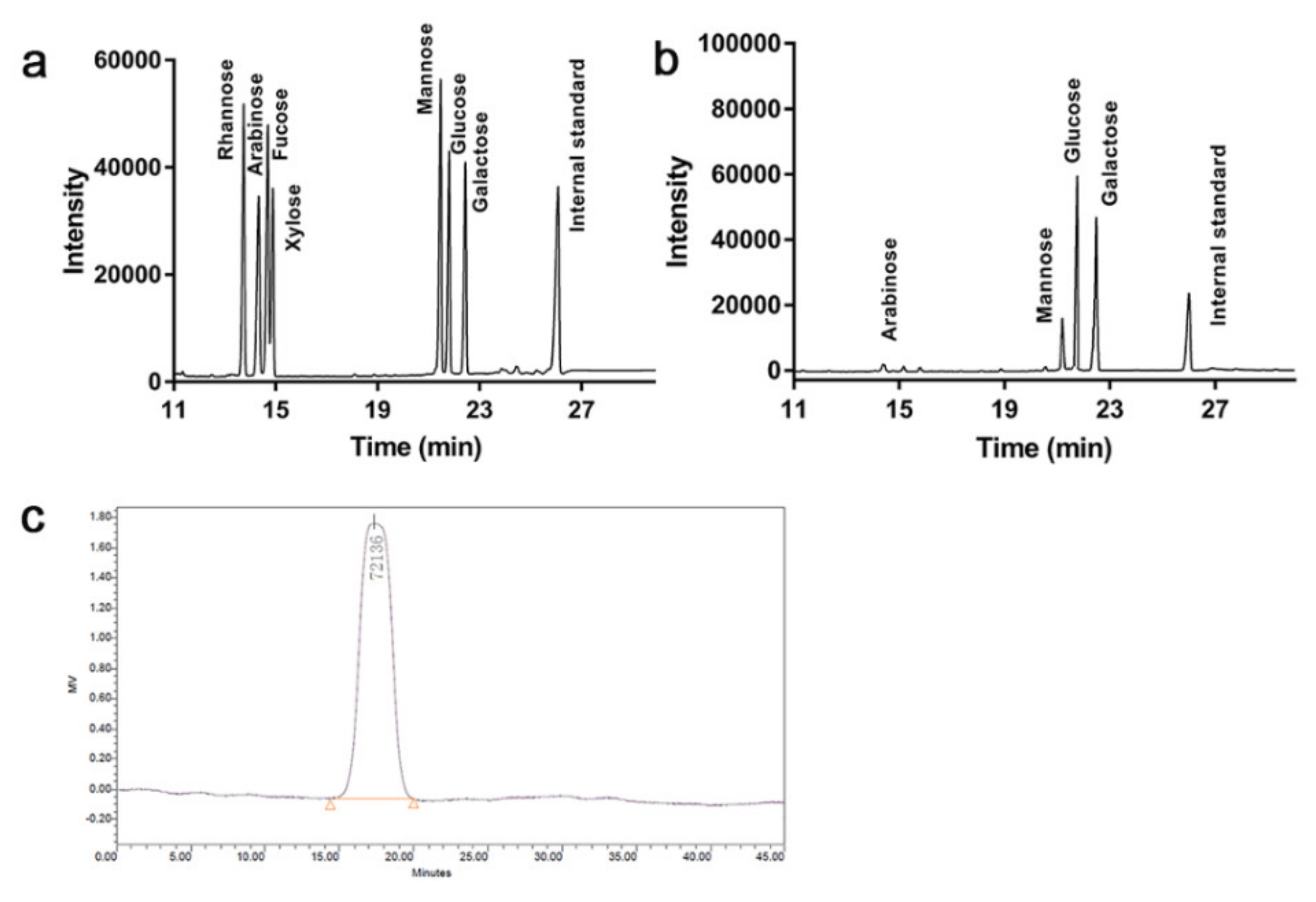
3.2. Monosaccharide Composition and Mw of Tmp
3.3. Methylation Analysis of Tmp
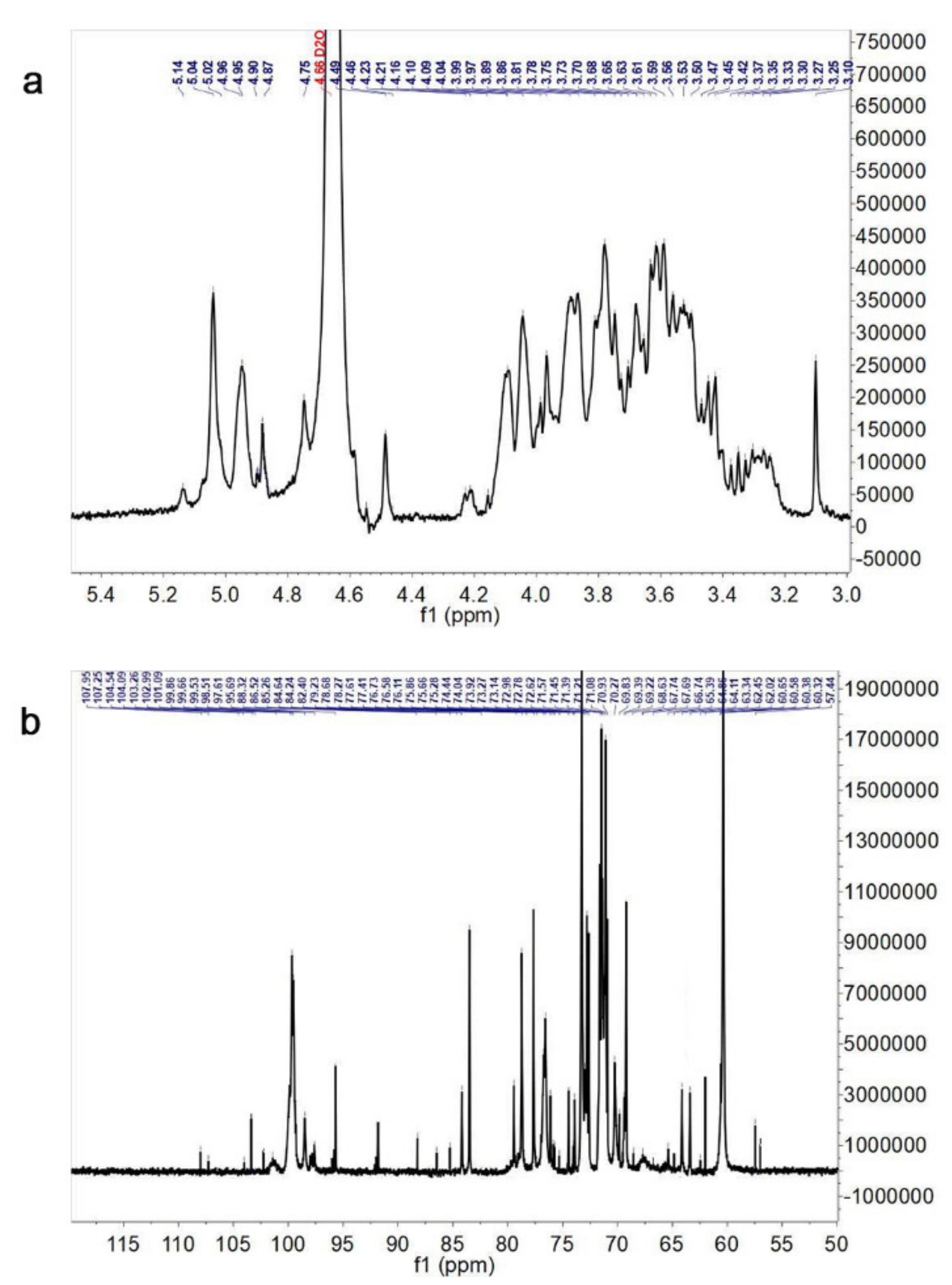
3.4. NMR Spectroscopy Analysis
3.5. Antioxidant Activity
3.6. Inhibitory Effect of Tmp on the α-Amylase and α-Glucosidase
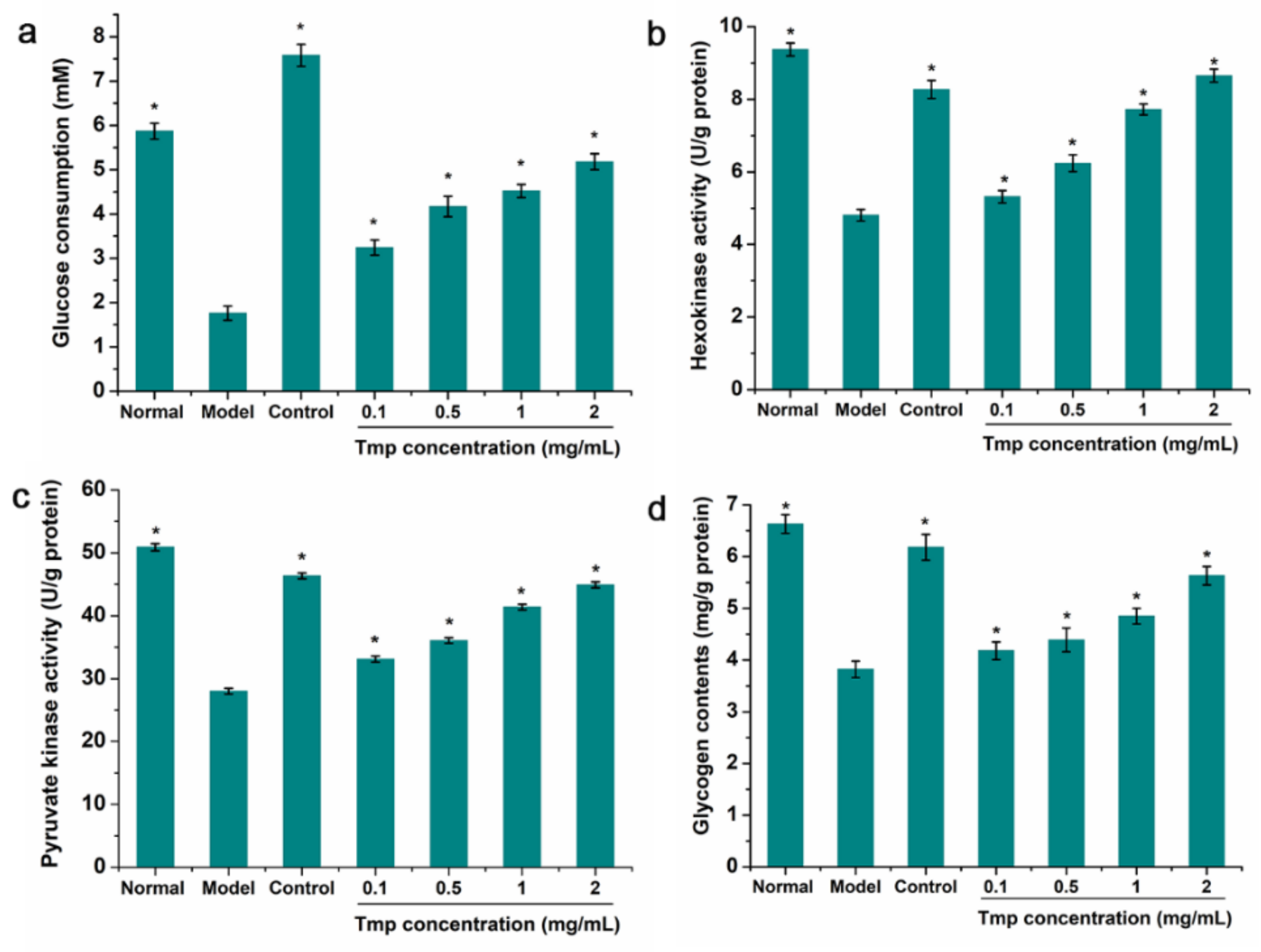
3.7. The Consumption of Glucose Analysis
3.8. Intracellular Enzyme Activity and Glycogen Content Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, X.; Hou, W.; Zhong, J.; Zhu, H.; Ma, B.; Xu, T.; Li, J.; Hou, Y. Anti-microorganism, anti-tumor, and immune activities of a novel polysaccharide isolated from Tricholoma matsutake. Pharmacogn. Mag. 2013, 9, 244–249. [Google Scholar] [CrossRef]
- Cheng, H.; Jia, Y.; Wang, L.; Liu, X.; Liu, G.; Li, L.; He, C. Isolation and structural elucidation of a novel homogenous polysaccharide from Tricholoma matsutake. Nat. Prod. Res. 2015, 30, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Q.; Ding, S.; Fan, L. Antioxidant activities of five polysaccharides from Inonotus obliquus. Int. J. Biol. Macromol. 2012, 50, 1183–1187. [Google Scholar] [CrossRef]
- Sun, L.; Feng, K.; Jiang, R.; Chen, J.; Zhao, Y.; Ma, R.; Tong, H. Water-soluble polysaccharide from Bupleurum chinense DC: Isolation, structural features and antioxidant activity. Carbohydr. Polym. 2010, 79, 180–183. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, Q.; Luo, H.; Xu, Z.; Huang, S.; Yang, F.; Liu, Y.; Mahaman, Y.A.R.; Ke, D.; Wang, Q.; et al. Codonopsis pilosula polysaccharide attenuates Aβ toxicity and cognitive defects in APP/PS1 mice. Aging 2020, 12, 13422–13436. [Google Scholar] [CrossRef]
- Liu, W.; Lv, X.; Huang, W.; Yao, W.; Gao, X. Characterization and hypoglycemic effect of a neutral polysaccharide extracted from the residue of Codonopsis Pilosula. Carbohydr. Polym. 2018, 197, 215–226. [Google Scholar] [CrossRef]
- Yin, X.; You, Q.; Jiang, Z. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 2011, 86, 1358–1364. [Google Scholar] [CrossRef]
- You, L.; Gao, Q.; Feng, M.; Yang, B.; Ren, J.; Gu, L.; Cui, C.; Zhao, M. Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem. 2013, 138, 2242–2249. [Google Scholar] [CrossRef]
- Yang, S.; Ren, X.; Sheng, J.; Lu, J.; Li, T.; Tang, F.; Wang, Y.; Meng, L.; Meng, Q.; Teng, L. Preparation and the antitumor activity in vitro of polysaccharides from Tricholoma matsutake. World J. Microbiol. Biotechnol. 2009, 26, 497–503. [Google Scholar] [CrossRef]
- Kim, J.Y.; Byeon, S.E.; Lee, Y.G.; Lee, J.Y.; Park, J.; Hong, E.K.; Cho, J.Y. Immunostimulatory activities of polysaccharides from liquid culture of pine-mushroom Tricholoma matsutake. J. Microbiol. Biotechnol. 2008, 18, 95–103. [Google Scholar]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef]
- Mojica, L.; Meyer, A.; Berhow, M.A.; Mejía, E.G. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Park, Y.-H.; Na, M.; Kang, S.C. α-Glucosidase and tyrosinase inhibitory effects of an abietane type diterpenoid taxoquinone from Metasequoia glyptostroboides. BMC Complement. Altern. Med. 2015, 15, 84. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Wang, C.; Li, S.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed. Pharmacother. 2017, 95, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Cao, J.J.; Zhang, B.; Chen, H.Q. Structural characterization, physicochemical properties and α-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohydr. Polym. 2019, 211, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhang, B.; Li, C.; Huang, Q.; Fu, X.; Liu, R.H. Structure and in vitro hypoglycemic activity of a homogenous polysaccharide purified from Sargassum pallidum. Food Funct. 2019, 10, 2828–2838. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Li, C. Structural characterization of a novel acidic polysaccharide from Rosa roxburghii Tratt fruit and its α-glucosidase inhibitory activity. Food Funct. 2018, 9, 3974–3985. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-J.; Yang, H.-R.; Wu, X.-L.; Peng, F.; Huang, Z.; Pu, L.; Zong, M.-H.; Yang, J.-G.; Lou, W.-Y. Structure and immunomodulatory activity of polysaccharides from Fusarium solani DO7 by solid-state fermentation. Int. J. Biol. Macromol. 2019, 137, 568–575. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; You, L.; Fu, X.; Liu, R.H. Fractionation, preliminary structural characterization and bioactivities of polysaccharides from Sargassum pallidum. Carbohydr. Polym. 2016, 155, 261–270. [Google Scholar] [CrossRef]
- Lin, L.; Zhuang, M.; Zou, L.; Lei, F.; Yang, B.; Zhao, M. Structural characteristics of water-soluble polysaccharides from Rabdosia serra (MAXIM.) HARA leaf and stem and their antioxidant capacities. Food Chem. 2012, 135, 730–737. [Google Scholar] [CrossRef]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure Characterization of a Novel Polysaccharide from Dictyophora indusiata and Its Macrophage Immunomodulatory Activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Chen, C.; You, L.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Zhao, S.; Nie, C.; Wang, N.; Du, X.; Zhou, Y. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 95, 809–817. [Google Scholar] [CrossRef]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. Comparison Study on Polysaccharide Fractions from Laminaria japonica: Structural Characterization and Bile Acid Binding Capacity. J. Agric. Food Chem. 2017, 65, 9790–9798. [Google Scholar] [CrossRef]
- Shashkov, A.S.; Zhang, W.; Perepelov, A.V.; Weintraub, A.; Liu, B.; Widmalm, G.; Knirel, Y.A. Structure of the O-polysaccharide of Escherichia coli O132. Carbohydr. Res. 2016, 427, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, C.; Lyu, X.; Hua, X.; Zhao, W.; Zhang, W.; Yang, R. Structure and physicochemical properties of arabinan-rich acidic polysaccharide from the by-product of peanut oil processing. Food Hydrocolloid 2021, 117, 106743. [Google Scholar] [CrossRef]
- Teng, C.; Qin, P.; Shi, Z.; Zhang, W.; Yang, X.; Yao, Y.; Ren, G. Structural characterization and antioxidant activity of alkali-extracted polysaccharides from quinoa. Food Hydrocolloid 2020, 113, 106392. [Google Scholar] [CrossRef]
- Wang, Y.X.; Yin, J.Y.; Zhang, T.; Xin, Y.; Huang, X.J.; Nie, S.P. Utilizing relative ordered structure theory to guide polysaccharide purification for structural characterization. Food Hydrocolloid 2021, 115, 106603. [Google Scholar] [CrossRef]
- Chang, C.-C.; Cheng, J.-J.; Lee, I.-J.; Lu, M.-K. Purification, structural elucidation, and anti-inflammatory activity of xylosyl galactofucan from Armillaria mellea. Int. J. Biol. Macromol. 2018, 114, 584–591. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, Y.-J.; Hu, C.-Y. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef]
- Fan, R.; Xie, Y.; Zhu, C.; Qiu, D.; Zeng, J.; Liu, Z. Structural elucidation of an acidic polysaccharide from Citrus grandis ‘Tomentosa’ and its anti-proliferative effects on LOVO and SW620 cells. Int. J. Biol. Macromol. 2019, 138, 511–518. [Google Scholar] [CrossRef]
- Mandal, S.; Patra, S.; Dey, B.; Bhunia, S.K.; Maity, K.K.; Islam, S.S. Structural analysis of an arabinan isolated from alkaline extract of the endosperm of seeds of Caesalpinia bonduc (Nata Karanja). Carbohydr. Polym. 2011, 84, 471–476. [Google Scholar] [CrossRef]
- Nandan, C.K.; Sarkar, R.; Bhanja, S.K.; Sikdar, S.R.; Islam, S.S. Isolation and characterization of polysaccharides of a hybrid mushroom (backcross mating between PfloVv12 and Volvariella volvacea). Carbohydr. Res. 2011, 346, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Wold, C.W.; Kjeldsen, C.; Corthay, A.; Rise, F.; Christensen, B.E.; Duus, J.; Inngjerdingen, K.T. Structural characterization of bioactive heteropolysaccharides from the medicinal fungus Inonotus obliquus (Chaga). Carbohydr. Polym. 2018, 185, 27–40. [Google Scholar] [CrossRef]
- Han, P.-P.; Shen, S.-G.; Guo, R.-J.; Zhao, D.-X.; Lin, Y.-H.; Jia, S.-R.; Yan, R.-R.; Wu, Y.-K. ROS Is a Factor Regulating the Increased Polysaccharide Production by Light Quality in the Edible Cyanobacterium Nostoc flagelliforme. J. Agric. Food Chem. 2019, 67, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, H.; Wang, H.; Lin, M.; Chen, Y.; Fan, Z.; Hung, Y.-C.; Lin, Y. Effects of hydrogen peroxide treatment on pulp breakdown, softening, and cell wall polysaccharide metabolism in fresh longan fruit. Carbohydr. Polym. 2020, 242, 116427. [Google Scholar] [CrossRef]
- Li, Z.; Mei, J.; Jiang, L.; Geng, C.; Li, Q.; Yao, X.; Cao, J. Chaga Medicinal Mushroom, Inonotus obliquus (Agaricomycetes) Polysaccharides Suppress Tacrine-induced Apoptosis by ROS-scavenging and Mitochondrial Pathway in HepG2 Cells. Int. J. Med. Mushrooms 2019, 21, 583–593. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Xue, C.; Sun, L. Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. Environ. Boil. Fishes 2011, 24, 295–300. [Google Scholar] [CrossRef]
- Meng, Y.; Su, A.; Yuan, S.; Zhao, H.; Tan, S.; Hu, C.; Deng, H.; Guo, Y. Evaluation of total flavonoids, myricetin, and quercetin from Hovenia dulcis Thunb. As inhibitors of α-amylase and α-glucosidase. Plant Food. Hum. Nutr. 2016, 71, 444–449. [Google Scholar] [CrossRef]
- Chen, C.; You, L.; Abbasi, A.M.; Fu, X.; Liu, R.H.; Li, C. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Funct. 2015, 7, 530–539. [Google Scholar] [CrossRef]
- Song, Y.H.; Kim, D.W.; Long, M.; Park, C.; Son, M.; Kim, J.Y.; Yuk, H.J.; Lee, K.W.; Park, K.H. Cinnamic acid amides from Tribulus terrestris displaying uncompetitive α-glucosidase inhibition. Eur. J. Med. Chem. 2016, 114, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, H.; Wang, J.; Wang, X.; Hu, B.; Lv, F. Involvement of the PI3K/Akt signal pathway in the hypoglycemic effects of tea polysaccharides on diabetic mice. Int. J. Biol. Macromol. 2015, 81, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, J.; He, X.; Wang, S.; Cao, S.; Qin, L.; Mao, W. A rhamnan-type sulfated polysaccharide with novel structure from Monostroma angicava Kjellm (Chlorophyta) and its bioactivity. Carbohydr. Polym. 2017, 173, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, Y.; Zhang, Z.; Yao, W.; Gao, X. Hypoglycemic, hypolipidemic and antioxidant effects of Sarcandra glabra polysaccharide in type 2 diabetic mice. Food Funct. 2014, 5, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, W.; Yu, J.; Zou, S.; Wang, J.; Yao, W.; Gao, X. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr. Polym. 2013, 98, 8–16. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Nie, S.-P.; Tan, L.-H.; Li, C.; Gong, D.-M.; Xie, M.-Y. A Polysaccharide from Ganoderma atrum Improves Liver Function in Type 2 Diabetic Rats via Antioxidant Action and Short-Chain Fatty Acids Excretion. J. Agric. Food Chem. 2016, 64, 1938–1944. [Google Scholar] [CrossRef]


| Carbohydrate Content (%) | Protein Content (%) | Sulfate Content (%) | |
|---|---|---|---|
| Tmp | 93.29 ± 1.79 | 6.92 ± 0.58 | 6.87 ± 1.24 |
| Methylated Sugar Resides | Mass Fragments (m/z) | Linkage Types | Molar Ratio (%) |
|---|---|---|---|
| 3-Me1-Araf | 43, 71, 87, 129, 189 | →2,5)-Araf-(1→ | 1.31 |
| 2-Me1-Araf | 43, 71, 85, 99, 115, 117, 127 | →3,5)-Araf-(1→ | 0.79 |
| 2,3,4,6-Me4-Glcp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | Glc-(1→ | 2.8 |
| 2,3,4,6-Me4-Manp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | Man-(1→ | 1.47 |
| 2,3,4,6-Me4-Galp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | Gal-(1→ | 2.92 |
| 2,3,6-Me3-Galp | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 | →4)-Galp-(1→ | 3.75 |
| 2,4,6-Me3-Glcp | 43, 71, 85, 87, 99, 101, 117, 129, 161 | →3)-Glc-(1→ | 25.23 |
| 2,4,6-Me3-Manp | 43, 71, 85, 87, 99, 101, 117, 129, 161 | →3)-Man-(1→ | 5.12 |
| 2,3,4-Me3-Manp | 43, 71, 87, 99, 101, 117, 129, 161, 173, 189, 233 | →6)-3-O-Me-Man-(1→ | 8.34 |
| 2,3,4-Me3-Galp | 43, 71, 87, 99, 101, 117, 129, 161, 173, 189, 233 | →6)-Gal-(1→ | 36.51 |
| 2,4-Me2-Glcp | 43, 71, 87, 99, 101, 117, 129, 161, 173, 189 | →3,6)-Glcp-(1→ | 12.08 |
| Resides | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | O-Me |
|---|---|---|---|---|---|---|---|
| →2,5)-α-L-Araf-(1→ | 5.14/107.95 | 4.09/88.32 | 4.16/76.93 | 3.97/84.24 | 3.81/67.74 | 3.63/— | |
| →3,5)-α-L-Araf-(1→ | 5.04/107.25 | 4.04/82.40 | 4.04/84.69 | 3.75/79.23 | 3.59/67.69 | 3.81/— | |
| β-d-Glcp-(1→ | 4.75/103.26 | 3.33/70.27 | 3.63/75.3 | 3.81/70.27 | ND 1 | 3.86/62.02 | |
| α-d-Manp-(1→ | 4.96/102.99 | 4.04/71.57 | 3.81/72.23 | 3.37/75.28 | 3.61/68.63 | 3.81/63.34 | |
| α-d-Galp-(1→ | 4.90/99.86 | 3.78/71.45 | 4.16/70.17 | 3.61/74.44 | ND | 3.61/64.11 | |
| →4)-β-d-Galp-(1→ | 4.46/103.26 | 3.42/74.04 | 3.61/73.88 | 4.04/78.27 | 3.53/74.44 | 3.73/64.86 | |
| →3)-β-d-Glcp-(1→ | 4.75/104.54 | 3.47/74.44 | 3.70/85.78 | 3.45/69.83 | 3.42/76.73 | 3.81/62.02 | |
| →3)- α-d-Manp-(1→ | 5.04/102.99 | 4.16/70.93 | 3.99/78.23 | 3.78/68.63 | 3.81/75.86 | 3.73/62.45 | |
| →6)-3-O-Me-α-d-Manp-(1→ | 4.96/103.26 | 3.81/71.39 | 3.81/79.3 | 4.21/66.74 | 3.53/74.44 | 3.78/70.27 | 3.37/57.44 |
| →6)-α-d-Galp-(1→ | 4.95/99.53 | 3.56/72.62 | 3.78/71.89 | ND | ND | 3.75/70.93 | |
| →3,6)-β-d-Glcp-(1→ | 4.49/104.09 | 3.30/74.44 | 3.68/85.97 | 3.30/70.93 | 3.42/77.41 | 3.81/70.27 | |
| →6)-α-d-Manp-(1→ | 4.49/101.09 | 3.35/72.62 | 3.63/72.62 | 3.70/68.63 | 3.97/71.57 | 3.78/70.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-R.; Chen, L.-H.; Zeng, Y.-J. Structure, Antioxidant Activity and In Vitro Hypoglycemic Activity of a Polysaccharide Purified from Tricholoma matsutake. Foods 2021, 10, 2184. https://doi.org/10.3390/foods10092184
Yang H-R, Chen L-H, Zeng Y-J. Structure, Antioxidant Activity and In Vitro Hypoglycemic Activity of a Polysaccharide Purified from Tricholoma matsutake. Foods. 2021; 10(9):2184. https://doi.org/10.3390/foods10092184
Chicago/Turabian StyleYang, Hui-Rong, Lian-Hong Chen, and Ying-Jie Zeng. 2021. "Structure, Antioxidant Activity and In Vitro Hypoglycemic Activity of a Polysaccharide Purified from Tricholoma matsutake" Foods 10, no. 9: 2184. https://doi.org/10.3390/foods10092184
APA StyleYang, H.-R., Chen, L.-H., & Zeng, Y.-J. (2021). Structure, Antioxidant Activity and In Vitro Hypoglycemic Activity of a Polysaccharide Purified from Tricholoma matsutake. Foods, 10(9), 2184. https://doi.org/10.3390/foods10092184






