Structure Differences of Water Soluble Polysaccharides in Astragalus membranaceus Induced by Origin and Their Bioactivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Water-Soluble Polysaccharides
2.2. Chrominance Analysis
2.3. Determination of Polysaccharide Content
2.4. Uronic Acid Content
2.5. Molecular Weight Determination
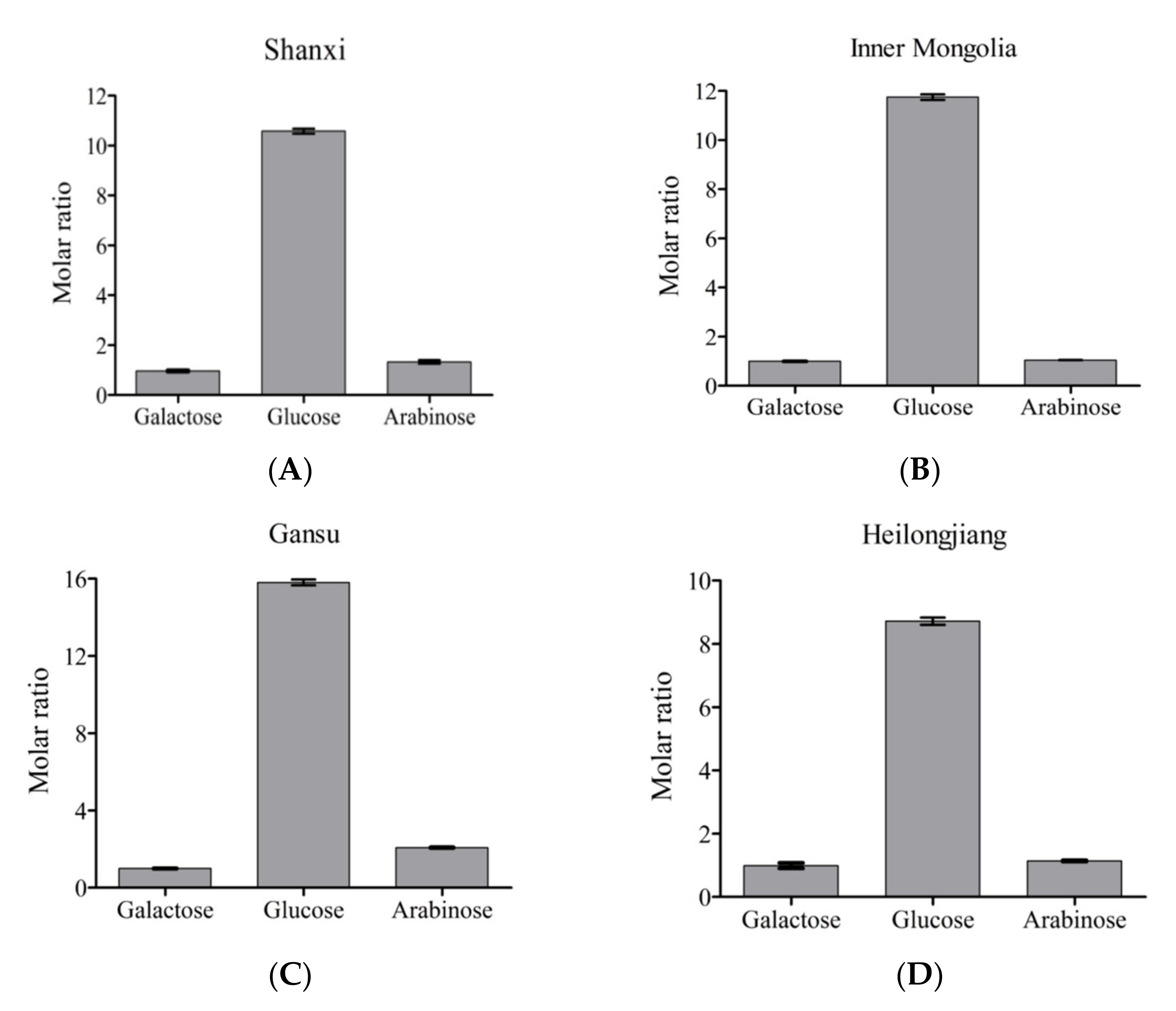
2.6. Analyses of Monosaccharide Composition
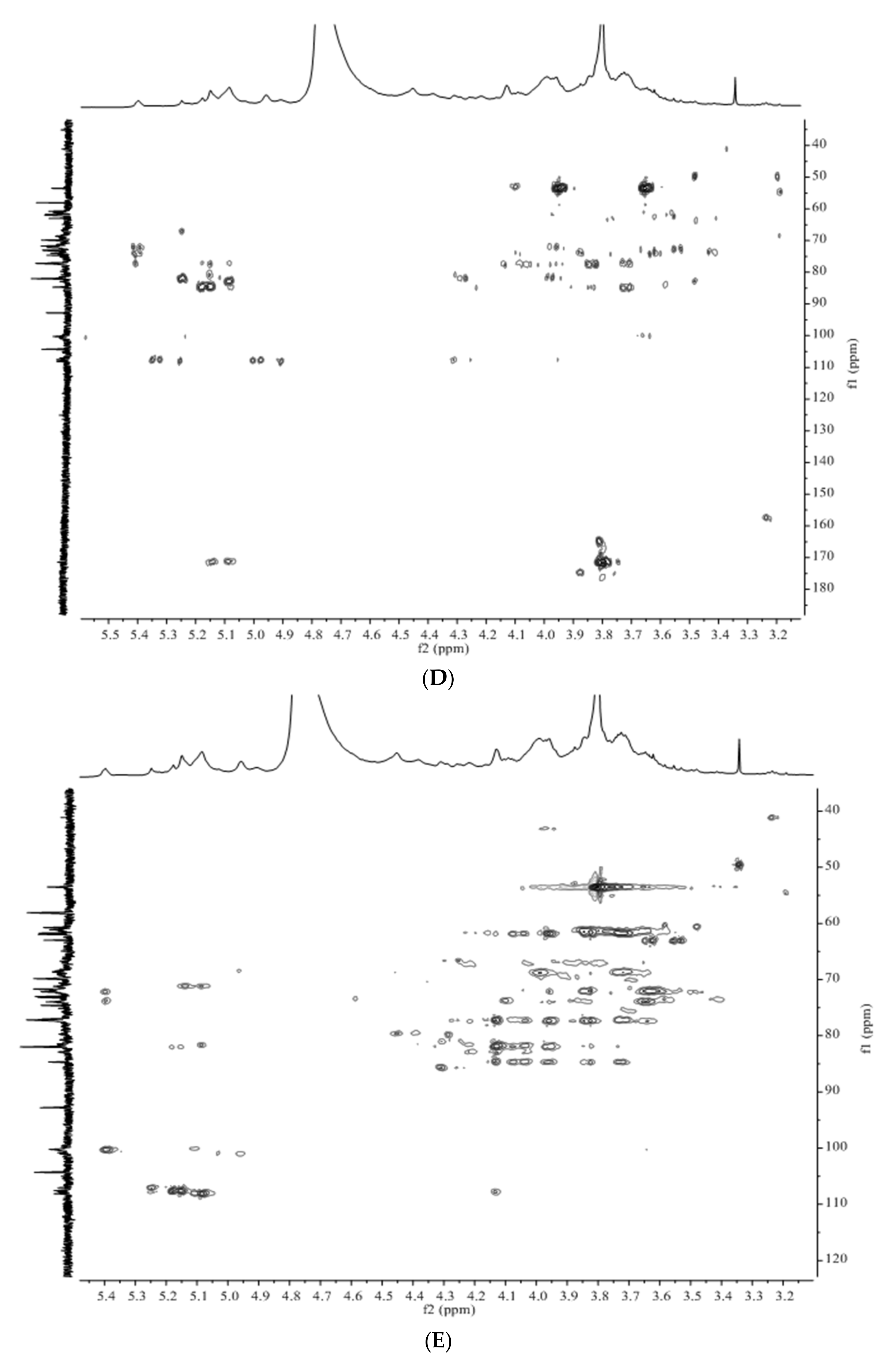
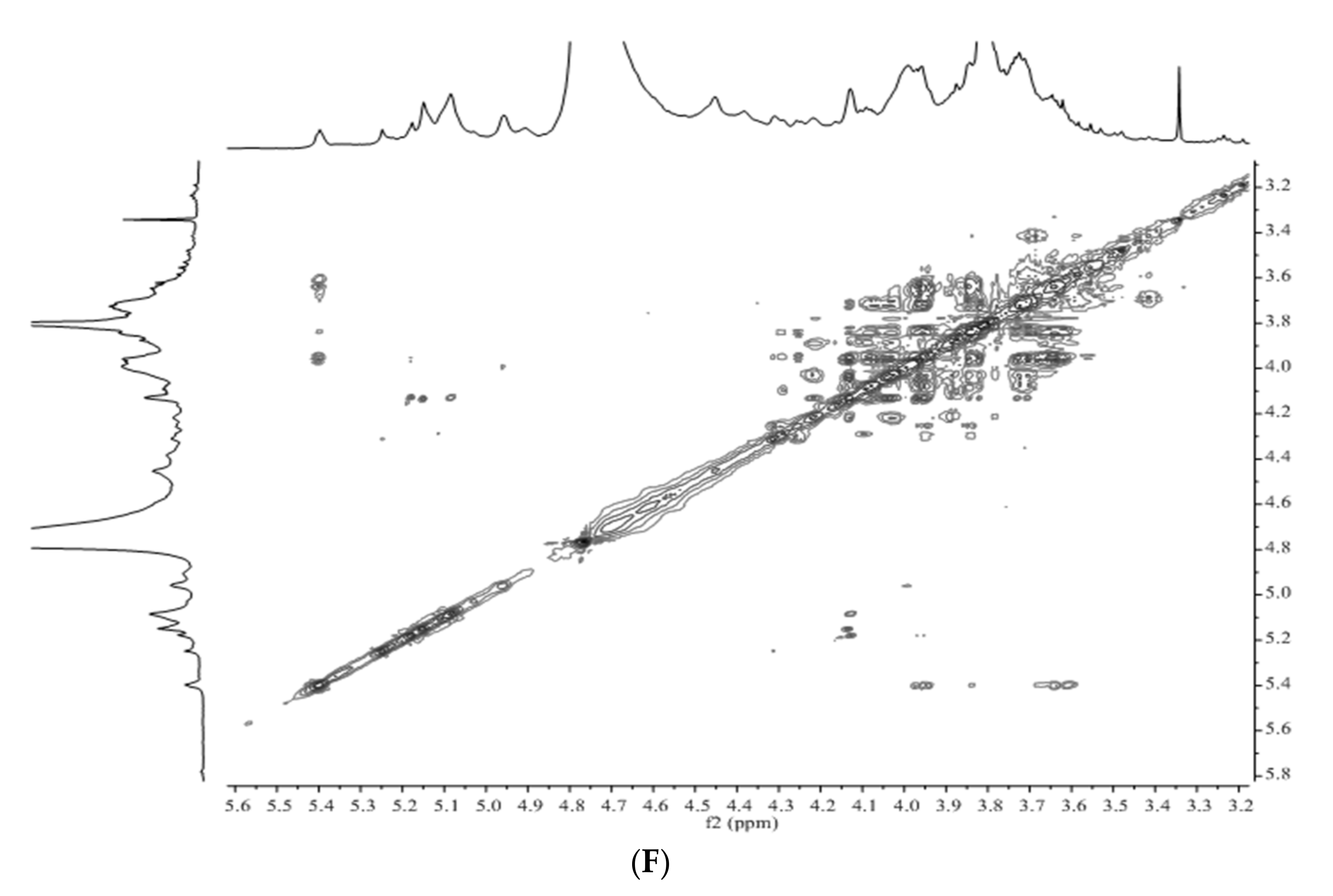
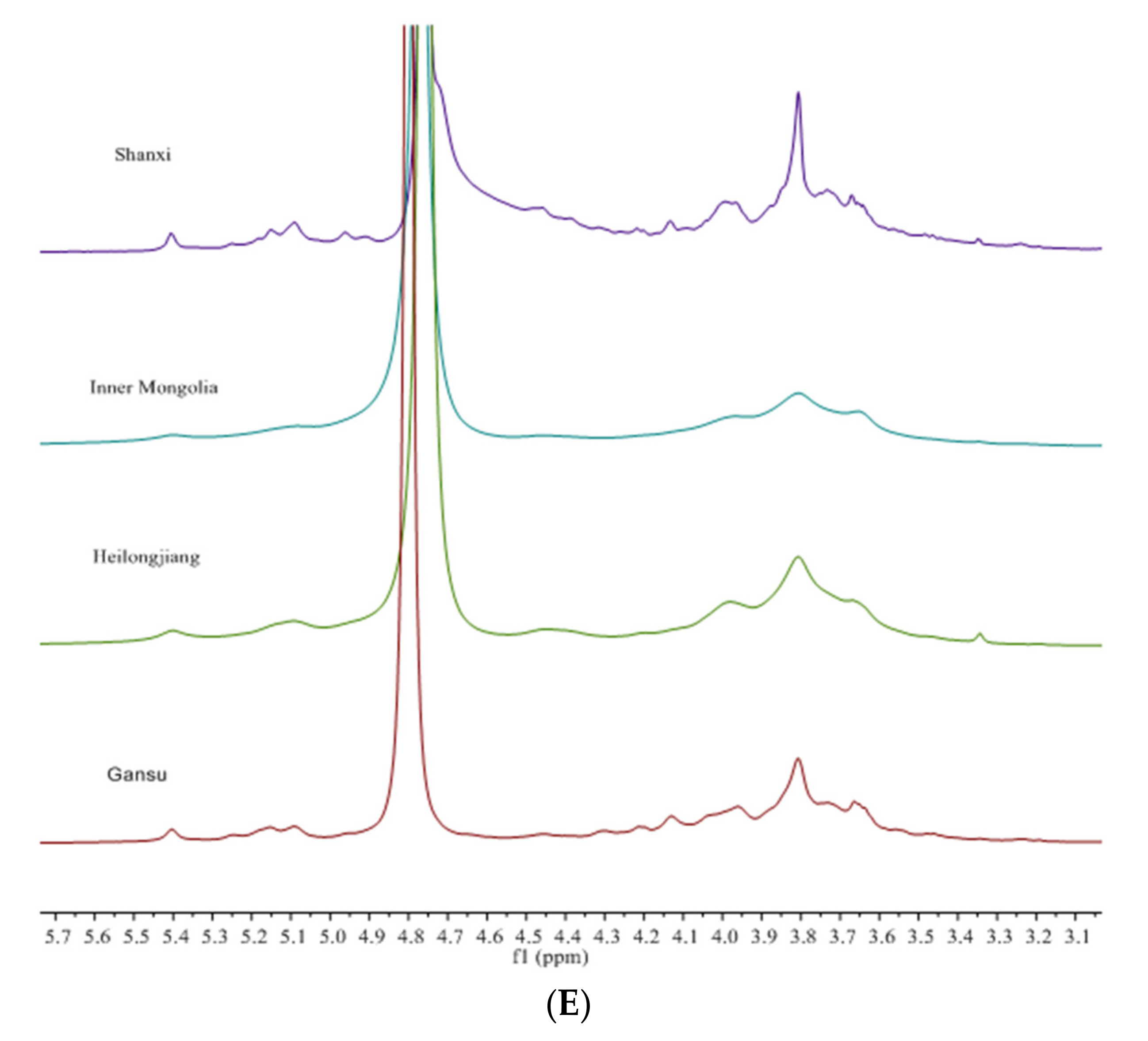
2.7. NMR Spectroscopy Analysis
2.8. Endotoxin Content Analyses
2.9. Determination of DPPH Radical Scavenging Activity
2.10. Determination of Oxygen Radical Absorbance Capacity (ORAC)
2.11. Statistical Analyses
3. Results and Discussion
3.1. Appearance, Content, and Monosaccharide Composition
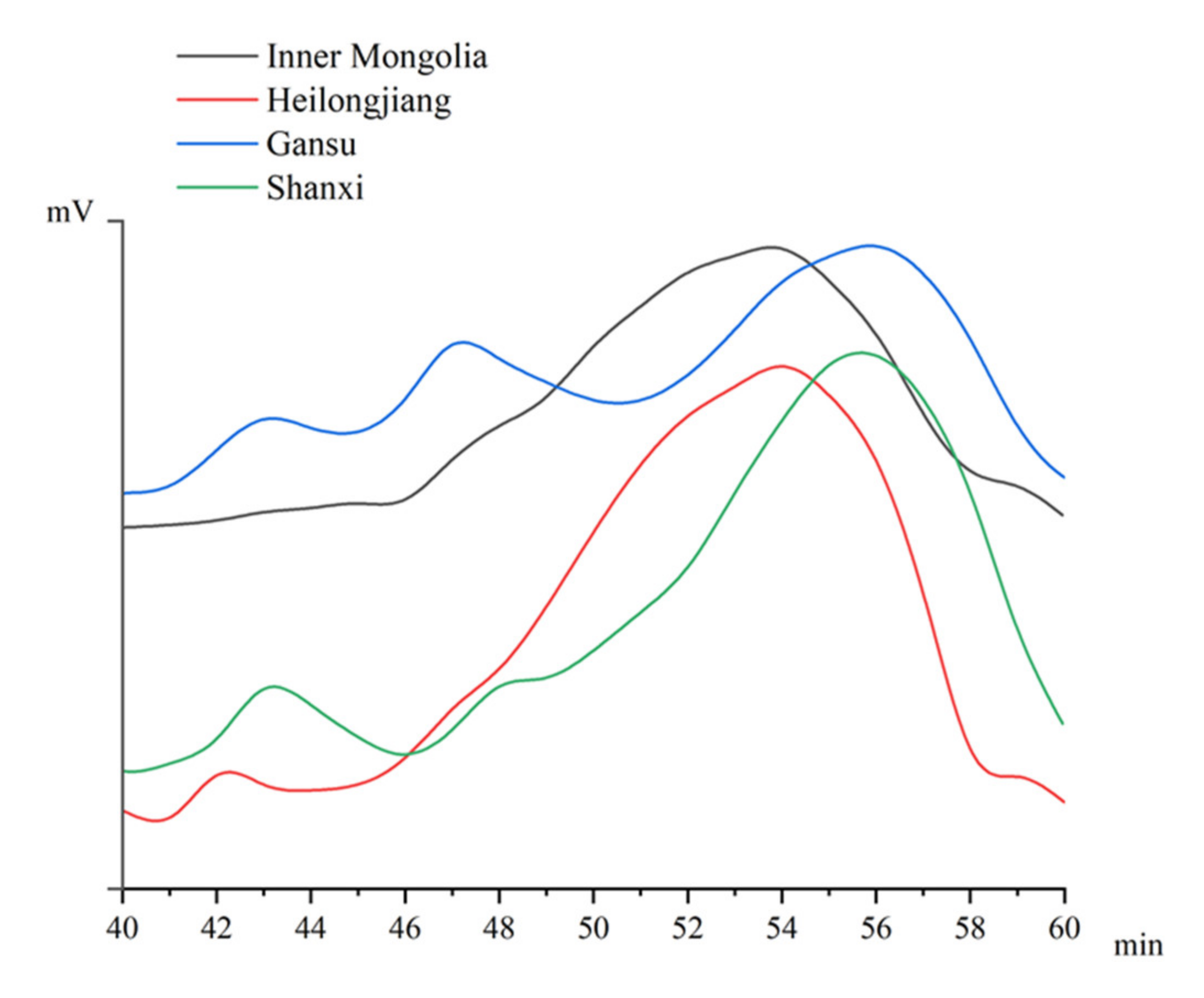
3.2. Molecular Weight Profile of APS
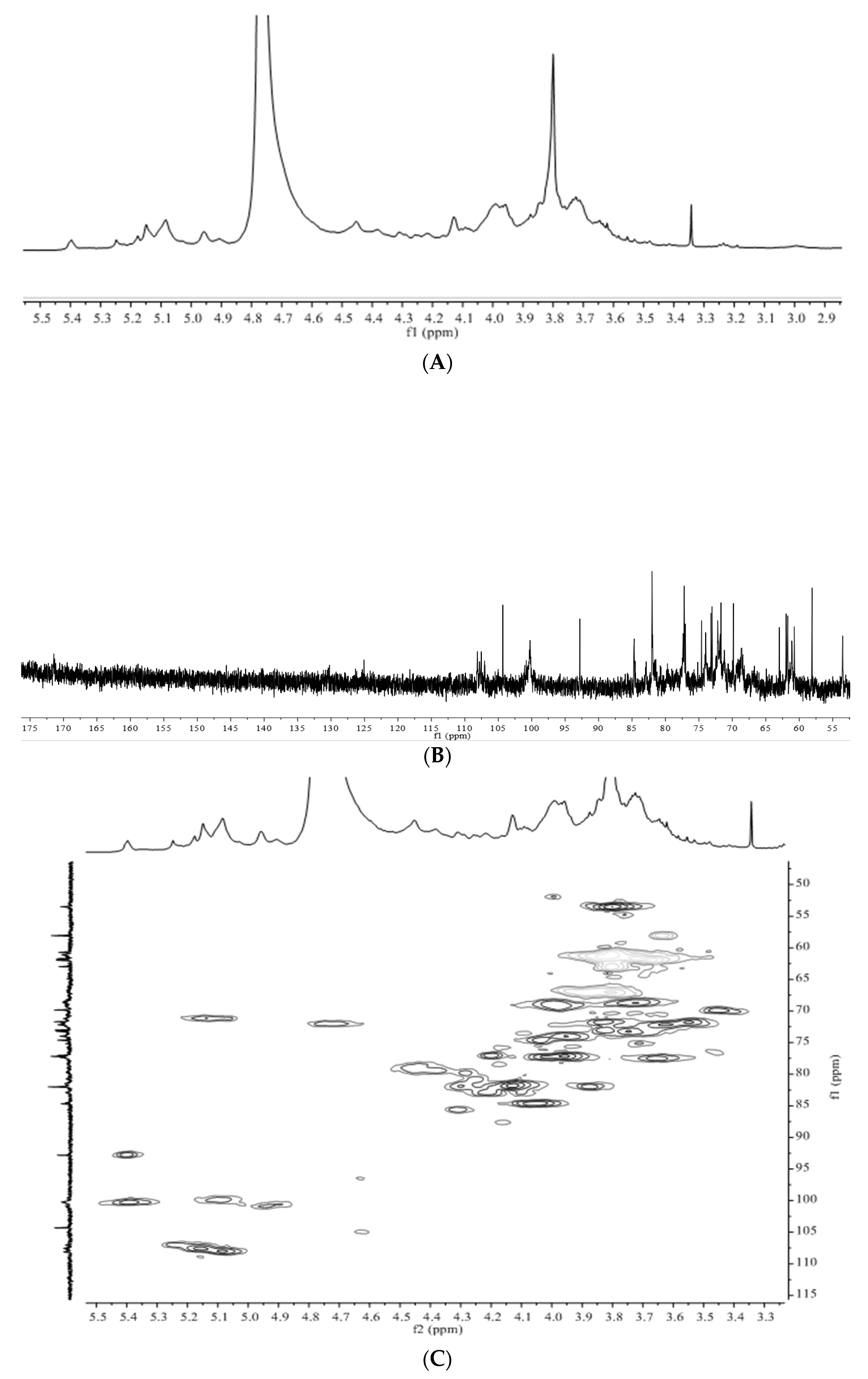
3.3. Structural Characteristics Identified by NMR
3.4. Structure Differences of APS from Four Origins
3.5. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ny, V.; Houška, M.; Pavela, R.; Tříska, J. Potential benefits of incorporating Astragalus membranaceus into the diet of people undergoing disease treatment: An overview. J. Funct. Foods 2021, 77, 104339. [Google Scholar] [CrossRef]
- Li, S.; Sun, Y.; Huang, J.; Wang, B.; Gong, Y.; Fang, Y.; Liu, Y.; Wang, S.; Guo, Y.; Wang, H. Anti-tumor effects and mechanisms of Astragalus membranaceus (AM) and its specific immunopotentiation: Status and prospect. J. Ethnopharmacol. 2020, 258, 112797. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Qiu, S.D.; Chen, H.X.; Tian, H.; Wang, H.X. The immunotherapeutic effects of Astragalus polysaccharide in type 1 diabetic mice. Biol. Pharm. Bull. 2007, 30, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jia, J.; Song, L.; Gong, X.; Xu, J.; Yang, M.; Li, M. Extraction, structure, and pharmacological activities of Astragalus polysaccharides. Appl. Sci. 2019, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, Y.; Zhao, J. Preparation and suppressive effect of astragalus polysaccharide in glomerulonephritis rats. Int. Immunopharmacol. 2007, 7, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A review of the pharmacological action of Astragalus polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Shen, Y.; Cen, M.; Hong, X.; Shao, Q.; Chen, Y.; Zheng, B. Polysaccharide and crocin contents, and antioxidant activity of saffron from different origins. Ind. Crop. Prod. 2019, 133, 111–117. [Google Scholar] [CrossRef]
- Nie, S.P.; Xie, M.Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Z.; Wang, P.; Wang, M. Extraction, structure and bioactivities of the polysaccharides from Ginkgo biloba: A review. Int. J. Biol. Macromol. 2020, 162, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Kakar, M.U.; Kakar, I.U.; Mehboob, M.Z.; Zada, S.; Soomro, H.; Umair, M.; Iqbal, I.; Umer, M.; Shaheen, S.; Syed, S.F. A review on polysaccharides from Artemisia sphaerocephala Krasch seeds, their extraction, modification, structure, and applications. Carbohydr. Polym. 2020, 256, 117113. [Google Scholar]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Med. Cell. Longev. 2016, 5692852. [Google Scholar]
- Im, S.A.; Wang, W.; Lee, C.K.; Lee, Y.N. Activation of macrophages by exopolysaccharide produced by MK1 bacterial strain isolated from neungee mushroom, Sarcodon aspratus. Immune Netw. 2010, 10, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, P.; Zhang, A.; Zhang, F.; Linhardt, R.J.; Sun, P. Structure and bioactivity of a polysaccharide containing uronic acid from Polyporus umbellatus sclerotia. Carbohydr. Polym. 2016, 152, 222–230. [Google Scholar] [CrossRef]
- Chen, H.M.; Yan, X.J. Antioxidant activities of agaro-oligosaccharides with different degrees of polymerization in cell-based system. Biochim. Et Biophys. Acta-Gen. Subj. 2005, 1722, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kang, Y.; Zhou, L.; Podlech, D. Phylogenetic origin of phyllolobium with a further implication for diversification of Astragalus in China. J. Integr. Plant Biol. 2009, 51, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.X.; Chen, T.L.; Li, M.; Chen, M.; Zhou, Y.Q.; Cui, G.H.; Zhao, A.H.; Jia, W.; Huang, L.Q.; Qi, X. Use of the metabolomics approach to characterize Chinese medicinal material Huangqi. Mol. Plant 2012, 5, 376–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zheng, S.; Brinckmann, J.A.; Fu, J.; Zeng, R.; Huang, L.; Chen, S. Chemical and genetic diversity of Astragalus mongholicus grown in different eco-climatic regions. PLoS ONE 2017, 12, e0184791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wen, L.; Zhao, Y.; Jiang, Y.; Tian, M.; Liu, H.; Liu, J.; Yang, B. Structure identification of an arabinogalacturonan in Citrus reticulata Blanco ‘Chachiensis’ peel. Food Hydrocoll. 2018, 84, 481–488. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Chen, Z.; Li, T.; Jiang, Y. Metabolic variations in the pulp of four litchi cultivars during pulp breakdown. Food Res. Int. 2020, 140, 110080. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef] [PubMed]
- Colodel, C.; de Oliveira Petkowicz, C.L. Acid extraction and physicochemical characterization of pectin from cubiu (Solanum sessiliflorum D.) fruit peel. Food Hydrocoll. 2019, 86, 193–200. [Google Scholar] [CrossRef]
- John, A.; Yang, J.; Liu, J.; Jiang, Y.; Yang, B. The structure changes of water-soluble polysaccharides in papaya during ripening. Int. J. Biol. Macromol. 2018, 115, 152–156. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211–216. [Google Scholar] [CrossRef]
- Yang, J.; Tu, J.; Liu, H.; Wen, L.; Jiang, Y.; Yang, B. Identification of an immunostimulatory polysaccharide in banana. Food Chem. 2019, 277, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Shi, D.; Wen, L.; Jiang, Y.; Zhao, Y.; Yang, J.; Liu, H.; Liu, G.; Yang, B. Identification of moracin N in mulberry leaf and evaluation of antioxidant activity. Food Chem. Toxicol. 2019, 132, 110730. [Google Scholar] [CrossRef] [PubMed]
- Alebie, G.; Urga, B.; Worku, A. Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: Trends and perspectives. Malar. J. 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Sánchez, B.; Fdez-Riverola, F.; Margolles, A.; Lourenço, A. In silico approach for unveiling the glycoside hydrolase activities in Faecalibacterium prausnitzii through a systematic and integrative large-scale analysis. Front. Microbiol. 2019, 10, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Li, S.; Du, Y.; Qin, X. Screening and structure study of active components of Astragalus polysaccharide for injection based on different molecular weights. J. Chromatogr. B 2020, 1152, 122255. [Google Scholar] [CrossRef]
- Kaur, A.; Yadav, M.P.; Singh, B.; Bhinder, S.; Simon, S.; Singh, N. Isolation and characterization of arabinoxylans from wheat bran and study of their contribution to wheat flour dough rheology. Carbohydr. Polym. 2019, 221, 166–173. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, T.; Jiang, B.; Mu, W.; Miao, M. Characterization and antioxidant activity of Ginkgo biloba exocarp polysaccharides. Carbohydr. Polym. 2012, 87, 40–45. [Google Scholar] [CrossRef]
- Zhu, Y.; He, Z.; Bao, X.; Wang, M.; Yin, S.; Song, L.; Peng, Q. Purification, in-depth structure analysis and antioxidant stress activity of a novel pectin-type polysaccharide from Ziziphus Jujuba cv. Muzaoresidue. J. Funct. Foods 2021, 80, 104439. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al Maleki, A.R.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef]
- Vannucci, L.; Krizan, J.; Sima, P.; Stakheev, D.; Caja, F.; Rajsiglova, L.; Horak, V.; Saieh, M. Immunostimulatory properties and antitumor activities of glucans (Review). Int. J. Oncol. 2013, 43, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Kitaguchi, K.; Yabe, T. Dietary fiber pectin is recognized in a structure-specific manner by intestinal cells. Trends Glycosci. Glycotechnol. 2019, 31, E91–E97. [Google Scholar] [CrossRef]
- Huo, J.; Lu, Y.; Xia, L.; Chen, D. Structural characterization and anticomplement activities of three acidic homogeneous polysaccharides from Artemisia annua. J. Ethnopharmacol. 2020, 247, 112281. [Google Scholar] [CrossRef]
- Noreen, A.; Nazli, Z.H.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. Int. J. Biol. Macromol. 2017, 101, 254–272. [Google Scholar] [CrossRef]
- Xu, D.J.; Xia, Q.; Wang, J.J.; Wang, P.P. Molecular weight and monosaccharide composition of Astragalus polysaccharides. Molecules 2008, 13, 2408–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F.; et al. The polysaccharide and low molecular weight components of Opuntia ficus indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, X.; Li, S.; Li, J.; Zhu, X.; Zhang, L.; Gao, F.; Zhou, G. Effect of gamma irradiation on structure, physicochemical and immunomodulatory properties of Astragalus polysaccharides. Int. J. Biol. Macromol. 2018, 120, 641–649. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Popov, K.I.; Sultanova, N.; Ronkkomaki, H.; Hannu Kuure, M.; Jalonen, J.; Lajunen, L.H.J.; Bugaenko, I.F.; Tuzhilkin, V.I. 13C NMR and electrospray ionization mass spectrometric study of sucrose aqueous solutions at high pH: NMR measurement of sucrose dissociation constant. Food Chem. 2006, 96, 248–253. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Groman, E.V.; Enriquez, P.M.; Jung, C.; Josephson, L. Arabinogalactan for Hepatic Drug-Delivery. Bioconjug. Chem. 1994, 5, 547–556. [Google Scholar] [CrossRef]
- Vos, A.P.; M’Rabet, L.; Stahl, B.; Boehm, G.; Garssen, J. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit. Rev. Immunol. 2007, 27, 97–140. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, J.M. Changes in cell growth, cyclin/kinase, endogenous phosphoproteins and nm23 gene expression in human prostatic JCA-1 cells treated with modified citrus pectin. Biochem. Mol. Biol. Int. 1995, 37, 833–841. [Google Scholar] [PubMed]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, 127. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Cui, C.; Lu, J.; Sun-Waterhouse, D.; Mu, L.; Sun, W.; Zhao, M.; Zhao, H. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. Lwt 2016, 73, 602–608. [Google Scholar] [CrossRef]
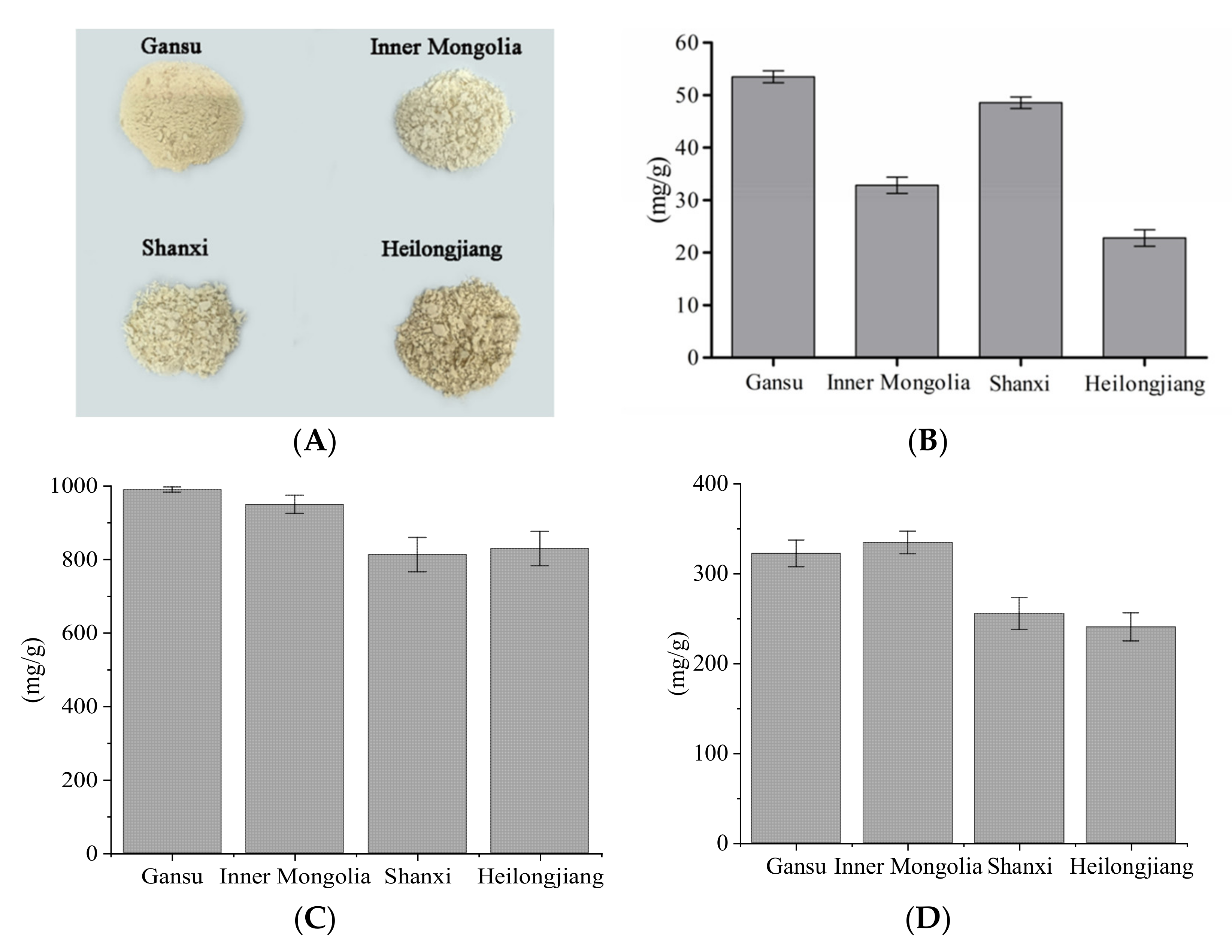


| Origins | Chrominance | |||
|---|---|---|---|---|
| L* | a* | b* | ΔE | |
| Inner Mongolia | 78.65 ± 2.46 a | 1.53 ± 0.10 a | 6.42 ± 0.34 a | 0 a |
| Shanxi | 80.51 ± 0.28 a | 1.79 ± 0.03 b | 8.35 ± 0.36 b | 2.69 ± 0.44 a |
| Gansu | 73.51 ± 2.03 b | 2.27 ± 0.04 c | 9.00 ± 0.87 b | 5.80 ± 1.97 b |
| Heilongjiang | 70.48 ± 3.92 b | 2.08 ± 0.02 d | 13.11 ± 0.45 c | 10.57 ± 2.61 c |
| Origins | Molecular Weight (kDa) | ||
|---|---|---|---|
| 20–40 | 160–300 | 700–1200 | |
| Gansu | 62.9% | 23.0% | 14.1% |
| Heilongjiang | 76.6% | 19.4% | 4.0% |
| Inner Mongolia | 75.7% | 22.1% | 2.2% |
| Shanxi | 80.4% | 7.4% | 12.2% |
| Origins | ORAC Values (μmol Trolox Equiv/g ASP) | IC50 Values Against DPPH (μmol Ascorbic Acid/g ASP) |
|---|---|---|
| Inner Mongolia | 192.03 ± 6.12 a | 1.48 ± 0.10 a |
| Shanxi | 43.66 ± 3.32 b | 2.82 ± 0.08 b |
| Gansu | 201.91 ± 8.33 a | 3.38 ± 0.12 c |
| Heilongjiang | 17.91 ± 2.09 c | 3.43 ± 0.06 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Z.; Liu, J.; Yang, B. Structure Differences of Water Soluble Polysaccharides in Astragalus membranaceus Induced by Origin and Their Bioactivity. Foods 2021, 10, 1755. https://doi.org/10.3390/foods10081755
Sheng Z, Liu J, Yang B. Structure Differences of Water Soluble Polysaccharides in Astragalus membranaceus Induced by Origin and Their Bioactivity. Foods. 2021; 10(8):1755. https://doi.org/10.3390/foods10081755
Chicago/Turabian StyleSheng, Zhili, Junmei Liu, and Bao Yang. 2021. "Structure Differences of Water Soluble Polysaccharides in Astragalus membranaceus Induced by Origin and Their Bioactivity" Foods 10, no. 8: 1755. https://doi.org/10.3390/foods10081755
APA StyleSheng, Z., Liu, J., & Yang, B. (2021). Structure Differences of Water Soluble Polysaccharides in Astragalus membranaceus Induced by Origin and Their Bioactivity. Foods, 10(8), 1755. https://doi.org/10.3390/foods10081755





