A Comprehensive Review of the Composition, Nutritional Value, and Functional Properties of Camel Milk Fat
Abstract
1. Introduction
2. The Health-Promoting Properties of CM Fat
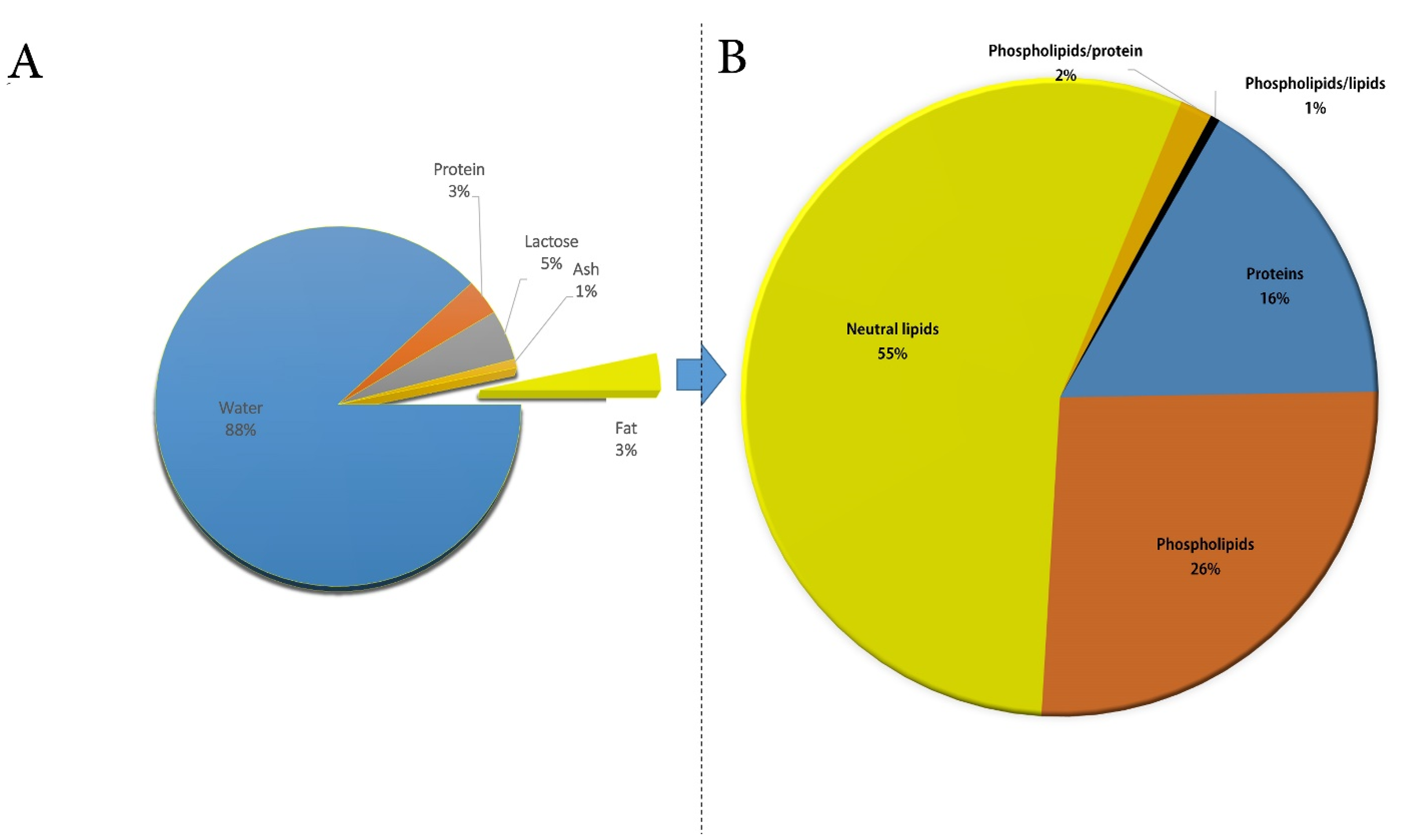
3. Composition and Functional Properties of CM Fat Globule and Membrane
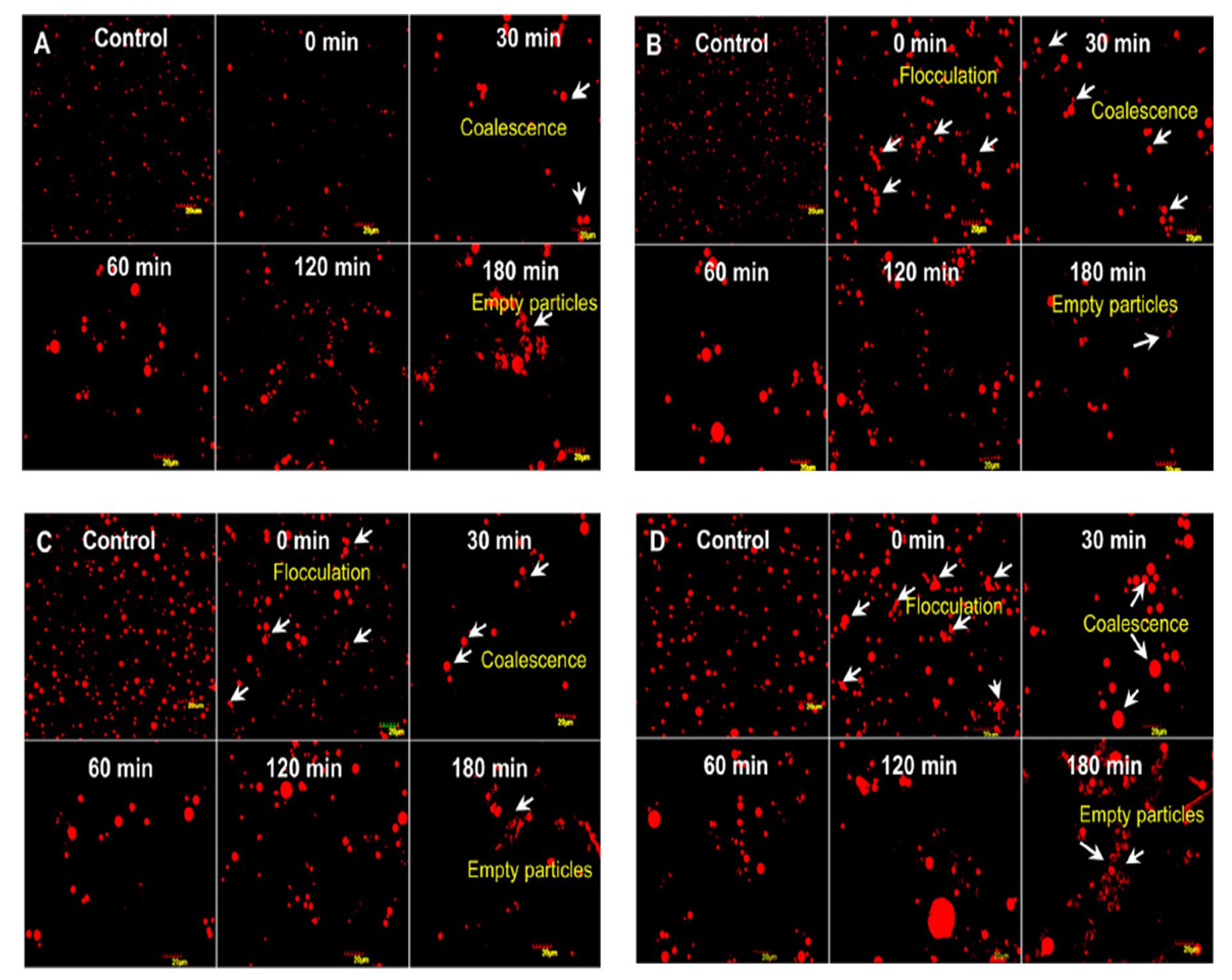
3.1. CM Fat Globule Membrane
3.2. CM Fat Globules
4. CM Fat Composition
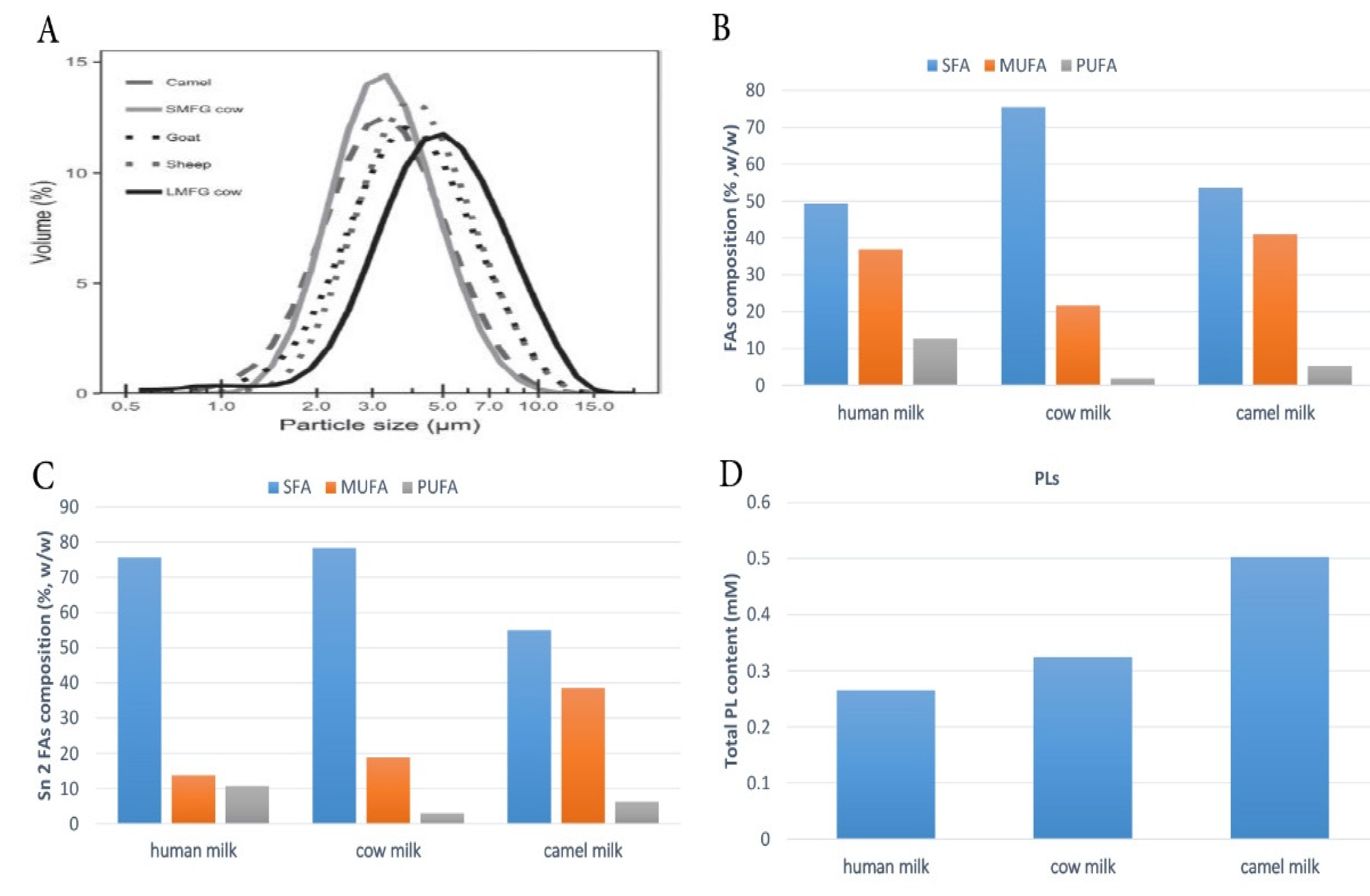
4.1. FAs Composition of CM Fat
4.2. Saturated Fatty Acids (SFAs)
| Locality/References | SE | L/Days | 4:0 | 6:0 | 8:0 | 10:0 | 12:0 | 13:0 | 14:0 | 14:1 | 15:0 | FAs 15:1 | 16:0 | 16:1 | 17:0 | 17:1 | 18:0 | 18:1 n-9 | 20:0 | 20:1 | ΣSFAs | ΣMUFAs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| China ** [36] | W | NS | 12.20 | 0.01 | 0.26 | 0.21 | 0.91 | 0.08 | 12.00 | 0.55 | 1.09 | 0.36 | 23.60 | 0.42 | 0.56 | 0.49 | 12.80 | 15.15 | 0.08 | 0.08 | 64.10 | 25.70 | |
| China ** [82] | NS | NS | ND | 0.10 | 0.10 | 0.10 | 0.89 | ND | 7.32 | 0.33 | 1.71 | ND | 18.80 | 3.51 | 0.89 | ND | 21.30 | 28.10 | 0.31 | 0.22 | 51.90 | 39.60 | |
| China ** [13] | Hovd | NS | NS | 0.02 | 0.20 | 0.09 | 0.01 | 0.81 | 0.01 | 10.18 | 0.27 | 1.94 | ND | 23.99 | 5.58 | 1.16 | 0.66 | 16.18 | 22.42 | 1.72 | ND | 57.53 | 34.15 |
| Sharga | NS | NS | 0.01 | 0.10 | 0.13 | 0.11 | 1.09 | 0.03 | 12.68 | 0.42 | 1.71 | ND | 30.08 | 5.37 | 1.21 | 0.56 | 16.49 | 15.79 | 0.89 | ND | 65.24 | 26.14 | |
| Tseel | NS | NS | 0.01 | 0.12 | 0.10 | 0.05 | 0.83 | ND | 12.24 | 0.42 | 2.14 | ND | 27.40 | 5.72 | 1.46 | 0.76 | 14.99 | 17.87 | 1.09 | ND | 61.12 | 29.16 | |
| Bulgan | NS | NS | 0.04 | 0.30 | 0.36 | 0.16 | 0.98 | 0.03 | 13.15 | 0.62 | 2.23 | ND | 30.48 | 7.53 | 1.29 | 0.79 | 11.13 | 18.13 | 0.39 | ND | 60.90 | 30.13 | |
| Tsogtovoo | NS | NS | 0.04 | 0.22 | 0.29 | 0.18 | 1.55 | 0.06 | 13.66 | 0.58 | 1.94 | ND | 30.72 | 7.40 | 1.22 | 0.63 | 12.27 | 17.06 | 0.56 | ND | 62.75 | 29.29 | |
| China ** [83] | NS | NS | ND | ND | ND | ND | 0.78 | ND | 11.49 | ND | ND | ND | 30.12 | ND | ND | ND | 15.15 | 26.05 | ND | ND | NS | NS | |
| China ** [84] | NS | NS | ND | ND | ND | ND | 0.96 | 0.05 | 12.50 | 0.58 | 1.23 | ND | 31.92 | 7.32 | ND | ND | 16.10 | 21.87 | ND | ND | NS | NS | |
| China ** [12] | W | NS | ND | ND | ND | ND | 0.83 | ND | 11.54 | 1.56 | 1.57 | ND | 31.51 | 5.17 | ND | ND | 18.67 | 23.59 | 0.31 | 0.08 | 64.42 | 30.40 | |
| Turkey [18] | NS | NS | 0.02 | 0.18 | 0.18 | 0.32 | 0.02 | ND | 10.84 | ND | ND | ND | 24.90 | 11.86 | ND | ND | 15.38 | 30.74 | 0.66 | ND | NS | NS | |
| Germany [35] | S | NS | ND | ND | ND | ND | 0.43 | 0.20 | 10.50 | 1.17 | 1.71 | 0.07 | 27.60 | 10.70 | 0.82 | 0.64 | 12.50 | 17.2 | 0.42 | 0.11 | 54.50 | 32.20 | |
| China ** [85] | NS | NS | ND | ND | ND | ND | 0.88 | ND | 13.04 | 1.49 | 1.06 | 1.64 | 34.13 | 8.85 | 0.92 | 0.82 | 14.58 | 18.96 | ND | ND | 64.61 | 31.76 | |
| China ** [20] | NS | NS | ND | ND | ND | ND | 1.05 | ND | 11.84 | 0.72 | ND | ND | 27.07 | 9.74 | ND | ND | 11.85 | 29.25 | 0.63 | 0.14 | 53.66 | 41.00 | |
| Kazakhstan ** [11] | NS | NS | 0.54 | 0.46 | 0.53 | 0.46 | 1.24 | 0.17 | 15.43 | 0.80 | 1.41 | ND | 32.05 | 7.01 | 0.65 | 0.33 | 14.75 | 18.78 | 0.01 | ND | NS | NS | |
| Kazakhstan * [11] | NS | NS | 0.34 | 0.29 | 0.27 | 0.27 | 0.80 | 0.03 | 10.10 | 0.57 | 1.24 | ND | 29.74 | 6.60 | 0.76 | 0.38 | 17.82 | 24.66 | 0.05 | 0.01 | NS | NS | |
| UAE * [19] | NS | NS | ND | ND | ND | 0.54 | 1.21 | 0.09 | 15.84 | 2.16 | 1.46 | ND | 35.97 | 10.07 | 0.80 | 0.49 | 11.55 | 16.34 | 0.46 | ND | 46.41 | 49.33 | |
| Saudi Arabia * [86] | W | 90 | 0.11 | 0.90 | 0.22 | 0.23 | 1.54 | ND | 15.89 | ND | 1.39 | ND | 34.65 | 11.87 | 0.58 | 0.64 | 8.88 | 15.44 | ND | 0.23 | 66.40 | 30.30 | |
| Jordan * [87] | AlKhalidyah | S | NS | 0.08 | 1.90 | 0.14 | 0.11 | 0.70 | 0.07 | 8.84 | 0.58 | 1.47 | 0.54 | 29.86 | 7.18 | 0.74 | 0.81 | 13.76 | 26.25 | 0.045 | 2.01 | 59.81 | NS |
| Al Hazeem | 0.10 | 2.90 | 1.40 | 1.09 | 3.42 | 2.50 | 11.23 | 1.96 | 0.40 | 0.30 | 18.16 | 5.93 | 0.64 | 1.26 | 9.18 | 24.74 | 0.045 | 2.64 | 53.62 | NS | |||
| Al Umari | 0.07 | 0.41 | 0.13 | 0.22 | 0.93 | 0.06 | 7.79 | 0.85 | 1.55 | 0.08 | 24.66 | 9.19 | 0.68 | 0.88 | 12.70 | 32.88 | 0.25 | 3.13 | 50.15 | NS | |||
| Al Safawi | 0.05 | 3.30 | 1.30 | 1.04 | 2.58 | 1.00 | 13.32 | 1.70 | 2.14 | 0.15 | 25.00 | 11.54 | 0.64 | 1.18 | 8.70 | 22.52 | 0.14 | 1.26 | 61.16 | NS | |||
| Al Hamra | 0.03 | 0.50 | 0.23 | 0.50 | 1.01 | 0.04 | 9.17 | 0.73 | 0.96 | 0.02 | 32.48 | 9.47 | 0.53 | 0.75 | 9.13 | 32.48 | 0.08 | 0.23 | 55.25 | NS | |||
| Al Qatrana | 0.10 | 3.80 | 1.58 | 1.25 | 0.90 | 0.09 | 14.28 | 2.14 | 1.01 | 0.07 | 32.43 | 16.02 | 0.04 | 0.53 | 6.96 | 16.64 | 0.14 | 0.17 | 64.12 | NS | |||
| Wadi Araba | 0.21 | 3.60 | 0.43 | 1.80 | 1.35 | 0.18 | 8.86 | 0.51 | 2.47 | 1.17 | 24.30 | 7.01 | 1.53 | 3.60 | 15.20 | 26.37 | 0.15 | 0.29 | 60.58 | NS | |||
| Al Jweideh | 0.22 | 1.90 | 2.44 | 1.88 | 1.58 | 0.20 | 7.64 | 1.14 | 1.33 | 0.61 | 23.80 | 10.47 | 0.60 | 1.32 | 11.92 | 20.36 | 3.95 | 1.98 | 58.66 | NS | |||
| Maghrebi * [88] | NS | 140 | ND | 0.10 | 0.10 | 0.10 | 0.70 | 0.10 | 9.90 | 0.70 | 1.50 | ND | 28.70 | 8.10 | 0.10 | ND | 13.40 | 25.30 | 0.50 | 0.20 | 56.00 | 39.00 | |
| 245 | ND | 0.10 | 0.20 | 0.20 | 0.90 | 0.10 | 14.60 | 1.20 | 1.40 | ND | 37.20 | 10.90 | 0.05 | ND | 9.60 | 15.20 | 0.40 | 0.20 | 66.00 | 31.00 | |||
| 329 | ND | 0.10 | 0.20 | 0.20 | 1.10 | 0.10 | 15.30 | 2.00 | 1.50 | ND | 31.20 | 13.40 | 0.06 | ND | 7.70 | 17.90 | 0.40 | 0.20 | 59.00 | 37.00 | |||
| Egypt * [12] | W | NS | ND | ND | ND | ND | 0.83 | ND | 10.55 | 2.33 | 1.64 | 0.75 | 27.90 | 7.56 | 0.79 | 0.75 | 12.99 | 29.83 | 0.28 | ND | 54.98 | 41.22 | |
| Egypt * [89] | NS | NS | 0.83 | 0.37 | 0.28 | 0.37 | 0.66 | ND | 10.98 | 1.49 | ND | ND | 29.05 | 10.13 | ND | ND | 12.38 | 24.45 | 0.70 | ND | NS | NS | |
| Sudan * [90] | S | NS | ND | 0.20 | ND | 0.12 | 0.41 | 0.13 | 8.43 | 0.58 | 1.05 | ND | 30.74 | 7.81 | 0.67 | 0.40 | 21.11 | 24.21 | 0.48 | 0.13 | 63.83 | 34.38 | |
| Tunisia * [91] | W | 100–180 | ND | ND | 0.50 | 0.30 | 1.66 | 0.06 | 14.91 | 0.90 | 2.03 | 0.28 | 28.50 | 6.34 | 0.91 | 0.57 | 10.52 | 18.39 | 0.24 | 0.66 | 62.84 | 33.35 | |
| Tunisia * [92] | MJ | 28–56 | 0.06 | 0.17 | 0.14 | 0.18 | 0.86 | ND | 11.65 | 1.49 | ND | ND | 31.26 | 10.03 | ND | ND | 10.30 | 17.38 | 0.06 | 0.06 | 60.31 | 32.88 | |
| 77–175 | 0.04 | 0.11 | 0.09 | 0.12 | 0.75 | ND | 9.05 | 1.34 | ND | ND | 27.63 | 7.54 | ND | ND | 12.45 | 23.78 | 0.09 | 0.09 | 55.49 | 37.17 | |||
| 196–366 | 0.05 | 0.13 | 0.15 | 0.20 | 1.02 | ND | 11.50 | 1.52 | ND | ND | 28.22 | 8.50 | ND | ND | 11.46 | 19.05 | 0.03 | 0.08 | 58.50 | 33.25 | |||
4.3. Monounsaturated Fatty Acids (MUFAs)
4.4. Polyunsaturated Fatty Acids (PUFAs)
| Locality/References | SE | L/days | 18:2 n-6tt | CLA | 18:2 n-6 | 18:3 n-6 | FAs 20:3 n-6 | 20:4 n-6 (AA) | 18:3 n-3 | 20:5 n-3 (EPA) | 22:5 n-3 | 22:6 n-3 (DHA) | ΣPUFAs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| China ** [36] | W | NS | 0.29 | 0.59 | 3.19 | 0.01 | 0.09 | 1.35 | 2.12 | 0.14 | 0.34 | 0.01 | 8.10 | |
| China ** [82] | NS | NS | 1.85 | 4.5 | 2.66 | 0.53 | 0.53 | ND | 1.81 | ND | ND | ND | 8.46 | |
| China ** [13] | Hovd | NS | NS | ND | ND | 2.54 | ND | ND | ND | 1.13 | ND | ND | ND | 4.42 |
| Sharga | NS | NS | ND | ND | 1.92 | ND | ND | ND | 1.21 | ND | ND | ND | 3.76 | |
| Tseel | NS | NS | ND | ND | 2.21 | ND | ND | ND | 1.03 | ND | ND | ND | 3.97 | |
| Bulgan | NS | NS | ND | ND | 1.73 | ND | ND | ND | 0.61 | ND | ND | ND | 2.94 | |
| Tsogtovoo | NS | NS | ND | ND | 1.68 | ND | ND | ND | 0.46 | ND | ND | ND | 2.70 | |
| China ** [83] | NS | NS | ND | ND | 2.04 | ND | ND | ND | 2.04 | ND | ND | ND | NS | |
| China ** [84] | NS | NS | ND | ND | 1.51 | ND | ND | ND | 1.51 | ND | ND | ND | NS | |
| China ** [12] | W | NS | 0.26 | ND | 4.09 | 0.66 | ND | ND | 0.05 | ND | ND | ND | 5.18 | |
| Turkey [18] | NS | NS | ND | ND | 2.12 | ND | ND | ND | 1.74 | ND | ND | ND | NS | |
| Germany [35] | S | NS | 0.47 | 0.31 | 3.07 | ND | 0.12 | 0.21 | 0.17 | 0.01 | ND | 0.02 | 3.91 | |
| China ** [85] | NS | NS | ND | ND | 2.28 | ND | ND | ND | 1.31 | ND | ND | ND | 3.69 | |
| China ** [20] | NS | NS | 0.18 | ND | 3.31 | 0.17 | 0.36 | ND | 1.37 | ND | ND | ND | 5.21 | |
| Kazakhstan ** [11] | NS | NS | ND | ND | 1.19 | ND | ND | ND | 0.60 | ND | ND | ND | NS | |
| Kazakhstan * [11] | NS | NS | ND | ND | 1.61 | ND | ND | ND | 0.51 | ND | ND | ND | NS | |
| UAE * [19] | NS | NS | 0.49 | ND | 1.73 | 0.21 | ND | ND | 0.26 | ND | ND | ND | 4.26 | |
| Saudi Arabia * [86] | W | 90 | ND | 0.23 | 2.14 | 0.28 | ND | ND | 0.51 | 0.05 | ND | 0.19 | 3.40 | |
| Jordan * [87] | AlKhalidyah | S | NS | 1.12 | ND | 2.00 | 0.01 | ND | ND | 1.63 | ND | ND | ND | NS |
| Al Hazeem | 1.25 | ND | 2.60 | 2.17 | ND | ND | 6.15 | ND | ND | ND | NS | |||
| Al Umari | 1.56 | ND | 3.10 | 0.10 | ND | ND | 1.19 | ND | ND | ND | NS | |||
| Al Safawi | 0.06 | ND | 1.20 | 0.04 | ND | ND | 0.33 | ND | ND | ND | NS | |||
| Al Hamra | 0.46 | ND | 0.23 | 0.005 | ND | ND | 0.58 | ND | ND | ND | NS | |||
| Al Qatrana | 0.15 | ND | 0.17 | 0.06 | ND | ND | 0.09 | ND | ND | ND | NS | |||
| Wadi Araba | 0.20 | ND | 0.29 | 0.11 | ND | ND | 0.16 | ND | ND | ND | NS | |||
| Al Jweideh | 1.25 | ND | 1.98 | 1.44 | ND | ND | 2.78 | ND | ND | ND | NS | |||
| Maghrebi * [88] | NS | 140 | 2.70 | 0.9 | 2.70 | ND | 0.1 | 0.3 | 0.50 | ND | ND | 0.2 | 5.10 | |
| 245 | 2.00 | 0.4 | 2.00 | ND | 0.1 | 0.2 | 0.50 | 0.1 | ND | 0.1 | 3.80 | |||
| 329 | 2.40 | 0.7 | 2.40 | ND | 0.1 | 0.2 | 0.50 | 0.1 | ND | 0.2 | 4.50 | |||
| Egypt * [12] | W | NS | 0.46 | ND | 3.21 | ND | ND | ND | ND | ND | ND | ND | 3.67 | |
| Egypt * [89] | NS | NS | ND | ND | 3.11 | ND | ND | ND | 1.39 | ND | ND | ND | NS | |
| Sudan * [90] | S | NS | 0.12 | ND | 1.40 | ND | ND | ND | 0.13 | ND | ND | ND | 1.78 | |
| Tunisia * [91] | W | 100–180 | 0.25 | 2.05 | 2.05 | ND | ND | 0.04 | 1.24 | ND | ND | ND | 3.58 | |
| Tunisia * [92] | MJ | 28–56 | ND | 0.21 | 2.07 | ND | 0.18 | 0.04 | ND | ND | ND | ND | 5.12 | |
| 77–175 | ND | 0.29 | 2.12 | ND | 0.22 | 0.09 | ND | ND | ND | ND | 5.44 | |||
| 196–366 | ND | 0.49 | 2.20 | ND | 0.18 | 0.1 | ND | ND | ND | ND | 5.97 | |||
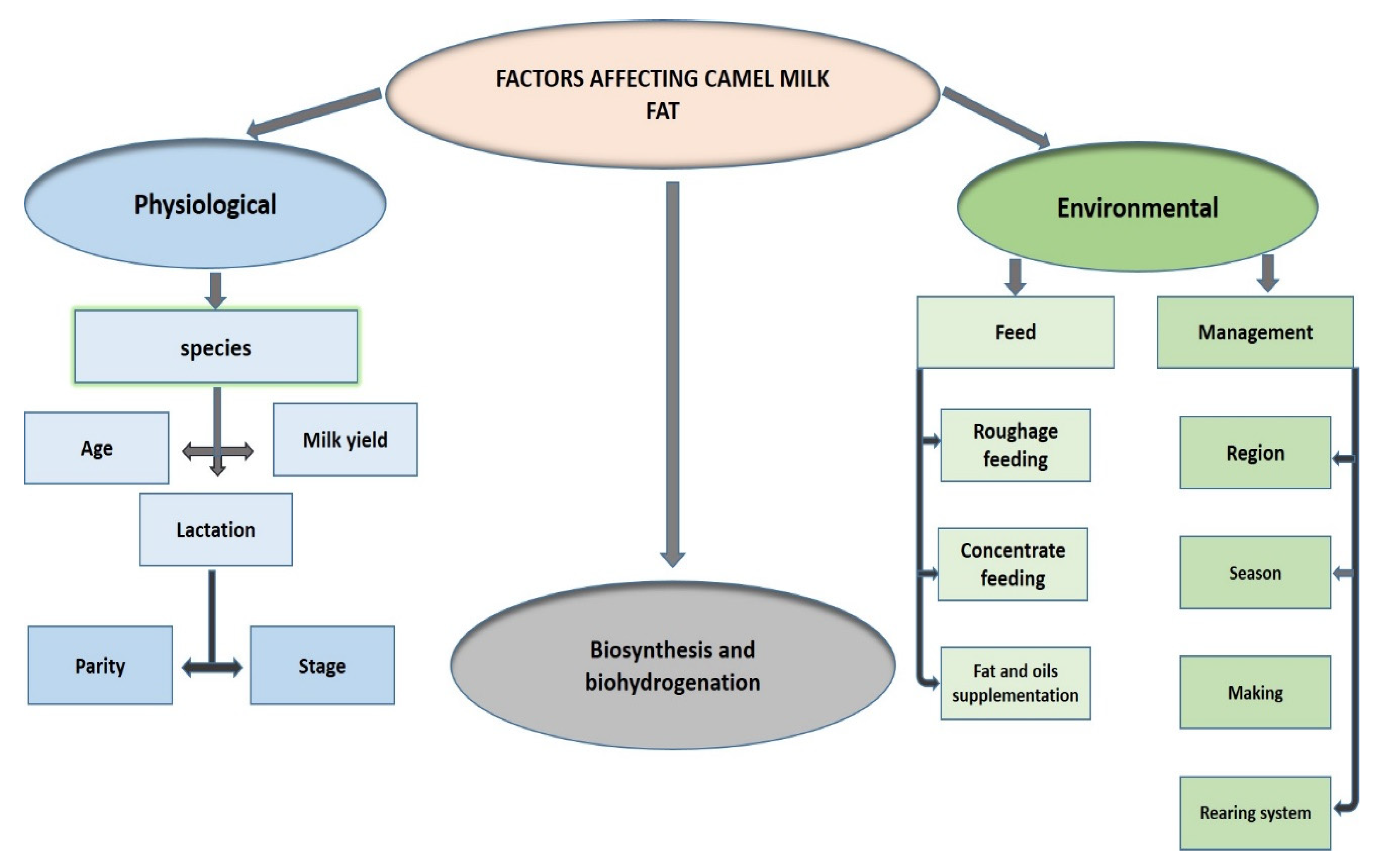
4.5. Environmental and Physiological Factors Affecting FA Composition in CM
4.6. Physical Characteristics of the CM Fat
5. Composition and Distributions of TAGs in the CM
5.1. TAGs Composition
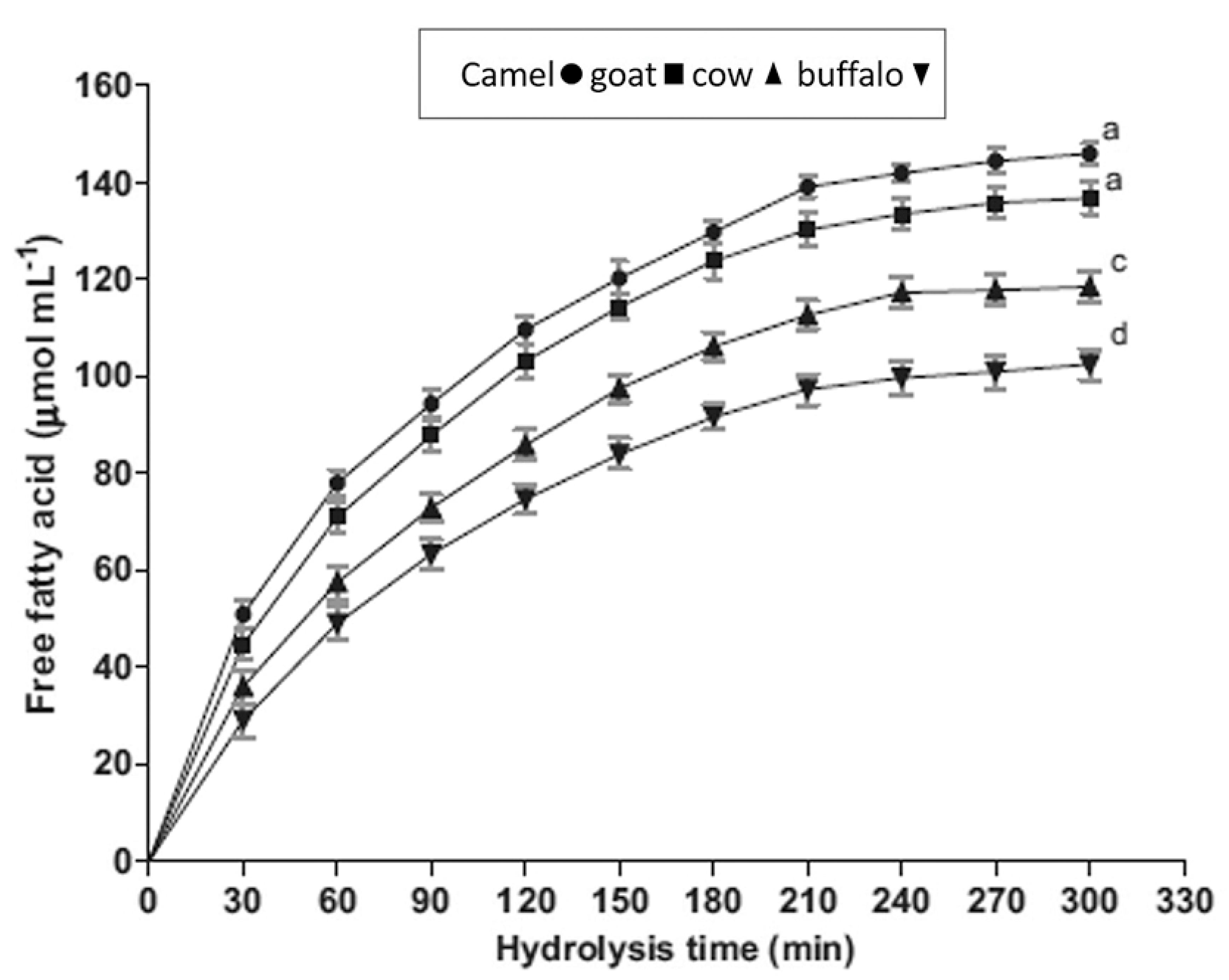
5.2. FA Distribution on TAGs
6. Composition and Nutritional Properties of CM Cholesterol
7. Composition and Nutritional Properties of CM Phospholipids
8. CM Butter and Its Production Challenges
8.1. CM Butter
8.2. Physicochemical Properties of the CM Butter
8.3. The Challenges of Industry Development of CM Butter
9. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CM | Camel milk |
| C. dromedarius | Camelus dromedarius |
| C. bactrianus | Camelus bactrianus |
| MFGs | Milk fat globules |
| MFGM | Milk fat globule membrane |
| FAs | Fatty acids |
| EFAs | Essential fatty acids |
| AA | Arachidonic acid |
| DHA | Docosahexaenoic acid |
| ALA | Alpha-linolenic acid |
| LA | Linoleic acid |
| EPA | Eicosapentaenoic acid |
| CLA | Conjugated linoleic acid |
| RA | Rumenic acid |
| LDL | Low-density lipoprotein |
| CN | Carbon number |
| TAGs | Triacylglycerols |
| SFAs | Saturated fatty acids |
| UFAs | Unsaturated fatty acids |
| MUFAs | Mono-unsaturated fatty acids |
| PUFAs | Poly-unsaturated fatty acids |
| SC-FAs | Short-chain fatty acids |
| MC-FAs | Medium-chain fatty acids |
| LC-FAs | Long-chain fatty acids |
| PLs | Phospholipids |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| SM | Sphingomyelin |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
References
- Khalesi, M.; Salami, M.; Moslehishad, M.; Winterburn, J.; Moosavi-Movahedi, A.A. Biomolecular content of camel milk: A traditional superfood towards future healthcare industry. Trends Food Sci. Technol. 2017, 62, 49–58. [Google Scholar] [CrossRef]
- Suliman, G.M.; Alowaimer, A.N.; Hussein, E.O.; Ali, H.S.; Abdelnour, S.A.; El-Hack, M.E.A.; Swelum, A.A. Chemical Composition and Quality Characteristics of Meat in Three One-Humped Camel (Camelus dromedarius) Breeds as Affected by Muscle Type and Post-Mortem Storage Period. Animals 2019, 9, 834. [Google Scholar] [CrossRef]
- FAO. Proceedings of the Gateway to Dairy Production and Products; Food and Agriculture Organisation of the United Nations (FAOSTAT): Rome, Italy, 2019; Available online: http://www.fao.org/dairy-production-products/en/ (accessed on 3 September 2021).
- Al-Sayyed, H.F. Historical Background and Population of Camels. In Handbook of Research on Health and Environmental Benefits of Camel Products; IGI Global: Hershey, PA, USA, 2020; pp. 1–14. [Google Scholar]
- El-Agamy, E.I. Camel milk. In Handbook of Milk of Non-Bovine Mammals; Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 297–344. [Google Scholar]
- Garcia, C.; Lutz, N.W.; Confort-Gouny, S.; Cozzone, P.J.; Armand, M.; Bernard, M. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: Towards specific interest in human health. Food Chem. 2012, 135, 1777–1783. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B. Recent Advances in Camel Milk Processing. Animals 2021, 11, 1045. [Google Scholar] [CrossRef]
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.S.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E. Nutritional, antimicrobial and medicinal properties of Camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef]
- Konuspayeva, G.S. Camel milk composition and nutritional value. In Handbook of Research on Health and Environmental Benefits of Camel Products; IGI Global: Hershey, PA, USA, 2020; pp. 15–40. [Google Scholar]
- Meena, S.; Rajput, Y.; Sharma, R. Comparative fat digestibility of goat, camel, cow and buffalo milk. Int. Dairy J. 2014, 35, 153–156. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Lemarie, É.; Faye, B.; Loiseau, G.; Montet, D. Fatty acid and cholesterol composition of camel’s (Camelus bactrianus, Camelus dromedarius and hybrids) milk in Kazakhstan. Dairy Sci. Techn. 2008, 88, 327–340. [Google Scholar] [CrossRef]
- Bakry, I.A.; Ali, A.H.; Abdeen, E.-S.; Ghazal, A.F.; Wei, W.; Wang, X. Comparative characterisation of fat fractions extracted from Egyptian and Chinese camel milk. Int. Dairy J. 2020, 105, 104691. [Google Scholar] [CrossRef]
- He, J.; Xiao, Y.; Orgoldol, K.; Ming, L.; Yi, L.; Ji, R. Effects of Geographic Region on the Composition of Bactrian Camel Milk in Mongolia. Animals 2019, 9, 890. [Google Scholar] [CrossRef]
- Smiddy, M.A.; Huppertz, T.; van Ruth, S.M. Triacylglycerol and melting profiles of milk fat from several species. Int. Dairy J. 2012, 24, 64–69. [Google Scholar] [CrossRef]
- Haddad, I.; Mozzon, M.; Strabbioli, R.; Frega, N.G. Stereospecific analysis of triacylglycerols in camel (Camelus dromedarius) milk fat. Int. Dairy J. 2010, 20, 863–867. [Google Scholar] [CrossRef]
- Bakry, I.A.; Korma, S.A.; Wei, W.; Nafea, A.E.; Mahdi, A.A.; Ziedan, N.I.; Wang, X. Changes in the fatty acid content of Egyptian human milk across the lactation stages and in comparison with Chinese human milk. Eur. Food Res. Technol. 2021, 247, 1035–1048. [Google Scholar] [CrossRef]
- Nyuar, K.; Min, Y.; Ghebremeskel, K.; Khalil, A.; Elbashir, M.; Cawford, M. Milk of northern Sudanese mothers whose traditional diet is high in carbohydrate contains low docosahexaenoic acid. Acta Paediatr. 2010, 99, 1824–1827. [Google Scholar] [CrossRef]
- Cardak, A.D.; Yetismeyen, A.; Bruckner, H. Quantitative comparison of camel, goat and cow milk fatty acids. Milchwissenschaft 2003, 58, 34–36. [Google Scholar]
- Maqsood, S.; Al-Dowaila, A.; Mudgil, P.; Kamal, H.; Jobe, B.; Hassan, H.M. Comparative characterization of protein and lipid fractions from camel and cow milk, their functionality, antioxidant and antihypertensive properties upon simulated gastro-intestinal digestion. Food Chem. 2018, 279, 328–338. [Google Scholar] [CrossRef]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.-Z.; Xu, X.; Wang, X. Lipid Composition Analysis of Milk Fats from Different Mammalian Species: Potential for Use as Human Milk Fat Substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef]
- Nikkhah, A. Science of Camel and Yak Milks: Human Nutrition and Health Perspectives. Food Nutr. Sci. 2011, 2, 667–673. [Google Scholar] [CrossRef]
- Mann, J. Diet and risk of coronary heart disease and type 2 diabetes. Lancet 2002, 360, 783–789. [Google Scholar] [CrossRef]
- Faye, B.; Bengoumi, M.; Al-Massaud, A.; Konuspayeva, G. Comparative milk and serum cholesterol content in dairy cow and camel. J. King Saud Univ. Sci. 2015, 27, 168–175. [Google Scholar] [CrossRef]
- Gorban, A.M.S.; Izzeldin, O.M. Study on cholesteryl ester fatty acids in camel and cow milk lipid. Int. J. Food Sci. Technol. 1999, 34, 229–234. [Google Scholar] [CrossRef]
- Alabdulkarim, B. Effect of camel milk on blood glucose, cholesterol, triglyceride and liver enzymes activities in female albino rats. World Appl. Sci. J. 2012, 17, 1394–1397. [Google Scholar]
- Sulieman, A.M.E.; Elayan, A.A.; Saleh, F. The Hypocholesterolemic Effect of Gariss and Gariss Containing Bifidobacteria in Rats Fed on a Cholesterol-Enriched Diet. Asian J. Biochem. 2008, 3, 43–47. [Google Scholar] [CrossRef][Green Version]
- Sboui, A.; Djegham, M.; Khorchani, T.; Hammadi, M.; Barhoumi, K.; Belhadj, O. Effect of camel milk on blood glucose, cholesterol and total proteins variations in alloxan-induced diabetic dogs. Int. J. Diab. Metabol. 2010, 18, 5–11. [Google Scholar] [CrossRef]
- Meena, S.; Rajput, Y.S.; Sharma, R.; Singh, R. Effect of goat and camel milk vis a vis cow milk on cholesterol homeostasis in hypercholesterolemic rats. Small Rumin. Res. 2018, 171, 8–12. [Google Scholar] [CrossRef]
- Karray, N.L.; Danthine, S.; Blecker, C.; Attia, H. Contribution to the study of camel milk fat globule membrane. Int. J. Food Sci. Nutr. 2006, 57, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Genetic Variation and Evolutionary Aspects of Diet. In Antioxidant Status, Diet, Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2019; pp. 64–89. [Google Scholar] [CrossRef]
- Eaton, S.B.; Konner, M. Paleolithic nutrition: A consideration of its nature and current implications. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef]
- Emken, E.A. Nutrition and biochemistry of trans and positional fatty acid isomers in hydrogenated oils. Annu. Rev. Nutr. 1984, 4, 339–376. [Google Scholar] [CrossRef] [PubMed]
- Shingfield, K.J.; Chilliard, Y.; Toivonen, V.; Kairenius, P.; Givens, D.I. Trans Fatty Acids and Bioactive Lipids in Ruminant Milk. Bioact. Compon. Milk 2008, 606, 3–65. [Google Scholar] [CrossRef]
- Jenkins, T.; McGuire, M. Major Advances in Nutrition: Impact on Milk Composition. Int. J. Dairy Sci. 2006, 89, 1302–1310. [Google Scholar] [CrossRef]
- Dreiucker, J.; Vetter, W. Fatty acids patterns in camel, moose, cow and human milk as determined with GC/MS after silver ion solid phase extraction. Food Chem. 2011, 126, 762–771. [Google Scholar] [CrossRef]
- Teng, F.; Wang, P.; Yang, L.; Ma, Y.; Day, L. Quantification of Fatty Acids in Human, Cow, Buffalo, Goat, Yak, and Camel Milk Using an Improved One-Step GC-FID Method. Food Anal. Methods 2017, 10, 2881–2891. [Google Scholar] [CrossRef]
- O’Shea, M.; Bassaganya-Riera, J.; Mohede, I.C.M. Immunomodulatory properties of conjugated linoleic acid. Am. J. Clin. Nutr. 2004, 79, 1199S–1206S. [Google Scholar] [CrossRef]
- Wang, Y.; Jones, P.J.H. Dietary conjugated linoleic acid and body composition. Am. J. Clin. Nutr. 2004, 79, 1153S–1158S. [Google Scholar] [CrossRef]
- Singh, R.; Mal, G.; Kumar, D.; Patil, N.V.; Pathak, K.M.L. Camel Milk: An Important Natural Adjuvant. Agric. Res. 2017, 6, 327–340. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; Jagla, A. Milk and diabetes. J. Am. Coll. Nutr. 2000, 19, 176S–190S. [Google Scholar] [CrossRef]
- Liu, C.; Gelius, E.; Liu, G.; Steiner, H.; Dziarski, R. Mammalian Peptidoglycan Recognition Protein Binds Peptidoglycan with High Affinity, Is Expressed in Neutrophils, and Inhibits Bacterial Growth. J. Biol. Chem. 2000, 275, 24490–24499. [Google Scholar] [CrossRef]
- Redwan, E.M.; Tabll, A. Camel Lactoferrin Markedly Inhibits Hepatitis C Virus Genotype 4 Infection of Human Peripheral Blood Leukocytes. J. Immunoass. Immunochem. 2007, 28, 267–277. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Agrawal, R.; Beniwal, R.; Kochar, D.; Tuteja, F.; Ghorui, S.; Sahani, M.; Sharma, S. Camel milk as an adjunct to insulin therapy improves long-term glycemic control and reduction in doses of insulin in patients with type-1 diabetes: A 1 year randomized controlled trial. Diabetes Res. Clin. Pr. 2005, 68, 176–177. [Google Scholar] [CrossRef]
- Walter, L.; Shrestha, P.; Fry, R.; Leury, B.; Logan, A. Lipid metabolic differences in cows producing small or large milk fat globules: Fatty acid origin and degree of saturation. J. Dairy Sci. 2020, 103, 1920–1930. [Google Scholar] [CrossRef]
- Månsson, H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, N.; Zhao, X.; Zhang, Y.; Han, R.; Ma, L.; Zhao, S.; Li, S.; Guo, T.; Wang, J. Proteomic characterization and comparison of mammalian milk fat globule proteomes by iTRAQ analysis. J. Proteom. 2015, 116, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sabha, B.H.; Masood, A.; Alanazi, I.O.; Alfadda, A.A.; Almehdar, H.A.; Benabdelkamel, H.; Redwan, E.M. Comparative Analysis of Milk Fat Globular Membrane (MFGM) Proteome between Saudi Arabia Camelus dromedary Safra and Wadha Breeds. Molecules 2020, 25, 2146. [Google Scholar] [CrossRef]
- Saadaoui, B.; Henry, C.; Khorchani, T.; Mars, M.; Martin, P.; Cebo, C. Proteomics of the milk fat globule membrane from C amelus dromedarius. Proteomics 2013, 13, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Evers, J.M. The milkfat globule membrane—compositional and structural changes post secretion by the mammary secretory cell. Int. Dairy J. 2004, 14, 661–674. [Google Scholar] [CrossRef]
- Lopez, C. Milk fat globules enveloped by their biological membrane: Unique colloidal assemblies with a specific composition and structure. Curr. Opin. Colloid Interface Sci. 2011, 16, 391–404. [Google Scholar] [CrossRef]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.W. Historical Perspective: Milk Lipid Globules and Their Surrounding Membrane: A Brief History and Perspectives for Future Research. J. Mammary Gland. Biol. Neoplasia 2001, 6, 365–371. [Google Scholar] [CrossRef]
- Attia, H.; Kherouatou, N.; Fakhfakh, N.; Khorchani, T.; Trigui, N. Dromedary Milk Fat: Biochemical, Microscopic and Rheological Characteristics. J. Food Lipids 2000, 7, 95–112. [Google Scholar] [CrossRef]
- Farah, Z.; Rüegg, M. The Creaming Properties and Size Distribution of Fat Globules in Camel Milk. J. Dairy Sci. 1991, 74, 2901–2904. [Google Scholar] [CrossRef]
- Attaie, R.; Richter, R. Size Distribution of Fat Globules in Goat Milk. J. Dairy Sci. 2000, 83, 940–944. [Google Scholar] [CrossRef]
- Manoni, M.; Di Lorenzo, C.; Ottoboni, M.; Tretola, M.; Pinotti, L. Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation. Foods 2020, 9, 1251. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.; Jakob, S.; Mosenthin, R. Principles of Physiology of Lipid Digestion. Asian-Australas. J. Anim. Sci. 2005, 18, 282–295. [Google Scholar] [CrossRef]
- El Fakharany, E.; El-Baky, N.A.; Linjawi, M.H.; AlJaddawi, A.A.; Saleem, T.H.; Nassar, A.Y.; Osman, A.; Redwan, E.M. Influence of camel milk on the hepatitis C virus burden of infected patients. Exp. Ther. Med. 2017, 13, 1313–1320. [Google Scholar] [CrossRef]
- Imam, A.; Drushella, M.M.; Taylor, C.R.; Tökés, Z.A. Preferential expression of a Mr 155,000 milk-fat-globule membrane glycoprotein on luminal epithelium of lobules in human breast. Cancer Res. 1986, 46, 6374–6379. [Google Scholar]
- Khatoon, H.; Ikram, R.; Anser, H.; Naeem, S.; Khan, S.S.; Fatima, S.; Sultana, N.; Sarfaraz, S. Investigation of anti-inflammatory and analgesic activities of camel milk in animal models. Pak. J. Pharm. Sci. 2019, 32, 1879–1883. [Google Scholar]
- Mulder, H.; Walstra, P. The Milk Fat Globule; Commonwealth Agricultural Bureaux Farnham Royal: Wallingford, UK, 1974; ISBN 0851982891. [Google Scholar]
- Heid, H.W.; Keenan, T.W. Intracellular origin and secretion of milk fat globules. Eur. J. Cell Biol. 2005, 84, 245–258. [Google Scholar] [CrossRef]
- Robenek, H.; Buers, I.; Hofnagel, O.; Robenek, M.J.; Troyer, D.; Severs, N.J. Compartmentalization of proteins in lipid droplet biogenesis. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2009, 1791, 408–418. [Google Scholar] [CrossRef]
- Masedunskas, A.; Chen, Y.; Stussman, R.; Weigert, R.; Mather, I.H. Kinetics of milk lipid droplet transport, growth, and secretion revealed by intravital imaging: Lipid droplet release is intermittently stimulated by oxytocin. Mol. Biol. Cell 2017, 28, 935–946. [Google Scholar] [CrossRef]
- El-Zeini, H.M. Microstructure, rheological and geometrical properties of fat globules of milk from different animal species. Polish J. Food Nutr. Sci. 2006, 15, 147–153. [Google Scholar]
- Habtegebriel, H.; Wawire, M.; Gaukel, V.; Taboada, M.L. Comparison of the viscosity of camel milk with model milk systems in relation to their atomization properties. J. Food Sci. 2020, 85, 3459–3466. [Google Scholar] [CrossRef]
- Zhao, D.B. Studies on the Chemical Composition and Chemical–Physical Properties of Alxa Bactrian Camels Milk in Inner Mongolia. Master’s Thesis, Inner Mongolia Agricultural University, Huhhot, China, 2006. [Google Scholar]
- Berton, A.; Rouvellac, S.; Robert, B.; Rousseau, F.; Lopez, C.; Crenon, I. Effect of the size and interface composition of milk fat globules on their in vitro digestion by the human pancreatic lipase: Native versus homogenized milk fat globules. Food Hydrocoll. 2012, 29, 123–134. [Google Scholar] [CrossRef]
- Favé, G.; Coste, T.; Armand, M. Physicochemical properties of lipids: New strategies to manage fatty acid bioavailability. Cell. Mol. Boil. 2004, 50, 815–831. [Google Scholar]
- D’Urso, S.; Cutrignelli, M.I.; Calabrò, S.; Bovera, F.; Tudisco, R.; Piccolo, V.; Infascelli, F. Influence of pasture on fatty acid profile of goat milk. J. Anim. Physiol. Anim. Nutr. 2008, 92, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Briard, V.; Leconte, N.; Michel, F.; Michalski, M.-C. The fatty acid composition of small and large naturally occurring milk fat globules. Eur. J. Lipid Sci. Technol. 2003, 105, 677–682. [Google Scholar] [CrossRef]
- Fauquant, C.; Leconte, N.; Michalski, M.-C. Differently sized native milk fat globules separated by microfiltration: Fatty acid composition of the milk fat globule membrane and triglyceride core. Eur. J. Lipid Sci. Technol. 2005, 107, 80–86. [Google Scholar] [CrossRef]
- Aoki, N.; Ishii, T.; Ohira, S.; Yamaguchi, Y.; Negi, M.; Adachi, T.; Nakamura, R.; Matsuda, T. Stage specific expression of milk fat globule membrane glycoproteins in mouse mammary gland: Comparison of MFG-E8, butyrophilin, and CD36 with a major milk protein, β-casein. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1997, 1334, 182–190. [Google Scholar] [CrossRef]
- Jenness, R. Composition and Characteristics of Goat Milk: Review 1968−1979. Int. J. Dairy Sci. 1980, 63, 1605–1630. [Google Scholar] [CrossRef]
- Zouari, A.; Schuck, P.; Gaucheron, F.; Triki, M.; Delaplace, G.; Gauzelin-Gaiani, C.; Lopez, C.; Attia, H.; Ayadi, M.A. Microstructure and chemical composition of camel and cow milk powders’ surface. LWT 2019, 117, 108693. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Loiseau, G. The composition of camel milk: A meta-analysis of the literature data. J. Food Compos. Anal. 2009, 22, 95–101. [Google Scholar] [CrossRef]
- Ohlsson, L. Dairy products and plasma cholesterol levels. Food Nutr. Res. 2010, 54. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Mursu, J.; Tuomainen, T.-P.; Voutilainen, S. Dietary fatty acids and risk of coronary heart disease in men: The Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2679–2687. [Google Scholar] [CrossRef]
- Karray, N.; Lopez, C.; Ollivon, M.; Attia, H. La matière grasse du lait de dromadaire: Composition, microstructure et polymorphisme. Une revue. Oléagineux Corps Gras Lipides 2005, 12, 439–446. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Zheng, N.; Wang, J.; Yang, Y. Comparative milk fatty acid analysis of different dairy species. Int. J. Dairy Technol. 2017, 71, 544–550. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, J.; Zhao, D.; Liu, H.; Li, J.; Guo, M. Changes in Chemical Composition of Alxa Bactrian Camel Milk During Lactation. J. Dairy Sci. 2005, 88, 3402–3410. [Google Scholar] [CrossRef]
- Yi, L.; Te, M.L.; Zheng, Z.Q.; Ming, L.; Er, D.M.T.; Chen, G.L.; Ji, R.M.T. Study on seasonal variation of fatty acid composition of Bactrian camel’s milk in Zhunger. Dairy Ind. China 2014, 3, 18–21. [Google Scholar]
- Ali, A.H.; El-Wahed, E.M.A.; Abed, S.M.; Korma, S.A.; Wei, W.; Wang, X. Analysis of triacylglycerols molecular species composition, total fatty acids, and sn-2 fatty acids positional distribution in different types of milk powders. J. Food Meas. Charact. 2019, 13, 2613–2625. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Mussaad, A. Some lipid components of the camel milk and blood in intensive farm insaudi arabia. Emir. J. Food Agric. 2014, 26, 349–353. [Google Scholar]
- Ereifej, K.I.; Alu’Datt, M.H.; AlKhalidy, H.A.; Alli, I.; Rababah, T. Comparison and characterisation of fat and protein composition for camel milk from eight Jordanian locations. Food Chem. 2011, 127, 282–289. [Google Scholar] [CrossRef]
- Ayadi, M.; Hammadi, M.; Casals, R.; Atigui, M.; Khorchani, T.; Samara, E.M.; Abdoun, K.A.; Al-Haidary, A.A.; Caja, G. Influence of management type and stage of lactation on the performance and milk fatty acid profile of dairy camels (Camelus dromedaries). J. Agric. Sci. 2018, 156, 1111–1122. [Google Scholar] [CrossRef]
- Abu-Lehia, I.H. Physical and chemical characteristics of camel milkfat and its fractions. Food Chem. 1989, 34, 261–271. [Google Scholar] [CrossRef]
- Mohamed, E.; Mustafa, A. Fatty Acids Content in Milk of Dromedary Camel (Camelus dromedarius) from Farming and Pastoral Systems in Sudan. Inter J. Sci. Res. 2013, 6, 382–390. [Google Scholar]
- Haddad, I.; Mozzon, M.; Strabbioli, R.; Frega, N.G. Electrospray ionization tandem mass spectrometry analysis of triacylglycerols molecular species in camel milk (Camelus dromedarius). Int. Dairy J. 2011, 21, 119–127. [Google Scholar] [CrossRef]
- Chamekh, L.; Calvo, M.; Khorchani, T.; Castro-Gómez, P.; Hammadi, M.; Fontecha, J.; Yahyaoui, M.H.; Latifa, C.; Marivi, C.; Touhami, K.; et al. Impact of management system and lactation stage on fatty acid composition of camel milk. J. Food Compos. Anal. 2020, 87, 103418. [Google Scholar] [CrossRef]
- Pérez-Jiménez, F.; Castro, P.; López-Miranda, J.; Paz-Rojas, E.; Blanco, A.; López-Segura, F.; Velasco, F.; Marin, C.; Fuentes, F.; Ordovás, J.M. Circulating levels of endothelial function are modulated by dietary monounsaturated fat. Atherosclerosis 1999, 145, 351–358. [Google Scholar] [CrossRef]
- Wei, W.; Jin, Q.; Wang, X. Human milk fat substitutes: Past achievements and current trends. Prog. Lipid Res. 2019, 74, 69–86. [Google Scholar] [CrossRef]
- Burlingame, B.; Nishida, C.; Uauy, R.; Weisell, R. Fats and Fatty Acids in Human Nutrition: Introduction. Ann. Nutr. Metab. 2009, 55, 5–7. [Google Scholar] [CrossRef]
- Wongtangtintharn, S.; Oku, H.; Iwasaki, H.; Toda, T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J. Nutr. Sci. Vitaminol. 2004, 50, 137–143. [Google Scholar] [CrossRef] [PubMed]
- MS Gorban, A.; Izzeldin, O.M. Fatty acids and lipids of camel milk and colostrum. Int. J. Food Sci. Nutr. 2001, 52, 283–287. [Google Scholar] [CrossRef]
- Dowelmadina, I.M.M.; El Zubeir, I.E.M.; Arabi, O.; Abakar, A.D. Omega-3 fatty acids in milk fat of some Sudanese camels. J. Dairy Res.Technol. 2019, 2. [Google Scholar] [CrossRef]
- Faye, B.; Konuspayeva, G.; Narmuratova, Z.; Serikbaeva, A.; Musaad, A.M.; Mehri, H. Effect of crude olive cake supplementation on camel milk production and fatty acid composition. Dairy Sci. Technol. 2013, 93, 225–239. [Google Scholar] [CrossRef][Green Version]
- Leparmarai, P.T.; Kunz, C.; Mwangi, D.M.; Gluecks, I.; Kreuzer, M.; Marquardt, S. Camels and cattle respond differently in milk phenol excretion and milk fatty acid profile to free ranging conditions in East-African rangelands. Sci. Afr. 2021, e00896. [Google Scholar] [CrossRef]
- Koletzko, B.; Bergmann, K.; Brenna, J.T.; Calder, P.C.; Campoy, C.; Clandinin, M.T.; Colombo, J.; Daly, M.; Decsi, T.; Demmelmair, H.; et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am. J. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Farah, Z.; Streiff, T.; Bachmann, M. Manufacture and characterization of camel milk butter. Milchwissenschaft 1989, 44, 412–414. [Google Scholar]
- Zhao, D.-B.; Bai, Y.-H.; Niu, Y.-W. Composition and characteristics of Chinese Bactrian camel milk. Small Rumin. Res. 2015, 127, 58–67. [Google Scholar] [CrossRef]
- Abu-Lehia, I.H. Composition of camel milk. Milchwissenschaft 1987, 42, 368–371. [Google Scholar]
- Buldo, P. Crystallization of Fat in and Outside Milk Fat Globules—Effect of Processing and Storage Conditions. Ph.D. Thesis, Department of Food Science, Aarhus University, Aarhus, Denmark, 2013. [Google Scholar]
- Purchase, H.S. Some Experiments in the Making of Butter, Ghee, and Cheese from Camels’ Milk. East. Afr. Agric. J. 1943, 9, 39–41. [Google Scholar] [CrossRef]
- Bharwade, M.; Balakrishnan, S.; Chaudhary, N.; Jain, A. Fatty Acid Profile and Physico-Chemical Characteristics of Milk Lipids of Kankrej Cow. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3035–3047. [Google Scholar] [CrossRef]
- Wright, A.J.; Marangoni, A.G. Crystallization and Rheological Properties of Milk Fat. In Advanced Dairy Chem Volume 2 Lipids; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Hansen, H.S.; Jensen, B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and α-linolenate. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1985, 834, 357–363. [Google Scholar] [CrossRef]
- Ali, A.; Zou, X.; Huang, J.; Abed, S.M.; Tao, G.; Jin, Q.; Wang, X. Profiling of phospholipids molecular species from different mammalian milk powders by using ultra-performance liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry. J. Food Compos. Anal. 2017, 62, 143–154. [Google Scholar] [CrossRef]
- Jenkins, B.; West, J.A.; Koulman, A. A Review of Odd-Chain Fatty Acid Metabolism and the Role of Pentadecanoic Acid (C15:0) and Heptadecanoic Acid (C17:0) in Health and Disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef]
- Gresti, J.; Bugaut, M.; Maniongui, C.; Bezard, J. Composition of Molecular Species of Triacylglycerols in Bovine Milk Fat. J. Dairy Sci. 1993, 76, 1850–1869. [Google Scholar] [CrossRef]
- Bornaz, S.; Fanni, J.; Parmentier, M. Butter texture: The prevalent triglycerides. J. Am. Oil Chem. Soc. 1993, 70, 1075–1079. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. The influence of the position of palmitate in infant formula triacylglycerols on health outcomes. Nutr. Res. 2017, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yoseph, F.; Lifshitz, Y.; Cohen, T. Review of sn-2 palmitate oil implications for infant health. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 139–143. [Google Scholar] [CrossRef]
- Farag, S.I.; Kebary, K.M.K. Chemical composition and physical properties of camel’s milk and milk fat. In Proceedings of the 5th Egyptian Conference for Dairy Science and Technology; Egyptian Society of Dairy Sciences: Cairo, Egypt, 1992; pp. 57–67. [Google Scholar]
- Parodi, P.W. Has the association between saturated fatty acids, serum cholesterol and coronary heart disease been over emphasized? Int. Dairy J. 2009, 19, 345–361. [Google Scholar] [CrossRef]
- Wasfi, I.; Hafez, A.; El Tayeb, F.; El Taher, A. Thyroid hormones, cholesterol and triglyceride levels in the camel. Res. Veter Sci. 1987, 42, 418. [Google Scholar] [CrossRef]
- Kamal, A.M.; Salama, O.A. Lipid Fractions and Fatty Acid Composition of Colostrums, Transitional and Mature She-Camel Milk During the First Month of Lactation. Asian J. Clin. Nutr. 2009, 1, 23–30. [Google Scholar] [CrossRef]
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional Value and Technological Suitability of Milk from Various Animal Species Used for Dairy Production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Li, H.; Papadopoulos, V. Peripheral-Type Benzodiazepine Receptor Function in Cholesterol Transport. Identification of a Putative Cholesterol Recognition/Interaction Amino Acid Sequence and Consensus Pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef]
- Buonopane, G.J.; Kilara, A.; Smith, J.S.; McCarthy, R.D. Effect of skim milk supplementation on blood cholesterol concentration, blood pressure, and triglycerides in a free-living human population. J. Am. Coll. Nutr. 1992, 11, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.R.; Chawan, C.B.; Pulusani, S.R. Influence of Milk and Thermophilus Milk on Plasma Cholesterol Levels and Hepatic Cholesterogenesis in Rats. J. Food Sci. 1981, 46, 1339–1341. [Google Scholar] [CrossRef]
- Morrison, W.R. The distribution of phospholipids in some mammalian milks. Lipids 1968, 3, 101–103. [Google Scholar] [CrossRef]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef]
- Nishimukai, M.; Wakisaka, T.; Hara, H. Ingestion of plasmalogen markedly increased plasmalogen levels of blood plasma in rats. Lipids 2003, 38, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Helmerich, G.; Koehler, P. Comparison of Methods for the Quantitative Determination of Phospholipids in Lecithins and Flour Improvers. J. Agric. Food Chem. 2003, 51, 6645–6651. [Google Scholar] [CrossRef]
- Yassin, A.M.; Hamid, M.I.A.; Farid, O.A.; Amer, H.; Warda, M. Dromedary milk exosomes as mammary transcriptome nano-vehicle: Their isolation, vesicular and phospholipidomic characterizations. J. Adv. Res. 2016, 7, 749–756. [Google Scholar] [CrossRef]
- Shirouchi, B.; Nagao, K.; Furuya, K.; Inoue, N.; Inafuku, M.; Nasu, M.; Otsubo, K.; Koga, S.; Matsumoto, H.; Yanagita, T. Effect of dietary phosphatidylinositol on cholesterol metabolism in Zucker (fa/fa) rats. J. Oleo Sci. 2009, 58, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Maxfield, F.R. Membrane domains. Annu. Rev. Cell Dev. Biol. 2004, 20, 839–866. [Google Scholar] [CrossRef] [PubMed]
- Yavin, E.; Brand, A.; Green, P. Docosahexaenoic Acid Abundance in the Brain: A biodevice to Combat Oxidative Stress. Nutr. Neurosci. 2002, 5, 149–157. [Google Scholar] [CrossRef]
- Labadaridis, I.; Moraitou, M.; Theodoraki, M.; Triantafyllidis, G.; Sarafidou, J.; Michelakakis, H. Plasmalogen levels in full-term neonates. Acta Paediatr. 2009, 98, 640–642. [Google Scholar] [CrossRef]
- Mourad, K.; Nour-Eddine, K. Physicochemical and microbiological study of “shmen”, a traditional butter made from camel milk in the Sahara (Algeria): Isolation and identification of lactic acid bacteria and yeasts. Grasas Aceites 2006, 57. [Google Scholar] [CrossRef]
- Yagil, R. Camels and Camel Milk: FAO Animal Production and Health; Publications Division, Food and Agriculture Organization of the United Nations: Rome, Italy, 1982; p. 26. [Google Scholar]
- Parmar, N.B. Characterization of Ghee Prepared from Camel Milk and Evaluation of Its Shelf Life During Storage. Ph.D. Thesis, Anand Agricultural University, Anand, India, 2013. [Google Scholar]
- Knoess, K.H.; Makhudum, A.J.; Rafiq, M.; Hafeez, M. Milk production potential of the dromedary with special reference to the province of Punjab, Pakistan. World Anim. Rev. 1986, 57, 11–21. [Google Scholar]
- Mtibaa, I.; Zouari, A.; Attia, H.; Ayadi, M.A.; Danthine, S. Effects of Physical Ripening Conditions and Churning Temperature on the Butter-Making Process and the Physical Characteristics of Camel Milk Butter. Food Bioprocess. Technol. 2021, 14, 1518–1528. [Google Scholar] [CrossRef]
- Berhe, T.; Seifu, E.; Kurtu, M.Y. Physicochemical properties of butter made from camel milk. Int. Dairy J. 2013, 31, 51–54. [Google Scholar] [CrossRef]
- Bylund, G. Dairy processing handbook: Tetra Pak Processing Systems AB; Sweden, AB: Lund, Sweden, 1995; pp. 13–36. [Google Scholar]
- Khan, K.U.; Appanna, T.C. Carotene and vitamin A in camel milk. Indian J. Nutr. Diet. 1967, 4, 17–20. [Google Scholar]

| Type | Camel Milk | Cow Milk c | |
|---|---|---|---|
| C. dromedariusa | C. bactrianusb | ||
| Acid value | 0.54 | 0.30–0.44 | 1.50 |
| Refractive point | 1.4490–1.4714 | 1.4563 | 1.4530 |
| Saponification value | 200.00–217.00 | 189.30–200.00 | 228.50 |
| Iodine value | 43.80–55.00 | 51.80–55.00 | 28.13–32.30 |
| Polenske value | 0.50–0.62 | NS | 1.56–1.61 |
| Reichert–Meissel value | 1.10–2.12 | NS | 28.40–29.56 |
| Melting point (°C) | 37.97–44.10 | 40.40–42.46 | 31.50–34.80 |
| Studies | Samples | Extraction Method | Analysis Method | Major TAGs/Concentration |
|---|---|---|---|---|
| [20] | C. dromedarius (n = 5) | Folch | RP-HPLC/-APCI-MS | PPL (13.67%), POM (12.78%), PPO (11.77%), POO (10.67%), PPM (6.76%) |
| [91] | C. dromedarius (n = 20) | Folch | HPLC/ESI-MS | MPO (6.71%), PPO (5.72%), SPO (5.30%) |
| [14] | C. dromedarius (n = 20) | Rose–Gottlieb | GC | CN52 (20.37%), CN48 (21.60%), CN46 (12.48%), CN50 (25.79%) |
| [85] | C. dromedarius | Folch | UPLC/Q-TOF–MS | OPM/PPaP/PaMS (10.96%), OPaP/OMO/PLP (8.91%), OPP/OSM/PSPa (8.80%) |
| [12] | C. dromedarius and C. bactrianus | Mojonnier | UPC2/Q-TOF–MS | C. dromedarius: MPaPa/LMM/OMMy (5.36%), MPO (11.73%), SPP/MSS (7.15%), PaPaO (8.37%), PSS (7.88%) C. bactrianus: MMO/MyMS/LaPO (7.25%), MSP (8.52%), LaLS/MHH (7.04%), SPP/MSS (8.22%), PSS (5.91%) |
| Studies | Samples | Extraction Method | Analysis Method | PLs Identified | Total PL Amounts |
|---|---|---|---|---|---|
| [124] | Israel, C. dromedarius | Folch | TLC | PI, PS, PE, PC, SM, | 52.30% |
| [97] | Saudi Arabia, (n = 8, C. dromedarius) | Rose–Gottlieb | Ultroscan laser densitometer | ND | 1.21% |
| [6] | Tunisia (n = 8, from 8 different C. dromedarius) | Folch | 31P NMR | PE, PS, PI, PC, SM, aaPC, LPC | 0.503 mmol/L |
| [20] | C. bactrianus (n = 20) | Folch | HPLC–ELSD | PI, PS, PE, PC, SM | 4.65 mg/g fat |
| [128] | C. dromedarius | Folch | HPLC–UV | PI, PE, PS, PC | 60–66 µg/mL |
| [110] | UAE, C. dromedarius | Folch | UPLC–ESI–QTOF–MS | PC, PE, PI, PS, SM, LPC | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakry, I.A.; Yang, L.; Farag, M.A.; Korma, S.A.; Khalifa, I.; Cacciotti, I.; Ziedan, N.I.; Jin, J.; Jin, Q.; Wei, W.; et al. A Comprehensive Review of the Composition, Nutritional Value, and Functional Properties of Camel Milk Fat. Foods 2021, 10, 2158. https://doi.org/10.3390/foods10092158
Bakry IA, Yang L, Farag MA, Korma SA, Khalifa I, Cacciotti I, Ziedan NI, Jin J, Jin Q, Wei W, et al. A Comprehensive Review of the Composition, Nutritional Value, and Functional Properties of Camel Milk Fat. Foods. 2021; 10(9):2158. https://doi.org/10.3390/foods10092158
Chicago/Turabian StyleBakry, Ibrahim A., Lan Yang, Mohamed A. Farag, Sameh A. Korma, Ibrahim Khalifa, Ilaria Cacciotti, Noha I. Ziedan, Jun Jin, Qingzhe Jin, Wei Wei, and et al. 2021. "A Comprehensive Review of the Composition, Nutritional Value, and Functional Properties of Camel Milk Fat" Foods 10, no. 9: 2158. https://doi.org/10.3390/foods10092158
APA StyleBakry, I. A., Yang, L., Farag, M. A., Korma, S. A., Khalifa, I., Cacciotti, I., Ziedan, N. I., Jin, J., Jin, Q., Wei, W., & Wang, X. (2021). A Comprehensive Review of the Composition, Nutritional Value, and Functional Properties of Camel Milk Fat. Foods, 10(9), 2158. https://doi.org/10.3390/foods10092158










