Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Bee Pollen
2.2. Determination of Botanical Origin-Melissopalynological Analysis
2.3. Physico-Chemical Analyses
2.3.1. Moisture Content (%)
2.3.2. Ash Content (%)
2.3.3. Protein Content (%)
2.3.4. Total Lipid Content (%)
2.3.5. Determination of Sugar Content (%) by HPLC Method
2.4. HS-SPME/GC-MS Analysis
2.5. FTIR-ATR Spectroscopy and Spectral Data Processing
2.6. Statistical Analyses
3. Results
3.1. Bee Pollen Classification According to Botanical Origin
3.2. Characterization of Bee Pollen by Physico-Chemical Analyses
3.3. The Headspace Composition of Unifloral Bee Pollen
3.4. FTIR-ATR Spectral Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid. Based Complementary Altern. Med. 2015. [Google Scholar] [CrossRef]
- Campos, M.; Markham, K.R.; Mitchell, K.A.; Da Cunha, A.P. An approach to the characterization of bee pollens via their flavonoid/phenolic profiles. Phytochem. Anal. 1997, 8, 181–185. [Google Scholar] [CrossRef]
- Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.; Barth, O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Compos. Anal. 2005, 18, 105–111. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardization of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient-The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef]
- Adaškevičiūtė, V.; Kaškonienė, V.; Kaškonas, P.; Barčauskaitė, K.; Maruška, A. Comparison of Physicochemical Properties of Bee Pollen with Other Bee Products. Biomolecules 2019, 9, 819. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef] [PubMed]
- Feás, X.; Vázquez-Tato, M.P.; Estevinho, L.; Seijas, J.A.; Iglesias, A. Organic bee pollen: Botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules 2012, 17, 8359–8377. [Google Scholar] [CrossRef]
- Sagona, S.; Pozzo, L.; Peiretti, P.G.; Biondi, C.; Giusti, M.; Gabriele, M.; Pucci, L.; Felicioli, A. Palynological origin, chemical composition, lipid peroxidation and fatty acid profile of organic Tuscanian bee-pollen. J. Apic. Res. 2017, 56, 136–143. [Google Scholar] [CrossRef]
- Oltica, C.; Mărghitas, L.A.; Dezmirean, D.S. Total Polyphenols, Flavonoids and Radical Scavenging Activity of Bee pollen and Beebread Collected from Transylvania Area. Bull. Univ. Agric. Sci. Vet. Med. 2006, 62, 149–152. [Google Scholar]
- Anjos, O.; Santos, A.J.A.; Dias, T.; Estevinho, L.M. Application of FTIR-ATR spectroscopy on the bee pollen characterization. J. Apic. Res. 2017, 56, 210–218. [Google Scholar] [CrossRef]
- Spulber, R.; Doğaroğlu, M.; Băbeanu, N.; Popa, O. Physicochemical characteristics of fresh bee pollen from different botanical origins. Rom. Biotechnol. Lett. 2018, 23, 13357–13365. [Google Scholar]
- Nicolson, W.S. Bee food: The chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr. Zool. 2011, 46, 197–204. [Google Scholar] [CrossRef]
- Código Argentino Alimentario–Actualizado, Dpto. De Salud Pública Veterinaria. In Artículo 785; Código Argentino Alimentario–Actualizado, Dpto: Buenos Aires, Argentina, 2001; pp. 443–444. [Google Scholar]
- Instrução Normativa n.3. Regulamentos Técnicos de Identidade e Qualidade de Apitoxina, Cera de Abelha, Geléia Real, Geléia Real Liofilizada, Pólen Apícola, Própolis e Extrato de Própolis; Ministério da Agricultura, Pecuária e Abastecimento: Rio de Janeiro, Brazil, 2001. [Google Scholar]
- Official Gazette 54. Ordinance №9 on the Ministry of Agriculture and Forest; The Ministry of Agriculture and Foestry: Sofia, Bulgaria, 2005.
- PN-R-78893 “Obnóza Pylkowe”—Polish Legislation for Bee-Pollen; Ministry of Agriculture and Rural Development: Warsaw, Poland, 2003.
- Swiss Food Manual: Pollen Bienenprodukte; BAG—Swiss Federal Office for Public Health: Bern, Switzerland, 2015.
- Bergström, G.; Dobson, H.E.M.; Groth, I. Spatial fragrance patterns within the flowers of Ranunculus acris (Ranunculaceae). Plant Syst. Evol. 1995, 195, 221–242. [Google Scholar] [CrossRef]
- Dobson, H.E.M.; Danielson, E.M.; van Wesep, I.D. Pollen odor chemicals as modu lators of bumble bee foraging on Rosa rugose Thunb. (Rosaceae). Plant Species Biol. 1999, 14, 153–166. [Google Scholar] [CrossRef]
- Moore, P.D.; Webb, J.A.; Collinson, M.E. Pollen Analysis; Blackwell: Oxford, UK, 1991. [Google Scholar]
- Dobson, H.E.M.; Groth, I.; Bergstrom, G. Pollen Advertisement: Chemical contrasts between whole-flower and pollen odors. Am. J. Bot. 1996, 83, 877–885. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L. Floral scent and intrafloral scent differentiation in Moneses and Pyrola (Pyrolaceae). Plant Syst. Evol. 1991, 177, 81–91. [Google Scholar] [CrossRef]
- Kasprzyk, I.; Depciuch, J.; Grabek-Lejko, D.; Parlinska-Wojtan, M. FTIR-ATR spectroscopy of pollen and honey as a tool for unifloral honey authentication: The case study of rape honey. Food Control 2018, 84, 33–40. [Google Scholar] [CrossRef]
- Castiglioni, S.; Astolfi, P.; Conti, C.; Monaci, E.; Stefano, M.; Carloni, P. Morphological, Physicochemical and FTIR Spectroscopic Properties of Bee Pollen Loads from Different Botanical Origin. Molecules 2019, 24, 3974. [Google Scholar] [CrossRef]
- Isopescu, R.D.; Spulber, R.; Josceanu, A.M.; Mihaiescu, D.E.; Popa, O. Romanian bee pollen classification and property modelling. J. Apic. Res. 2020, 59, 443–451. [Google Scholar] [CrossRef]
- Barth, O.M.; Freitas, A.S.; Oliveira, E.S.; Silva, R.A.; Maester, F.M.; Andrella, R.R.S.; Cardozo, G.M.B.Q. Evaluation of the botanical origin of commercial dry bee pollen load batches using pollen analysis: A proposal for tehnical standardization. An. Acad. Bras. Ciências 2010, 82, 893–902. [Google Scholar] [CrossRef]
- Von der Ohe, W.; Persano Oddo, L.; Piana, L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynological analysis. Apidologie 2004, 35, 18–25. [Google Scholar] [CrossRef]
- Ricciardelli D’Albore, G. Mediterranean Melissopalynology; Università degli studi di Perugia, Facoltà di agraria, Istituto di entomologia Agrarian: Perugia, Italy, 1998. [Google Scholar]
- Von der Ohe, W.; von der Ohe, K. Pollen-und Nektargehalt von Rapblüten restaurierter Winterrapshybriden und Liniensorten. Dtsch. Bienen 2002, 10, 10–11. [Google Scholar]
- Freitas, A.S.; Arruda, V.A.S.; Almeida-Muradian, L.B.; Barth, O.M. The Botanical Profiles of Dried Bee Pollen Loads Collected by Apis mellifera (Linnaeus) in Brazil. Sociobiology 2013, 60, 56–64. [Google Scholar] [CrossRef][Green Version]
- AOAC 969.38. Moisture in Honey. In AOAC Official Methods, Suppl; Association of Official Analytical Chemists: Rockville, MD, USA, 2000; Chapter 44; p. 23. [Google Scholar]
- AOAC 923.03. Ash in Flour. In AOAC Official Methods, Suppl; Association of Official Analytical Chemists: Rockville, MD, USA, 2000; Chapter 32; p. 2. [Google Scholar]
- AOAC 2001.11. Protein in Animal Feed, Forage, Grain and Oilseeds. In AOAC Official Methods; Association of Official Analytical Chemists: Rockville, MD, USA, 2000; Chapter 4; pp. 29, 30, 30A, 30B. [Google Scholar]
- AOAC 963.15. Fat in Cacao Products. In AOAC Official Methods, Suppl; Association of Official Analytical Chemists: Rockville, MD, USA, 2000; Chapter 31; p. 10. [Google Scholar]
- Resolutions Pro, Version 5.3.0, FTIR Software; Agilent Technologies: Palo Alto, CA, USA, 2015.
- Origin, Version 8.1, Data Analysis and Graphing Software; OriginLab Corporation: Northampton, MA, USA, 2009.
- Statistica, Version 7, Data Analysis Software System; StatSoft Inc.: Tulsa, OK, USA, 2004.
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Max, J.J.; Chapados, C. Glucose and fructose hydrates in aqueous solution by IR spectroscopy. J. Phys. Chem. 2007, 111, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kliks, M.M.; Jun, S.; Jackson, M.; Li, Q.X. Rapid analysis of glucose, fructose, sucrose, and maltose in honeys from different geographic regions using Fourier transform infrared spectroscopy and multivariate analysis. J. Food Sci. 2010, 75, 208–214. [Google Scholar] [CrossRef]
- Svečnjak, L.; Prđun, S.; Rogina, J.; Bubalo, D.; Jerković, I. Characterization of Satsuma mandarin (Citrus unshiu Marc.) nectar-to-honey transformation pathway using FTIR-ATR spectroscopy. Food Chem. 2017, 232, 286–294. [Google Scholar] [CrossRef] [PubMed]
- De Melo, A.A.M.; Estevinho, M.L.M.F.; Sattler, J.A.G.; Souza, B.R.; da Silva Freitas, A.; Barth, O.M.; Almeida-Muradian, L.B. Effect of prcessing conditions on characteristics of dehydrated bee-pollen and correlation between quality parameters. LWT Food Sci. Technol. 2016, 65, 808–815. [Google Scholar] [CrossRef]
- Isik, A.; Ozdemir, M.; Doymaz, I. Effect of hot air drying on quality characteristics and physicochemical properties of bee pollen. Food Sci. Technol. 2019, 39, 224–231. [Google Scholar] [CrossRef]
- Siuda, M.; Wilde, J.; Bak, T. The effect of various storage methods on organoleptic quality of bee pollen loads. J. Apic. Sci. 2012, 56, 71–79. [Google Scholar] [CrossRef]
- Castagna, A.; Benelli, G.; Conte, G.; Sgherri, C.; Signorini, F.; Nicolella, C.; Ranieri, A.; Canale, A. Drying Techniques and Storage: Do They Affect the Nutritional Value of Bee-Collected Pollen? Molecules 2020, 25, 4925. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Bayram, N.E.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Asmae, E.G.; Nawal, E.M.; Bakour, M.; Lyoussi, B. Moroccan Monofloral Bee Pollen: Botanical Origin, Physicochemical Characterization, and Antioxidant Activities. J. Food Qual. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Formicki, G.; Greń, A.; Stawarz, R.; Zyśk, B.; Gał, A. Metal content in honey, propolis, wax, and bee pollen and implications for metal pollution monitoring. Pol. J. Environ. Stud. 2013, 22, 99–106. [Google Scholar]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Liolios, V.; Tananaki, C.; Papaioannou, A.; Kanelis, D.; Rodopoulou, M.A.; Argena, A. Mineral content in monofloral bee pollen: Investigation of the effect of the botanical and geographical origin. J. Food Meas. Charact. 2019, 13, 1674–1682. [Google Scholar] [CrossRef]
- Carpes, S.T.; Cabral, I.S.R.; Luz, C.F.P.; Capeletti, J.P.; Alencar, S.M.; Masson, M.L. Palynological and physicochemical characterization of Apis mellifera L. bee pollen in the Southern region of Brazil. J. Food Agric. Environ. 2009, 7, 667–673. [Google Scholar]
- Nogueira, C.; Iglesias, A.; Feás, X.; Estevinho, L. Commercial bee pollen with different geographical origins: A comprehensive approach. Int. J. Mol. Sci. 2012, 13, 11173–11187. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Barać, M.B.; Stanojević, S.P.; Milojković-Opsenica, D.M.; Tešić, Ž.L.J.; Šikoparija, B.; Radišić, P.; Prentović, M.; Pešić, M.B. Physicochemical composition and techno-functional properties of bee pollen collected in Serbia. LWT Food Sci. Technol. 2015, 62, 301–309. [Google Scholar] [CrossRef]
- Barajas, J.; Cortes-Rodriguez, M.; Rodríguez-Sandoval, E. Effect of temperature on the drying process of bee pollen from two zones of Colombia. J. Food Process Eng. 2012, 35, 134–148. [Google Scholar] [CrossRef]
- Taha, E.K.A.; Al-Kahtani, S.; Taha, R. Protein content and amino acids composition of bee-pollens from major floral sources in Al-Ahsa, eastern Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 232–237. [Google Scholar] [CrossRef]
- Aličić, D.; Flanjak, I.; Ačkar, Đ.; Jašić, M.; Babić, J.; Šubarić, D. Physicochemical properties and antioxidant capacity of bee pollen collected in Tuzla Canton (B&H). J. Cent. Eur. Agric. 2020, 21, 42–50. [Google Scholar]
- Orzaez Villanueva, M.T.; Díaz Marquina, A.; Bravo Serrano, R.; Blazquez Abellán, G. The importance of bee-collected pollen in the diet: A study of its composition. Int. J. Food Sci. 2002, 53, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.M.; Rodrigues, S.; Pereira, A.P.; Feás, X. Portuguese bee pollen: Palynological study nutritional and microbiological evaluation. Int. J. Food Sci. 2012, 47, 429–435. [Google Scholar] [CrossRef]
- Liolios, V.; Tananaki, C.; Dimou, M.; Kanelis, D.; Goras, G.; Karazafiris, E.; Thrasyvoulou, A. Ranking pollen from bee plants according to their protein contribution to honey bees. J. Apic. Res. 2015, 54, 582–592. [Google Scholar] [CrossRef]
- Qian, W.L.; Khan, Z.; Watson, D.G.; Fearnley, J. Analysis of sugars in bee pollen and propolis by ligand exchange chromatography in combination with pulsed amperometric detection and mass spectrometry. J. Food Compost. Anal. 2008, 21, 78–83. [Google Scholar] [CrossRef]
- Bobis, O.; Marghitas, L.A.; Dezmirean, D.; Morar, O.; Bonta, V.; Chirila, F. Quality parameters and nutritional value of different commercial bee products. J. Anim. Sci. Biotechnol. 2010, 67, 91–96. [Google Scholar] [CrossRef]
- Martins, M.C.T.; Morgano, A.M.; Vicente, E.; Baggio, S.R.; Rodriguez-Amaya, D.B. Physicochemical composition of bee pollen from eleven Brazilian states. J. Apic. Res. 2011, 55, 107–116. [Google Scholar]
- Bertoncelj, J.; Polak, T.; Pucihar, T.; Lilek, N.; Kandolf Borovšak, A.; Korošec, M. Carbohydrate composition of Slovenian bee pollens. Int. J. Food Sci. Tech. 2018, 53, 1880–1888. [Google Scholar] [CrossRef]
- Lilek, N.; Pereyra Gonzales, A.; Božič, J.; Kandolf Borovšak, A.; Bertoncelj, J. Chemical composition and content of free tryptophan in Slovenian bee pollen. J. Food Nutr. Res. 2015, 54, 323–333. [Google Scholar]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błazejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Frigerio, C.; Lopes, J.; Bogdanov, S. What is the future of bee pollen. JAAS 2010, 2, 131–144. [Google Scholar] [CrossRef]
- Collin, S.; Vanhavre, T.; Bodart, E.; Bouseta, A. Heat treatment of pollens: Impact on their volatile flavor constituents. J. Agric. Food Chem. 1995, 43, 444–448. [Google Scholar] [CrossRef]
- Schmitt, T.; Herzner, G.; Weckerle, B.; Schreier, P.; Strohm, E. Volatiles of foraging honeybees Apis mellifera (Hymenoptera: Apidae) and their potential role as semiochemicals. Apidologie 2007, 38, 164–170. [Google Scholar] [CrossRef]
- Frankel, E. Lipid Oxidation; The Oily Press Ltd.: Dundee, UK, 1998. [Google Scholar]
- Bartelt, R.; Cossé, A.; Petroski, R.; Weaver, D. Cuticular hydrocarbons and novel alkenediol diacetates from wheat stem sawfly (Cephus cinctus): Natural oxidation to pheromone components. J. Chem. Ecol. 2002, 28, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Ogden, J.; Pardo, C.; Tchapla, A. Method development for the analysis of bee cuticular hydrocarbons and esters by capillary gas chromatograph. J. Chromatogr. Sci. 1998, 36, 287–292. [Google Scholar] [CrossRef][Green Version]
- Fröhlich, B.; Riederer, M.; Tautz, J. Honeybee discriminate cuticular waxes based on esters and polar components. Apidologie 2001, 32, 265–274. [Google Scholar] [CrossRef]
- Jerković, I. Volatile benzene derivatives as honey biomarkers. Synlett 2013, 24, 2331–2334. [Google Scholar] [CrossRef]
- De la Fuente, E.; Sanz, M.L.; Martínez-Castro, I.; Sanz, J.; Ruiz-Matute, A.I. Volatile and carbohydrate composition of rare unifloral honeys from Spain. Food Chem. 2007, 105, 84–93. [Google Scholar] [CrossRef]
- Jerković, I.; Kuś, P.M.; Tuberoso, C.I.G.; Šarolić, M. Phytochemical and physical–chemical analysis of Polish willow (Salix spp.) honey: Identification of the marker compounds. Food Chem. 2014, 145, 8–14. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Malenica Staver, M. Screening of Natural Organic Volatiles from Prunus mahaleb L. Honey: Coumarin and Vomifoliol as Nonspecific Biomarkers. Molecules 2011, 16, 2507–2518. [Google Scholar] [CrossRef]
- Mastelić, J.; Jerković, I.; Mesić, M. Volatile constituents from flowers, leaves, bark and wood of Prunus mahaleb L. Flavour Fragr. J. 2006, 21, 306–313. [Google Scholar] [CrossRef]
- Wen, Y.Q.; He, F.; Zhu, B.Q.; Lan, Y.B.; Pan, Q.H.; Li, C.Y.; Reeves, M.J.; Wang, J. Free and glycosidically bound aroma compounds in cherry (Prunus avium L.). Food Chem. 2014, 152, 29–36. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z. Volatile Composition Screening of Salix spp. Nectar Honey: Benzenecarboxylic Acids, Norisoprenoids, Terpenes, and Others. Chem. Biodivers. 2010, 7, 2309–2325. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Toia, R.F. Biosynthesis of 6-Methylhept-5-en-2-one in the Australian Meat Ant, Iridomyrmex purpureus. J. Nat. Prod. 1989, 52, 63–66. [Google Scholar] [CrossRef]
- Ustun, O.; Sezik, E.; Kurkcuoglu, M.; Baser, K.H.C. Study of The Essential Oil Composition Of Pinus Sylvestris From Turkey. Chem. Nat. Compd. 2006, 42, 26–31. [Google Scholar] [CrossRef]
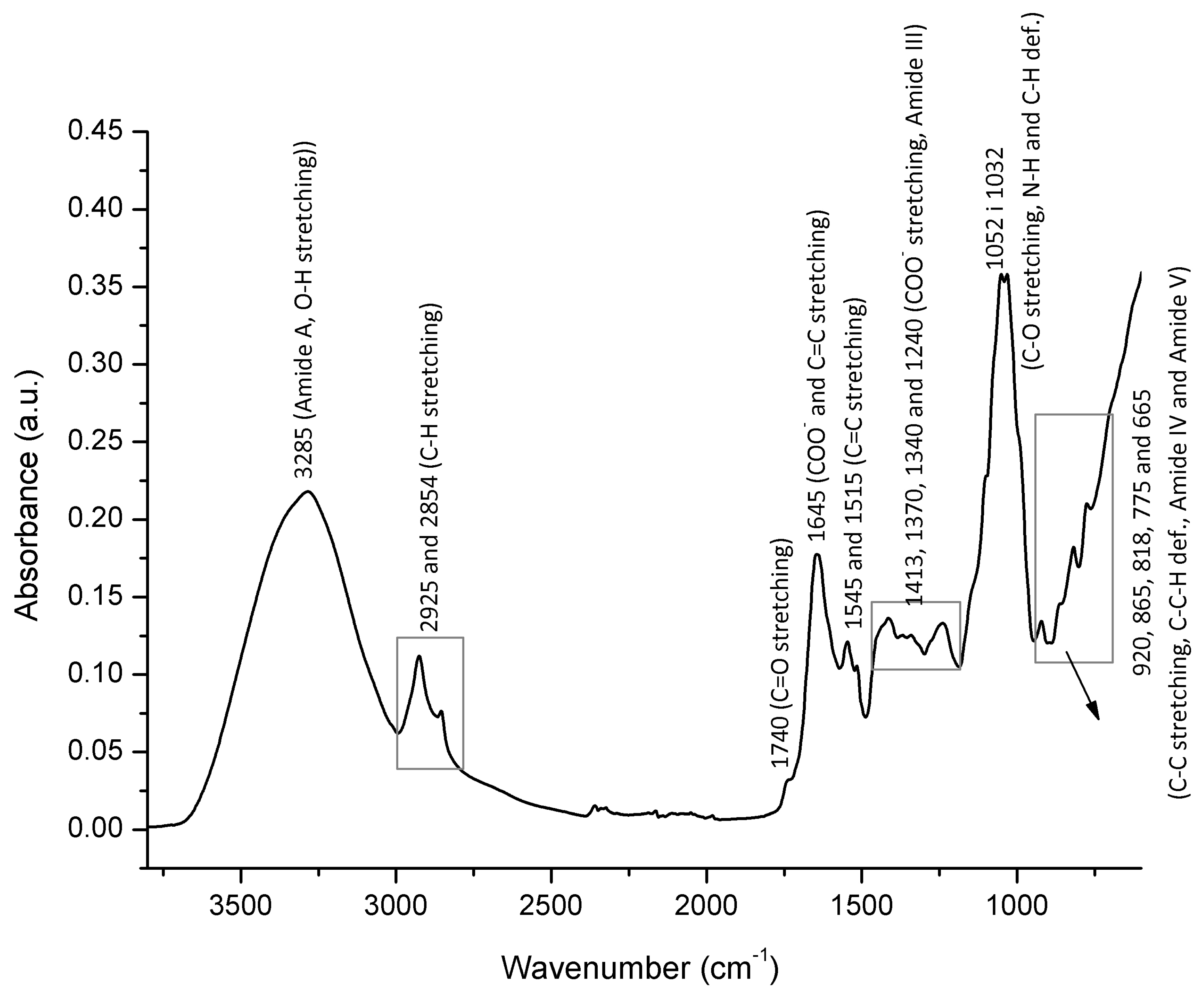
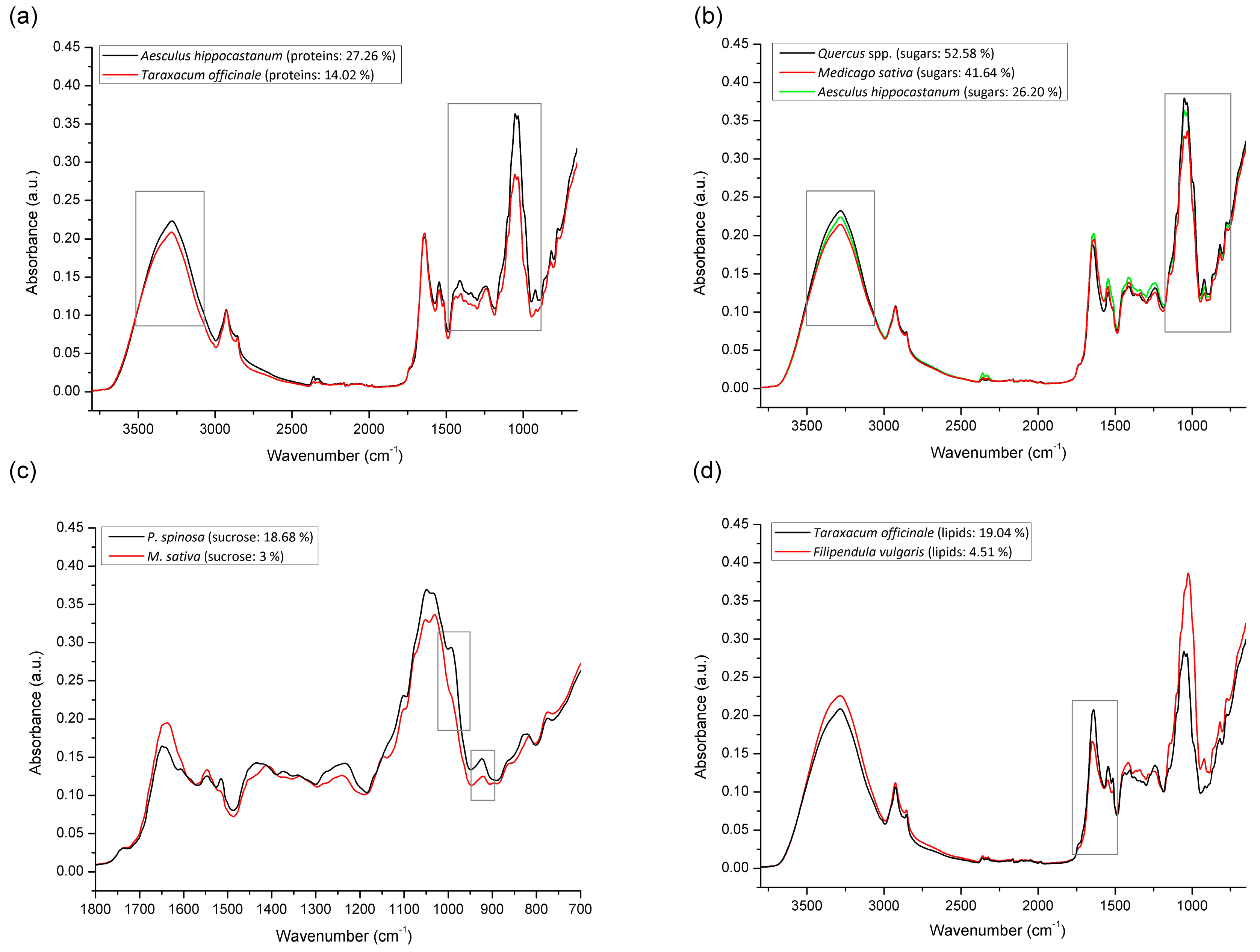

| Region | Botanical Origin |
|---|---|
| Continental region–CR | willow (Salix spp.), oak (Quercus spp.), horse chestnut (Aesculus hippocastanum L.), blackthorn (Prunus spinosa L.), common dandelion (Taraxacum officinale L.), viburnum (Viburnum spp.), Persian/English walnut (Juglans regia L.), common ash (Fraxinus excelsior L.), rough hawk’s beard (Crepis biennis L.), meadow buttercup (Ranunculus acris L.), chives (Allium schoenoprasum L.), Scots pine (Pinus sylvestris L.), common poppy (Papaver rhoeas L.), common dogwood (Cornus sanguinea L.), black locust (Robinia pseudoacacia L.), blackberry (Rubus spp.) |
| Mountain region–MR | blackthorn (Prunus spinosa L.), common dandelion (Taraxacum officinale L.), wild cherry (Prunus avium L.), willow (Salix spp.), rough hawk’s beard (Crepis biennis L.), downy oak (Quercus pubescens Willd.), Persian/English walnut (Juglans regia L.), Scots pine (Pinus sylvestris L.), dropwort (Filipendula vulgaris Moench), alfalfa (Medicago sativa L.), fiddleneck (Phacelia tanacetifolia Benth.) |
| Adriatic region–AR | rough hawk’s beard (Crepis biennis L.), downy oak (Quercus pubescens Willd.), mahaleb cherry/St. Lucie’s cherry (Prunus mahaleb L.), common poppy (Papaver rhoeas L.), perfoliate alexanders (Smyrnium perfoliatum L.), yellow sweet clover (Melilotus officinalis (L.) Pall.), spiny plumeless thistle (Carduus acanthoides L.), mouse-ear chickweed (Cerastium spp.), wild/yellow mignonette (Reseda lutea L.), bladder campion (Silene vulgaris (Moench) Garcke), apple (Malus spp.) |
| Sample | Moisture | Ash | Proteins | Total Lipids | Total Sugars | Fructose | Glucose | Sucrose | Maltose | Melezitose | Raffinose | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Continental region Multifloral | mCR1 | 15.940 ± 0.028 | 2.796 ± 0.034 | 23.19 ± 0.16 | 8.536 ± 0.059 | 43.25 ± 0.69 | 10.79 ± 0.12 | 8.915 ± 0.081 | 22.04 ± 0.00 | 0.650 ± 0.025 | 0.853 ± 0.057 | - |
| mCR2 | 16.045 ± 0.021 | 2.561 ± 0.051 | 19.20 ± 0.57 | 9.47 ± 0.10 | 38.76 ± 1.14 | 13.99 ± 0.20 | 11.913 ± 0.010 | 10.99 ± 0.96 | 1.555 ± 0.030 | 0.302 ± 0.039 | - | |
| mCR3 | 15.010 ± 0.127 | 1.75 ± 0.00 | 14.73 ± 0.17 | 18.36 ± 0.28 | 49.361 ± 0.020 | 19.69 ± 0.36 | 15.87 ± 0.21 | 8.91 ± 0.41 | 3.116 ± 0.042 | 1.54 ± 0.11 | 0.23 ± 0.11 | |
| mCR4 | 22.395 ± 0.049 | 2.577 ± 0.055 | 16.75 ± 0.23 | 9.928 ± 0.010 | 45.01 ± 0.29 | 20.05 ± 0.69 | 14.94 ± 0.16 | 5.87 ± 0.66 | 3.85 ± 0.15 | 0.298 ± 0.045 | - | |
| mCR5 | 18.325 ± 0.049 | 2.736 ± 0.061 | 17.24 ± 0.12 | 8.74 ± 0.22 | 45.872 ± 0.061 | 20.03 ± 0.38 | 14.05 ± 0.31 | 8.30 ± 0.12 | 3.21 ± 0.53 | 0.267 ± 0.016 | 0.02 ± 0.00 | |
| mCR6 | 16.050 ± 0.057 | 2.752 ± 0.034 | 18.91 ± 0.14 | 8.827 ± 0.051 | 44.14 ± 1.11 | 19.09 ± 0.55 | 14.61 ± 0.67 | 8.52 ± 0.23 | 1.878 ± 0.074 | - | 0.055 ± 0.041 | |
| Mean | 17.29 ± 0.06 | 2.53 ± 0.04 | 18.34 ± 0.23 | 10.64 ± 0.12 | 44.40 ± 5.55 | 17.27 ± 0.38 | 13.38 ± 0.24 | 10.77 ± 0.40 | 2.38 ± 0.14 | 0.65 ± 0.05 | 0.10 ± 0.05 | |
| Minimum | 15.01 ±0.13 | 1.75 ± 0.00 | 14.73 ±0. 17 | 8.54 ± 0.06 | 38.75 ± 1.14 | 10.79 ± 0.12 | 8.92 ± 0.08 | 5.87 ± 0.66 | 0.65 ± 0.03 | 0.27 ± 0.02 | 0.02 ± 0.00 | |
| Maximum | 22.40 ± 0.05 | 2.80 ± 0.03 | 23.19 ± 0.16 | 18.36 ± 0.28 | 49.36 ± 0.02 | 20.05 ± 0.69 | 15.87 ± 0.21 | 22.04 ± 0.00 | 3.85 ± 0.15 | 1.54 ± 0.11 | 0.23 ± 0.08 | |
| Continental region Unifloral | T. officinale | 21.395 ± 0.035 | 1.151 ± 0.010 | 14.019 ± 0.011 | 19.038 ± 0.027 | 43.61 ± 0.20 | 18.53 ± 0.31 | 18.803 ± 0.025 | 1.615 ± 0.023 | 4.33 ± 0.10 | 0.247 ± 0.027 | 0.085 ± 0.013 |
| Salix spp. | 15.27 ± 0.11 | 2.971 ± 0.021 | 20.409 ± 0.083 | 7.475 ± 0.029 | 49.49 ± 0.53 | 16.71 ± 0.37 | 13.95 ± 0.36 | 15.09 ± 0.13 | 1.58 ± 0.17 | 2.154 ± 0.053 | 0.01 ± 0.00 | |
| P. spinosa | 14.005 ± 0.035 | 2.925 ± 0.025 | 23.93 ± 0.80 | 8.57 ± 0.12 | 41.77 ± 1.84 | 10.97 ± 0.12 | 9.06 ± 0.68 | 20.33 ± 0.51 | 0.54 ± 0.11 | 0.874 ± 0.056 | - | |
| Viburnum spp. | 13.795 ± 0.011 | 2.836 ± 0.025 | 23.52 ± 0.65 | 8.32 ± 0.11 | 45.52 ± 0.24 | 14.31 ± 0.38 | 11.23 ± 0.45 | 17.32 ± 0.82 | 1.12 ± 0.34 | 1.540 ± 0.079 | - | |
| A. hippocastanum | 14.175 ± 0.078 | 2.94 ± 0.11 | 27.26 ± 0.31 | 6.199 ± 0.082 | 26.20 ± 1.05 | 10.87 ± 0.45 | 8.96 ± 0.59 | 5.196 ± 0.016 | 0.95 ± 0.00 | 0.224 ± 0.078 | - | |
| Quercus spp. | 16.39 ± 0.00 | 2.918 ± 0.034 | 21.08 ± 0.33 | 7.98 ± 0.12 | 52.58 ± 0.60 | 17.75 ± 0.23 | 14.644 ± 0.013 | 15.79 ± 0.43 | 1.648 ± 0.041 | 2.154 ± 0.046 | 0.596 ± 0.079 | |
| J. regia | 13.900 ± 0.042 | 1.829 ± 0.025 | 14.367 ± 0.082 | 17.04 ± 0.26 | 50.20 ± 0.19 | 19.875 ± 0.060 | 16.001 ± 0.058 | 9.582 ± 0.088 | 2.79 ± 0.13 | 1.688 ± 0.018 | 0.265 ± 0.075 | |
| P. rhoeas | 18.150 ± 0.028 | 2.071 ± 0.026 | 22.72 ± 0.26 | 7.495 ± 0.043 | 49.96 ± 0.42 | 25.653 ± 0.091 | 18.101 ± 0.064 | 4.08 ± 0.33 | 2.13 ± 0.12 | - | - | |
| C. sanguinea | 16.580 ± 0.057 | 3.081 ± 0.034 | 17.74 ± 0.19 | 10.231 ± 0.042 | 40.14 ± 0.78 | 16.37 ± 0.58 | 12.22 ± 0.67 | 8.47 ± 0.59 | 2.70 ± 0.13 | 0.353 ± 0.037 | 0.03 ± 0.02 | |
| Mean | 15.96 ± 0.04 | 2.52 ± 0.03 | 20.56 ± 0.30 | 10.26 ± 0.09 | 44.39 ± 0.65 | 16.78 ± 0.29 | 13.66 ± 0.32 | 10.83 ± 0.33 | 1.98 ± 0.13 | 1.15 ± 0.05 | 0.20 ± 0.04 | |
| Minimum | 13.80 ± 0.01 | 1.15 ± 0.01 | 14.02 ± 0.01 | 6.20 ± 0.08 | 26.20 ± 1.05 | 10.87 ± 0.45 | 8.96 ± 0.59 | 1.62 ± 0.02 | 0.54 ± 0.11 | 0.22 ± 0.08 | 0.01 ± 0.00 | |
| Maximum | 21.40 ± 0.04 | 3.08 ± 0.03 | 27.26 ± 0.31 | 19.04 ± 0.03 | 52.58 ± 0.60 | 25.65 ± 0.09 | 18.80 ± 0.03 | 20.33 ± 0.51 | 4.33 ± 0.10 | 2.16 ± 0.05 | 0.60 ± 0.08 | |
| Mountain region Multifloral | mMR1 | 14.655 ± 0.064 | 2.888 ± 0.075 | 23.29 ± 0.17 | 8.887 ± 0.041 | 41.75 ± 1.93 | 13.96 ± 0.73 | 10.67 ± 1.27 | 15.85 ± 0.15 | 0.902 ± 0.060 | 0.368 ± 0.021 | - |
| mMR2 | 16.460 ± 0.014 | 2.45 ± 0.00 | 17.40 ± 0.26 | 13.71 ± 0.36 | 41.28 ± 1.43 | 16.38 ± 0.40 | 13.60 ± 0.56 | 8.74 ± 0.30 | 2.23 ± 0.12 | 0.327 ± 0.043 | - | |
| mMR3 | 13.780 ± 0.028 | 2.08 ± 0.00 | 17.31 ± 0.22 | 13.35 ± 0.43 | 49.42 ± 0.13 | 18.64 ± 0.38 | 14.302 ± 0.015 | 11.95 ± 0.28 | 3.129 ± 0.057 | 1.158 ± 0.010 | 0.242 ± 0.083 | |
| mMR4 | 22.210 ± 0.085 | 1.84 ± 0.00 | 15.59 ± 0.20 | 14.57 ± 0.14 | 51.29 ± 0.82 | 19.88 ± 0.36 | 18.70 ± 0.50 | 7.60 ± 0.33 | 4.98 ± 0.29 | - | 0.13 ± 0.00 | |
| mMR5 | 19.780 ± 0.042 | 2.375 ± 0.011 | 16.76 ± 0.20 | 6.13 ± 0.19 | 43.68 ± 1.04 | 18.841 ± 0.046 | 15.36 ± 0.94 | 5.75 ± 0.13 | 3.56 ± 0.10 | 0.010 ± 0.021 | 0.156 ± 0.022 | |
| mMR6 | 17.150 ± 0.085 | 2.25 ± 0.00 | 18.171 ± 0.043 | 6.94 ± 0.12 | 45.07 ± 0.71 | 18.40 ± 0.27 | 15.31 ± 0.25 | 8.41 ± 0.15 | 2.81 ± 0.39 | 0.017 ± 0.015 | 0.122 ± 0.041 | |
| Mean | 17.34 ± 0.05 | 2.33 ± 0.01 | 18.09 ± 0.18 | 10.60 ± 0.21 | 45.42 ± 1.01 | 17.68 ± 0.36 | 14.66 ±0.59 | 9.72 ± 0.22 | 2.94 ± 0.17 | 0.38 ± 0.02 | 0.16 ± 0.04 | |
| Minimum | 13.78 ± 0.03 | 1.84 ± 0.00 | 15.59 ± 0.20 | 6.13 ± 0.19 | 40.85 ± 1.93 | 13.96 ± 0.73 | 10.67 ± 1.27 | 5.75 ± 0.13 | 2.23 ± 0.12 | 0.01 ± 0.02 | 0.12 ± 0.04 | |
| Maximum | 22.21 ± 0.09 | 2.89 ± 0.08 | 23.29 ± 0.17 | 14.57 ± 0.14 | 51.29 ± 0.82 | 19.88 ± 0.36 | 18.70 ± 0.50 | 15.85 ± 0.15 | 4.98 ± 0.29 | 1.16 ± 0.01 | 0.24 ± 0.08 | |
| Mountain region Unifloral | P. spinosa | 11.295 ± 0.011 | 3.021 ± 0.016 | 23.719 ± 0.48 | 8.534 ± 0.080 | 42.32 ± 0.10 | 12.28 ± 0.23 | 10.61 ± 0.14 | 18.68 ± 0.43 | 0.438 ± 0.076 | 0.315 ± 0.033 | - |
| P. avium | 15.525 ± 0.021 | 3.81 ± 0.00 | 21.97 ± 0.58 | 9.10 ± 0.23 | 43.65 ± 0.82 | 12.39 ± 0.43 | 9.82 ± 0.23 | 17.84 ± 0.26 | 3.228 ± 0.046 | 0.206 ± 0.071 | 0.159 ± 0.017 | |
| Salix spp. | 16.760 ± 0.014 | 2.39 ± 0.00 | 17.35 ± 0.42 | 12.07 ± 0.19 | 45.56 ± 0.48 | 18.63 ± 0.53 | 16.01 ± 0.52 | 9.60 ± 0.21 | 1.17 ± 0.30 | 0.155 ± 0.025 | - | |
| T. officinale | 16.175 ± 0.049 | 1.18 ± 0.00 | 13.90 ± 0.17 | 17.531 ± 0.025 | 42.11 ± 1.12 | 19.19 ± 0.14 | 17.20 ± 0.41 | 3.79 ± 0.62 | 1.483 ± 0.086 | 0.36 ± 0.00 | 0.09 ± 0.13 | |
| Q. pubescens | 26.475 ± 0.106 | 2.047 ± 0.029 | 16.423 ± 0.067 | 11.55 ± 0.13 | 51.70 ± 0.11 | 24.21 ± 0.21 | 19.856 ± 0.025 | 4.09 ± 0.13 | 3.31 ± 0.23 | 0.05 ± 0.00 | 0.176 ± 0.028 | |
| J. regia | 20.515 ± 0.064 | 2.057 ± 0.019 | 16.39 ± 0.12 | 13.73 ± 0.31 | 49.26 ± 0.45 | 20.353 ± 0.010 | 18.286 ± 0.071 | 8.14 ± 0.36 | 1.756 ± 0.065 | 0.638 ± 0.022 | 0.18 ± 0.00 | |
| F. vulgaris | 17.680 ± 0.014 | 2.08 ± 0.00 | 14.33 ± 0.29 | 4.51 ± 0.11 | 45.06 ± 0.97 | 19.291 ± 0.036 | 16.02 ± 0.75 | 7.36 ± 0.00 | 2.22 ± 0.11 | 0.106 ± 0.099 | 0.061 ± 0.011 | |
| M. sativa | 16.57 ± 0.11 | 2.823 ± 0.059 | 23.56 ± 0.24 | 8.863 ± 0.025 | 41.64 ± 0.62 | 18.73 ± 0.12 | 14.14 ± 0.47 | 3.00 ± 0.10 | 5.480 ± 0.012 | - | 0.289 ± 0.086 | |
| P. tanacetifolia | 17.645 ± 0.021 | 2.714 ± 0.010 | 26.32 ± 0.11 | 5.847 ± 0.026 | 50.86 ± 0.69 | 24.284 ± 0.042 | 18.49 ± 0.22 | 4.35 ± 0.54 | 3.350 ± 0.088 | 0.300 ± 0.023 | 0.087 ± 0.066 | |
| Mean | 17.63 ± 0.05 | 2.46 ± 0.01 | 19.33 ± 0.28 | 11.76 ± 0.13 | 45.80 ± 0.60 | 18.82 ± 0.19 | 15.60 ± 0.32 | 8.54 ± 0.29 | 2.49 ± 0.11 | 0.27 ± 0.03 | 0.15 ± 0.05 | |
| Minimum | 11.30 ± 0.01 | 1.18 ± 0.00 | 13.90 ± 0.17 | 4.51 ± 0.11 | 41.64 ± 0.62 | 12.28 ± 0.23 | 9.82 ± 0.23 | 3.00 ± 0.10 | 0.44 ± 0.08 | 0.05 ± 0.00 | 0.06 ± 0.01 | |
| Maximum | 26.48 ± 0.11 | 3.81 ± 0.00 | 26.32 ± 0.11 | 17.53 ± 0.03 | 51.70 ± 0.11 | 24.28 ± 0.04 | 19.86 ± 0.03 | 18.68 ± 0.43 | 5.48 ± 0.01 | 0.64 ± 0.02 | 0.29 ± 0.09 | |
| Adriatic region Multifloral | mAR1 | 15.005 ± 0.049 | 2.49 ± 0.00 | 19.536 ± 0.075 | 8.883 ± 0.083 | 35.79 ± 0.31 | 14.23 ± 0.28 | 11.809 ± 0.061 | 8.50 ± 0.54 | 0.96 ± 0.11 | 0.286 ± 0.066 | 0.01 ± 0.00 |
| mAR2 | 22.84 ± 0.14 | 2.080 ± 0.027 | 17.211 ± 0.092 | 10.80 ± 0.24 | 46.66 ± 0.080 | 22.602 ± 0.019 | 16.799 ± 0.032 | 3.17 ± 0.13 | 3.881 ± 0.077 | - | 0.212 ± 0.012 | |
| mAR3 | 14.18 ± 0.23 | 2.26 ± 0.016 | 21.190 ± 0.041 | 8.52 ± 0.11 | 43.19 ± 0.64 | 23.48 ± 0.12 | 14.27 ± 0.48 | 2.62 ± 0.34 | 2.730 ± 0.026 | - | 0.097 ± 0.087 | |
| mAR4 | 19.31 ±0.12 | 2.06 ± 0.00 | 20.02 ± 0.10 | 9.790 ± 0.053 | 47.90 ± 0.25 | 23.88 ± 0.43 | 16.378 ± 0.018 | 5.15 ± 0.23 | 2.270 ± 0.015 | 0.137 ± 0.089 | 0.09 ± 0.00 | |
| Mean | 17.83 ± 0.13 | 2.22 ± 0.01 | 19.49 ± 0.08 | 9.50 ± 0.12 | 43.39 ± 0.32 | 21.05 ± 0.21 | 14.81 ± 0.15 | 4.86 ± 0.31 | 2.46 ± 0.06 | 0.21 ± 0.08 | 0.10 ± 0.02 | |
| Minimum | 14.18 ± 0.23 | 2.06 ± 0.00 | 17.21 ± 0.09 | 8.52 ± 0.11 | 35.79 ± 0.31 | 14.23 ± 0.28 | 11.81 ± 0.06 | 2.62 ± 0.34 | 0.96 ± 0.11 | 0.14 ± 0.09 | 0.01 ± 0.00 | |
| Maximum | 22.84 ± 0.14 | 2.49 ± 0.00 | 21.19 ± 0.04 | 10.80 ± 0.24 | 47.90 ± 0.25 | 23.88 ± 0.43 | 16.80 ± 0.03 | 8.50 ± 0.54 | 3.88 ± 0.08 | 0.29 ± 0.07 | 0.21 ± 0.01 | |
| Adriatic region Unifloral | C. biennis | 17.49 ± 0.17 | 1.563 ± 0.051 | 16.60 ± 0.00 | 14.49 ± 0.25 | 43.83 ± 1.12 | 20.08 ± 0.99 | 15.51 ± 0.80 | 4.45 ± 0.24 | 3.38 ± 0.90 | 0.23 ± 0.00 | 0.186 ± 0.010 |
| P. mahaleb | 12.745 ± 0.04 | 3.089 ± 0.024 | 22.23 ± 0.16 | 8.62 ± 0.13 | 43.32 ± 2.61 | 14.17 ± 0.83 | 11.95 ± 0.67 | 14.37 ± 0.78 | 2.55 ± 0.30 | 0.089 ± 0.032 | 0.194 ± 0.061 | |
| Q. pubescens | 16.14 ± 0.26 | 2.217 ± 0.014 | 18.12 ± 0.25 | 10.21 ± 0.11 | 46.73 ± 1.30 | 19.26 ± 0.56 | 14.55 ± 0.45 | 10.17 ± 0.34 | 2.23 ± 0.13 | 0.367 ± 0.021 | 0.149 ± 0.040 | |
| Mean | 15.46 ± 0.16 | 2.29 ± 0.03 | 18.98 ± 0.14 | 11.11 ± 0.16 | 44.63 ± 1.68 | 17.84 ± 0.79 | 14.00 ± 0.64 | 9.66 ± 0.45 | 2.72 ± 0.44 | 0.23 ± 0.02 | 0.18 ± 0.04 | |
| Minimum | 12.75 ± 0.04 | 1.56 ± 0.05 | 16.60 ± 0.00 | 8.62 ± 0.13 | 43.32 ± 2.61 | 14.17 ± 0.83 | 11.95 ± 0.67 | 4.45 ± 0.24 | 2.23 ± 0.13 | 0.09 ± 0.03 | 0.15 ± 0.04 | |
| Maximum | 17.49 ± 0.17 | 3.09 ± 0.02 | 22.23 ± 0.16 | 14.49 ± 0.25 | 46.73 ± 1.30 | 20.08 ± 0.99 | 15.51 ± 0.80 | 14.37 ± 0.78 | 3.38 ± 0.90 | 0.37 ± 0.02 | 0.19 ± 0.06 | |
| p | 0.92 | 0.80 | 0.63 | 0.85 | 0.75 | 0.52 | 0.72 | 0.41 | 0.28 | 0.04 | 0.80 |
| No. | Compound | Sample * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RI | A | B | C | D | E | F | G | H | I | ||
| 1. | Butanal | <900 | - | - | - | - | - | - | - | 20.96 | 5.54 |
| 2. | 3-Methylbutanal | <900 | - | - | - | 3.29 | - | - | 3.38 | 8.85 | 2.54 |
| 3. | 2-Methylbutanal | <900 | - | - | - | - | - | - | 4.59 | - | 2.43 |
| 4. | 3-Hydroxybutan-2-one (acetoin) | <900 | - | - | - | - | 5.06 | - | - | - | - |
| 5. | 3-Methylbutan-1-ol | <900 | - | - | 2.54 | - | - | - | 1.46 | - | 0.65 |
| 6. | Dimethyl disulfide | <900 | - | - | - | 2.49 | - | - | - | - | - |
| 7. | Pentanal | <900 | 0.18 | - | - | - | 4.21 | - | 2.08 | 4.74 | 0.79 |
| 8. | 3-Methylpentanal ** | <900 | - | - | - | - | - | - | - | - | 0.34 |
| 9. | Butanoic acid | <900 | 3.33 | 4.51 | - | - | - | - | - | - | - |
| 10. | Butan-1,3-diol | <900 | - | - | - | 2.72 | - | - | - | - | - |
| 11. | Butan-2,3-diol | <900 | - | - | - | 3.37 | - | - | - | - | - |
| 12. | Hexanal | <900 | 0.84 | 1.14 | 1.64 | 3.20 | 4.59 | 7.34 | 2.11 | 5.40 | 1.55 |
| 13. | (E)-Hex-2-enal | <900 | - | - | - | - | - | - | - | - | - |
| 14. | Hexan-1-ol | <900 | - | - | 4.95 | - | - | - | - | - | - |
| 15. | Cyclopent-2-ene-1,4-dione | <900 | 1.06 | 2.23 | - | 6.14 | - | - | - | - | - |
| 16. | Heptanal | 904 | 0.75 | 1.97 | - | 2.12 | - | - | 1.21 | - | 0.78 |
| 17. | Methyl allyl disulfide | 925 | - | - | - | 3.07 | - | - | - | - | - |
| 18. | Methyl hexanoate | 928 | - | - | - | - | 1.90 | - | - | - | - |
| 19. | α-Pinene | 942 | - | - | 1.85 | - | - | - | - | - | - |
| 20. | Methyl (Z)-prop-1-enyl disulfide | 948 | - | - | - | 1.02 | - | - | - | - | - |
| 21. | γ-Valerolactone | 960 | 1.59 | 1.61 | - | - | - | - | - | - | - |
| 22. | Benzaldehyde | 969 | 1.09 | 1.61 | - | 29.11 | 1.44 | - | 40.34 | 18.68 | 27.46 |
| 23. | Dimethyl trisulfide | 979 | - | - | - | 3.83 | - | - | - | - | - |
| 24. | β-Pinene | 985 | - | - | 1.69 | - | - | - | - | - | - |
| 25. | Hexanoic acid | 986 | 1.20 | 3.15 | - | - | 24.46 | - | - | - | - |
| 26. | 6-Methylhept-5-en-2-one | 989 | 12.39 | 21.45 | - | 3.16 | - | 8.61 | 0.51 | 3.07 | - |
| 27. | Octanal | 1005 | - | - | - | - | - | - | - | 1.46 | - |
| 28. | δ-3-Carene | 1016 | - | - | 6.72 | - | - | - | - | - | - |
| 29. | (E,E)-Hepta-2,4-dienal | 1015 | - | - | - | - | - | - | - | - | - |
| 30. | p-Cymene | 1031 | - | - | 1.85 | - | - | - | - | - | - |
| 31. | 2-Ethylhexan-1-ol | 1035 | - | - | - | - | - | - | 1.16 | 6.15 | 1.38 |
| 32. | γ-Terpinene | 1036 | - | - | 7.02 | - | - | - | |||
| 33. | Benzyl alcohol | 1046 | 0.23 | - | - | 1.21 | - | - | - | - | 3.28 |
| 34. | Phenylacetaldehyde | 1050 | - | - | - | - | - | - | 1.16 | - | 3.07 |
| 35. | (E)-β-Ocymene | 1053 | - | - | - | - | - | 5.04 | - | - | - |
| 36. | (E,E)-Octa-3,5-dien-2-one | 1075 | - | - | - | 0.41 | 2.55 | - | - | 4.74 | - |
| 37. | Diallyl disulfide | 1084 | - | - | - | 2.77 | - | - | - | - | - |
| 38. | Nonan-2-one | 1093 | - | - | - | - | - | - | - | - | - |
| 39. | (E,Z)-Octa-3,5-dien-2-one | 1096 | - | - | - | 0.30 | - | - | - | 2.68 | - |
| 40. | Undecane | 1100 | - | - | 2.03 | - | - | - | 0.53 | 1.43 | - |
| 41. | Linalool | 1103 | - | - | - | - | - | - | 2.25 | - | 4.06 |
| 42. | Nonanal | 1105 | 8.92 | 15.22 | 46.70 | 14.38 | 8.37 | 59.76 | 6.98 | 7.84 | 7.53 |
| 43. | 2-Phenylethanol | 1122 | - | - | - | - | - | - | 1.35 | - | 1.68 |
| 44. | Methyl octanoate | 1128 | - | - | - | - | 4.46 | - | - | - | - |
| 45. | Lilac aldehyde A | 1147 | - | - | - | - | - | - | 2.57 | - | - |
| 46. | Phenylacetonitrile | 1148 | - | - | - | - | - | - | - | - | 0.44 |
| 47. | Lilac aldehyde B | 1155 | - | - | - | - | - | - | 2.94 | - | - |
| 48. | Lilac aldehyde D | 1169 | - | - | - | - | - | - | 1.27 | - | - |
| 49. | Octanoic acid | 1186 | - | - | - | - | 8.78 | - | - | - | - |
| 50. | Decanal | 1207 | - | 0.92 | 7.74 | - | - | - | - | - | - |
| 51. | Verbenone | 1211 | - | - | - | - | - | - | 13.11 | - | 21.44 |
| 52. | 4-Methoxybenzaldehyde | 1261 | - | - | - | 0.07 | - | - | - | - | - |
| 53. | Tetradecane | 1400 | 0.48 | 1.05 | - | - | - | - | - | - | 0.83 |
| 54. | 1,3,5-Trimethoxybenzene | 1411 | 0.62 | 1.30 | - | 1.71 | - | - | - | - | - |
| 55. | (E)-Geranyl acetone | 1455 | 5.46 | 16.19 | - | - | - | - | - | - | - |
| 56. | Pentadecane | 1500 | - | 9.76 | - | 9.63 | - | - | - | - | - |
| 57. | β-Tumerone | 1668 | - | 0.87 | - | - | - | - | - | - | - |
| 58. | Heptadecane | 1700 | 0.62 | 1.41 | - | - | - | - | - | - | - |
| 59. | Nonadecane | 1900 | 1.66 | 3.17 | - | - | - | 3.26 | - | - | - |
| 60. | (E,E)-Geranyl linalool | 2038 | 49.27 | - | - | - | - | - | - | - | - |
| 61. | Heneicosane | 2100 | 2.14 | 5.42 | 2.28 | - | 20.17 | - | - | - | - |
| 62. | Docosane | 2200 | 0.17 | - | - | - | - | - | - | - | - |
| No. | Compound | Sample * | ||||||
|---|---|---|---|---|---|---|---|---|
| RI | A | B | C | D | E | F | ||
| 1. | Acetic acid | <900 | - | - | - | 10.10 | 21.34 | 21.69 |
| 2. | Ethanol | <900 | - | - | - | 6.93 | 7.17 | 9.44 |
| 3. | Butanal | <900 | - | - | 4.66 | - | - | - |
| 4. | 3-Methylbutanal | <900 | - | 3.73 | 4.57 | - | - | - |
| 5. | 2-Methylbutanal | <900 | - | 3.71 | 5.42 | - | - | - |
| 6. | 3-Hydroxybutan-2-one (acetoin) | <900 | - | 5.47 | 3.05 | - | 11.87 | 7.07 |
| 7. | 3-Methylbutan-1-ol | <900 | - | 3.08 | 2.84 | 0.29 | - | - |
| 8. | Pentanal | <900 | - | 4.14 | - | - | - | 11.85 |
| 9. | Butane-1,3-diol | <900 | - | - | - | - | 1.52 | - |
| 10. | Butane-2,3-diol | <900 | - | - | - | - | 1.80 | - |
| 11. | (E)-Hex-2-enal | <900 | - | - | - | 0.12 | - | 0.94 |
| 12. | 3-Methylpentanal ** | <900 | - | - | 10.37 | - | - | - |
| 13. | Hexanal | <900 | 0.40 | 7.02 | 1.62 | 1.22 | 2.89 | - |
| 14. | Hexan-1-ol | <900 | - | - | - | 1.46 | 1.62 | 2.63 |
| 15. | Heptanal | 904 | - | 5.17 | - | 0.49 | - | 5.37 |
| 16. | γ-Butyrolactone | 922 | 0.09 | - | - | 0.43 | 0.66 | - |
| 17. | Methyl hexanoate | 928 | - | - | 0.53 | - | 0.48 | 0.60 |
| 18. | α-Pinene | 942 | - | - | - | 0.45 | - | - |
| 19. | 3-Methylpentanoic acid | 954 | - | - | 3.12 | - | - | - |
| 20. | γ-Valerolactone | 960 | - | - | - | 0.37 | - | - |
| 21. | Benzaldehyde | 969 | 0.65 | 11.93 | 28.98 | 0.63 | 2.73 | 1.39 |
| 22. | β-Pinene | 985 | - | - | - | 0.94 | 0.89 | - |
| 23. | 6-Methylhept-5-en-2-one | 989 | 0.25 | 2.68 | 2.20 | 0.80 | 2.05 | 2.96 |
| 24. | β-Myrcene | 993 | - | - | - | 1.46 | - | - |
| 25. | Octanal | 1005 | 0.14 | 1.90 | 0.48 | 0.55 | 0.91 | 1.35 |
| 26. | (E,E)-Hepta-2,4-dienal | 1015 | - | - | - | 0.34 | 0.39 | 2.09 |
| 27. | β-Phellandrene | 1034 | - | - | - | 5.69 | - | - |
| 28. | 2-Ethylhexan-1-ol | 1035 | - | - | - | 0.11 | - | - |
| 29. | 1,8-Cineole | 1039 | - | - | - | - | 0.77 | - |
| 30. | Benzyl alcohol | 1046 | - | - | 1.17 | - | - | - |
| 31. | Phenylacetaldehyde | 1050 | 1.04 | 0.38 | 3.95 | 0.25 | 2.28 | 1.35 |
| 32. | (E)-β-Ocymene | 1053 | - | 0.92 | - | - | - | - |
| 33. | (E,E)-Octa-3,5-diene-2-one | 1075 | - | - | 0.54 | 2.46 | 3.52 | 5.08 |
| 34. | (E,Z)-Octa-3,5-diene-2-one | 1096 | - | - | - | 0.45 | 0.44 | 1.85 |
| 35. | Linalool | 1103 | - | - | - | - | 0.91 | - |
| 36. | Nonanal | 1105 | 1.53 | 9.28 | 1.24 | 8.09 | 3.81 | 15.57 |
| 37. | Methyl octanoate | 1128 | - | - | 0.89 | - | 1.87 | 0.38 |
| 38. | Lilac aldehyde A | 1147 | 14.84 | 6.06 | 3.85 | - | - | - |
| 39. | Lilac aldehyde B | 1155 | 29.30 | 10.43 | 6.42 | - | - | - |
| 40. | Lilac aldehyde D | 1169 | 13.37 | 3.94 | 2.48 | - | - | - |
| 41. | Ethyl octanoate | 1198 | - | - | - | - | 0.48 | - |
| 42. | Dodecane | 1200 | - | - | 0.66 | - | 0.80 | - |
| 43. | Lilac alcohol A | 1205 | 11.37 | 0.24 | - | - | - | - |
| 44. | Decanal | 1207 | - | - | - | 1.11 | - | - |
| 45. | Verbenone | 1211 | - | - | - | - | 1.22 | - |
| 46. | Lilac alcohol B | 1214 | 13.76 | 0.59 | - | - | - | - |
| 47. | β-Cyclocitral | 1223 | 1.90 | - | - | - | - | - |
| 48. | Lilac alcohol D | 1232 | 4.21 | - | - | - | - | - |
| 49. | Bornyl acetate | 1287 | - | - | - | 0.82 | - | - |
| 50. | Tetradecane | 1400 | - | - | - | 0.61 | 0.36 | 0.39 |
| 51. | α-Ionone | 1429 | 0.53 | - | - | - | - | - |
| 52. | Pentadecane | 1500 | - | 10.29 | - | 51.16 | - | - |
| 53. | (E)-β-Ionone | 1512 | 1.43 | - | - | - | - | - |
| 54. | Heneicosane | 2100 | - | - | - | - | 18.23 | - |
| No. | Compound | Sample * | ||||||
|---|---|---|---|---|---|---|---|---|
| RI * | A | B | C | D | E | F | ||
| 1. | Acetaldehyde | <900 | 5.63 | - | - | - | - | - |
| 2. | Ethanol | <900 | 4.39 | 3.01 | 3.14 | 2.99 | - | - |
| 3. | Dimetyl sulfide | <900 | - | 4.30 | - | 6.48 | - | - |
| 4. | Acetic acid | <900 | 6.93 | 9.30 | 9.27 | 19.09 | - | - |
| 5. | 3-Methylbutanal | <900 | 2.87 | 1.86 | - | - | - | - |
| 6. | 3-Hydroxy-butan-2-one (acetoin) | <900 | 5.05 | 3.99 | 2.68 | 3.67 | 19.41 | - |
| 7. | Pentanal | <900 | - | 1.46 | 1.24 | - | 5.83 | - |
| 8. | Butane-1,3-diol | <900 | 0.53 | 1.36 | 0.27 | 1.17 | - | - |
| 9. | Butane-2,3-diol | <900 | 0.62 | 1.23 | 0.43 | 1.12 | - | - |
| 10. | Hexanal | <900 | 4.88 | 3.56 | 2.54 | 2.45 | 5.22 | 1.44 |
| 11. | 4-Methylpentan-1-ol | <900 | - | - | 2.75 | - | - | - |
| 12. | (E)-Hex-2-enal | <900 | 0.30 | - | 0.24 | 0.52 | - | - |
| 13. | Hexan-1-ol | <900 | - | 0.67 | 0.15 | - | - | - |
| 14. | Heptan-2-one | <900 | - | - | 2.08 | - | - | - |
| 15. | Heptan-2-ol | 902 | - | - | 30.63 | - | - | - |
| 16. | Heptanal | 904 | 4.46 | 2.19 | - | 1.50 | - | - |
| 17. | γ-Butyrolactone | 922 | - | - | 2.13 | 0.34 | 0.93 | 0.30 |
| 18. | Methyl hexanoate | 928 | - | - | - | 0.45 | 0.70 | - |
| 19. | α-Pinene | 942 | - | 0.39 | - | 0.19 | - | - |
| 20. | Benzaldehyde | 969 | 4.88 | 6.36 | 3.62 | 5.60 | 3.14 | 2.09 |
| 21. | Sabinene | 982 | - | - | - | - | 1.21 | - |
| 22. | β-Pinene | 985 | 2.16 | 2.37 | 0.86 | 1.57 | - | - |
| 23. | Hexanoic acid | 986 | - | - | - | - | - | - |
| 24. | 6-Methylhept-5-en-2-one | 989 | 5.81 | 8.33 | 1.91 | 9.56 | 2.50 | 1.53 |
| 25. | β-Myrcene | 993 | - | - | - | - | - | - |
| 26. | Octanal | 1005 | - | 0.89 | 0.33 | - | - | 0.57 |
| 27. | (E,E)-Hepta-2,4-dienal | 1015 | - | - | 0.65 | - | - | |
| 28. | 2-Ethylhexan-1-ol | 1035 | - | - | - | 1.23 | 5.41 | 0.87 |
| 29. | 1,8-Cineole | 1039 | - | 0.99 | - | 2.45 | - | - |
| 30. | Benzyl alcohol | 1046 | - | 1.04 | - | 3.26 | - | - |
| 31. | Phenylacetaldehyde | 1050 | 5.46 | 5.69 | 3.67 | 11.79 | 1.57 | 0.44 |
| 32. | (E)-β-Ocymene | 1053 | 4.06 | 5.27 | - | - | - | |
| 33. | (E,E)-Octa-3,5-diene-2-one | 1075 | 0.42 | 2.40 | 5.03 | 1.77 | 1.82 | 0.33 |
| 34. | (E,Z)-Octa-3,5-diene-2-one | 1096 | 0.30 | 0.29 | - | - | - | - |
| 35. | Undecane | 1100 | - | - | - | - | 0.98 | 0.54 |
| 36. | Linalool | 1103 | 1.79 | 1.94 | 0.45 | 0.87 | - | - |
| 37. | Nonanal | 1105 | 28.67 | 15.53 | 4.60 | 8.14 | 3.65 | 5.19 |
| 38. | β-Thujone | 1109 | - | - | - | 1.21 | - | - |
| 39. | 2-Phenylethanol | 1122 | - | 2.60 | - | 0.91 | - | - |
| 40. | Methyl octanoate | 1128 | - | 0.99 | - | 0.64 | 4.35 | - |
| 41. | Lilac aldehyde A | 1146 | - | - | 3.12 | - | 5.61 | 14.69 |
| 42. | Lilac aldehyde B | 1155 | - | - | 5.20 | - | 9.00 | 25.04 |
| 43. | Lilac aldehyde D | 1169 | - | - | 2.16 | - | 3.28 | 14.51 |
| 44. | Ethyl octanoate | 1198 | - | - | - | - | 1.68 | - |
| 45. | Dodecane | 1200 | - | 0.51 | 0.39 | 0.40 | 0.62 | - |
| 46. | Lilac alcohol A | 1205 | - | - | - | - | - | 2.99 |
| 47. | Decanal | 1207 | 1.35 | 0.77 | - | - | - | - |
| 48. | Verbenone | 1211 | - | - | - | - | 1.09 | - |
| 49. | Lilac alcohol B | 1214 | - | - | 0.24 | - | - | 5.32 |
| 50. | β-Cyclocitral | 1223 | - | - | - | - | - | 3.28 |
| 51. | Lilac alcohol D | 1232 | - | - | - | - | - | 1.10 |
| 52. | Nerol | 1234 | - | - | 1.22 | - | - | - |
| 53. | 4-Methoxybenzaldehyde | 1264 | - | - | - | - | - | 5.66 |
| 54. | Methyl decanoate | 1327 | - | 0.49 | - | - | 0.81 | - |
| 55. | Tetradecane | 1400 | - | - | - | 0.96 | - | 0.27 |
| 56. | α-Ionone | 1429 | - | - | - | - | - | 0.92 |
| 57. | (E)-Geranyl acetone | 1455 | 2.44 | 3.51 | - | 4.07 | - | - |
| 58. | (E)-β-Ionone | 1512 | - | - | - | - | - | 2.24 |
| 59. | β-Tumerone | 1668 | - | - | - | 0.69 | - | - |
| 60. | Heneicosane | 2100 | - | 1.74 | - | 0.90 | 6.17 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prđun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods 2021, 10, 2103. https://doi.org/10.3390/foods10092103
Prđun S, Svečnjak L, Valentić M, Marijanović Z, Jerković I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods. 2021; 10(9):2103. https://doi.org/10.3390/foods10092103
Chicago/Turabian StylePrđun, Saša, Lidija Svečnjak, Mato Valentić, Zvonimir Marijanović, and Igor Jerković. 2021. "Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles" Foods 10, no. 9: 2103. https://doi.org/10.3390/foods10092103
APA StylePrđun, S., Svečnjak, L., Valentić, M., Marijanović, Z., & Jerković, I. (2021). Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods, 10(9), 2103. https://doi.org/10.3390/foods10092103








