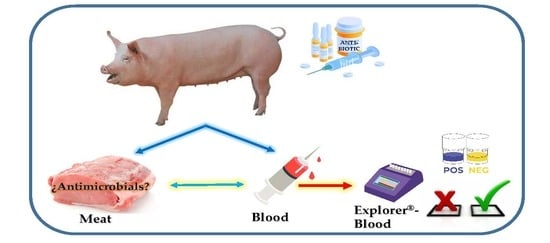
Optimization and Validation of a New Microbial Inhibition Test for the Detection of Antimicrobial Residues in Living Animals Intended for Human Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Serum Samples
2.1.1. Antibiotic-Free Blood Serum Samples
2.1.2. Antimicrobials Choice for Validation
2.1.3. Antimicrobial Standards and Spiked Blood Serum Samples
2.1.4. Blood Samples with Antibiotics Injected In Vivo
2.2. Sample Preparation
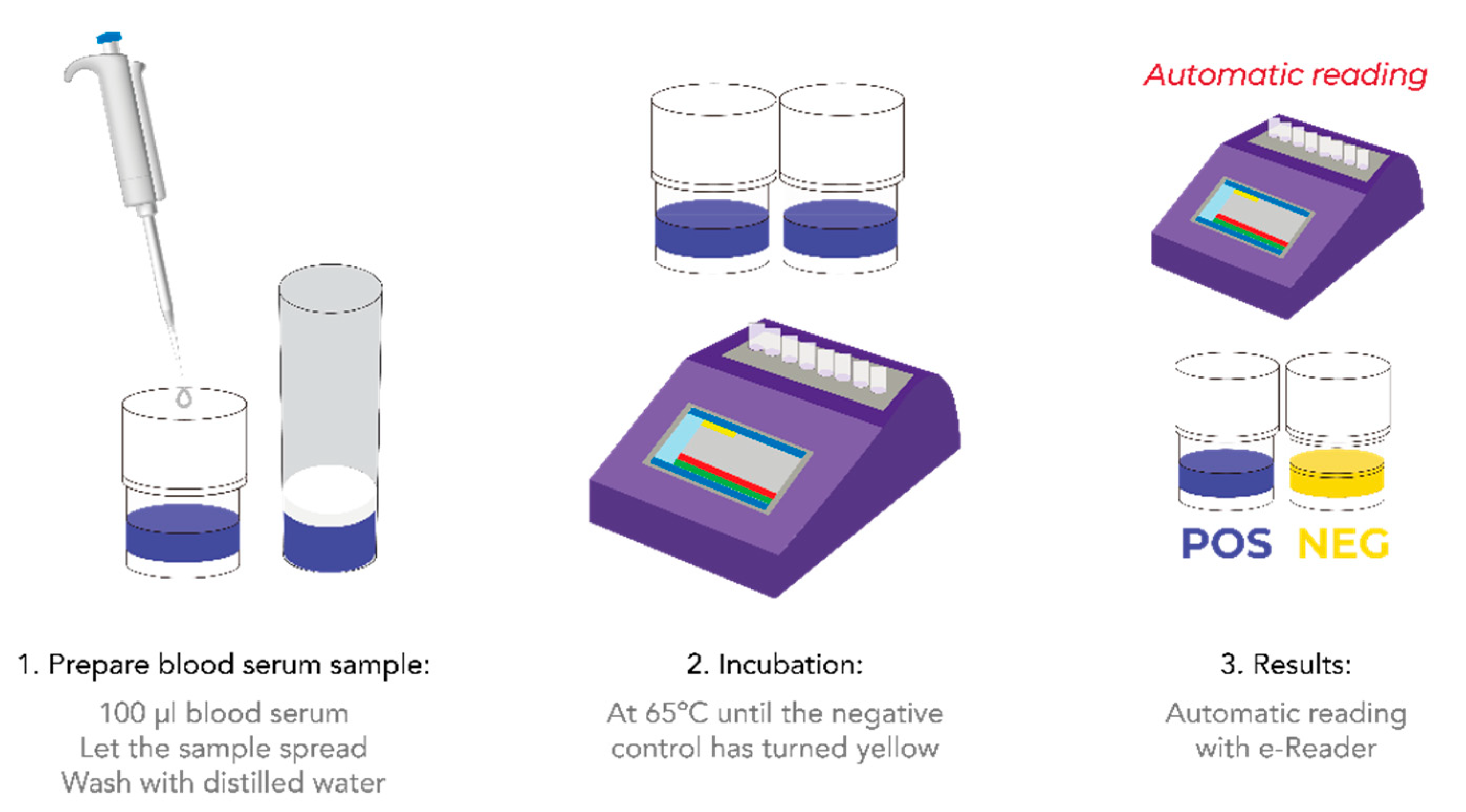
2.3. Explorer®-Blood Test
2.3.1. Description
2.3.2. Procedure
2.4. Parameter Setting and Validation of the Explorer®-Blood Test
2.4.1. Cut-Off Level
2.4.2. Limits of Detection (LoD)
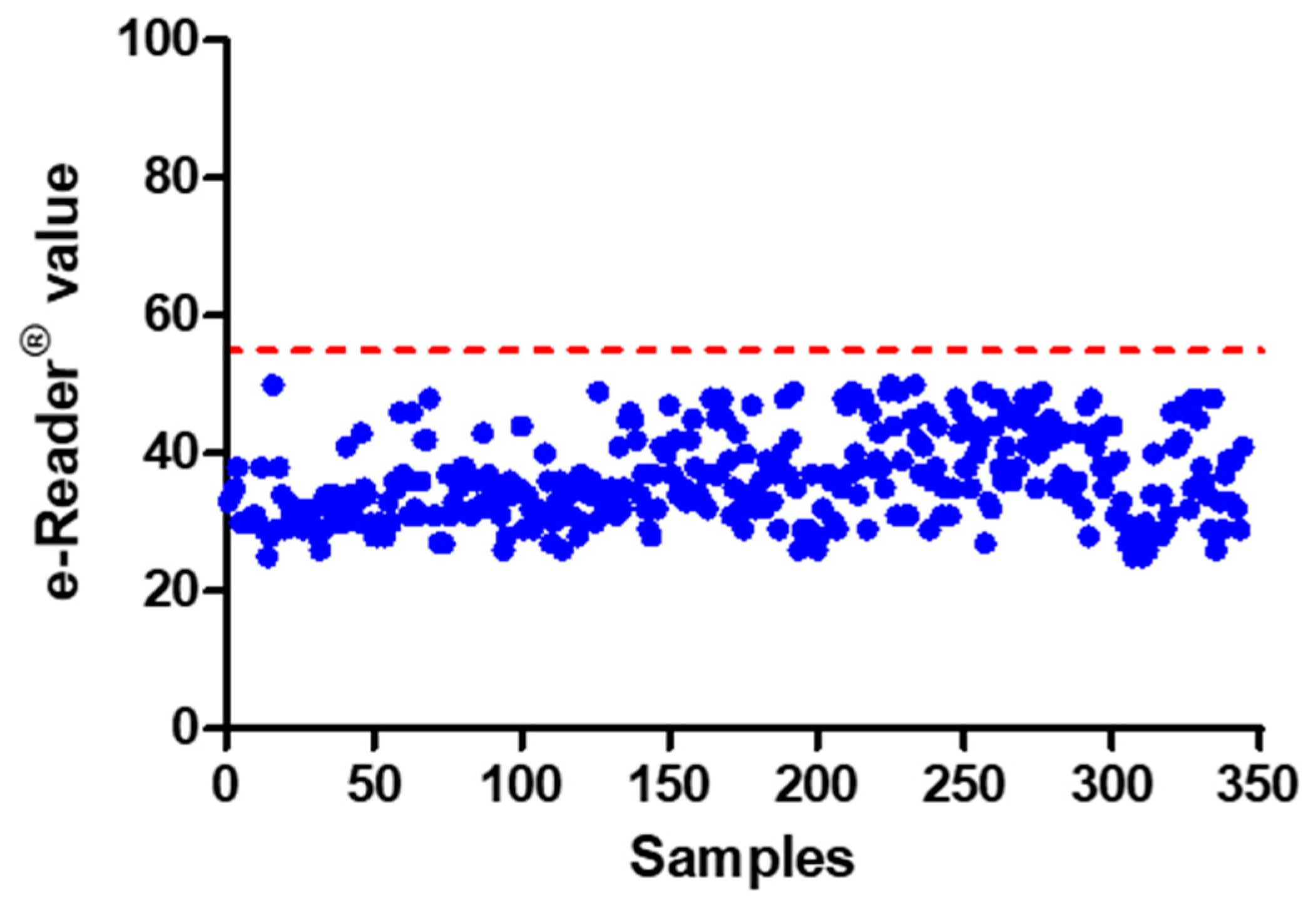
2.4.3. False Positive Rate
2.4.4. Validation with Blood Samples Containing Antibiotics Injected In Vivo
2.5. Statistical Analysis
3. Results and Discussion
3.1. Matrix Preparation and Test Procedure
3.2. Parameter Settings
3.2.1. End-Time
3.2.2. Cut-Off Level
3.2.3. False Positive Rate
3.3. Limits of Detection (LoDs) in Swine Blood Serum
3.4. Validation with Blood Samples Injected In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Cheng, G.; Ning, J.; Ahmed, S.; Huang, J.; Ullah, R.; An, B.; Hao, H.; Dai, M.; Huang, L.; Wang, X.; et al. Selection and dissemination of antimicrobial resistance in Agri-food production. Antimicrob. Resist. Infect. Control. 2019, 8, 1–13. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, H.J.; Ryu, P.D. Public health risks: Chemical and antibiotic residues—Review. Asian-Australas. J. Anim. Sci. 2001, 14, 402–413. [Google Scholar] [CrossRef]
- Zdziarski, P.; Simon, K.; Majda, J.A.C.E.K. Overuse of high stability antibiotics and its consequences in public and environmental health. Acta Microbiol. Pol. 2003, 52, 5–13. [Google Scholar]
- Reig, M.; Toldrá, F. Veterinary drug residues in meat: Concerns and rapid methods for detection. Meat Sci. 2008, 78, 60–67. [Google Scholar] [CrossRef]
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Ghering, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food. Chem. Toxicol. 2016, 88, 112–122. [Google Scholar] [CrossRef]
- Nguyen, V.; Nguyen, V.; Li, C.; Zhou, G. The degradation of oxytetracycline during thermal treatments of chicken and pig meat and the toxic effects of degradation products of oxytetracycline on rats. J. Food Sci. Technol. 2015, 52, 2842–2850. [Google Scholar] [CrossRef]
- Elzagallaai, A.A.; Sultan, E.A.; Bend, J.R.; Abuzgaia, A.M.; Loubani, E.; Rieder, M.J. Role of oxidative stress in hypersensitivity reactions to sulfonamides. J. Clin. Pharmacol. 2020, 60, 409–421. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No. 726/2004 of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. Off. J. Eur. Union 2004, L. 136, 1–33. [Google Scholar]
- Commission Regulation (EU). No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, L. 15, 1–72. [Google Scholar]
- Directive 2001/82/EC of the European parliament and of the council of 6 November 2001 on the Community code relating to veterinary medicinal products. Off. J. Eur. Community 2001, L. 311, 1–66.
- Council Directive (EEC). No. 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products. Off. J. Eur. Community 1996, L. 125, 1–32. [Google Scholar]
- Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and interpretation of results. Off. J. Eur. Community 2002, L. 221, 1–36.
- Pikkemaat, M.G. Microbial screening methods for detection of antibiotic residues in slaughter animals. Anal. Bioanal. Chem. 2009, 395, 893–905. [Google Scholar] [CrossRef]
- Mata, L.; Sanz, D.; Razquin, P. Validation of the Explorer® 2.0 test coupled to e-Reader® for the screening of antimicrobials in muscle from different animal species. Food Addit. Contam. Part A 2014, 31, 1496–1505. [Google Scholar] [CrossRef]
- Gaudin, V.; Rault, A.; Hedou, C.; Soumet, C.; Verdon, E. Strategies for the screening of antibiotic residues in eggs: Comparison of the validation of the classical microbiological method with an immunobiosensor method. Food Addit. Contam. Part A 2017, 34, 1510–1527. [Google Scholar] [CrossRef]
- Djekic, I.; Radović, Č.; Lukić, M.; Stanišić, N.; Lilić, S. Environmental life-cycle assessment in production of pork products. Meso 2015, 17, 345–351. [Google Scholar]
- Jones, S.A.; Salter, R.S.; Goldsmith, T.; Quintana, J.; Rapnicki, P.; Shuck, K.; Wells, J.E.; Schneider, M.J.; Griffin, D. Development and model testing of antemortem screening methodology to predict required drug withholds in heifers. J. Food Prot. 2014, 77, 292–298. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, Q.; Shabbir, M.A.B.; Sattar, A.; Peng, D.; Tao, Y.; Chen, D.; Yuan, Z.; Wang, Y. The search for a microbiological inhibition method for the rapid, broad-spectrum and high-throughput screening of six kinds of antibiotic residues in swine urine. Food Chem. 2021, 339, 127580. [Google Scholar] [CrossRef]
- Hernández, E.; Rey, R.; Puig, M.; Garcia, M.A.; Solans, C.; Bregante, M.A. Pharmacokinetics and residues of a new oral amoxicillin formulation in piglets: A preliminary study. Vet. J. 2005, 170, 237–242. [Google Scholar] [CrossRef]
- Reyes-Herrera, I.; Schneider, M.J.; Cole, K.; Farnell, M.B.; Blore, P.J.; Donoghue, D.J. Concentrations of antibiotic residues vary between different edible muscle tissues in poultry. Research note. J. Food Prot. 2005, 68, 2217–2219. [Google Scholar] [CrossRef]
- Castellari, M.; Gratacos-Cubarsi, M.; Garcia-Regueiro, J.A. Detection of tetracycline and oxytetracycline residues in pig and calf hair by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8096–8100. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Antibiotic use in heavy pigs: Comparison between urine and muscle samples from food chain animals analysed by HPLC-MS/MS. Food Chem. 2017, 235, 111–118. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Suitability of feathers as control matrix for antimicrobial treatments detection compared to muscle and liver of broilers. Food Control 2018, 91, 268–275. [Google Scholar] [CrossRef]
- Serrano, M.J.; Mitjana, O.; Bonastre, C.; Laborda, A.; Falceto, M.V.; García-Gonzalo, D.; Abilleira, E.; Elorduy, J.; Bousquet-Melou, A.; Mata, L.; et al. Is Blood a Good Indicator for Detecting Antimicrobials in Meat? Evidence for the Development of In Vivo Surveillance Methods. Antibiotics 2020, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, V.; Hedou, C.; Rault, A.; Verdon, E. Validation of a Five Plate Test, the STAR protocol, for the screening of antibiotic residues in muscle from different animal species according to European Decision 2002/657/EC. Food Addit. Contam. Part A 2010, 27, 935–952. [Google Scholar] [CrossRef] [PubMed]
- CIMAVET Centro de Información Online de Medicamentos Veterinarios de la AEMPS (CIMA Vet). Available online: https://cimavet.aemps.es/cimavet/publico/home.html (accessed on 28 July 2020).
- ISO 13969:2003/IDF 183:2003. Milk and Milk Products-Guidelines for a Standardized Description of Microbial Inhibitor Tests; International Organization for Standarization: Geneva, Switzerland; International Dairy Federation: Brussels, Belgium, 2003; Available online: https://www.iso.org/standard/35327.html (accessed on 28 July 2020).
- Gaudin, V.; Hedou, C.; Verdon, E. Validation of a wide spectrum microbiological tube test, the EXPLORER® test, for the detection of antimicrobials in muscle from different animal species. Food Addit. Contam. Part A 2009, 26, 1162–1171. [Google Scholar] [CrossRef]
- Mata, L.; Sanz, D.; Razquin, P. Performance of Eclipse farm test coupled to e-Reader for antibiotic residues detection in raw milk. Food Anal. Methods 2016, 9, 519–527. [Google Scholar] [CrossRef]
- Razquin, P.; Sanz, D.; Marco, A.; Carrascón, V.; Andaluz, S.; Soler, L.; Antón, A.; Mata, L. Validation of the Eclipse Farm 4G & COMET for Detection of Antibiotics in Raw Bovine Milk: AOAC Performance Tested MethodSM 022101. J. AOAC Int. 2021, qsab061. [Google Scholar] [CrossRef]
- Community Reference Laboratories Residues (CRLs). Guidelines for the Validation of Screening Methods for Residues of Veterinary Medicines. Initial Validation and Transfer. 2010. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_vet-med-residues_guideline_validation_screening_en.pdf (accessed on 17 September 2020).
- Verdon, E.; Fuselier, R.; Hurtaud-Pessel, D.; Couëdor, P.; Cadieu, N.; Laurentie, M. Stability of penicillin antibiotic residues in meat during storage: Ampicillin. J. Chromatogr. A 2000, 882, 135–143. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Romero-González, R.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.; Garrido-Frenich, A. Stability of antibacterial and coccidiostat drugs on chicken meat burgers upon cooking and in vitro digestion. Food Chem. 2020, 316, 126367. [Google Scholar] [CrossRef]


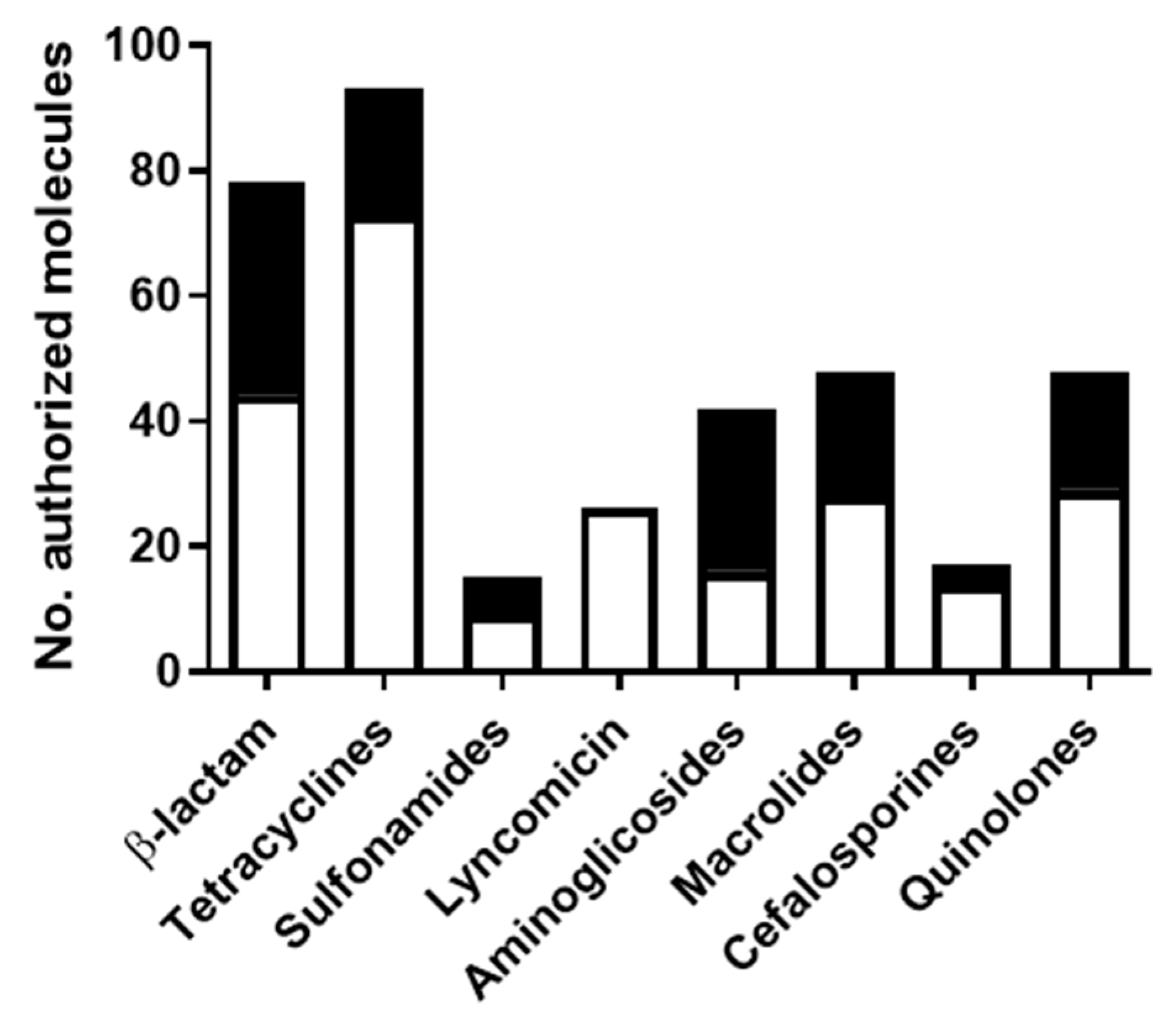
| Molecule | Concentration Level Tested (µg/L) | MRL (Muscle) |
|---|---|---|
| Amoxicillin | 10, 15, 20 | 50 |
| Cefalexin | 100, 200, 250, 300, 400 | 200 |
| Ceftiofur | 100, 200, 300, 400 | 1000 |
| Sulfamethazine | 100, 150, 200 | 100 |
| Sulfadiazine | 50, 100, 150 | 100 |
| Oxitetracycline | 100, 200, 300 | 100 |
| Doxicyclin | 100, 200, 300, 400 | 100 |
| Neomycin | 5, 15, 25, 50 | 500 |
| Apramycin | 500, 1000, 1500 | 1000 |
| Tylosin | 25, 50, 75 | 100 |
| Lincomycin | 200, 300, 400 | 100 |
| Molecule | e-Reader® Value | LoD Serum | LoD Muscle | MRL (Muscle) |
|---|---|---|---|---|
| Amoxicillin | 121 ± 7 | 10 | 10 | 50 |
| Cefalexin | 67 ± 5 | 200–250 | 200 | 200 |
| Ceftiofur | 103 ± 9 | 300 | 200 | 1000 |
| Sulfamethazine | 76 ± 7 | 100 | 100 | 100 |
| Sulfadiazine | 89 ± 4 | 100 | 50 | 100 |
| Oxitetracycline | 80 ± 6 | 200 | 200 | 100 |
| Doxicyclin | 80 ± 7 | 100 | 100 | 100 |
| Neomycin | 99 ± 6 | 25 | ≤200 | 500 |
| Apramycin | 123 ± 4 | ≤500 | 900 | 1000 |
| Tylosin | 92 ± 3 | 25–50 | 100 | 100 |
| Lincomycin | 65 ± 3 | 200 | 300 | 100 |
| Sample | LC-MS/MS (µg Kg−1) | e-Reader® Value | Qualitative Result |
|---|---|---|---|
| 1 | <10 | 45 | − |
| 2 | <10 | 47 | − |
| 3 | 73/ND * | 41 | − |
| 4 | <10 | 45 | − |
| 5 | <10 | 48 | − |
| 6 | <10 | 37 | − |
| 7 | 58/ND * | 36 | − |
| 8 | <10 | 37 | − |
| 9 | <10 | 39 | − |
| 10 | 262 | 129 | + |
| 11 | 2005 | 121 | + |
| 12 | 872 | 126 | + |
| 13 | 1484 | 121 | + |
| Sample | LC-MS/MS (µg Kg−1) | e-Reader® Value | Qualitative Result |
|---|---|---|---|
| 1 | 403 | 94 | + |
| 2 | <10 | 40 | − |
| 3 | 375 | 87 | + |
| 4 | 234 | 70 | + |
| 5 | 220 | 86 | + |
| 6 | 71 | 57 | + |
| 7 | 83 | 53 | − |
| 8 | 89 | 50 | − |
| 9 | 46 | 52 | − |
| 10 | 24 | 33 | − |
| 11 | 37 | 35 | − |
| 12 | 102 | 57 | + |
| 13 | 55 | 48 | − |
| 14 | 41 | 50 | − |
| 15 | 32 | 32 | − |
| 16 | 58 | 34 | − |
| Sample | LC-MS/MS (µg Kg−1) | e-Reader® Value | Qualitative Result |
|---|---|---|---|
| 1 | 1095 | 115 | + |
| 2 | 133 | 94 | + |
| 3 | 51 | 74 | + |
| 4 | 11 | 35 | − |
| 5 | 4098 | 117 | + |
| 6 | 773 | 112 | + |
| 7 | 569 | 114 | + |
| 8 | 115 | 89 | + |
| 9 | 25 | 102 | + |
| 10 | 3462 | 111 | + |
| 11 | 1113 | 109 | + |
| 12 | 229 | 101 | + |
| 13 | 74 | 82 | + |
| 14 | 35 | 65 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, M.J.; Mata, L.; García-Gonzalo, D.; Antón, A.; Razquin, P.; Condón, S.; Pagán, R. Optimization and Validation of a New Microbial Inhibition Test for the Detection of Antimicrobial Residues in Living Animals Intended for Human Consumption. Foods 2021, 10, 1897. https://doi.org/10.3390/foods10081897
Serrano MJ, Mata L, García-Gonzalo D, Antón A, Razquin P, Condón S, Pagán R. Optimization and Validation of a New Microbial Inhibition Test for the Detection of Antimicrobial Residues in Living Animals Intended for Human Consumption. Foods. 2021; 10(8):1897. https://doi.org/10.3390/foods10081897
Chicago/Turabian StyleSerrano, María Jesús, Luis Mata, Diego García-Gonzalo, Alejandra Antón, Pedro Razquin, Santiago Condón, and Rafael Pagán. 2021. "Optimization and Validation of a New Microbial Inhibition Test for the Detection of Antimicrobial Residues in Living Animals Intended for Human Consumption" Foods 10, no. 8: 1897. https://doi.org/10.3390/foods10081897
APA StyleSerrano, M. J., Mata, L., García-Gonzalo, D., Antón, A., Razquin, P., Condón, S., & Pagán, R. (2021). Optimization and Validation of a New Microbial Inhibition Test for the Detection of Antimicrobial Residues in Living Animals Intended for Human Consumption. Foods, 10(8), 1897. https://doi.org/10.3390/foods10081897