Beneficial Effects of Holothuria leucospilota Polysaccharides on Fermentability In Vivo and In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Polysaccharides
2.2. In-Vitro Fermentation of HLP
2.2.1. Preparation of Human Intestinal Microbiota
2.2.2. In-Vitro Fermentation Procedure
2.2.3. Determination of Total Sugar, Reducing Sugar, and Free Monosaccharides in the Fermented Products
2.2.4. Determination of pH and SCFA Content
2.3. In Vivo Fermentation of HLP
2.4. Liver Antioxidant Activity Assay
2.5. Histological Examinations
2.6. Statistical Analysis
3. Results and Discussion
3.1. Changes in Total Sugar, Reducing Sugar, and Free Monosaccharide Contents during In-Vitro Fermentation
3.2. Changes in pH Values and SCFA Contents during In-Vitro Fermentation
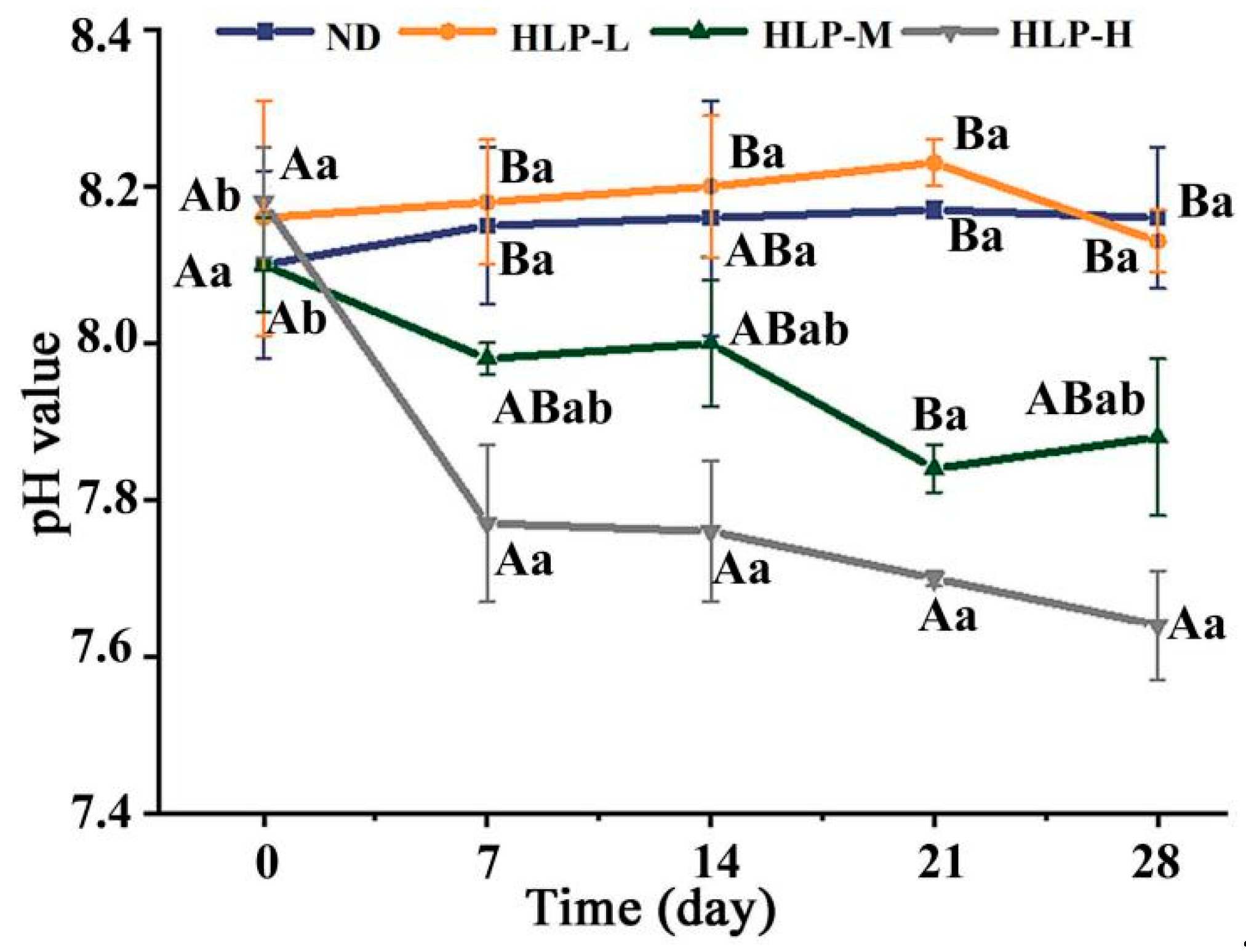
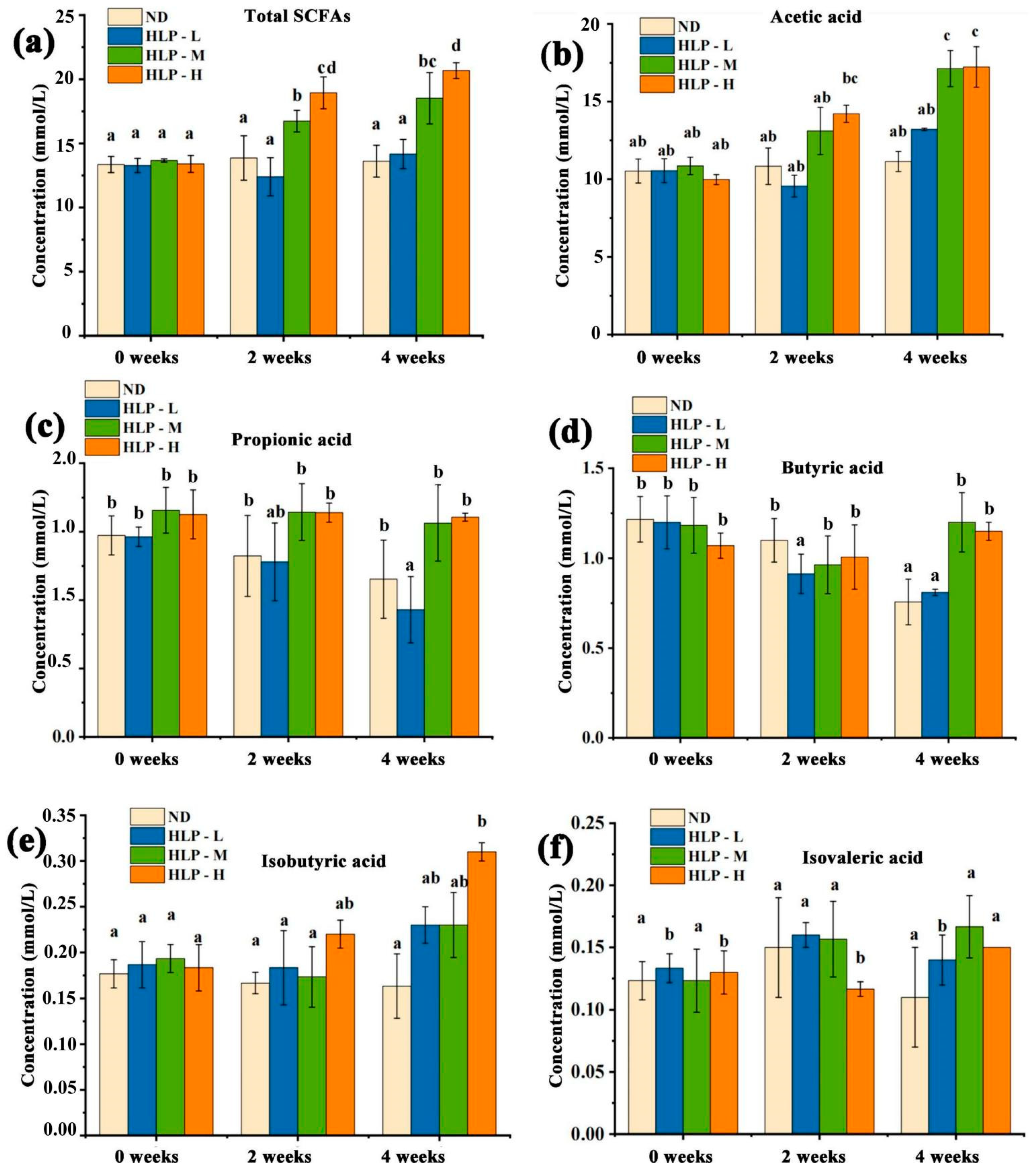
3.3. Changes in pH Values and SCFA Contents in Mouse Feces
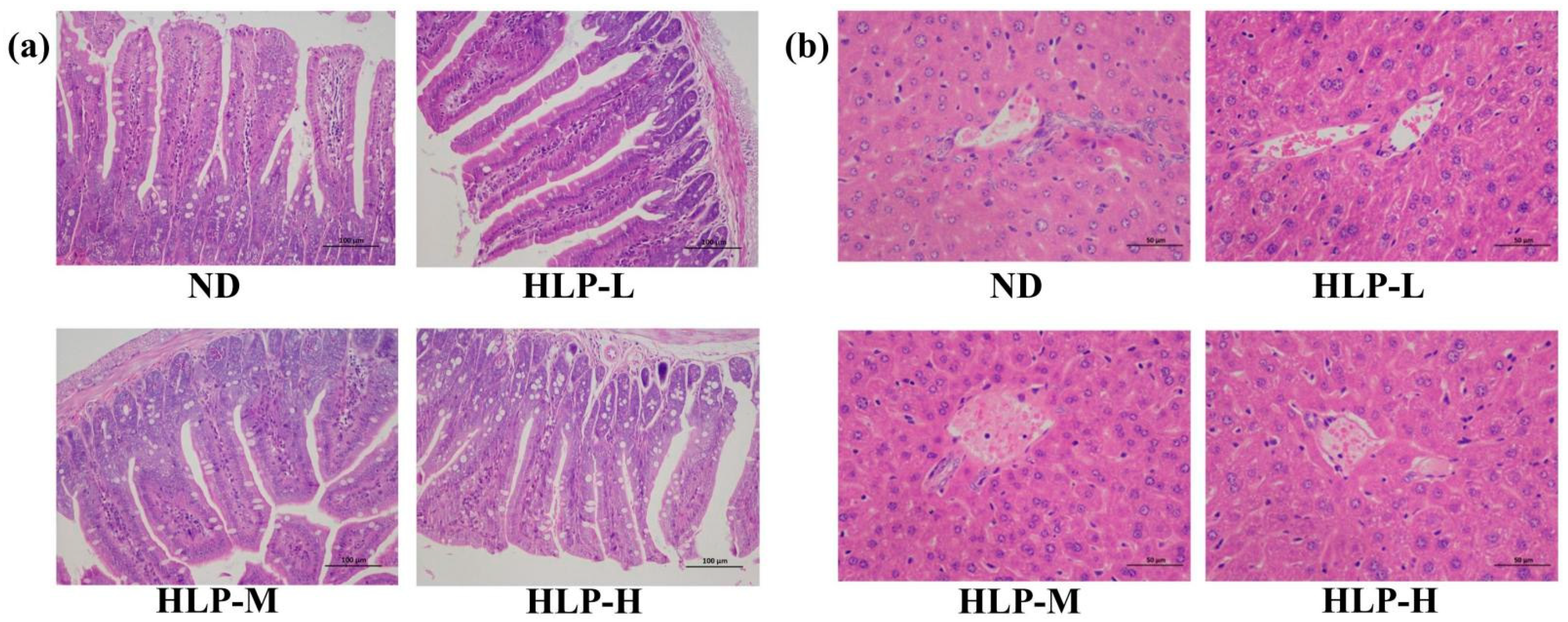
3.4. Histological Analysis of the Ileum and Liver
3.5. Effects of HLP on Oxidative Stress Parameters in Mouse Livers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Chen, J.; Guo, Z.; Zheng, Y.; Rea, M.C.; Su, H.; Zheng, X.; Zheng, B.; Miao, S. Using polysaccharides for the enhancement of functionality of foods: A review. Trends Food Sci. Technol. 2019, 86, 311–327. [Google Scholar] [CrossRef]
- Xie, J.-H.; Jin, M.-L.; Morris, G.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2015, 56, S60–S84. [Google Scholar] [CrossRef] [PubMed]
- Rakariyatham, K.; Zhou, D.; Rakariyatham, N.; Shahidi, F. Sapindaceae (Dimocarpus longan and Nephelium lappaceum) seed and peel by-products: Potential sources for phenolic compounds and use as functional ingredients in food and health applications. J. Funct. Foods 2020, 67, 103846. [Google Scholar] [CrossRef]
- Van Dam, J.E.G.; Van Den Broek, L.A.; Boeriu, C.G. Polysaccharides in Human Health Care. Nat. Prod. Commun. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Aweya, J.J.; Huang, Z.-X.; Kang, Z.-Y.; Bai, Z.-H.; Li, K.-H.; He, X.-T.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Erasmus, S.W.; Liu, X.; Van Ruth, S.M. Study on the Relations between Hyperspectral Images of Bananas (Musa spp.) from Different Countries, their Compositional Traits and Growing Conditions. Sensors 2020, 20, 5793. [Google Scholar] [CrossRef]
- Li, Y.; Shabani, K.I.; Liu, H.; Guo, Q.; Liu, X. Structural, physicochemical and rheological properties of a novel native starch obtained from Rhizoma Gastrodiae. Food Struct. 2020, 25, 100148. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Y.; Zhang, R.; Bai, Y.; Dong, L.; Liu, L.; Jia, X.; Wang, G.; Zhang, M. Structural characterization and in vitro gastrointestinal digestion and fermentation of litchi polysaccharide. Int. J. Biol. Macromol. 2019, 140, 965–972. [Google Scholar] [CrossRef]
- Mou, J.; Li, Q.; Shi, W.; Qi, X.; Song, W.; Yang, J. Chain conformation, physicochemical properties of fucosylated chondroitin sulfate from sea cucumber Stichopus chloronotus and its in vitro fermentation by human gut microbiota. Carbohydr. Polym. 2019, 228, 115359. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, Q.; Sun, L.; Ye, Y.; Ji, G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J. Nutr. Biochem. 2018, 57, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huo, D.; Yu, Z.; Ren, C.; Jiang, X.; Luo, P.; Chen, T.; Hu, C. Spawning, larval development and juvenile growth of the tropical sea cucumber Holothuria leucospilota. Aquaculture 2018, 488, 22–29. [Google Scholar] [CrossRef]
- Wang, G. Studies on chemical constituents from Holothuria leucospilota; Hainan University: Haikou, China, 2018. [Google Scholar]
- Qiu, P.; Wu, F.; Yi, L.; Chen, L.; Jin, Y.; Ding, X.; Ouyang, Y.; Yao, Y.; Jiang, Y.; Zhang, Z. Structure characterization of a heavily fucosylated chondroitin sulfate from sea cucumber (H. leucospilota) with bottom-up strategies. Carbohydr. Polym. 2020, 240, 116337. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, C.; Zheng, Q.; Wu, J.; Zhu, K.; Shen, X.; Cao, J. Effect of simulated gastrointestinal digestion in vitro on the antioxidant activity, molecular weight and microstructure of polysaccharides from a tropical sea cucumber (Holothuria leucospilota). Food Hydrocoll. 2018, 89, 735–741. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Q.; Cao, J.; Xu, Y.; Pei, Z.; Fan, H.; Yuan, Y.; Shen, X.; Li, C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food Chem. Toxicol. 2019, 135, 110886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Zhang, L.; Yuan, Y.; Yu, D. Codonopsis lanceolata polysaccharide CLPS alleviates high fat/high sucrose diet-induced insulin resistance via anti-oxidative stress. Int. J. Biol. Macromol. 2019, 145, 944–949. [Google Scholar] [CrossRef]
- Su, A.; Ma, G.; Xie, M.; Ji, Y.; Li, X.; Zhao, L.; Hu, Q. Characteristic of polysaccharides from Flammulina velutipes in vitro digestion under salivary, simulated gastric and small intestinal conditions and fermentation by human gut microbiota. Int. J. Food Sci. Technol. 2019, 54, 2277–2287. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Ke, Y.; Li, C.; Zhang, Z.; Wu, Y.; Hu, B.; Liu, A.; Luo, Q.; Wu, W. In vitro saliva-gastrointestinal digestion and fecal fermentation of Oudemansiella radicata polysaccharides reveal its digestion profile and effect on the modulation of the gut microbiota. Carbohydr. Polym. 2020, 251, 117041. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; Fu, X.; Liu, R.H. In vitro fermentation of mulberry fruit polysaccharides by human fecal inocula and impact on microbiota. Food Funct. 2016, 7, 4637–4643. [Google Scholar] [CrossRef]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 2017, 183, 230–239. [Google Scholar] [CrossRef]
- Xie, J.-H.; Tang, W.; Jin, M.-L.; Li, J.-E.; Xie, M.-Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Salvador, V.; Cherbut, C.; Barry, J.-L.; Bertrand, D.; Bonnet, C.; Delort-Laval, J. Sugar composition of dietary fibre and short-chain fatty acid production during in vitro fermentation by human bacteria. Br. J. Nutr. 1993, 70, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Cockburn, D.; Koropatkin, N.M. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Chen, K.; Xiao, C.; Fan, L.; Zhang, B.; Ren, J.; Fang, B. An in vitro fermentation study on the effects of Dendrobium officinale polysaccharides on human intestinal microbiota from fecal microbiota transplantation donors. J. Funct. Foods 2018, 53, 44–53. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, Q.; Zhang, K.; Wang, N.; Li, C.; Li, Z.; Zhang, A.; Wang, C. Polysaccharide from Pleurotus nebrodensis: Physicochemical, structural characterization and in vitro fermentation characteristics. Int. J. Biol. Macromol. 2020, 165, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2018, 125, 751–760. [Google Scholar] [CrossRef]
- Besten, G.D.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.M.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg Effect Dictates the Mechanism of Butyrate-Mediated Histone Acetylation and Cell Proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, A.; Duncan, S.; Leitch, C.; Child, M.W.; Flint, H.J. pH and Peptide Supply Can Radically Alter Bacterial Populations and Short-Chain Fatty Acid Ratios within Microbial Communities from the Human Colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Ho, S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Kuang, H.; Hu, J.; Chen, Q. Structural characterization and anti-hypoxia activities of polysaccharides from the sporocarp, fermentation broth and cultured mycelium of Agaricus bitorquis (Quél.) Sacc. Chaidam in mice. J. Funct. Foods 2018, 51, 75–85. [Google Scholar] [CrossRef]
- Cui, J.-J.; Yuan, J.-F.; Zhang, Z.-Q. Anti-oxidation activity of the crude polysaccharides isolated from Polygonum Cillinerve (Nakai) Ohwi in immunosuppressed mice. J. Ethnopharmacol. 2010, 132, 512–517. [Google Scholar] [CrossRef]
- Li, Q.; Chen, G.; Chen, H.; Zhang, W.; Ding, Y.; Yu, P.; Zhao, T.; Mao, G.; Feng, W.; Yang, L.; et al. Se-enriched G. frondosa polysaccharide protects against immunosuppression in cyclophosphamide-induced mice via MAPKs signal transduction pathway. Carbohydr. Polym. 2018, 196, 445–456. [Google Scholar] [CrossRef] [PubMed]

| Time (h) | Total Sugar (mg/mL) | Reducing Sugar (mg/mL) | Fucose (mg/mL) | Xylose (mg/mL) | Galactosamine (mg/mL) |
|---|---|---|---|---|---|
| 0 | 1.099 ± 0.177 d | 0.432 ± 0.014 c | ND | ND | ND |
| 3 | 0.928 ± 0.051 c | 0.833 ± 0.025 d | 0.174 ± 0.004 c | 0.063 ± 0.009 b | 0.017 ± 0.002 a |
| 6 | 0.475 ± 0.066 b | 0.274 ± 0.034 b | 0.134 ± 0.001 b | 0.086 ± 0.004 c | 0.016 ± 0.001 a |
| 12 | 0.265 ± 0.018 a | 0.104 ± 0.004 a | 0.119 ± 0.009 a | 0.029 ± 0.001 a | 0.029 ± 0.004 b |
| 24 | 0.234 ± 0.019 a | 0.102 ± 0.004 a | ND | 0.036 ± 0.001 a | 0.016 ± 0.007 a |
| 48 | 0.218 ± 0.009 a | 0.097 ± 0.002 a | ND | ND | ND |
| Time (h) | 0 | 6 | 12 | 24 | 48 |
|---|---|---|---|---|---|
| pH | 6.87 ± 0.01 e | 4.59 ± 0.02 f | 4.49 ± 0.00 a | 5.55 ± 0.01 c | 5.91 ± 0.02 d |
| Acetic acid (mmol/mL) | 9.65 ± 0.26 a | 39.00 ± 5.90 b,c | 70.71 ± 1.48 d | 34.99 ± 1.3 b | 45.53 ± 9.86 b |
| Propionic acid (mmol/mL) | 2.93 ± 0.05 a | 7.04 ± 1.01 b | 16.00 ± 0.92 c | 16.26 ± 0.92 c | 18.26 ± 3.93 c |
| Isobutyric acid (mmol/mL) | 0.26 ± 0.01a | 0.36 ± 0.04 a | 0.15 ± 0.02 a | 2.32 ± 0.13 c | 3.15 ± 0.58 d |
| Butyric acid (mmol/mL) | 2.70 ± 0.31 a | 3.09 ± 0.84 a | 3.53 ± 0.43 a | 28.30 ± 1.61 b | 33.76 ± 7.20 c |
| Isovaleric acid (mmol/mL) | 0.40 ± 0.04 a | 0.63 ± 0.02 a | 0.32 ± 0.03 a | 2.99 ± 0.18 b | 3.93 ± 0.68 c |
| Pentanoic acid (mmol/mL) | 0.64 ± 0.08 a | 0.42 ± 0.02 a | 0.41 ± 0.07 a | 4.62 ± 0.18 b | 6.51 ± 1.34 c |
| Total (mmol/mL) | 16.58 ± 0.74 a | 50.54 ± 5.99 b | 91.12 ± 2.88 c | 89.47 ± 4.30 c | 111.13 ± 23.19 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yuan, Y.; Cao, J.; Shen, X.; Li, C. Beneficial Effects of Holothuria leucospilota Polysaccharides on Fermentability In Vivo and In Vitro. Foods 2021, 10, 1884. https://doi.org/10.3390/foods10081884
Wang W, Yuan Y, Cao J, Shen X, Li C. Beneficial Effects of Holothuria leucospilota Polysaccharides on Fermentability In Vivo and In Vitro. Foods. 2021; 10(8):1884. https://doi.org/10.3390/foods10081884
Chicago/Turabian StyleWang, Wanting, Yiqiong Yuan, Jun Cao, Xuanri Shen, and Chuan Li. 2021. "Beneficial Effects of Holothuria leucospilota Polysaccharides on Fermentability In Vivo and In Vitro" Foods 10, no. 8: 1884. https://doi.org/10.3390/foods10081884
APA StyleWang, W., Yuan, Y., Cao, J., Shen, X., & Li, C. (2021). Beneficial Effects of Holothuria leucospilota Polysaccharides on Fermentability In Vivo and In Vitro. Foods, 10(8), 1884. https://doi.org/10.3390/foods10081884