Abstract
Papaya-associated foodborne illness outbreaks have been frequently reported worldwide. The goal of this study was to evaluate the behavior of Salmonella Typhimurium and Listeria monocytogenes on whole papaya during storage and sanitizing process. Fresh green papayas were inoculated with approximately 7 log CFU of S. Typhimurium and L. monocytogenes and stored at 21 or 7 °C for 14 days. Bacteria counts were determined on day 0, 1, 7, 10 and 14. Fresh green papayas inoculated with approximately 8 log CFU of the bacteria were treated for 5 min with 2.5, 5 and 10 ppm aqueous chlorine dioxide (ClO2). The ClO2 solutions were generated by mixing sodium chlorite with an acid, which was HCl, lactic acid or malic acid. The detection limit of the enumeration method was 2.40 log CFU per papaya. At the end of storage period, S. Typhimurium and L. monocytogenes grew by 1.88 and 1.24 log CFU on papayas at 21 °C, respectively. Both bacteria maintained their initial population at inoculation on papayas stored at 7 °C. Higher concentrations of ClO2 reduced more bacteria on papaya. 10 ppm ClO2, regardless the acid used to generate the solutions, inactivated S. Typhimurium to undetectable level on papaya. 10 ppm ClO2 generated with HCl, lactic acid and malic acid reduced L. monocytogenes by 4.40, 6.54 and 8.04 log CFU on papaya, respectively. Overall, ClO2 generated with malic acid showed significantly higher bacterial reduction than ClO2 generated with HCl or lactic acid. These results indicate there is a risk of survival and growth for S. Typhimurium and L. monocytogenes on papaya at commercial storage conditions. Aqueous ClO2 generated with malic acid shows effectiveness in inactivating the pathogenic bacteria on papaya.
1. Introduction
Papaya (Carica papaya) is one of the major tropical agricultural commodities amongst banana, mango, avocado and pineapple [1]. Annual global papaya production has increased by approximately 90% since 2000 and reached 13.7 million metric tons in 2019 [2]. The top three papaya-producing countries are India, Brazil and Mexico, among which 99% of Mexican papayas are exported to the United States [2]. However, along with the increased papaya demand and production worldwide, foodborne illness outbreaks linked to papaya have also been emerging in recent years [3,4]. In particular, outbreaks associated with whole fresh papaya have been frequently reported in the U.S. from 2011 to 2019, which affected the papaya industry in both US and Mexico [4,5]. Papaya grows best in tropic environments at 21–33 °C where the survival and growth of pathogenic bacteria are favored [6]. Microbial contamination of papaya might happen at any step of the production chain where the fruits are in contact with water, soil, harvest equipment and human handling [7]. Salmonella Litchfield was detected on whole papayas associated with an outbreak in Australia between 2006 and 2007, and other Salmonella serotypes of Chester, Eastborne and Poona were detected in farm water samples [3]. In multiple cases reported in the U.S., whole papayas were contaminated by Salmonella serotypes of Agona, Uganda, Newport, etc. [5]. Therefore, papaya seems to be susceptible to Salmonella contamination. In addition, Listeria monocytogenes is one of the concerned foodborne pathogenic bacteria associated with fresh produce due to its nature of being present in the environment and its ability to grow at refrigeration temperature [8]. L. monocytogenes-caused multistate outbreaks in the U.S. were linked to whole cantaloupe and caramel apple [9,10]. L. monocytogenes was also found to be able to survive or grow on the surfaces of apple, mango, kiwifruit and cherry tomato under various storage conditions [11,12,13,14].
Studies have reported the survival and growth of foodborne pathogenic bacteria in fresh-cut papaya and papaya pulp [15,16,17,18,19]. However, little is known regarding whole fresh papaya. There are differences between fresh-cut and whole fruits in terms of pH, nutrient availability and native microflora composition. For example, S. Typhimurium and L. monocytogenes decreased by approximately 2–2.5 log CFU over 20 days on whole mango at 25 °C; however, these bacteria grew on cut mango [12]. The growth of L. monocytogenes was inhibited on intact jalapeño pepper stored at 7 °C for 14 days, but it grew in the internal cavity of jalapeño pepper at the same storage condition [20]. It is important to note that even when the skin part of fruit is inedible or usually not eaten, pathogenic bacteria surviving on the surface may further cross-contaminate wash water and other fruits that are rinsed in the same batch, internalize into the flesh or transfer to fruit flesh during cutting [21,22]. Information of pathogenic bacteria behavior on whole papaya would assist regulatory and industrial agencies in the assessment and prevention of papaya microbiological safety issues.
Once contaminated, fresh fruits cannot be thermally disinfected and would likely be distributed to the market. Therefore, washing and sanitizing is a critical step in the post-harvest process to prevent cross-contamination and reduce pathogens. Chlorine-based bleach at a concentration of 50–200 ppm is the most widely used sanitizer in fresh produce handling and processing [23]. However, the effectiveness of chlorine varies at different pH and is reduced significantly in the presence of organics, and there are concerns regarding the carcinogenetic by-products such as trihalomethanes formed in the reactions between chlorine and organics [24]. Chlorine dioxide (ClO2) is approved by FDA for fresh produce washing with a maximum residue of 3 ppm in the wash water [24]. The antimicrobial efficacy of ClO2 is less prone to low pH and the presence of organics than chlorine [25]. ClO2 also forms fewer carcinogenetic by-products than chlorine when chlorinated [24]. Despite the advantages, ClO2 is reduced to chlorite (ClO2−), chlorate (ClO3−) and chloride (Cl−) to some extend [26]. The United State Environmental Protection Agency (EPA) sets the Maximum Residual Disinfectant Level (MRDL) of ClO2 in public drinking water to be 0.8 mg/L and the Maximum Contaminant Level (MCL) of ClO2− to be 1.0 mg/L [27]. ClO2 has been studied in sanitizing a wide variety of fresh produce, such as lettuce, cantaloupe, alfalfa sprouts and blueberries [23,28,29,30]. No ClO2, ClO2− or ClO3− residues were detected in Mulberry fruit treated by 60 ppm aqueous ClO2 for 15 min [31]. Cantaloupes, oranges, tomatoes and apples treated with 5 ppm gaseous ClO2 for 10 min showed very minimal ClO2− residue on the fruits with a maximum of 0.36 mg/kg; however, lettuce and alfalfa sprouts had high ClO2− residue of 16.5–1259.6 mg/kg [32]. Acidified sodium chlorite was used to reduce microbial contamination in shredded green papaya [33]. Ozone was used to reduce the microbial load and improve the nutritional values of fresh-cut papaya [34]. Gu et al. investigated the efficiency of chlorine or peracetic acid in the inactivation and cross-contamination prevention of Salmonella spp. on Maradol papayas [35]. Inactivation of pathogenic bacteria by ClO2 has not been investigated on whole papayas.
Aqueous ClO2 can be made by mixing an acid with sodium chlorite (NaClO2) [36]. Hydrochloric acid (HCl) is a commonly used acid in ClO2 generation [30,31,32,36]. Kim et al. [37] reported ClO2 solutions formed from organic acids, including acetic acid, citric acid and lactic acid, were more stable and more lethal to Bacillus cereus spores than ClO2 formed using HCl. Our previous study has also shown that aqueous ClO2 generated by mixing NaClO2 with organic acids, including citric acid, lactic acid and malic acid, had higher antimicrobial efficacy against common foodborne pathogenic bacteria on Romaine lettuce than ClO2 generated with inorganic acids [38]. For example, 5 min treatments with 5 ppm ClO2 generated with lactic acid, citric acid and malic acid reduced S. Typhimurium on Romaine lettuce by 0.92, 1.39 and 1.37 log CFU/g, respectively, whereas lettuce treated with ClO2 generated with HCl and sodium bisulfate reduced S. Typhimurium by 0.71 and 1.14 log CFU/g, respectively [38].
In numerous studies investigating the survival of foodborne pathogenic bacteria on fresh produce or decontamination of fresh produce using sanitizers, procedures used to recover and quantify bacteria cells from fresh produce vary. The ununiformed procedures make it difficult to compare and accurately interpret results of different studies [39]. For example, pummeling using a stomacher resulted in higher bacteria recovery than pulsifying, sonication and shaking by hand from iceberg lettuce, perilla leaves, cucumber and green pepper, while a lower level of bacteria was recovered from cherry tomato due to its acidity [40]. Sample preparation method, bacteria type and produce type may affect the efficiency of bacteria recovery and hence further affect the accuracy of a microbiological method. So far, there has been no recommendation of sample preparation methods specifically for whole papaya.
This study aimed to optimize homogenization parameters and enumeration methods for recovering S. Typhimurium and L. monocytogenes from papaya surface. It also sought to evaluate the behaviors of these pathogenic bacteria on whole papaya during storage and sanitizing process. Obtaining information in this regard would assist the papaya industry in selecting optimal sanitizer type, usage concentration and treatment time for papaya washing and sanitizing.
2. Materials and Methods
2.1. Bacterial Strains and Cell Cultures
Salmonella Typhimurium (ATCC 14028) and Listeria monocytogenes (F2365) were obtained from Food Microbiology Lab at the University of Hawaii at Manoa and stored in trypticase soy broth (TSB; Becton Dickinson, Franklin Lakes, NJ, USA) containing 50% glycerol at −80 °C. Working cultures were prepared by transferring 50 µL of stock culture into 5 mL of sterile TSB and incubating at 37 °C for 24 h. Working cultures were transferred twice in TSB before each experiment.
2.2. Preparation of Papayas and Inocula
Fresh papayas (Carica papaya L.cv. Rainbow Solo) were purchased on the day of experimentations on separate occasions from local grocery stores in Honolulu, USA. Non-injured whole papayas at mature green/color break stage were selected according to the maturity chart [41]. Papayas were rinsed with tap water and dried on a lab bench at room temperature for 1 h. Then an area of 2.5 × 2.5 cm2 on the middle part of the fruit surface was marked with a thin-line non-toxic marker (Sharpie, Oak Brook, IL, USA). The marked whole papayas were placed on sterile Petri dishes in a biosafety hood before experimenting. S. Typhimurium and L. monocytogenes cultures were diluted with 0.1% peptone water (Becton Dickinson, Franklin Lakes, NJ, USA) to desired concentrations. 100 µL of the inoculum was spot inoculated on the marked area and the papayas were dried under a biosafety hood. For Section 2.3 and Section 2.4, approximately 107 log CFU of S. Typhimurium or L. monocytogenes inocula were used, and the papayas were dried for 1 h to initiate the attachment before every experiment [42]. For Section 2.5, approximately 108 log CFU of the inocula were used, and the papayas were dried for two hours to ensure attachment and initiate colonization before being washed with sanitizer solutions [42].
2.3. Optimization of Recovery Method for Counting Bacteria Cells on Papaya Surface
2.3.1. Recovery Method
Optimization of homogenization parameters is essential for accurate assessment of bacterial behavior on fruit surfaces. The goal of this experiment was to maximize the number of bacteria cells recovered from the papaya surface. After inoculation and drying as described above, the skin of the inoculated area was excised with a sterile knife and placed in a sterile stomacher bag. Bacterial cells were collected by homogenizing the skin under different conditions described as follows. Tested homogenization buffers included phosphate buffered saline (PBS, pH 7.4), 0.1% peptone water (PEPT), PBS + 0.2% Tween 80 (PBS + T) and 0.1% peptone water + 0.2% Tween 80 (PEPT + T). 25 mL of each buffer was separately added into the stomacher bag containing the excised skin and homogenized at 150 or 250 rpm for 1 or 5 min using a stomacher (Seward Stomacher®, Model 400 Circulator, West Sussex, UK). After homogenization, the homogenate was serially diluted with 0.1% peptone water and plated on selective agar or using the agar overlay method. The agar overlay method was to plate the serially diluted homogenate on Plate Count Agar (PCA, Becton Dickinson, Franklin Lakes, NJ, USA) and incubating the plate at 37 °C for 1 h to ensure the recovery of injured cells, followed by pouring warm selective agar at 55 °C over the PCA [43]. The agar plates were incubated at 37 °C for 24 h and then analyzed for bacterial counts. The selective agar for S. Typhimurium and L. monocytogenes were xylose lysine deoxycholate agar (XLD, Becton Dickinson, Franklin Lakes, NJ, USA) and modified oxford agar (MOX, Becton Dickinson, Franklin Lakes, NJ, USA), respectively. Bacterial colonies were counted and populations were expressed as log CFU/papaya. The detection limit was 2.40 log CFU/papaya.
2.3.2. PH of Papaya Skin Homogenate as Affected by Homogenization Parameters
Papayas were prepared as described in Section 2.2 except that they were not inoculated with pathogenic bacteria. The skin of the marked area was cut and homogenized with buffer in a stomacher bag under the conditions described above. Papaya skin was also homogenized with water as a control. pH of the homogenate was measured using a pH meter (Model pH 6+, Oakton Instruments, Vernon Hills, IL, USA).
2.4. Behavior of Pathogenic Bacteria on Whole Papayas Stored at Different Temperatures
After harvesting and packing, papayas are usually stored at 7–13 °C before being distributed to grocery stores [44]. At grocery stores and customers’ homes, papayas are usually placed at room temperature (21–25 °C). Hence, we selected 21 and 7 °C to simulate the two papaya storage scenarios. Inoculated whole papayas were individually placed in large sterile beakers and stored at 21 and 7 °C for 14 days. One papaya was randomly sampled, with the skin of the inoculated area being sterilely excised and collected for bacteria count on storage days 0, 7, 10 and 14. The papaya that was inoculated and dried for 1 h on the day of inoculation was considered as the sample on day 0. To determine bacterial population on papaya, the excised skin was homogenized using the optimized method from Section 2.3, which was homogenizing in PBS + T buffer at 250 rpm for 1 min for both S. Typhimurium and L. monocytogenes. Subsequently, the homogenates were serially diluted with 0.1% peptone water and plated using the agar overlay method described above. After incubation, bacterial colonies were counted and populations were expressed as log CFU/papaya.
2.5. ClO2 Treatment on Whole Papayas
2.5.1. Preparation of Aqueous ClO2
Aqueous ClO2 solutions were made on-site using a previous method [38]. Briefly, ClO2 stock solutions were prepared by mixing 10 mL of 4.0% NaClO2 (Fisher Scientific, Waltham, MA, USA) with 10 mL of 1 M HCl (Fisher Scientific, Waltham, MA, USA), lactic acid (VWR Chemicals, Radnor, PA, USA) or malic acid (Fisher Scientific) in aluminum foil-covered bottles. After reacting for 1 min, 100 mL of distilled water was added into the bottles. The final mixture was set at 21 °C for 20 min before being placed in a refrigerator at 4 °C. We previously investigated the generation kinetics and the stability of ClO2 [38]. As organic acids release hydrogen ions slowly, it took one week to achieve equilibrium. During the 14-day-experimentation, the ClO2 concentration increased till up to day seven and then remained stable for those generated with organic acids. For ClO2 generated with HCl, the reaction was quick and the concentration remained stable for up to eight days and eventually decreased. Therefore, the stock solutions were all stored for seven days to allow the completion of the reaction in malic acid- and lactic acid-produced ClO2 solutions and ensure no loss of the effectiveness of HCl-produced ClO2 solutions. On the day of experimentation, the concentration of ClO2 in each stock solution was measured using Chlordioxid-Test kit (EMD Millipore Corp., Burlington, MA, USA). The stock solutions were diluted with distilled water to 2.5, 5 and 10 ppm to treat papayas. The pH of each diluted solution was determined.
2.5.2. Washing Papayas with Aqueous ClO2 and Individual Acid Solutions
To wash artificially contaminated papayas, each papaya was inoculated with S. Typhimurium or L. monocytogenes as described in Section 2.2 and then submerged into a sterile container containing 1 L of ClO2 made with HCl, lactic acid or malic acid at concentrations of 2.5, 5 and 10 ppm. The submerged papayas were mildly stirred at a rate of 150 rpm for 5 min [45]. Subsequently, the washed fruits were dried under a biosafety hood for 15 min. After drying, the marked surface was sterilely cut and homogenized in 25 mL of PBS + T buffer at 250 rpm for 1 min. The homogenate was serially diluted and plated by the agar overlay method with XLD and MOX agar for the selection of S. Typhimurium and L. monocytogenes, respectively. Bacterial populations were expressed as log CFU/papaya, and the detection limit was 2.40 log CFU/papaya. Washing with distilled water and 200 ppm bleach (sodium hypochlorite (NaClO), pH 6.5) diluted from Clorox® (6.0% NaClO, The Clorox Company, Oakland, CA, USA) served as the control treatments.
Acid solutions were prepared by adjusting 1 L of distilled water individually with 1 M HCl, 1 M lactic acid or 1 M malic acid to the pH of 10 ppm ClO2 made with the corresponding acid. Papayas inoculated with S. Typhimurium or L. monocytogenes were washed with the acid solutions, and the remaining bacteria were collected and enumerated following the procedures described above.
2.5.3. ClO2 Residue on Papaya Surface after Washing
Papayas were washed with tap water and dried on a lab bench for 1 h. Subsequently, the papayas were washed with 1 L of ClO2 made with HCl, lactic acid or malic acid at concentrations of 5, 10 and 20 ppm. After drying for 15 min, the papayas were placed in 1-gallon Ziploc bags containing 100 mL distilled water. The papayas surfaces were hand massaged and rinsed thoroughly for 2 min, followed by filtering the rinse water into a flask [46]. 10 mL of the filtrate was collected and measured for ClO2 concentration using Chlordioxid-Test kit. The detection limit was 0.02 mg/L in the undiluted filtrate. The ClO2 concentration was converted into mg/kg papaya.
2.6. Statistical Analysis
All experiments were conducted in three independent replicates. Bacterial cultures were separately grown following the same procedure for each replicate. ClO2 solutions were prepared freshly for each replicate. Data were reported as mean ± standard deviation (SD). Analysis of variance and Tukey’s multiple comparison test were performed using SSPS software (IBM® SPSS® Statistics 24.0 for Windows, IBM Corp., Armonk, NY, USA). A significance level of 0.05 was used to determine the differences between the means of treatment groups.
3. Results and Discussion
3.1. Recovery of S. Typhimurium and L. monocytogenes Cells from Whole Papaya Surface as Affected by Homogenization Parameters and Enumeration Methods
Statistical analysis revealed no interactions among homogenization parameters, and only buffer significantly affected the bacterial count (p < 0.05). For S. Typhimurium (Table 1), papayas homogenized in buffers with the non-ionic surfactant Tween 80 resulted in significantly higher bacteria counts than those homogenized in peptone water alone. Tween 80 interrupts the hydrophobic interactions between bacteria cells and papaya surface and promotes the detachment of cells [47]. Papayas homogenized in the combination of PBS and Tween 80 (PBS + T) had the highest S. Typhimurium counts; an average of 5.36 log CFU was recovered from the initial inoculum of approximately 7 log CFU. Among all treatments, homogenization at 150 rpm for 5 min using XLD plating resulted in the highest recovery of 5.64 log CFU from papaya surface. For L. monocytogenes (Table 2), homogenization in PBS + T collected significantly more cells than in PBS alone (p < 0.05). Homogenization time, speed or plating method did not play a significant role in the collection. Homogenization at 150 rpm for 5 min by the agar overlay method resulted in the highest count of 5.09 log CFU. However, homogenization at 250 rpm for 1 min also resulted in relatively high L. monocytogenes counts. Homogenization at 250 rpm for 1 min was chosen for collecting S. Typhimurium and L. monocytogenes from papaya surface to maintain the time efficiency and consistency of the experiment. Even though the agar overlay method did not result in significantly higher bacteria counts than using selective agar alone, incubating on non-selective media before adding selective media would help recover bacteria cells injured by sanitizers [43]. It is an essential step to avoid over-estimation of the antimicrobial efficiency of sanitizers. Therefore, homogenizing the inoculated papaya piece in PBS + T at 250 rpm for 1 min was chosen, and the homogenate was decided to be plated by overlaying selective agar on PCA.

Table 1.
S. Typhimurium population (log CFU) recovered from papaya surface as affected by homogenization buffer, time (min), speed (rpm) and enumeration methods *.

Table 2.
L. monocytogenes population (log CFU) recovered from papaya surface as affected by homogenization buffer, time (min), speed (rpm) and enumeration methods *.
pH values of the above-mentioned homogenates were measured with uninoculated samples to compare buffering capacity between homogenization buffers. Even with careful excision, papaya flesh attached to the skin could acidify the homogenate. Papaya flesh has a pH of 4.87–5.7 [16,18]. This pH range does not inhibit the growth of S. Typhimurium or L. monocytogenes; however, it could influence the recovery of cells injured by desiccation [43]. Tian et al. incubated sublethally injured E. coli O157:H7 cells in nutrient broth at pH 4.0, 5.0, 6.0, 7.2 and 8.0. They found that the cells showed no significant recovery at pH 4.0 and 8.0 whereas the cells recovered by 0.48, 0.49 and 0.72 log CFU/mL in pH 5.0, 6.0 and 7.2, respectively, indicating that pH even at relatively high levels (5.0 and 6.0) did affect the recovery of sublethally injured cells [48]. Shown in Table 3, homogenizing papaya skin in different buffers resulted in significant differences in homogenate acidity in a descent order of PBS, PBS + T, PEPT, water and PEPT + T (p < 0.05). The initial pH value of each buffer was measured with PBS, PBS + T and water being neutral whereas PEPT and PEPT + T being slightly acidic (pH = 6.5–6.7). PBS is known for its high buffering capacity, whereas water and peptone water have little buffering capacity. When mixed with the papaya juice, the pH of water and peptone water decreased to 5.89–6.26. The pH of the homogenate may affect the state of cells, and this is consistent with the higher cell counts observed in PBS + T. Peptone water is often used in studies involving fresh produce [20,23,49]. Researchers should carefully select homogenization buffers since peptone water alone may lead to experimental errors in studies with acidic produce.

Table 3.
pH of papaya skin homogenate as affected by homogenization buffer type, time (min) and speed (rpm) *.
3.2. Behavior of Pathogenic Bacteria on Whole Papayas Stored at Different Temperatures
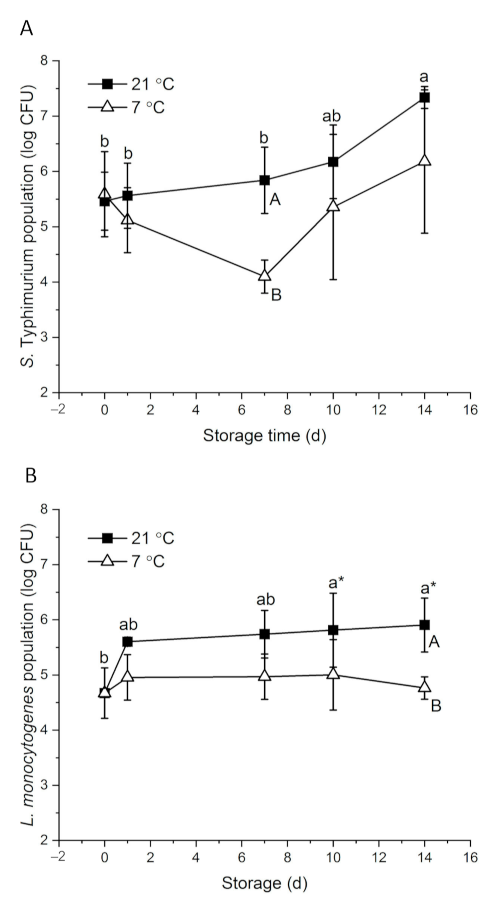
With about 7 log CFU of initial inocula, 5.46 and 4.67 log CFU S. Typhimurium and L. monocytogenes were detected on papaya surfaces on day 0, respectively (Figure 1). Bacteria response to environmental stress differently. Salmonella showed higher desiccation tolerance than L. monocytogenes in powdered infant formula and desiccated shredded coconut [50,51]. S. Typhimurium had an interesting survival and growth pattern. At 21 °C, the population increased gradually to 7.34 log CFU on day 14. At 7 °C, S. Typhimurium level decreased to 4.10 log CFU on day 7 and then increased to 6.18 log CFU at the end of the storage period (Figure 1A). Intrinsic factors of fruit, including surface roughness, surface hydrophobicity, nutrient and moisture availability and background flora, may affect the behavior of foodborne pathogenic bacteria on the fruit [8]. At ambient temperature, S. enterica level remained stable on whole mangos stored at 20–22 °C for nine days [52]. Salmonella was reduced by about 5 and 2 log CFU at high (~7 log) and low (~4 log) inoculation levels, respectively, on whole kiwifruits stored at room temperatures for 10 days [14]. On whole cucumbers stored at 23 °C, Salmonella level significantly increased by 1.7 log CFU within the first day of inoculation and remained stable for four days [53]. Looking at the fruit type alone, at commercial cold storage conditions (7–12 °C), S. Typhimurium level did not significantly change on whole papaya or mango at the end of the storage period [54]. However, Salmonella tended to decrease over time on other fruits, such as passionfruit, strawberry, cucumber and peppers [53,54,55,56]. Different from other tropical fruits, sugar accumulates on papaya surfaces as ripening progresses, which provides more nutrients for the attached microorganisms. Naturally present yeast may also aid the growth of S. Typhimurium by their saccharolytic interactions with the compounds permeated through papaya skin [57].
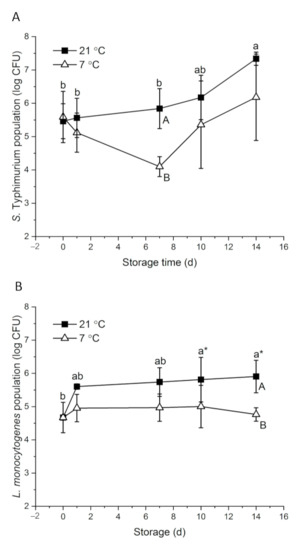
Figure 1.
Behavior of S. Typhimurium (A) and L. monocytogenes (B) on whole papayas at 21 and 7 °C. Error bars are standard deviations (n = 3). Different lower-case letters horizontally indicate significant differences between the means of different time points at each temperature (p < 0.05). Different upper-case letters vertically indicate significant differences (p < 0.05) between the means of different temperatures at the same time point. “a*” means p values were marginal, which were 0.058 and 0.059 on day 10 and day 14, respectively, compared with day 0.
L. monocytogenes showed a major increase from 4.67 to 5.60 log CFU during the 1st day of storage at 21 °C, and then gradually grew to 5.91 log CFU in the following 13 days. At 7 °C, L. monocytogenes level remained stable on papayas for 14 days (Figure 1B). The behavior of L. monocytogenes on fruits varies. L. monocytogenes grew on whole cucumbers stored at 4 °C and grew on fresh Gala apples stored at 5 °C and 25 °C [53,57]. However, on Granny Smith apples, 1.5 log CFU and 0.5–1.2 log CFU reductions were observed at 25 and 22 °C, respectively, in two studies [13,57]. The reductions of L. monocytogenes on whole cantaloupe and mango were also reported [12,49]. Aside from the intrinsic differences of the fruits, initial inoculation levels and the carrying capacity of the fruit may contribute to the varied behavior of L. monocytogenes [8,18]. Approximately three-fold more L. monocytogenes died on whole kiwi fruits inoculated with 7 log CFU than those inoculated with 4 log CFU at room temperature over 10 days [14]. In the case of organic Granny Smith apples, L. monocytogenes decreased by 1.8 and 0.7 log CFU at inoculation levels of 6.3 and 3.0 log CFU, respectively, at 22 °C over two weeks [13]. Papayas could have a higher carrying capacity than the above-mentioned fruits, leading to the growth of L. monocytogenes on papayas even at a relatively high inoculation level. Regardless, L. monocytogenes is known for its ability to adapt to cold temperatures through mechanisms of alternating membrane fatty acid composition, synthesizing cold shock proteins and cold acclimation proteins and activating energy providing pathways such as glycolysis [58].
S. Typhimurium and L. monocytogenes showed abilities to survive and grow on papaya, and hence effective sanitation methods are essential for papaya production.
3.3. Inactivation of S. Typhimurium and L. monocytogenes on Whole Papayas Using Aqueous ClO2
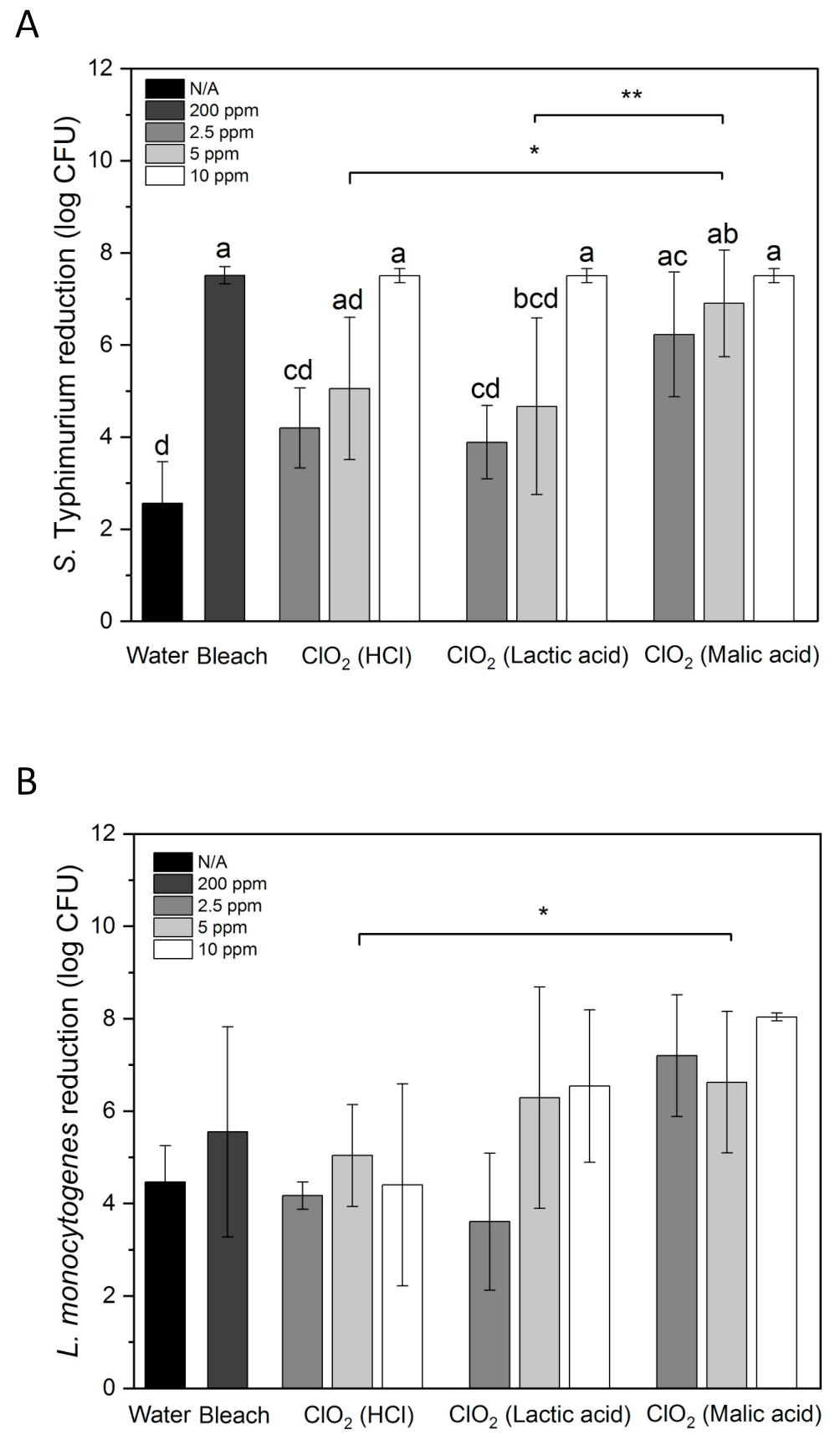
Figure 2A shows S. Typhimurium reduction by water, aqueous ClO2, and bleach on whole papayas. 10 ppm of ClO2 was significantly more effective than 2.5 and 5 ppm (p < 0.05). 10 ppm of ClO2 reduced S. Typhimurium from the initial inoculation level of 7.5 log CFU to an undetectable level. 200 ppm of bleach achieved the same result. Malic acid-produced ClO2 reduced S. Typhimurium by 6.23 and 6.90 log CFU at 2.5 and 5 ppm, respectively. HCl- and lactic acid-produced ClO2 reduced S. Typhimurium by 4.20 and 5.05 log CFU, and 3.89 and 4.67 log CFU at 2.5 and 5 ppm, respectively. Overall, ClO2 solutions generated with malic acid inactivated significantly higher numbers of S. Typhimurium than the solutions generated with HCl or lactic acid (p < 0.05). 1.74–2.01 and 0.86–1.97 log CFU/cm2 Salmonella was inactivated in 100 ppm free chlorine and 80 ppm peracetic acid with scrubbing by sponges/microfiber, respectively [35]. Comparing with these results, the microbial reduction on papayas achieved by immersing in ClO2 for 5 min seems more effective.
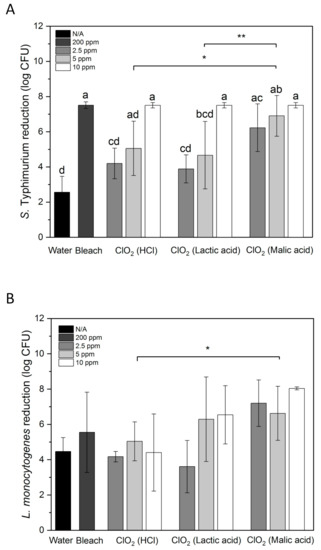
Figure 2.
S. Typhimurium (A) and L. monocytogenes (B) reduction by water, 200 ppm bleach, and aqueous ClO2 generated by mixing NaClO2 with HCl, lactic acid or malic acid on whole papayas. Error bars are standard deviations (n = 3). Bars labeled with different letters indicate significant differences between the means of treatments (p < 0.05). Lines labeled with “*” indicate significant differences between ClO2 groups made with different acids (“*”, p < 0.05; “**”, p < 0.01).
Water treatment only removed 2.56 log CFU of S. Typhimurium from papaya surface, whereas 4.47 log CFU of L. monocytogenes was removed by water (Figure 2). This may be partially due to that S. Typhimurium attached stronger to papaya surfaces than L. monocytogenes. In a study conducted by Collignon and Korsten [42], S. Typhimurium adhered to peach immediately after contact, whereas L. monocytogenes required 60 s for the adhesion. Higher numbers of S. Typhimurium cells were observed in one hour than L. monocytogenes on peach.
ClO2 produced with HCl did not show higher effectiveness in reducing L. monocytogenes than water (Figure 2B). ClO2 produced using lactic acid had increased bacterial reductions than HCl-produced ClO2 at 5 and 10 ppm but with large variations. Malic acid-produced ClO2 showed the highest L. monocytogenes reduction among all ClO2 treatments. However, there was no significant difference between the three tested concentrations. The group treated with ClO2 made with malic acid showed statistically higher bacterial reduction than the group treated with ClO2 made with HCl (p < 0.05). 2.5, 5 and 10 ppm of malic acid-generated ClO2 reduced L. monocytogenes by 7.20, 6.63 and 8.04 log CFU, respectively. These reductions were higher than the L. monocytogenes reductions on apples, lettuce, strawberries and cantaloupe treated with 5 ppm ClO2 made with phosphoric acid (~5.6 log CFU) [59]. L. monocytogenes-contaminated papayas treated with 200 ppm bleach also showed a relatively large variation with an average reduction of 5.5 log CFU, which was lower than all samples treated with malic acid-generated ClO2. However, the concentration of bleach was much higher than that of ClO2, indicating the high antimicrobial efficiency of ClO2. This result agrees with the higher reduction of L. monocytogenes on blueberries treated with 10 ppm ClO2 (1.7 log CFU/g) than those treated with 200 ppm chlorine (1.0 log CFU/g) for 5 min [23].
ClO2 generated with malic acid inactivated significantly more S. Typhimurium and L. monocytogenes than ClO2 generated with HCl. This result is consistent with our previous observation of the high antimicrobial efficiency of ClO2 generated with organic acids. In particular, malic acid-generated ClO2 had higher efficacy in killing S. Typhimurium and L. monocytogenes than HCl-, sodium bisulfate-, citric acid- and lactic acid- generated ClO2 [38]. This conclusion was drawn from experiments conducted on bacteria cell suspensions and Romaine lettuce. We hypothesized that synergistic effects between ClO2 and the excessive organic acids in the ClO2 solutions may contribute to the high antimicrobial efficiency of organic acid-generated ClO2. We treated contaminated papayas with individual acid solutions to confirm this hypothesis. Since the pH of ClO2 decreased with the increase of its concentration (data not shown), pH values corresponding to 10 ppm ClO2 were selected for the decontamination experiments with acids alone. This means the pH of HCl, lactic acid and malic acid solutions were adjusted to 3.15, 3.42 and 3.36, respectively. S. Typhimurium on papayas treated with the acids was reduced by 2.45–3.01 log CFU, which was not significantly different from the samples treated with water (Table 4, p > 0.05). Similarly, L. monocytogenes on papayas treated with the acids was reduced by 3.58–3.91 log CFU and was not significantly different from the samples treated with water (p > 0.05). Hence these results confirmed the high antimicrobial effect of ClO2 solutions made with malic acid and lactic acid was contributed little by the excessive organic acids, but rather a synergistic effect between ClO2 and organic acids. The combination treatment of 2.0% lactic acid and 10 ppm ClO2 resulted in higher reductions of S. Typhimurium and L. monocytogenes on blueberries than the treatments by each sanitizer alone [60]. On papaya, ClO2 produced with lactic acid interestingly had similar killing effects to ClO2 produced with HCl, yet ClO2 produced with malic acid still performed better than that with HCl. In many studies, lactic acid was either better or as good as malic acid in the inactivation of pathogens when used alone as the sanitizers [61,62]. The synergistic effect somehow altered the antimicrobial efficiency of lactic acid and malic acid. Another factor may contribute to the altered antimicrobial efficacy of the organic-acid-generated ClO2 compared with HCl-generated ClO2 is the intermediate compounds produced in the ClO2 solutions. ClO2 solution is a mixture of pure ClO2 and oxidative chlorine compounds such as ClO2−, ClO3−, free chlorine (Cl2), hypochlorous acid (HOCl) and hypochlorite ion (OCl−) [32]. These oxy-species varies in oxidation capacity and stability. Since the pKa values of lactic acid and malic acid are different, ClO2 solutions generated with the two organic acids reach equilibrium differently and have different intermediate compound compositions. Measures of the intermediate compound compositions and their chemical oxygen demand would help further understand the mechanisms underlining the different antimicrobial efficacies between various ClO2 solutions.

Table 4.
S. Typhimurium and L. monocytogenes reduction (log CFU) by water, HCl, lactic acid and malic acid on whole papayas *.
Additionally, CFR Sec. 173.300 specifies that ClO2 can be used in fresh produce wash with a rinse procedure, and ClO2 residue in the wash water of the applied fresh produce should not exceed 3 ppm [25]. EPA also specifies that ClO2 is allowed to rinse fruits and vegetables at a concentration of 5 ppm [63]. Some literature also suggests that the residue on the washed produce should not exceed 3 ppm [64,65]. In this study, the ClO2 residue on papayas after being treated with 5, 10 and 20 ppm ClO2 solutions ranged from 8.0 × 10−5 to 6.2 × 10−3 mg/kg, which were far below 3 ppm (Table 5). These numbers were also far below the EPA regulation of 0.8 mg/L ClO2 residue in public drinking water [27]. Tomatoes and strawberries treated with 0.5 ppm gaseous ClO2 for 10 min had 0.09 and 0.37 mg/kg ClO2 residue [29]. ClO2 residue on produce treated with gaseous ClO2 was much higher than ClO2 residue on papayas treated with aqueous ClO2, providing insights into safety concerns in the application of ClO2. However, future studies of ClO2− reside on food matrix treated with ClO2 should be carried out as ClO2− and ClO3− are harmful disinfection by-products (DPBs) that can cause anemia and thyroid dysfunction in animals [26].

Table 5.
ClO2 residue (mg/kg) on papaya surface after being washed with ClO2 *.
4. Conclusions
To provide potential solutions to the emerging issue of foodborne illness outbreaks associated with whole papayas, this study investigated the survival of S. Typhimurium and L. monocytogenes on whole papaya during storage at 21 and 7 °C and determined the efficiency of aqueous ClO2 in inactivating the two pathogenic bacteria on whole papaya. Temperature played a significant role in the survival and growth of the bacteria on the fruit. S. Typhimurium grew by 1.88 log CFU on whole papaya in 14 days at 21 °C and remained at the initial inoculation level at 7 °C. L. monocytogenes grew by 0.93 log CFU on papaya during the 1st day of storage at 21 °C; the level remained stable thereafter at 21 °C and at 7 °C. The acid used to produce aqueous ClO2 influenced the killing efficiency of ClO2 against these pathogenic bacteria. ClO2 solutions generated with malic acid reduced significantly higher levels of S. Typhimurium and L. monocytogenes than the solution generated with HCl. Methodology wise, we optimized the methods for recovering pathogenic bacteria cells from papaya surface, which was a crucial step evaluating bacterial behavior on fresh produce. This study also provided information on ClO2 residue on the washed papayas. These results give insights on risk assessment and management of microbiological safety issues associated with whole papaya. Further studies including the intermediate compound compositions in various ClO2 solutions and the residue of DPBs on ClO2 treated food matrix are suggested to better understand the antimicrobial mechanisms and safety concerns regarding using aqueous ClO2.
Author Contributions
Conceptualization, L.D. and Y.L.; methodology, L.D. and Y.L.; investiga-tion, L.D.; formal analysis, L.D.; resources, Y.L.; writing—original draft preparation, L.D.; writ-ing—review and editing, L.D. and Y.L.; supervision, Y.L. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the State of Hawaii Department of Agriculture and the Hawaii Farm Bureau Grant No. 64921 and the United States Department of Agriculture-Agricultural Research Service Agreement No. 58-2040-8-010.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Evans, E.A.; Ballen, F.H.; Crane, J.H. An Overview of US Papaya Production, Trade, and Consumption; Electronic Data Information Source (EDIS) FE914; University of Florida: Gainesville, FL, USA, 2012; Volume 9, pp. 1–8. [Google Scholar]
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 23 May 2021).
- Gibbs, R.; Pingault, N.; Mazzucchelli, T.; O’Reilly, L.; MacKenzie, B.; Green, J.; Mogyorosy, R.; Stafford, R.; Bell, R.; Hiley, L.; et al. An Outbreak of Salmonella enterica Serotype Litchfield Infection in Australia Linked to Consumption of Contaminated Papaya. J. Food Prot. 2009, 72, 1094–1098. [Google Scholar] [CrossRef]
- Hassan, R.; Whitney, B.; Williams, D.L.; Holloman, K.; Grady, D.; Thomas, D.; Omoregie, E.; Lamba, K.; Leeper, M.; Gieraltowski, L.; et al. Multistate outbreaks of Salmonella infections linked to imported Maradol papayas—United States, December 2016–September 2017. Epidemiol. Infect. 2019, 147, e265. [Google Scholar] [CrossRef]
- FDA. Letter to Papaya Grower, Harvesters, Packers, Distributors, Exporters, Importers, and Retailers Concerning Food-Borne Illness Outbreaks Tied to Papayas. 2019. Available online: https://www.fda.gov/media/130271/download (accessed on 23 May 2021).
- de Oliveira, J.G.; Vitória, A.P. Papaya: Nutritional and pharmacological characterization, and quality loss due to physiological disorders. An overview. Food Res. Int. 2011, 44, 1306–1313. [Google Scholar] [CrossRef]
- Strawn, L.K.; Schneider, K.R.; Danyluk, M.D. Microbial Safety of Tropical Fruits. Crit. Rev. Food Sci. Nutr. 2011, 51, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Marik, C.M.; Zuchel, J.; Schaffner, D.W.; Strawn, L.K. Growth and Survival of Listeria monocytogenes on Intact Fruit and Vegetable Surfaces During Postharvest Handling: A Systematic Literature Review. J. Food Prot. 2019, 83, 108–128. [Google Scholar] [CrossRef] [PubMed]
- CDC. Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms. Colorado|Listeria|CDC. 2012. Available online: https://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/index.html (accessed on 23 May 2021).
- CDC. Multistate Outbreak of Listeriosis Linked to Commercially Produced, Prepackaged Caramel Apples Made from Bidart Bros. Apples|Listeria|CDC. 2015. Available online: https://www.cdc.gov/listeria/outbreaks/caramel-apples-12-14/index.html (accessed on 23 May 2021).
- Poimenidou, S.V.; Chatzithoma, D.-N.; Nychas, G.-J.; Skandamis, P.N. Adaptive Response of Listeria monocytogenes to Heat, Salinity and Low pH, after Habituation on Cherry Tomatoes and Lettuce Leaves. PLoS ONE 2016, 11, e0165746. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Vargas, E.; Luna-Rojo, A.M.; Cadena-Ramírez, A.; Torres-Vitela, R.; Gomez-Aldapa, C.A.; Villarruel-López, A.; Téllez-Jurado, A.; Villagómez-Ibarra, J.R.; Reynoso-Camacho, R.; Castro-Rosas, J. Behavior of 11 Foodborne Bacteria on Whole and Cut Mangoes var. Ataulfo and Kent and Antibacterial Activities of Hibiscus sabdariffa Extracts and Chemical Sanitizers Directly onto Mangoes Contaminated with Foodborne Bacteria. J. Food Prot. 2018, 81, 743–753. [Google Scholar] [CrossRef]
- Sheng, L.; Edwards, K.; Tsai, H.-C.; Hanrahan, I.; Zhu, M.-J. Fate of Listeria monocytogenes on Fresh Apples under Different Storage Temperatures. Front. Microbiol. 2017, 8, 1396. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, L. Survival of Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes on Fresh and Sliced Green and Golden Kiwifruits. Foodborne Pathog. Dis. 2018, 15, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hu, W.; Jiang, A.; Xu, Y.; Sarengaowa; Li, X.; Bai, X. Growth Potential of Listeria Monocytogenes and Staphylococcus Aureus on Fresh-Cut Tropical Fruits. J. Food Sci. 2015, 80, M2548–M2554. [Google Scholar] [CrossRef] [PubMed]
- Penteado, A.L.; Leitão, M.F. Growth of Listeria monocytogenes in melon, watermelon and papaya pulps. Int. J. Food Microbiol. 2004, 92, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Penteado, A.L.; Leitão, M.F. Growth of Salmonella Enteritidis in melon, watermelon and papaya pulp stored at different times and temperatures. Food Control 2004, 15, 369–373. [Google Scholar] [CrossRef]
- Strawn, L.K.; Danyluk, M.D. Fate of Escherichia coli O157:H7 and Salmonella spp. on fresh and frozen cut mangoes and papayas. Int. J. Food Microbiol. 2010, 138, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yemmireddy, V. Fate of Salmonella spp. in fresh-cut papaya (Carica papaya L.) at different storage temperature and relative humidity. LWT 2021, 148, 111810. [Google Scholar] [CrossRef]
- Huff, K.; Boyer, R.; Denbow, C.; O’Keefe, S.; Williams, R. Effect of Storage Temperature on Survival and Growth of Foodborne Pathogens on Whole, Damaged, and Internally Inoculated Jalapeños (Capsicum annuum var. annuum). J. Food Prot. 2012, 75, 382–388. [Google Scholar] [CrossRef]
- Penteado, A.L.; Eblen, B.S.; Miller, A.J. Evidence of Salmonella Internalization into Fresh Mangos during Simulated Postharvest Insect Disinfestation Procedures. J. Food Prot. 2004, 67, 181–184. [Google Scholar] [CrossRef]
- Perez-Rodriguez, F.; Begum, M.; Johannessen, G. Study of the cross-contamination and survival of Salmonella in fresh apples. Int. J. Food Microbiol. 2014, 184, 92–97. [Google Scholar] [CrossRef]
- Tadepalli, S.; Bridges, D.F.; Driver, R.; Wu, V.C. Effectiveness of different antimicrobial washes combined with freezing against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes inoculated on blueberries. Food Microbiol. 2018, 74, 34–39. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. CFR173.300. CFR—Code of Federal Regulations Title 21. 2020. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=173.300 (accessed on 23 May 2021).
- Gómez-López, V.M.; Rajkovic, A.; Ragaert, P.; Smigic, N.; Devlieghere, F. Chlorine dioxide for minimally processed produce preservation: A review. Trends Food Sci. Technol. 2009, 20, 17–26. [Google Scholar] [CrossRef]
- Van Haute, S.; Tryland, I.; Escudero, C.; Vanneste, M.; Sampers, I. Chlorine dioxide as water disinfectant during fresh-cut iceberg lettuce washing: Disinfectant demand, disinfection efficiency, and chlorite formation. LWT 2017, 75, 301–304. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#one (accessed on 11 July 2021).
- Adhikari, A.; Chhetri, V.; Bhattacharya, D.; Cason, C.; Luu, P.; Suazo, A. Effectiveness of daily rinsing of alfalfa sprouts with aqueous chlorine dioxide and ozonated water on the growth of Listeria monocytogenes during sprouting. Lett. Appl. Microbiol. 2019, 69, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Banach, J.; van Overbeek, L.; Groot, M.N.; van der Zouwen, P.; van der Fels-Klerx, H. Efficacy of chlorine dioxide on Escherichia coli inactivation during pilot-scale fresh-cut lettuce processing. Int. J. Food Microbiol. 2018, 269, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ni Tan, J.; Hwang, C.-A.; Huang, L.; Wu, V.C.H.; Hsiao, H.-I. In Situ Generation of Chlorine Dioxide for Decontamination of Salmonella, Listeria monocytogenes, and Pathogenic Escherichia coli on Cantaloupes, Mung Beans, and Alfalfa Seeds. J. Food Prot. 2020, 83, 287–294. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C.; Han, Z. Effects of aqueous chlorine dioxide treatment on nutritional components and shelf-life of mulberry fruit (Morus alba L.). J. Biosci. Bioeng. 2011, 111, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; Vaidya, N.; Linton, R.; Morgan, M. Evaluation of Chlorine Dioxide Gas Residues on Selected Food Produce. J. Food Sci. 2010, 76, T11–T15. [Google Scholar] [CrossRef] [PubMed]
- Boonyaritthongchai, P.; Techavuthiporn, C.; Cumsingnok, T. Effect of Acidified Sodium Chlorite and Packaging on Microbial Reduction and Quality Maintenance of Shredded Green Papaya; Acta Hortic: Istanbul, Turkey, 2018; Volume 1292, pp. 287–292. [Google Scholar] [CrossRef]
- Yeoh, W.K.; Ali, A.; Forney, C. Effects of ozone on major antioxidants and microbial populations of fresh-cut papaya. Postharvest Biol. Technol. 2014, 89, 56–58. [Google Scholar] [CrossRef]
- Gu, G.; Bolten, S.; Mendes-Oliveira, G.; Zhou, B.; Teng, Z.; Pearlstein, D.; Luo, Y.; Millner, P.; Nou, X. Salmonella inactivation and sponge/microfiber mediated cross-contamination during papaya wash with chlorine or peracetic acid as sanitizer. Food Microbiol. 2020, 95, 103677. [Google Scholar] [CrossRef]
- Gordon, G.; Rosenblatt, A.A. Chlorine Dioxide: The Current State of the Art. Ozone Sci. Eng. 2005, 27, 203–207. [Google Scholar] [CrossRef]
- Kim, H.; Kang, Y.; Beuchat, L.R.; Ryu, J.-H. Production and stability of chlorine dioxide in organic acid solutions as affected by pH, type of acid, and concentration of sodium chlorite, and its effectiveness in inactivating Bacillus cereus spores. Food Microbiol. 2008, 25, 964–969. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y. Comparison of aqueous chlorine dioxide generated with different acids on reducing foodborne pathogenic bacteria. In Proceedings of the International Association for Food Protection 2020 Annual Meeting, Des Moines, IA, USA, 26–28 October 2020. [Google Scholar]
- Lang, M.M.; Harris, L.J.; Beuchat, L.R. Evaluation of Inoculation Method and Inoculum Drying Time for Their Effects on Survival and Efficiency of Recovery of Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes Inoculated on the Surface of Tomatoes. J. Food Prot. 2004, 67, 732–741. [Google Scholar] [CrossRef]
- Kim, S.-R.; Yoon, Y.; Kim, W.-I.; Park, K.-H.; Yun, H.-J.; Chung, D.H.; Yun, J.C.; Ryu, K.Y. Comparison of Sample Preparation Methods for the Recovery of Foodborne Pathogens from Fresh Produce. J. Food Prot. 2012, 75, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Suzuki, J.Y.; Carr, J.B.; McQuate, G.T.; Ferreira, S.A.; Manshardt, R.M.; Pitz, K.Y.; Wall, M.M.; Gonsalves, D. Nutritional composition of Rainbow papaya, the first commercialized transgenic fruit crop. J. Food Compos. Anal. 2011, 24, 140–147. [Google Scholar] [CrossRef]
- Collignon, S.; Korsten, L. Attachment and Colonization by Escherichia coli O157:H7, Listeria monocytogenes, Salmonella enterica subsp. enterica serovar Typhimurium, and Staphylococcus aureus on Stone Fruit Surfaces and Survival through a Simulated Commercial Export Chain. J. Food Prot. 2010, 73, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.H. A review of microbial injury and recovery methods in food. Food Microbiol. 2008, 25, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Paull, R.E.; Chen, N.J. Papaya: Postharvest Quality-Maintenance Guidelines. Fruit, Nut, and Beverage Crops; UH–CTAHR Extension Publication: Honolulu, HI, USA, 2014; Volume 34, pp. 2–4. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/F_N-34.pdf (accessed on 23 May 2021).
- Visvalingam, J.; Holley, R.A. Evaluation of chlorine dioxide, acidified sodium chlorite and peroxyacetic acid for control of Escherichia coli O157:H7 in beef patties from treated beef trim. Food Res. Int. 2018, 103, 295–300. [Google Scholar] [CrossRef]
- Wu, V.C.; Rioux, A. A simple instrument-free gaseous chlorine dioxide method for microbial decontamination of potatoes during storage. Food Microbiol. 2010, 27, 179–184. [Google Scholar] [CrossRef]
- Brandl, M.T.; Huynh, S. Effect of the Surfactant Tween 80 on the Detachment and Dispersal of Salmonella enterica Serovar Thompson Single Cells and Aggregates from Cilantro Leaves as Revealed by Image Analysis. Appl. Environ. Microbiol. 2014, 80, 5037–5042. [Google Scholar] [CrossRef]
- Tian, X.; Yu, Q.; Shao, L.; Li, X.; Dai, R. Sublethal injury and recovery of Escherichia coli O157:H7 after ohmic heating. Food Control 2018, 94, 85–92. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zhong, Q. Quality attributes and microbial survival on whole cantaloupes with antimicrobial coatings containing chitosan, lauric arginate, cinnamon oil and ethylenediaminetetraacetic acid. Int. J. Food Microbiol. 2016, 235, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Koseki, S.; Nakamura, N.; Shiina, T. Comparison of Desiccation Tolerance among Listeria monocytogenes, Escherichia coli O157:H7, Salmonella enterica, and Cronobacter sakazakii in Powdered Infant Formula. J. Food Prot. 2015, 78, 104–110. [Google Scholar] [CrossRef]
- Dhowlaghar, N.; Tang, J.; Zhu, M.-J. Thermal inactivation of Salmonella, Listeria monocytogenes and Enterococcus faecium NRRL B-2354 in desiccated shredded coconut. LWT 2021, 149, 111851. [Google Scholar] [CrossRef]
- Mathew, E.N.; Muyyarikkandy, M.S.; Kuttappan, D.; Amalaradjou, M.A. Attachment of Salmonella enterica on Mangoes and Survival Under Conditions Simulating Commercial Mango Packing House and Importer Facility. Front. Microbiol. 2018, 9, 1519. [Google Scholar] [CrossRef]
- Bardsley, C.A.; Truitt, L.N.; Pfuntner, R.C.; Danyluk, M.D.; Rideout, S.L.; Strawn, L.K. Growth and Survival of Listeria monocytogenes and Salmonella on Whole and Sliced Cucumbers. J. Food Prot. 2019, 82, 301–309. [Google Scholar] [CrossRef]
- Behrsing, J.; Jaeger, J.; Horlock, F.; Kita, N.; Franz, P.; Premier, R. Survival of Listeria innocua, Salmonella salford and Escherichia coli on the surface of fruit with inedible skins. Postharvest Biol. Technol. 2003, 29, 249–256. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Gomez-Aldapa, C.; Acevedo-Sandoval, O.A.; Ramírez, C.A.G.; Villagomez-Ibarra, J.R.; Hernández, N.C.; Villarruel-Lopez, A.; Torres-Vitela, M.D.R. Frequency and Behavior of Salmonella and Escherichia coli on Whole and Sliced Jalapeño and Serrano Peppers. J. Food Prot. 2011, 74, 874–881. [Google Scholar] [CrossRef]
- Knudsen, D.M.; Yamamoto, S.A.; Harris, L.J. Survival of Salmonella spp. and Escherichia coli O157:H7 on Fresh and Frozen Strawberries. J. Food Prot. 2001, 64, 1483–1488. [Google Scholar] [CrossRef]
- Salazar, J.K.; Carstens, C.K.; Bathija, V.M.; Narula, S.S.; Parish, M.; Tortorello, M.L. Fate of Listeria monocytogenes in Fresh Apples and Caramel Apples. J. Food Prot. 2016, 79, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Wiedmann, M. Physiology and Genetics of Listeria Monocytogenes Survival and Growth at Cold Temperatures. Crit. Rev. Food Sci. Nutr. 2008, 49, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S.L.; Cash, J.N.; Siddiq, M.; Ryser, E.T. A Comparison of Different Chemical Sanitizers for Inactivating Escherichia coli O157:H7 and Listeria monocytogenes in Solution and on Apples, Lettuce, Strawberries, and Cantaloupe. J. Food Prot. 2004, 67, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, S.; Bridges, D.F.; Anderson, R.; Zhang, R.; Wu, V.C. Synergistic effect of sequential wash treatment with two different low-dosage antimicrobial washes in combination with frozen storage increases Salmonella Typhimurium and Listeria monocytogenes reduction on wild blueberries. Food Control 2019, 102, 87–93. [Google Scholar] [CrossRef]
- Almasoud, A.; Hettiarachchy, N.; Rayaprolu, S.; Babu, D.; Kwon, Y.M.; Mauromoustakos, A. Inhibitory effects of lactic and malic organic acids on autoinducer type 2 (AI-2) quorum sensing of Escherichia coli O157:H7 and Salmonella Typhimurium. LWT 2016, 66, 560–564. [Google Scholar] [CrossRef]
- Mohan, A.; Pohlman, F. Role of organic acids and peroxyacetic acid as antimicrobial intervention for controlling Escherichia coli O157:H7 on beef trimmings. LWT 2016, 65, 868–873. [Google Scholar] [CrossRef]
- EPA. Reregistration Eligibility Decision (RED) for Chlorine Dioxide and Sodium Chlorite (Case 4023). 2006. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-020503_3-Aug-06.pdf (accessed on 23 May 2021).
- Mathew, E.N.; Muyyarikkandy, M.S.; Bedell, C.; Amalaradjou, M.A. Efficacy of Chlorine, Chlorine Dioxide, and Peroxyacetic Acid in Reducing Salmonella Contamination in Wash Water and on Mangoes Under Simulated Mango Packinghouse Washing Operations. Front. Sustain. Food Syst. 2018, 2, 18. [Google Scholar] [CrossRef]
- Pao, S.; Kelsey, D.F.; Long, W. Spray washing of tomatoes with chlorine dioxide to minimize Salmonella on inocu-lated fruit surfaces and cross-contamination from revolving brushes†. J. Food Prot. 2009, 72, 2448–2452. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).