Abstract
In meat processing, changes in the myofibrillar protein (MP) structure can affect the quality of meat products. High hydrostatic pressure (HHP) has been widely utilized to change the conformational structure (secondary, tertiary and quaternary structure) of MP so as to improve the quality of meat products. However, a systematic summary of the relationship between the conformational structure (secondary and tertiary structure) changes in MP, gel properties and product quality under HHP is lacking. Hence, this review provides a comprehensive summary of the changes in the conformational structure and gel properties of MP under HHP and discusses the mechanism based on previous studies and recent progress. The relationship between the spatial structure of MP and meat texture under HHP is also explored. Finally, we discuss considerations regarding ways to make HHP an effective strategy in future meat manufacturing.
1. Introduction
Protein is one of the most important elements of nutrition for humans, providing energy and essential amino acids. Meat is the most valuable livestock product and, therefore, the preferred protein source of many people [1]. In addition, meat can be part of a balanced diet, providing valuable nutrients that are beneficial to health and essential for human growth and development [2,3]. Consumers seek meat products that are of a high quality, are safe to eat and are fresh. Safety and natural flavors with no additives (i.e., preservatives and moisturizers) are their primary requirements [4]. Based on this demand, a new processing technology was developed in the recent years known as high hydrostatic pressure (HHP), which shows the possibility for achieving better processing and preservation of meat, poultry and seafood [5].
HHP treatment is a non-thermal processing method that has been widely used in the fields of seafood, fruit, vegetable and meat products [5]. HHP is a completely physical process in which food is subject to uniform pressure treatment from all directions.
Treatment with HHP is not limited by the shape or size of the material, and the original shape of the solid food can be maintained [6]. Meat is mainly composed of water, protein and lipids [7]. The relatively high protein content (i.e., 16–40%) in meat plays an important role in food flavor, nutrition, and health [8,9]. Compared to the traditional heating process, HHP technology only affects the non-covalent structure in foods [10,11]. HHP can preserve freshness and nutritional values of foods by preserving vitamins and free amino acids and reducing losses of desirable compounds [12,13]. Therefore, the effect of HHP on protein in meat products is central to the importance of HHP technology in the manufacture of meat products.
Muscle protein mainly consists of myofibril protein, comprising two-thirds of the total proteins in lean muscle. In meat product processing, changes in MP will alter the quality of meat, including its appearance, hardness, springiness, chewiness, juiciness, taste and other texture characteristics [14,15,16]. Therefore, the effect of HHP on the structure of MP has been studied for many years. In this paper, research on the changes in MP under HHP in recent years is summarized. The relationship between MP structure and meat quality is discussed. The challenges and prospects for future applications of HHP in meat industry are also outlined.
1.1. Myofibrillar Protein (MP)
Myofibrillar protein, a salt-soluble protein, accounts for approximately 55% of muscle protein content and mainly includes tropomyosin, myosin, myogenic protein, and actin [17]. Myosin and actin are the most important functional and structural MP components that contribute to meat texture [18]. Myosin is a macromolecular protein with a shape similar to that of a bean sprout, and with a molecular weight of approximately 520 kDa, consists of two heavy chains and four light chains [17]. The myosin heavy chain forms the stem of the bean sprout, and the light chain forms the petals of the bean sprout [18]. In addition, the ATPase activity of myosin is correlated with muscle shortening speed [19]. Actin is an important component of thin filaments in MP, with a molecular weight of about 42 kDa [20], it exists in two forms: free monomer G-actin (spherical) and linear polymer F-actin (filamentous) [21]. Muscle contraction is actually the interaction between myosin and actin, in that actin provides the molecular motor, myosin hydrolyses ATP, to release energy, while filamentous actin acts as the track for myosin movement [18].
1.2. Changes in MP Structure and Gel Properties under HHP
1.2.1. Effect of HHP on MP Structure
HHP can dissociate the non-covalent, ionic, hydrophobic, and hydrogen bonds to change the secondary, tertiary, and quaternary structure of MP [22]. Changes in pH, ionic strength, pressure and temperature can cause the denaturation, aggregation, or gelation of proteins [23]. The pressure level induces either local or global changes in protein, and eventually leads to denaturation by altering the delicate equilibrium of the interactions that stabilize the folded conformation of native proteins [24,25]. For example, the quaternary structure dissociates when treated at moderate pressures (100–200 MPa), the tertiary structure is significantly affected at a pressure level above 200 MPa, and secondary structure changes take place at higher pressures (300–700 MPa) [26]. According to Le Chatelier’s principle, pressure induces a shift of the equilibrium toward the state with the smallest volume [25]. The current study suggests that the main reason for the volume differences between folded and unfolded proteins is the solvent excluded void volumes that results upon unfolding [25]. Thus, the effect of pressure on any given protein varies depending on the specificity of its folding state (i.e., its internal packing density and pattern). Because hole volumes are not uniformly distributed in the folded polypeptide, homologous proteins with the same size and structure may have different volume properties and, thus different sensitivity to pressure perturbations [25]. HHP induces variable alterations on protein conformational structures depending on the applied pressure level [26]. Table 1 provides recent reports on the changes in MP structures with HHP treatment.

Table 1.
Changes in myofibrillar protein structure under HHP, including source, reaction conditions and relevant results.
1.2.2. Secondary Structure
By using available computational methods, detailed pressure-induced changes in protein secondary structure from characteristic shifts in the band frequencies that are recorded via various techniques, such as Fourier-transform infrared spectroscopy (FTIR), Raman spectra and circular dichroism (CD) [27]. The secondary structure of protein includes α-helix, the β-sheet, β-turn, random coil [28]. The α-helix is stabilized by intramolecular hydrogen bonds between the carbonyl oxygen and amino hydrogen of the polypeptide chain and is buried in the interior site of the protein [29]. The β-sheet is organized through intermolecular hydrogen bonds. The β-turn structure is usually distributed on the surface of a protein molecule, where the change in direction of the polypeptide chain encounters less resistance, and protein stability is maintained through the relationship between the amino acids and the electrostatic gravitational forces. A random coil can originate from the unfolding of any α-helix, β-sheet and β-turn structures and contributes to protein flexibility [30,31].
Most studies have shown that 100 MPa pressure can change the secondary structure of MP in meat products, with specific details according to various levels of pressure outlined in Table 1. However, some experiments have also demonstrated that the secondary structures of MP and tropomyosin are not significantly affected when the pressure is 100 MPa [32]. Schiaffino et al. pointed out that MPs from different sources have slight diversities within their amino acid sequences, leading to different structures and differences in the contractile and biochemical properties of the muscle, which may affect their response to pressure [33].
In a previous report, the α-helix structure of MP was significantly reduced by HHP, with corresponding increases in the contents of β-turn and random coil [26]. Some studies have suggested that HHP induces water infiltration into protein cavities, strengthening the hydrogen bonds between the protein and water molecules, wakening the intramolecular hydrogen bonds, resulting in changes to protein configuration [34,35]. With an increasing in pressure level, a gradual increase in β-sheets content is normally observed, but in the case of tropomyosin, the β-sheets content decreased at lower pressures and increased at higher pressures [36]. Li et al. attributed this to the fact that, at a relatively lower pressure, intermolecular hydrogen bonds could be broken, rather than intramolecular hydrogen bonds [36]. With an increase in pressure, the intramolecular hydrogen bonds would be destroyed, leading to the transformation of α-helix to β-sheet, β-turn, and random coil, thus resulting in an increase of β-sheet content. It has also been found that abalone proteins are predominantly of β-sheet structure [28]. HHP could disrupt the intermolecular β-sheet structure in abalone proteins, which was compensated for by forming new intramolecular β-sheet interactions [28]. Chen et al. found that the β-turn was reduced with the treatment of pressure [37]. In general, highly ordered structures (α-helix, β-sheet and β-turn) unfold under HHP, resulting in an increase in random coil structure [26]. Decreased α-helix is more common under HHP, but the pressure threshold for the reduction is closely related to the environment and the protein source [26]. HHP changes the MP secondary structure because the high pressure breaks its intermolecular hydrogen bonds structure and thus weakens the intermolecular interactions, while intermolecular and intramolecular interactions can be reformed in various combinations, resulting in the reduction, increase or retention of secondary structure conformation.
1.2.3. Tertiary and Quaternary Structure
Changes in surface hydrophobicity (H0) and the contents of the free sulfhydryl group (−SH) reflect changes in the tertiary structure of proteins and are closely related to their functional properties [38]. In an aqueous environment, nonpolar solutes repel water, which is one of the properties of hydrophobic groups [39]. The hydrophobic interaction between the side chains of nonpolar amino acid residues is one of the main forces that maintains the tertiary structure of proteins, and it plays an important role in protein functions [40]. The ANS fluorescence probe method is a method to evaluate protein surface hydrophobicity [41]. According to Table 1, the surface hydrophobicity and reactive sulfhydryl groups (−SH) of MP increase under HHP. A possible reason for this is that the tertiary structure of MP is destroyed, resulting in the internal exposure of hydrophobic groups under HHP [42]. Since the disulfide bond destruction requires 213.1 kJ/mol of energy, and the 10,000 MPa pressure treatment can only provide 8.37 kJ/mol of energy, the disulfide bond will not be destroyed by HHP treatment [43,44]. Thus, the increasing in the content of reactive −SH may be due to the unfolding the protein structure and the exposure of internal sulfhydryl groups [26,44]. In our work [9], it was found that when the pressure exceeded 400 MPa, the surface hydrophobicity content of MP began to decrease, possibly due to the aggregation of proteins that are covered by random amino acid residues. Bolumar et al. decribed the mechanism of MP aggregation in detail as follows: (a) Dissociation of the thin and thick filaments caused MP solubilization increased; (b) rupturing of non-covalent interactions inside the molecules results in protein denaturation; and (c) formation of new intra- and/or intermolecular bonds [18].
The quaternary structure of proteins is mainly stabilized by pressure-sensitive hydrophobic forces, which disassemble oligomeric proteins of pressures at 100–200 MPa, accompanied by a reduction in volume [45]. Thus, HHP treatment causes protein depolymerization and disruption of the hydrophobic bonds between the linked polypeptide chains, leading to the dissociation of numerous polypeptide chains [45]. Under HHP treatment, water molecules enter the binding region of each subunit, affecting non-covalent bonds (especially hydrophobic interactions) and destabilizing the connections between subunits, resulting in depolymerization, and affecting the quaternary structure of the proteins [26]. The dissociation of oligomeric proteins can be promoted by pressure treatment below 150 MPa. Pressure above 150 MPa can cause the protein to dissociate and the oligomeric subunits to recombine after separation [46].
Figure 1 shows the mechanism of MP structural changes under HHP. HHP provokes changes in molecular interactions (hydrogen bonds, hydrophobic interactions, and electrostatic bonds). This leads to significant conformational changes of in the myofibrillar protein structure (secondary, tertiary and quaternary) based on the volume reduction between folded and unfolded MP, as well as the dissociation of MP. The α-helix undergoes pressure-driven transformation into a random coil and β-turn, and higher pressures induced greater denaturation and unfolding of MP. The increase in H0 is attributed to the HHP-induced unfolding of MP. Mild and moderate pressure during HHP treatment exposure hydrophobic and sulfhydryl groups, which is beneficial for protein–protein interactions. Excessive pressure (above 400 MPa) causes MP aggregation due to the intermolecular interactions that dominate [40,47,48]. Overall, the reduction of free volume in the MP network under HHP contributes to the conformational rearrangement of secondary, tertiary and quaternary structures.
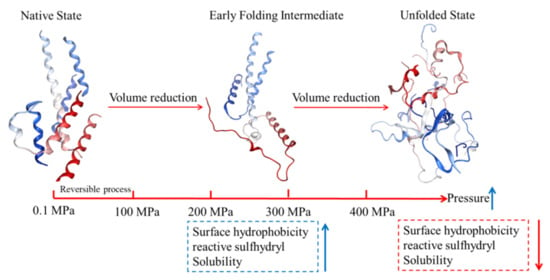
Figure 1.
Schematic diagram of the mechanism of pressure-induced protein denaturation.
1.3. Effect of HHP on Myofibrillar Protein Gel
The gel properties of MP determine the texture, adhesion and water retention of meat products. The formation of protein gels usually involves denaturation and aggregation, that is, the formation of a network structure, through physical and chemical reactions, that is capable of retaining water, fat, or other components [14]. In order to improve the quality of meat products and their gelatinous forms, polysaccharides are normally added [56]. Furthermore, new processing technologies, such as HHP, are also applied [16]. Table 2 summarizes recent studies on the mechanism of HHP-induced myofibrillar protein gelation.

Table 2.
Effects of HHP on myofibrillar gel.
As discussed in Section 1.2, HHP treatment induces MP unfolding. Moderate high pressure and a low concentration of CaCl2 can change the physical properties of meat products, mainly because, at pressures under 200 MPa, a large amount of myosin heavy chains and actin are dissolved, protein aggregation ability is reduced, and the tyrosine and tryptophan residues are exposed due to the unfolding of the tertiary structure [57]. Hsu et al. measured the aggregation and viscoelasticity of myosin in tilapia (Oreochromis niloticus) at 0° [65]. At 500 atmospheres, myosin unfolds; at 1000 atmospheres, they cling together, while at pressures higher than 1500 atmospheres, myosin forms aggregates and gels [65]. Chen et al. investigated the relationship between the gel properties of surimi and the structure of MP under high pressure (300~450MPa) [37]. The results showed that the secondary structure of MP in the surimi gels was significantly correlated with the whiteness, strength and structural characteristics of the gels. In general, pressure induces the unfolding of the protein, exposing more hydrophobic amino acids, enhancing the hydrophobic interaction and forming a three-dimensional network structure [26]. The enhanced solubility of the MP helped to improve its gel properties [66]. Zhang found that the content of disulfide bonds in MP gels was positively correlated with the treatment pressure [44]. Some studies showed that the pressure-induced protein gelation is also based on the formation of disulfide bonds [58,61]. However, Wang et al. reported that high pressure induced the hydrophobic rearrangement of MP and the cross-linking of disulfide bonds in the myosin S-1 subsegment [57]. This resulted in the formation of large protein aggregates and a decrease in MP solubility, which indicated a weaker gel and a lower water-holding capacity. For cod and turkey muscle, the gelation mechanism is similar to that of thermal gelation, and both rely on the formation of disulfide bonds and hydrophobic groups [58]. It is generally believed that the formation of a protein gel network is caused by the interaction between proteins and solvents, as well as the attraction and repulsion between polypeptide chains, which is related to hydrogen bonds, hydrophobic interaction, electrostatic interaction and disulfide bond. For example, the high content of −SH groups and S-S groups is conducive to the formation of an intermolecular network structure, and the high content of hydrophobic groups tends to result in the formation of a firm network structure [67,68,69].
HHP treatment can lead to protein unfolding [25]. Figure 2 showed the mechanism of pressure-induced protein gel. The unfolding of protein is more likely to expose the reactive groups (especially hydrophobic groups), facilitating the enhancement of hydrophobic interactions, hydrogen bonding, and electrostatic interactions [26]. Therefore, proteins with a large molecular weight and a high hydrophobic amino acid content are easily able to form a stable three-dimensional network structure. HHP treatment can also expose the sulfhydryl groups inside the protein molecules, which is conducive to the formation or exchange of disulfide bonds [9]. The existence of a large number of hydrophobic groups and disulfide bonds can strengthen the intermolecular network and facilitate the formation of irreversible gel. Angsupanich et al. noted that, although the pressure-induced myosin denaturation generated very different gels compared to the thermal process, the gelation mechanisms were similar [64]. Meanwhile, protein gelation also depends on the difference between the denaturation rate and the aggregation rate of myosin in the presence of HHP or heat. If the denaturation rate is slow, the functional groups in the protein molecules can fully expand and interact with each other to form a complete and ordered gel network. However, when the aggregation rate is relatively fast, the formation of stable chemical bonds between the peptides is difficult, resulting in disordered and rough gel products [70].
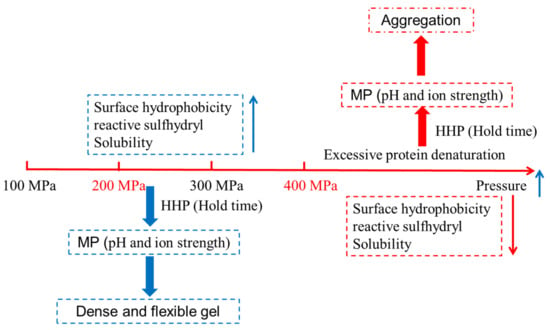
Figure 2.
Schematic diagram of the mechanism of pressure-induced protein gel.
2. Effect of HHP on Meat Quality
HHP treatment of meat has no significant effect on its flavor compounds, amino acids, vitamins or other small molecular substances when compared with traditional thermal processing [11,71]. As a promising emerging technology, the natural color, aroma, taste and nutrient contents of foods are retained to the greatest extent by reducing the degree of food processing as far as possible with HHP. Furthermore, the quality of foods can be improved, and the storage period can be prolonged [6].
The use of HHP as a non-thermal sterilization technique, with minimal impact on sensory quality and nutritional value, has received wide approval, and a variety of meat products have benefited from the application of HHP to ensure food safety and the extension of shelf life [72,73]. The deactivation of vegetative microbial cells usually occurs in the pressure range of 200 to 600 MPa at room temperature or in freezing facilities, which is the process often used in commercial and industrial condition [74]. The ability of HHP to disrupt microbes in meat products has been well documented in past studies [5]. High pressure can destroy the cell membranes of microorganisms and thereby deactivate them [75,76]. Endogenous (matrix properties) and exogenous (processing conditions) factors largely determine the inactivation efficiency of spoilage and pathogenic bacteria in meat product manufacturing. Pressure, temperature and duration time are considered to be the most important process parameters [74].
Color is considered to be the most important factor influencing consumers to judge the freshness of food products. There are three main parameters for evaluating color, namely L* value (brightness), A* value (redness) and B* value (yellowness). Under the condition of 4 °C and 100–200 MPa for 15 min, the muscle brightness of flounder increased continuously, and the brightness of cod also increased continuously under the conditions of 220–300 MPa for 5 min [77]. The treatment of carp muscle at 300 MPa and 20 °C for 10 min reduced its transparency and increased its L * value; while it had a similar appearance to cooked carp muscle after treated being treated at 500 MPa and 20 °C for 10 min [78]. HHP treatment causes color changes in meat products, depending on the effect of stress on the denaturation of proteins (mainly myoglobin). Generally speaking, soft pressure treatment will increase the brightness of meat products and decrease the redness. The color change after pressure treatment differs from meat to meat. At present, the effect of the mechanism of HHP on the color change of meat products is not clear and may be related to the degeneration of myoglobin and myofibrillar protein and the oxidation of lipid and protein [13].
Texture is one of the important indexes of meat products, among which hardness and elasticity are the most studied [79]. Hardness is considered to be the most important index for consumer recognition of fish [80]. It is well known that MPs are mainly responsible for the textural properties of processed meat products [81]. With increasing pressure, the hardness, springiness and chewiness values of red swamp crayfish (Procambarus clarkia) initially increased sharply, then decreased slightly, but all were higher than those of the control [82]. In our work, the texture parameters including hardness, springiness, chewiness and cohesiveness of eel balls were significantly improved with HHP treatment, and the optimum treating pressure was 400 MPa [9,36]. As for the eel surimi, the hardness, chewiness, gel strength and water-holding capacity increased significantly as well under HHP treatment, while the viscosity decreased [9,36]. The hardness, elasticity, mastic ability and cohesiveness increased by 3.00, 1.61, 5.96 and 1.23 times, respectively, when treated at 400 MPa for 15 min. In our work [9,36], further exploration of the protein structure revealed that the texture parameters of eel products were negatively correlated with the α-helix and β-sheet of proteins, but positively correlated with the random coil and β-turn of proteins (Figure 3). These results indicated that food textures can be directly related to the secondary and tertiary structures of MP.
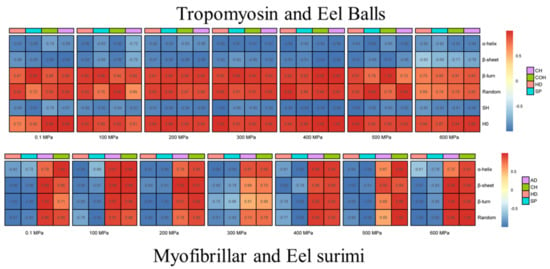
Figure 3.
Correlation analysis of tropomyosin and Eel balls/myofibrillar protein and eel surimi (in our work).
Protein oxidation not only leads to the deterioration of food color and texture, but also to the loss of nutrients (e.g., essential amino acids) and a reduction in protein digestibility [83,84]. In a recent review [85], the mechanism of HHP on lipid oxidation and protein oxidation in meat products was examined in detail. Pressure seems to be critical for the initiation of lipid oxidation, which is probably related to effects on the non-heme iron in meat [85]. The oxidation of lipids and proteins can be closely related depending on the type of meat, applied treatment and the method used to evaluate the reaction [85,86]. As observed, the effects of HHP may be greater for chilled meat products than for cooked meat products [4,23]. Better control of the quality of the meat products subjected to HHP treatments requires strict control of the entire process, from raw materials to preservation and consumption.
HHP has been proven to be effective in improving the color and texture of meat products (Table 3). Generally, excessive salt consumption causes cardiovascular problems and hypertension [87]. Muscle-gelled foods normally contain 2–3% NaCl [87]. In recent investigations, HHP has been found to be an effective method to reduce the addition of salt [88]. Cando et al. found that with HHP pre-treatment prior to heating, the addition of salt could be reduced to 0.3% (NaCl) compared to 3% (NaCl) addition in the single thermal treatment technique, with both achieving similarly high quality surimi gel [87]. Orel et al. suggested that HHP developed sodium-reduced chicken breasts [89]. Yang et al. reported that HHP not only decreased the salt content, but also reduced the fat concentration in sausages with maintained textural qualities [90]. Furthermore, HHP is also considered to be a potential method for developing low phosphate meat products [88] HHP can increase digestive susceptibility with the exposure of cleaving sites and the acceleration of proteolysis during aging [91], in contrast with thermal treatment (excessive heating) which hinders the digestion of meat proteins [92,93].

Table 3.
Effect of HHP on meat quality.
3. Conclusions and Prospect
Although HHP is considered to be an effective technology for improving the quality of meat as well as for the production of muscle-gelled foods, a certain threshold of treatment intensity is required for meat processing. However, the change in MP structure is revealed to be the main factor underlying the improvement in the quality of meat products. The spatial conformation of MP changes under HHP treatment, leading to changes in the product color and texture, and the mechanisms demonstrate the interdependence of the processes. However, meat is a complex system, containing not only MP but also other macromolecular proteins, polysaccharides and small molecules. Thus, the interactions between the different components under HHP treatment, together with their effects on the quality of meat products, require further in-depth research, particularly with regard to the molecular mechanism of the process.
Author Contributions
Data curation and formal analysis, H.L. and Y.X.; funding acquisition, Q.W. and N.H.; writing—original draft, H.L., Y.X and S.Z.; writing—review and editing, X.W., A.S., J.Z., Q.W. and N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Plan of China (2016YFD0400205).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Jiménez-Colmenero, F.; Carballo, J.; Cofrades, S. Healthier meat and meat products: Their role as functional foods. Meat Sci. 2001, 59, 5–13. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, 5324–5332. [Google Scholar] [CrossRef] [Green Version]
- Childers, A.B.; Heidelbaugh, N.D.; Carpenter, Z.L.; Smith, G.C.; White, S. Technologic advances in the food industry: Their influence on public health. J. Am. Vet. Med. A 1979, 175, 1291–1295. [Google Scholar]
- Hugas, M.; Garriga, M.; Monfort, J.M. New mild technologies in meat processing: High pressure as a model technology. Meat Sci. 2002, 62, 359–371. [Google Scholar] [CrossRef]
- Campus, M. High Pressure Processing of Meat, Meat Products and Seafood. Food Eng. Rev. 2010, 2, 256–273. [Google Scholar] [CrossRef]
- Truong, B.Q.; Buckow, R.; Stathopoulos, C.E.; Nguyen, M.H. Advances in High-Pressure Processing of Fish Muscles. Food Eng. Rev. 2015, 7, 109–129. [Google Scholar] [CrossRef]
- Cobos, Á.; Díaz, O. Chemical Composition of Meat and Meat Products. In Handbook of Food Chemistry; Cheung, P.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–32. [Google Scholar]
- Sun-Waterhouse, D.; Zhao, M.; Waterhouse, G.I.N. Protein Modification During Ingredient Preparation and Food Processing: Approaches to Improve Food Processability and Nutrition. Food Bioprocess Technol. 2014, 7, 1853–1893. [Google Scholar] [CrossRef]
- Ma, R.; Liu, H.; Li, Y.; Atem, B.J.A.; Ling, X.; He, N.; Che, L.; Wu, X.; Wang, Y.; Lu, Y. Effects of High Hydrostatic Pressure Treatment: Characterization of Eel (Anguilla japonica) Surimi, Structure, and Angiotensin-Converting Enzyme Inhibitory Activity of Myofibrillar Protein. Food Bioprocess Technol. 2021. [Google Scholar] [CrossRef]
- Marciniak, A.; Suwal, S.; Naderi, N.; Pouliot, Y.; Doyen, A. Enhancing enzymatic hydrolysis of food proteins and production of bioactive peptides using high hydrostatic pressure technology. Trends Food Sci. Technol. 2018, 80, 187–198. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Luo, S.; Li, P.; Mu, D.; Zhao, Y.; Zhong, X.; Jiang, S.; Zheng, Z. Effects of High Hydrostatic Pressure on the Properties of Heat-Induced Wheat Gluten Gels. Food Bioprocess Technol. 2019, 12, 220–227. [Google Scholar] [CrossRef]
- O’Reilly, C.E.; Kelly, A.L.; Murphy, P.M.; Beresford, T.P. High pressure treatment: Applications in cheese manufacture and ripening. Trends Food Sci. Technol. 2001, 12, 51–59. [Google Scholar] [CrossRef]
- Oliveira, F.A.d.; Neto, O.C.; Santos, L.M.R.d.; Ferreira, E.H.R.; Rosenthal, A. Effect of high pressure on fish meat quality—A review. Trends Food Sci. Technol. 2017, 66, 1–19. [Google Scholar] [CrossRef]
- Zhou, A.; Lin, L.; Liang, Y.; Benjakul, S.; Shi, X.; Liu, X. Physicochemical properties of natural actomyosin from threadfin bream (Nemipterus spp.) induced by high hydrostatic pressure. Food Chem. 2014, 156, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Guo, B.; Zhou, A.; Xiao, S.; Liu, X. Effect of high pressure treatment on gel characteristics and gel formation mechanism of bighead carp (Aristichthys nobilis) surimi gels. J. Food Process. Preserv. 2017, 41, 13155–13163. [Google Scholar] [CrossRef]
- Feng, D.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of Microwave Radiation and Water Bath Heating on the Physicochemical Properties of Actomyosin from Silver Carp (Hypophthalmichthys molitrix) during Setting. J. Food Process. Preserv. 2017, 41, 13031–13041. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 2009, 122, 105–118. [Google Scholar] [CrossRef]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stübler, A.-S.; de Lamballerie, M.; Hertel, C.; Brüggemann, D.A. High-pressure processing of meat: Molecular impacts and industrial applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 332–368. [Google Scholar] [CrossRef]
- Baáraány, M. ATPase Activity of Myosin Correlated with Speed of Muscle Shortening. J. Gen. Physiol. 1967, 50, 197–218. [Google Scholar] [CrossRef] [Green Version]
- Mudalal, S.; Babini, E.; Cavani, C.; Petracci, M. Quantity and functionality of protein fractions in chicken breast fillets affected by white striping. Poultry Sci. 2014, 93, 2108–2116. [Google Scholar] [CrossRef]
- Kim, T.; Hwang, W.; Kamm, R.D. Computational Analysis of a Cross-linked Actin-like Network. Exp. Mech. 2009, 49, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M. Functionality of food components and emerging technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. High hydrostatic pressure effects on the texture of meat and meat products. J. Food Sci. 2010, 75, R17–R23. [Google Scholar] [CrossRef]
- Prehoda, K.E.; Mooberry, E.S.; Markley, J.L. Pressure denaturation of proteins: Evaluation of compressibility effects. Biochemistry 1998, 37, 5785–5790. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Royer, C.A. Lessons from pressure denaturation of proteins. J. R. Soc. Interface 2018, 15, 20180244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chem. 2017, 217, 678–686. [Google Scholar] [CrossRef]
- Guo, M.; Liu, S.; Ismail, M.; Farid, M.M.; Ji, H.; Mao, W.; Gao, J.; Li, C. Changes in the myosin secondary structure and shrimp surimi gel strength induced by dense phase carbon dioxide. Food Chem. 2017, 227, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Cepero-Betancourt, Y.; Opazo-Navarrete, M.; Janssen, A.E.M.; Tabilo-Munizagab, G.; Perez-Won, M. Effects of high hydrostatic pressure (HHP) on protein structure and digestibility of red abalone (Haliotis rufescens) muscle. Innov. Food Sci. Emerg. 2020, 60, 102282–102291. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Tang, X.; Ni, W.; Zhou, L. Effects of pulsed ultrasound on rheological and structural properties of chicken myofibrillar protein. Ultrasonics Sonochem. 2017, 38, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, R.; Zhang, W.; Hu, Z.; Zhao, W. Structural characterization and physicochemical properties of protein extracted from soybean meal assisted by steam flash-explosion with dilute acid soaking. Food Chem. 2017, 219, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Neucere, N.J.; Jacks, T.J.; Sumrell, G. Interactions of globular protein with simple polyphenols. J. Agric. Food Chem. 1978, 26, 214–216. [Google Scholar] [CrossRef]
- Qiu, C.; Xia, W.; Jiang, Q. Pressure-induced changes of silver carp (Hypophthalmichthys molitrix) myofibrillar protein structure. Eur. Food Res. Technol. 2014, 238, 753–761. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol. Rev. 1996, 76, 371–423. [Google Scholar] [CrossRef] [PubMed]
- Villamonte, G.; Jury, V.; Jung, S.; de Lamballerie, M. Influence of Xanthan Gum on the Structural Characteristics of Myofibrillar Proteins Treated by High Pressure. J. Food Sci. 2015, 80, C522–C531. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, B.B.; Park, C.B.; Clark, D.S. Pressure effects on intra- and intermolecular interactions within proteins. BBA Protein Struct. Mol. Enzymol. 2002, 1595, 235–249. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Ma, R.; Tang, B.; Pan, D.; Peng, Y.; Ling, X.; Wang, Y.; Wu, X.; Che, L.; et al. Changes to the tropomyosin structure alter the angiotensin-converting enzyme inhibitory activity and texture profiles of eel balls under high hydrostatic pressure. Food Funct. 2018, 9, 6535–6543. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Zhang, J.; Xu, D.; Yang, W. Myofibrillar Protein Structure and Gel Properties of Trichiurus Haumela Surimi Subjected to High Pressure or High Pressure Synergistic Heat. Food Bioprocess Technol. 2020, 13, 589–598. [Google Scholar] [CrossRef]
- Ikeuchi, Y.; Tanji, H.; Kim, K.; Suzuki, A. Mechanism of heat-induce gelation of pressurized actomyosin-pressure-induces changes in actin and myosin in actomyosin. J. Agric. Food Chem. 1992, 40, 1756–1761. [Google Scholar] [CrossRef]
- Alizadeh-Pasdar, N.; Li-Chan, E.C.Y. Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef]
- Winter, R.; Dzwolak, W. Exploring the temperature-pressure configurational landscape of biomolecules: From lipid membranes to proteins. Phys. Trans. R. Soc. A 2005, 363, 537–562. [Google Scholar] [CrossRef] [PubMed]
- Balange, A.K.; Benjakul, S. Cross-linking activity of oxidised tannic acid towards mackerel muscle proteins as affected by protein types and setting temperatures. Food Chem. 2010, 120, 268–277. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, T.; Zhou, G.; Xu, X. The mechanism of high pressure-induced gels of rabbit myosin. Innov. Food Sci. Emerg. 2012, 16, 41–46. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Zhou, Y.; Li, P.; Ma, F.; Nishiumi, T.; Suzuki, A. Effects of high pressure processing on the thermal gelling properties of chicken breast myosin containing κ-carrageenan. Food Hydrocolloid. 2014, 40, 262–272. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Tang, X.; Chen, Y.; You, Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015, 188, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Ramaswamy, H.S.; Kasapis, S.; Boye, J.I. Novel Food Processing: Effects on Rheological and Functional Properties; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Peng, X.; Jonas, J.; Silva, J.L. High-pressure NMR study of the dissociation of Arc repressor. Biochemistry 1994, 33, 8323–8329. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W. Effects of High Hydrostatic Pressure on Some Functional and Nutritional Properties of Soy Protein Isolate for Infant Formula. J. Agric. Food Chem. 2011, 59, 12028–12036. [Google Scholar] [CrossRef]
- Messens, W.; Van Camp, J.; Huyghebaert, A. The use of high pressure to modify the functionality of food proteins. Trends Food Sci. Technol. 1997, 8, 107–112. [Google Scholar] [CrossRef]
- Grossi, A.; Olsen, K.; Bolumar, T.; Rinnan, Å.; Øgendal, L.H.; Orlien, V. The effect of high pressure on the functional properties of pork myofibrillar proteins. Food Chem. 2016, 196, 1005–1015. [Google Scholar] [CrossRef]
- Xue, S.; Yang, H.; Liu, R.; Qian, C.; Wang, M.; Zou, Y.; Xu, X.; Zhou, G. Applications of high pressure to pre-rigor rabbit muscles affect the functional properties associated with heat-induced gelation. Meat Sci. 2017, 129, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Cando, D.; Moreno, H.M.; Tovar, C.A.; Herranz, B.; Borderias, A.J. Effect of High Pressure and/or Temperature over Gelation of Isolated Hake Myofibrils. Food Bioprocess Technol. 2014, 7, 3197–3207. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhou, R.; Xu, X.; Zhou, G.; Liu, D. Structural modification by high-pressure homogenization for improved functional properties of freeze-dried myofibrillar proteins powder. Food Res. Int. 2017, 100, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Villamonte, G.; Pottier, L.; de Lamballerie, M. Influence of high-pressure processing on the physicochemical and the emulsifying properties of sarcoplasmic proteins from hake (Merluccius merluccius). Eur. Food Res. Technol. 2016, 242, 667–675. [Google Scholar] [CrossRef]
- Bai, Y.; Zeng, X.; Zhang, C.; Zhang, T.; Wang, C.; Han, M.; Zhou, G.; Xu, X. Effects of high hydrostatic pressure treatment on the emulsifying behavior of myosin and its underlying mechanism. LWT-Food Sci. Technol. 2021, 146, 111397–111408. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Moreno-Osorio, L.; Villalobos-Carvajal, R.; Pérez-Won, M. Protein Changes Caused by High Hydrostatic Pressure (HHP): A Study Using Differential Scanning Calorimetry (DSC) and Fourier Transform Infrared (FTIR) Spectroscopy. Food Eng. Rev. 2015, 7, 222–230. [Google Scholar] [CrossRef]
- Hong, G.; Min, S.; Ko, S.; Choi, M. Effect of high pressure treatments combined with various levels of κ-carrageenan on cold-set binding in restructured pork. Int. J. Food Sci. Technol. 2008, 43, 1484–1491. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, X.X.; Ma, F.; Xu, B.C.; Li, P.J.; Chen, C.G. Origin of high-pressure induced changes in the properties of reduced-sodium chicken myofibrillar protein gels containing CaCl2: Physicochemical and molecular modification perspectives. Food Chem. 2020, 319, 126535–126543. [Google Scholar] [CrossRef]
- Angsupanich, K.; Edde, M.; Ledward, D.A. Effects of high pressure on the myofibrillar proteins of cod and turkey muscle. J Agric. Food Chem. 1999, 47, 92–99. [Google Scholar] [CrossRef]
- Chapleau, N.J.; de Lamballerie-Anton, M.I. Changes in myofibrillar proteins interactions and rheological properties induced by high-pressure processing. Eur. Food Res. Technol. 2003, 216, 470–476. [Google Scholar] [CrossRef]
- Tan, F.; Lai, K.; Hsu, K. A comparative study on physical properties and chemical interactions of gels from tilapia meat pastes induced by heat and pressure. J. Texture Stud. 2010, 41, 153–170. [Google Scholar] [CrossRef]
- Lu, W.; Qin, Y.; Ruan, Z. Effects of high hydrostatic pressure on color, texture, microstructure, and proteins of the tilapia (Orechromis niloticus) surimi gels. J. Texture Stud. 2021, 52, 177–186. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Effects of high pressure processing on gelation properties and molecular forces of myosin containing deacetylated konjac glucomannan. Food Chem. 2019, 291, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhou, A.; Liu, G.; Ying, D.; Xiao, J.; Miao, J. Changes of physicochemical properties of greater lizardfish (Saurida tumbil) surimi gels treated with high pressure combined with microbial transglutaminase. J. Food Process. Preserv. 2019, 43, 14150–14160. [Google Scholar] [CrossRef]
- Zheng, H.; Han, M.; Bai, Y.; Xu, X.; Zhou, G. Combination of high pressure and heat on the gelation of chicken myofibrillar proteins. Innov. Food Sci. Emerg. 2019, 52, 122–130. [Google Scholar] [CrossRef]
- Hsu, K.C.; Ko, W.C. Effect of Hydrostatic Pressure on Aggregation and Viscoelastic Properties of Tilapia (Orechromis niloticus) Myosin. J. Food Sci. 2001, 66, 1158–1162. [Google Scholar] [CrossRef]
- Souza, C.M.; Boler, D.D.; Clark, D.L.; Kutzler, L.W.; Holmer, S.F.; Summerfield, J.W.; Cannon, J.E.; Smit, N.R.; McKeith, F.K.; Killefer, J. Varying the Temperature of the Liquid Used for High-Pressure Processing of Prerigor Pork: Effects on Fresh Pork Quality, Myofibrillar Protein Solubility, and Frankfurter Textural Properties. J. Food Sci. 2012, 77, S54–S61. [Google Scholar] [CrossRef]
- Ferry, J.D. Protein gels. Adv. Protein Chem. 1948, 4, 1–78. [Google Scholar] [PubMed]
- Yamamoto, K. Electron microscopy of thermal aggregation of myosin. J. Biochem. 1990, 108, 896–898. [Google Scholar] [CrossRef]
- Chan, J.K.; Gill, T.A.; Paulson, A.T. Thermal Aggregation of Myosin Subfragments from Cod and Herring. J. Food Sci. 1993, 58, 1057–1061. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, D.; Shao, Y.; Xiong, C.; Li, J.; Tu, Y. Changes in physico-chemical properties, microstructures, molecular forces and gastric digestive properties of preserved egg white during pickling with the regulation of different metal compounds. Food Hydrocolloid. 2020, 98, 105281–105291. [Google Scholar] [CrossRef]
- He, R.; He, H.; Chao, D.; Ju, X.; Aluko, R. Effects of High Pressure and Heat Treatments on Physicochemical and Gelation Properties of Rapeseed Protein Isolate. Food Bioprocess Technol. 2014, 7, 1344–1353. [Google Scholar] [CrossRef]
- Morris, C.; Brody, A.L.; Wicker, L. Non-thermal food processing/preservation technologies: A review with packaging implications. Packag. Technol. Sci. Int. J. 2007, 20, 275–286. [Google Scholar] [CrossRef]
- Li, X.; Farid, M. A review on recent development in non-conventional food sterilization technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Georget, E.; Sevenich, R.; Reineke, K.; Mathys, A.; Heinz, V.; Callanan, M.; Rauh, C.; Knorr, D. Inactivation of microorganisms by high isostatic pressure processing in complex matrices: A review. Innov. Food Sci. Emerg. 2015, 27, 1–14. [Google Scholar] [CrossRef]
- Campus, M.; Flores, M.; Martinez, A.; Toldrá, F. Effect of high pressure treatment on colour, microbial and chemical characteristics of dry cured loin. Meat Sci. 2008, 80, 1174–1181. [Google Scholar] [CrossRef]
- Cardone, F.; Brown, P.; Meyer, R.; Pocchiari, M. Inactivation of transmissible spongiform encephalopathy agents in food products by ultra high pressure–temperature treatment. BBA Proteins Proteom. 2006, 1764, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Angsupanich, K.; Ledward, D.A. High pressure treatment effects on cod (Gadus morhua) muscle. Food Chem. 1998, 63, 39–50. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Marshall, M.R. Effect of high pressure treatment on the quality of rainbow trout (Oncorhynchus mykiss) and mahi mahi (Coryphaena hippurus). J. Food Sci. 2007, 72, C509–C515. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.; Petersen, B.; Møller, A. Impact of salt, phosphate and temperature on the effect of a transglutaminase (F XIIIa) on the texture of restructured meat. Meat Sci. 1995, 41, 293–299. [Google Scholar] [CrossRef]
- Jain, D.; Pathare, P.B.; Manikantan, M.R. Evaluation of texture parameters of Rohu fish (Labeo rohita) during iced storage. J. Food Eng. 2007, 81, 336–340. [Google Scholar] [CrossRef]
- Li, C.T. Myofibrillar protein extracts from spent hen meat to improve whole muscle processed meats. Meat Sci. 2006, 72, 581–583. [Google Scholar] [CrossRef]
- Shao, Y.; Xiong, G.; Ling, J.; Hu, Y.; Shi, L.; Qiao, Y.; Yu, J.; Cui, Y.; Liao, L.; Wu, W.; et al. Effect of ultra-high pressure treatment on shucking and meat properties of red swamp crayfish (Procambarus clarkia). LWT-Food Sci. Technol. 2018, 87, 234–240. [Google Scholar] [CrossRef]
- Abraha, B.; Admassu, H.; Mahmud, A.; Tsighe, N.; Shui, X.W.; Fang, Y. Effect of processing methods on nutritional and physico-chemical composition of fish: A review. Food Process. Technol. 2018, 6, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Guyon, C.; Meynier, A.; de Lamballerie, M. Protein and lipid oxidation in meat: A review with emphasis on high-pressure treatments. Trends Food Sci. Technol. 2016, 50, 131–143. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Cando, D.; Herranz, B.; Borderías, A.J.; Moreno, H.M. Effect of high pressure on reduced sodium chloride surimi gels. Food Hydrocoll. 2015, 51, 176–187. [Google Scholar] [CrossRef]
- Chen, X.; Tume, R.K.; Xiong, Y.; Xu, X.; Zhou, G.; Chen, C.; Nishiumi, T. Structural modification of myofibrillar proteins by high-pressure processing for functionally improved, value-added, and healthy muscle gelled foods. Crit. Rev. Food Sci. 2018, 58, 2981–3003. [Google Scholar] [CrossRef] [PubMed]
- Orel, R.; Tabilo-Munizaga, G.; Cepero-Betancourt, Y.; Reyes-Parra, J.E.; Badillo-Ortiz, A.; Pérez-Won, M. Effects of high hydrostatic pressure processing and sodium reduction on physicochemical properties, sensory quality, and microbiological shelf life of ready-to-eat chicken breasts. LWT-Food Sci. Technol. 2020, 127, 109352–109362. [Google Scholar] [CrossRef]
- Yang, H.; Han, M.; Bai, Y.; Han, Y.; Xu, X.; Zhou, G. High pressure processing alters water distribution enabling the production of reduced-fat and reduced-salt pork sausages. Meat Sci. 2015, 102, 69–78. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.S.; Jo, K.; Yong, H.I.; Jeong, H.G.; Jung, S. Improvement of meat protein digestibility in infants and the elderly. Food Chem. 2021, 356, 129707–129718. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, G.; Bai, Y.; Wang, C.; Zhu, S.; Xu, X.; Li, C. The effect of meat processing methods on changes in disulfide bonding and alteration of protein structures: Impact on protein digestion products. RSC Adv. 2018, 8, 17595–17605. [Google Scholar] [CrossRef] [Green Version]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.D.A.; Kumar, S.; Bhat, H.F. Non-thermal processing has an impact on the digestibility of the muscle proteins. Crit. Rev. Food Sci. 2021, 1–28. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, W.; Ma, F.; Li, P.; Chen, C. High-Pressure Pretreatment to Improve the Water Retention of Sodium-Reduced Frozen Chicken Breast Gels with Two Organic Anion Types of Potassium Salts. Food Bioprocess Technol. 2018, 11, 526–535. [Google Scholar] [CrossRef]
- Cap, M.; Paredes, P.F.; Fernández, D.; Mozgovoj, M.; Vaudagna, S.R.; Rodriguez, A. Effect of high hydrostatic pressure on Salmonella spp inactivation and meat-quality of frozen chicken breast. LWT-Food Sci. Technol. 2020, 118, 108873–108878. [Google Scholar] [CrossRef]
- Ros-Polski, V.; Koutchma, T.; Xue, J.; Defelice, C.; Balamurugan, S. Effects of high hydrostatic pressure processing parameters and NaCl concentration on the physical properties, texture and quality of white chicken meat. Innov. Food Sci. Emerg. 2015, 30, 31–42. [Google Scholar] [CrossRef]
- De Alba, M.; Pérez-Andrés, J.M.; Harrison, S.M.; Brunton, N.P.; Burgess, C.M.; Tiwari, B.K. High pressure processing on microbial inactivation, quality parameters and nutritional quality indices of mackerel fillets. Innov. Food Sci. Emerg. 2019, 55, 80–87. [Google Scholar] [CrossRef]
- Kaur, B.P.; Kaushik, N.; Rao, P.S.; Chauhan, O.P. Effect of High-Pressure Processing on Physical, Biochemical, and Microbiological Characteristics of Black Tiger Shrimp (Penaeus monodon). Food Bioprocess Technol. 2013, 6, 1390–1400. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.K.; Wang, X.; Liu, D.H. The effects of high pressure on the myofibrillar structure and meat quality of marinating Tan mutton. J. Food Process Eng. 2019, 42, 13138–13152. [Google Scholar] [CrossRef]
- Kaur, L.; Astruc, T.; Venien, A.; Loison, O.; Cui, J.; Irastorza, M.; Boland, M. High pressure processing of meat: Effects on ultrastructure and protein digestibility. Food Funct. 2016, 7, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Wang, H.; Yang, H.; Yu, X.; Bai, Y.; Tendu, A.A.; Xu, X.; Ma, H.; Zhou, G. Effects of high-pressure treatments on water characteristics and juiciness of rabbit meat sausages: Role of microstructure and chemical interactions. Innov. Food Sci. Emerg. 2017, 41, 150–159. [Google Scholar] [CrossRef]
- Cava, R.; Higuero, N.; Ladero, L. High-pressure processing and storage temperature on Listeria monocytogenes, microbial counts and oxidative changes of two traditional dry-cured meat products. Meat Sci. 2021, 171, 108273–108284. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.L.G.; Mársico, E.T.; Cunha, L.C.M.; Rosenthal, A.; Deliza, R.; Conte-Junior, C.A. Application of emerging non-thermal technologies to sodium reduction in ready-to-eat fish products. Innov. Food Sci. Emerg. 2021, 71, 102710–102717. [Google Scholar] [CrossRef]
- Han, G.; Chen, Q.; Xia, X.; Liu, Q.; Kong, B.; Wang, H. High hydrostatic pressure combined with moisture regulators improves the tenderness and quality of beef jerky. Meat Sci. 2021, 181, 108617–108626. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).