Thermosonication Combined with Natural Antimicrobial Nisin: A Potential Technique Ensuring Microbiological Safety and Improving the Quality Parameters of Orange Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Orange Juice Preparation
2.2. Chemicals and Reagents
2.3. Preparation of Nisin Solution
2.4. TS, TSN, and CTS Treatment
2.5. Microbiological Assay
2.6. Determination of the Activities of the Polyphenol Oxidase (PPO), Peroxidase (POD), and Pectin Methylesterase (PME)
2.7. Physicochemical Indexes
2.8. Functional Indices and Antioxidant Activity
2.9. Sensory Evaluation
2.9.1. Electronic Nose (E-Nose) Assay
2.9.2. Color Determination
2.9.3. Artificial Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
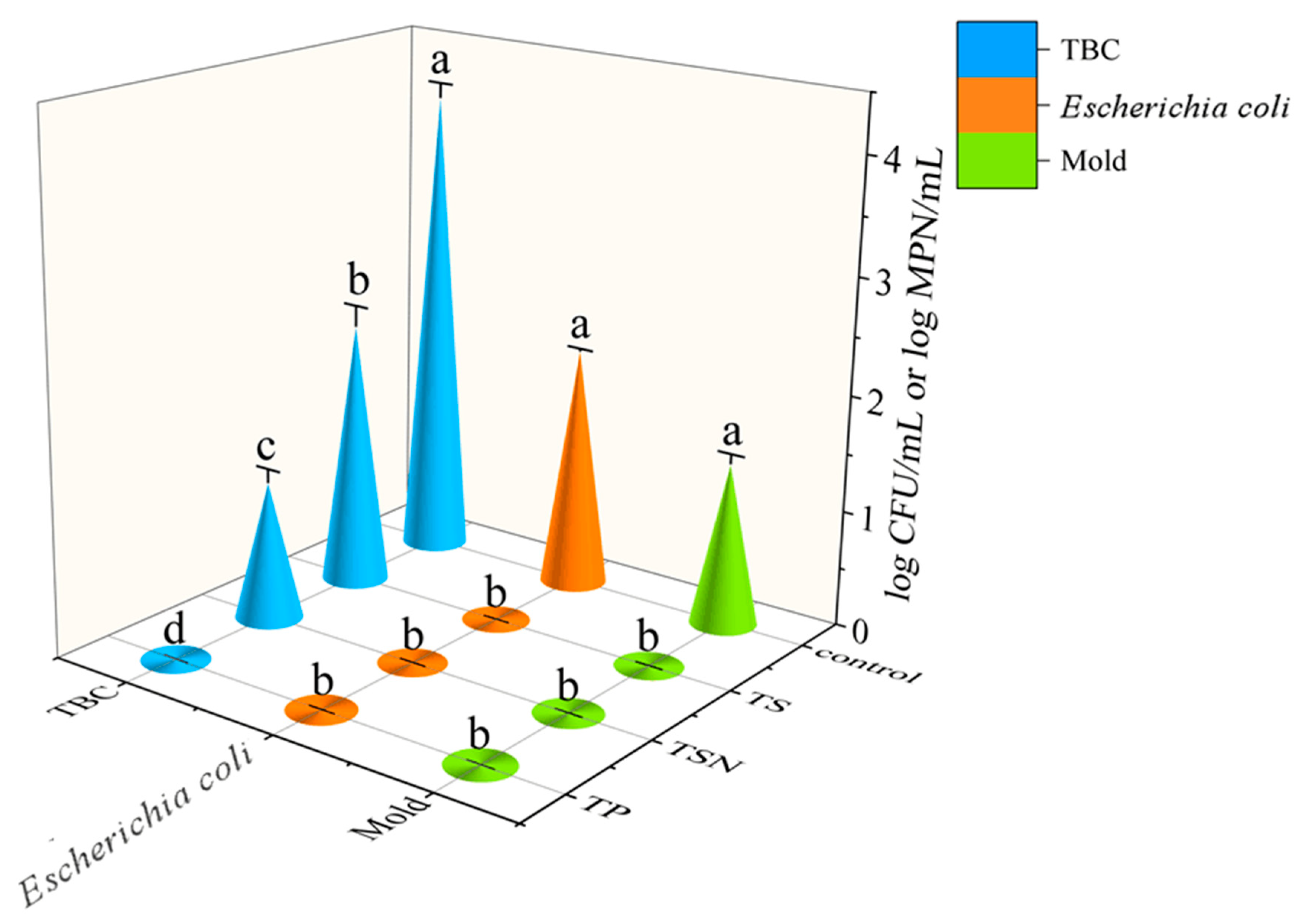
3.1. Microbial Inactivation of Different Treatments
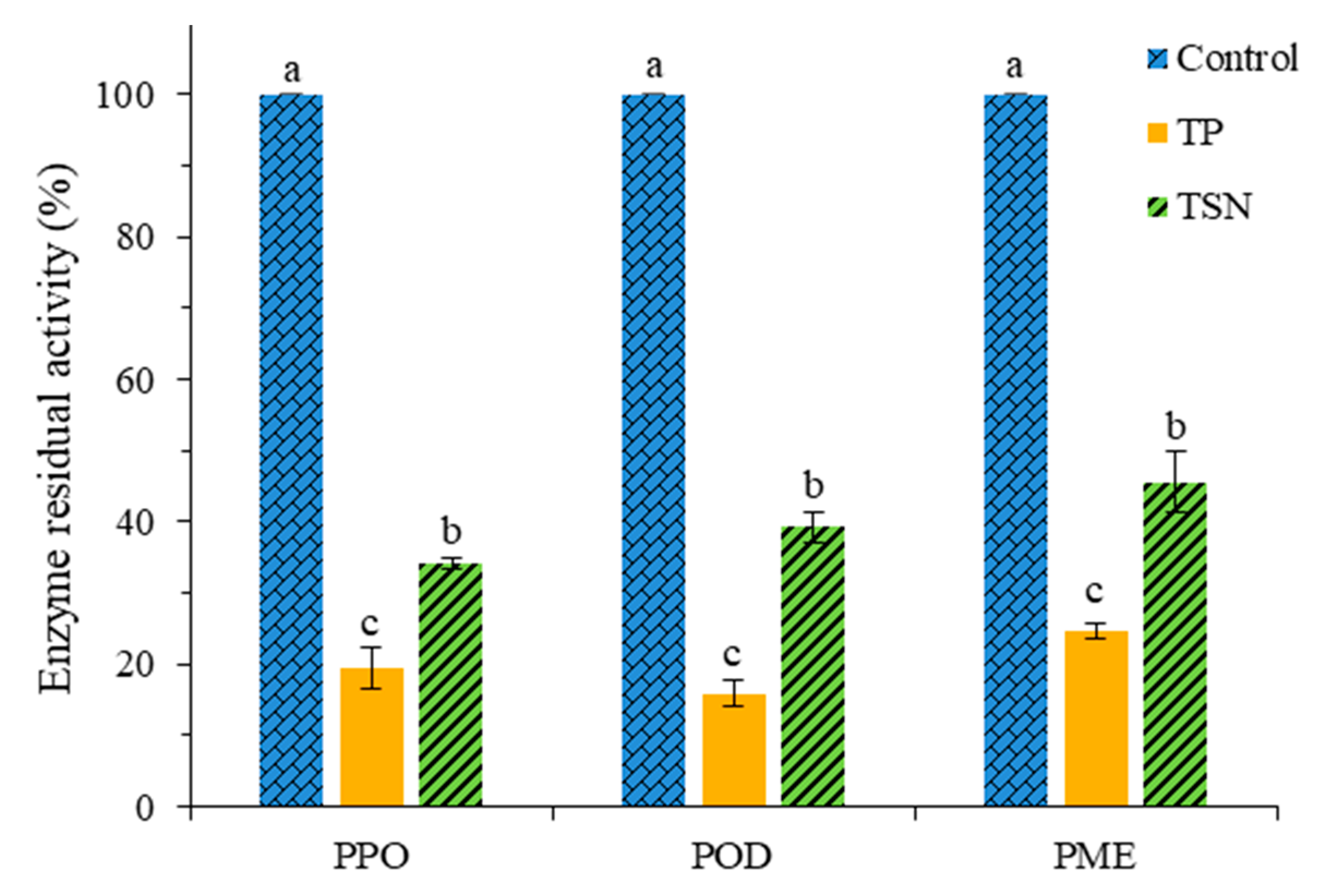
3.2. Residual Enzyme Activities of Different Treatments
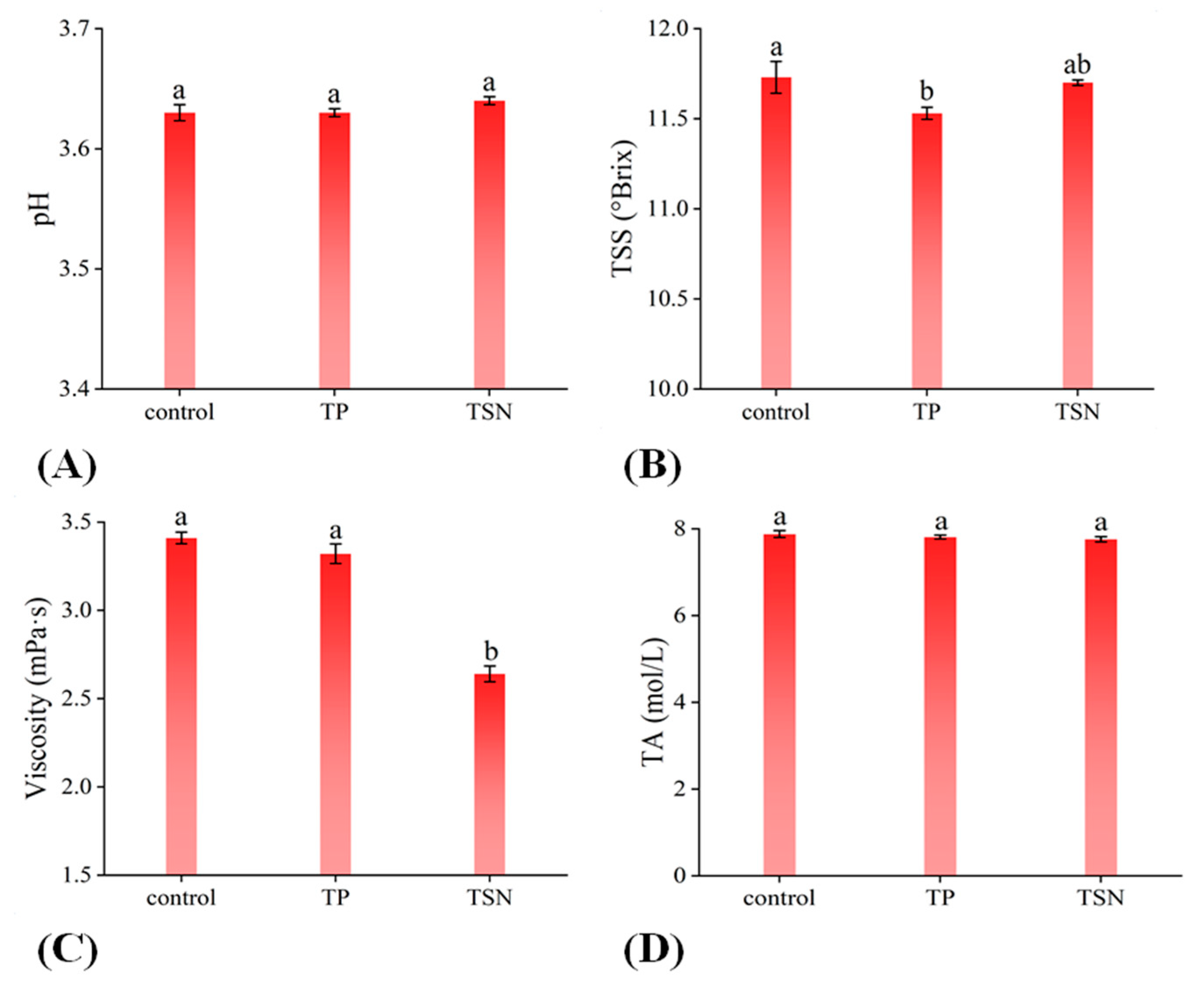
3.3. Physicochemical Properties of Different Treatments
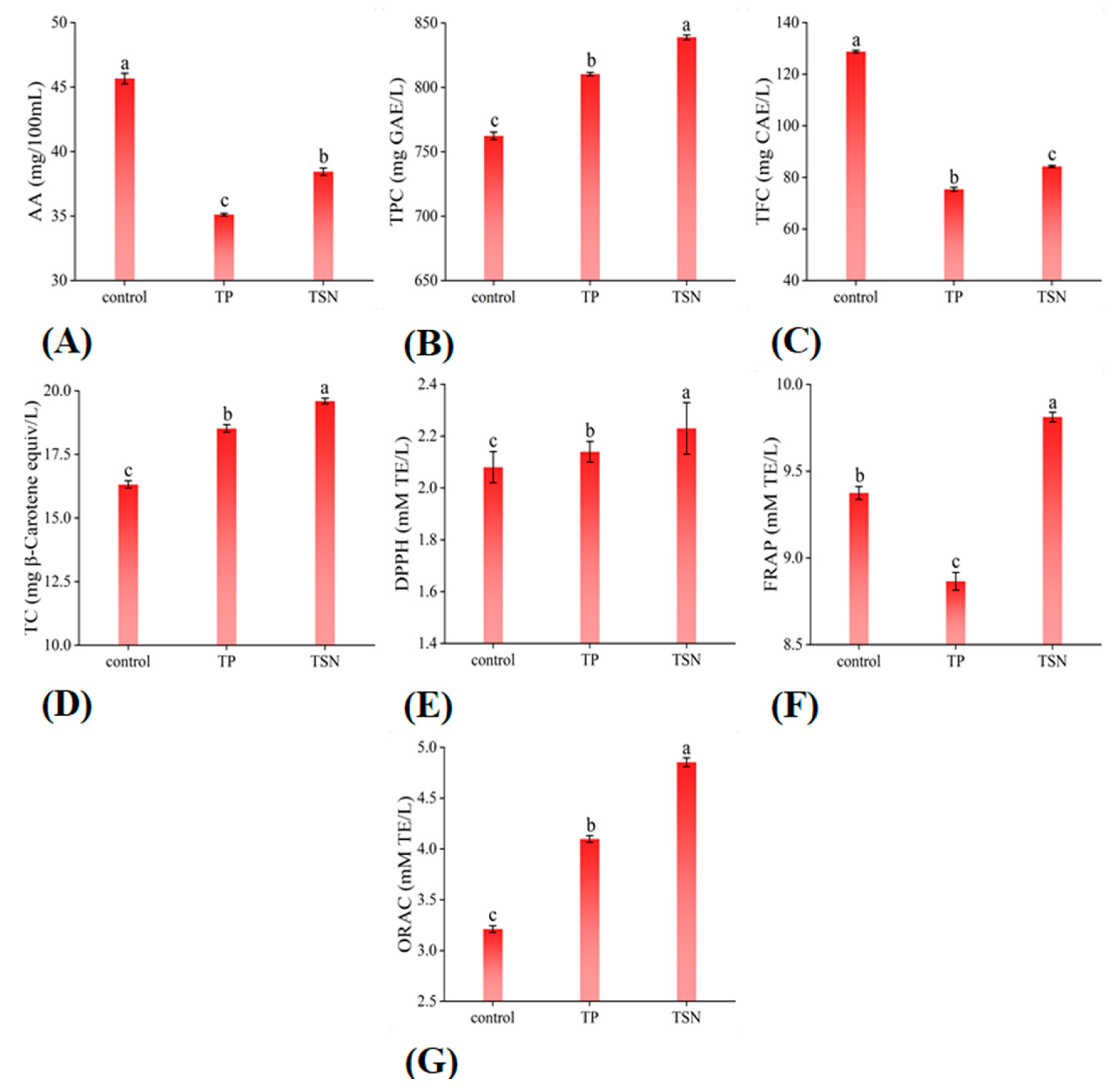
3.4. Functional Indices of Different Treatments
3.4.1. AA and TFC
3.4.2. TPC and TC
3.4.3. Antioxidant Activity
3.5. Correlation Analysis of Antioxidant Activity
3.6. Effects of Different Sterilization Treatments on the Sensory Quality
3.6.1. Color Analysis
3.6.2. Artificial Sensory Evaluation
3.6.3. E-Nose Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wibowo, S.; Grauwet, T.; Santiago, J.S.; Tomic, J.; Vervoort, L.; Hendrickx, M.; Van Loey, A. Quality changes of pasteurised orange juice during storage: A kinetic study of specific parameters and their relation to colour instability. Food Chem. 2015, 187, 140–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agcam, E.; Akyıldız, A.; Evrendilek, G.A. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurisation. Food Chem. 2014, 143, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ohrvik, V.; Witthoft, C. Orange juice is a good folate source in respect to folate content and stability during storage and simulated digestion. Eur. J. Nutr. 2008, 47, 92–98. [Google Scholar] [CrossRef]
- Rampersaud, G.C.; Valim, M.F. 100% citrus juice: Nutritional contribution, dietary benefits, and association with anthropometric measures. Crit. Rev. Food Sci. Nutr. 2017, 57, 129–140. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, A.; Goncalves, D.; Ferreira, P.; Baldwin, E.; Cesar, T. Postprandial effect of fresh and processed orange juice on the glucose metabolism, antioxidant activity and prospective food intake. J. Funct. Foods 2019, 52, 302–309. [Google Scholar] [CrossRef]
- Foroudi, S.; Potter, A.S.; Stamatikos, A.; Patil, B.S.; Deyhim, F. Drinking Orange juice increases total antioxidant status and decreases lipid peroxidation in adults. J. Funct. Foods 2014, 17, 612–617. [Google Scholar] [CrossRef]
- Alhabeeb, H.; Sohouli, M.H.; Lari, A.; Fatahi, S.; Shidfar, F.; Alomar, O.; Salem, H.; Al-Badawi, I.A.; Abu-Zaid, A. Impact of orange juice consumption on cardiovascular disease risk factors: A systematic review and meta-analysis of randomized-controlled trials. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Department of Agriculture. Citrus: World Market and Trade. 2021. Available online: http://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 1 July 2021).
- Kim, M.K.; Jang, H.W.; Lee, K.G. Sensory and instrumental volatile flavor analysis of commercial orange juices prepared by different processing methods. Food Chem. 2018, 30, 217–222. [Google Scholar] [CrossRef]
- Mastello, R.B.; Capobiango, M.; Chin, S.; Monteiro, M.; Marriott, P.J. Identification of odour-active compounds of pasteurised orange juice using multidimensional gas chromatography techniques. Food Res. Int. 2015, 75, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cacho, P.R.; Rouseff, R. Processing and storage effects on orange juice aroma: A Review. J. Agric. Food Chem. 2008, 56, 9785–9796. [Google Scholar] [CrossRef]
- Khandpur, P.; Gogate, P.R. Understanding the effect of novel approaches based on ultrasound on sensory profile of orange juice. Ultrason. Sonochem. 2015, 27, 87–95. [Google Scholar] [CrossRef]
- Hocine, R.; Christian, M.; Amine, B.; Nawel, A.; Manuel, D.; Khodir, M. Degradation kinetic modelling of ascorbic acid and colour intensity in pasteurised blood orange juice during storage. Food Chem. 2015, 173, 665–673. [Google Scholar] [CrossRef]
- Khandpur, P.; Gogate, P.R. Effect of novel ultrasound based processing on the nutrition quality of different fruit and vegetable juices. Ultrason. Sonochem. 2015, 27, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Velazquez-Estrada, R.M.; Roig, A.X.; Garcia-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-Gonzalez, E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Santhirasegaram, V.; Razali, Z.; Somasundram, C. Effects of thermal treatment and sonication on quality attributes of Chokanan mango (Mangifera indica L.) juice. Ultrason. Sonochem. 2013, 20, 1276–1282. [Google Scholar] [CrossRef]
- Dias, D.D.R.C.; Barros, Z.M.P.; Carvalho, C.B.O.D.; Honorato, F.A.; Guerra, N.B.; Azoubel, P.M. Effect of sonication on soursop juice quality. LWT Food Sci. Technol. 2015, 62, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Abid, M.; Jabba, S.; Hu, B.; Hashim, M.M.; Wu, T.; Lei, S.C.; Khan, M.A.; Zeng, X.X. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason. Sonochem. 2014, 21, 984–990. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, Q.D.; Xie, B.J.; Sun, Z.D. Effect of ultrasound combined with ultraviolet treatment on microbial inactivation and quality properties of mango juice. Ultrason. Sonochem. 2020, 64, 105000. [Google Scholar] [CrossRef] [PubMed]
- Aadil, R.M.; Zeng, X.A.; Sun, D.W.; Wang, M.S.; Liu, Z.; Zhang, Z.H.; Liu, Z.W. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT Food Sci. Technol. 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Paniagua-Martinez, I.; Mulet, A.; Garcia-Alvarado, M.A.; Benedito, J. Orange juice processing using a continuous flow ultrasound-assisted supercritical CO2 system: Microbiota inactivation and product quality. Innov. Food Sci. Emerg. 2018, 47, 362–370. [Google Scholar] [CrossRef]
- Park, J.S.; Ha, J.W. Ultrasound treatment combined with fumaric acid for inactivating food-borne pathogens in apple juice and its mechanisms. Food Microbiol. 2019, 84, 103277.1–103277.9. [Google Scholar] [CrossRef]
- Ma, T.T.; Wang, J.Q.; Wang, L.K.; Yang, Y.H.; Yang, W.Y.; Wang, H.L.; Lan, T.; Zhang, Q.W.; Sun, X.Y. Ultrasound-combined sterilization technology: An effective sterilization technique ensuring the microbial safety of grape juice and significantly improving its quality. Foods 2020, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.M.; Jiang, L.F.; Cheng, Y.L.; Liao, X.J.; Zhang, R.R. Application of nisin-assisted thermosonication processing for preservation and quality retention of fresh apple juice. Ultrason. Sonochem. 2018, 42, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Mok, J.H.; Pyatkovskyy, T.; Yousef, A.; Sastry, S.K. Synergistic effects of shear stress, moderate electric field, and nisin for the inactivation of Escherichia coli K12 and Listeria innocua in clear apple juice. Food Control 2020, 113, 107209. [Google Scholar] [CrossRef]
- Mastello, R.B.; Janzantti, N.S.; Bisconsin, A.; Monteiro, M. Impact of HHP processing on volatile profile and sensory acceptance of Pera-Rio orange juice. Innov. Food Sci. Emerg. 2018, 45, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Guerrouja, K.; Sánchez-Rubiob, M.; Taboada-Rodríguezc, A.; Cava-Rodac, R.M.; Marín-Iniestab, F. Sonication at mild temperatures enhances bioactive compounds and microbiological quality of orange juice. Food Bioprod. Process. 2016, 99, 20–28. [Google Scholar] [CrossRef]
- Alves, E.G.; Rodrigues, T.H.S.; Fernandes, F.A.N.; de Brito, E.S.; Cullen, P.J.; Frias, J.M.; Bourk, P.; Cavalcante, R.S.; Almeida, F.D.L.; Rodrigues, S. An untargeted chemometric evaluation of plasma and ozone processing effect on volatile compounds in orange juice. Innov. Food Sci. Emerg. 2019, 53, 63–69. [Google Scholar] [CrossRef]
- Cheng, R.M.; Churey, J.J.; Worobo, R.W. Inactivation of Salmonella enterica and spoilage microorganisms in orange juice treated with dimethyl dicarbonate (DMDC). Int. J. Food Microbiol. 2018, 285, 152–157. [Google Scholar] [CrossRef]
- Ma, T.T.; Wang, J.Q.; Wang, H.L.; Lan, T.; Liu, R.H.; Gao, T.; Yang, W.Y.; Yuan, Z.; Ge, Q.; Fang, Y.L.; et al. Is Overnight Fresh Juice Drinkable? The Shelf Life Prediction of Non-Industrial Fresh Watermelon Juice Based on the Nutritional Quality, Microbial Safety Quality, and Sensory Quality. Food Nutr. Res. 2020, 64, 4237. [Google Scholar] [CrossRef]
- Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J.; Tiwari, B.K. Inactivation kinetics of pectin methylesterase and cloud retention in sonicated orange juice. Innov. Food Sci. Emerg. 2009, 10, 166–171. [Google Scholar] [CrossRef]
- The National Standard of China. GB/T 12456-2008. Determination of Total Acid in Food; CN-GB: Beijing, China, 2008. [Google Scholar]
- Ma, T.T.; Lan, T.; Ju, Y.L.; Cheng, G.; Que, Z.L.; Geng, T.H.; Fang, Y.L.; Sun, X.Y. Comparison on the nutritional properties and biological activities of kiwifruit (Actinidia) and their different forms products: Towards making kiwifruit more nutritious and functional. Food Funct. 2019, 10, 1317–1329. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Han, Z.; Sun, D.W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.C.; Zeng, X.X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Esteve, M.J.; Frigola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Sulaiman, A.; Farid, M.; Silva, F.V. Quality stability and sensory attributes of apple juice processed by thermosonication, pulsed electric field and thermal processing. Int. J. Food Sci. Technol. 2017, 23, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.M.; Castro-Leite-Júnior, B.R.; Martins, E.M.F.; Cristianini, M.; Martins, M.L.; Vieira, É.N.R.; Binoti, M.L.; Almeida-Paula, D.; Ramos, A.M. Impact of high pressure and thermal processing on probiotic mixed mango and carrot juices. J. Food Process. Pres. 2020, 44, 1–11. [Google Scholar] [CrossRef]
- Walkling-Ribeiro, M.; Noci, F.; Cronin, D.A.; Lyng, J.G.; Morgan, D.J. Shelf life and sensory evaluation of orange juice after exposure to thermosonication and pulsed electric fields. Food Bioprod. Process. 2009, 87, 102–107. [Google Scholar] [CrossRef]
- Oner, M.E. The effect of high-pressure processing or thermosonication in combination with nisin on microbial inactivation and quality of green juice. J. Food Process. Pres. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Marsellés-Fontanet, R.; Puig-Pujol, A.; Olmos, P.; Mínguez-Sanz, S.; Martín-Belloso, O. A Comparison of the Effects of Pulsed Electric Field and Thermal Treatments on Grape Juice. Food Bioprocess Technol. 2013, 6, 978–987. [Google Scholar] [CrossRef]
- Gabriel, A.A.; Cayabyab, J.E.C.; Tan, A.L.K.L.; Corook, M.L.F.; Ables, E.J.O.; Tiangson-Bayaga, C.L.P. Development and validation of a predictive model for the influences of selected product and process variables on ascorbic acid degradation in simulated fruit juice. Food Chem. 2015, 177, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Peng, Y.; Zhu, C.H.; Pan, S.Y. Effect of thermal treatment on carotenoids, flavonoids and ascorbic acid in juice of orange cv. Cara Cara. Food Chem. 2018, 265, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Adebola, O.O.; Folasade, O.A.; Titilola, A.O.; Olaide, R.A. Effect of thermosonication on quality attributes of hog plum (Spondias mombin L.) juice. Ultrason. Sonochem. 2021, 70, 105316. [Google Scholar] [CrossRef]
- Nayak, P.K.; Chandrasekar, C.M.; Kesavan, R.K. Effect of thermosonication on the quality attributes of star fruit juice. J. Food Process Eng. 2018, 41, e12857. [Google Scholar] [CrossRef]
- Lemmens, L.; Colle, I.; Buggenhout, S.V.; Palmero, P.; Loey, A.V.; Hendrickx, M. Carotenoid bioaccessibility in fruit- and vegetable-based food products as affected by product (micro)structural characteristics and the presence of lipids: A review. Trends Food Sci. Technol. 2014, 38, 125–135. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ye, J.H.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.k.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Zhao, F.; Mei, X.; Zhang, Y.; Liao, X.; Wang, Y. Effect of High Hydrostatic Pressure and Heat Sterilization on the Quality of Lycium barbarum Juice. J. Chin. Inst. Food Sci. Technol. 2018, 18, 169–178. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Tedeschi, P.; Brandolini, V.; Frígola, A. A Comparative Study of the Analysis of Antioxidant Activities of Liquid Foods Employing Spectrophotometric, Fluorometric, and Chemiluminescent Methods. Food Anal. Method 2013, 6, 317–327. [Google Scholar] [CrossRef]
- Wei, S.T.; Ou, L.C.; Luo, M.R.; Hutchings, J.B. Optimisation of food expectations using product colour and appearance. Food Qual. Prefer. 2012, 23, 49–62. [Google Scholar] [CrossRef]
- Fernández-Vázquez, R.; Stinco, C.M.; Hernanz, D.; Heredia, F.J.; Vicario, I.M. Colour training and colour differences thresholds in orange juice. Food Qual. Prefer. 2013, 30, 320–327. [Google Scholar] [CrossRef]

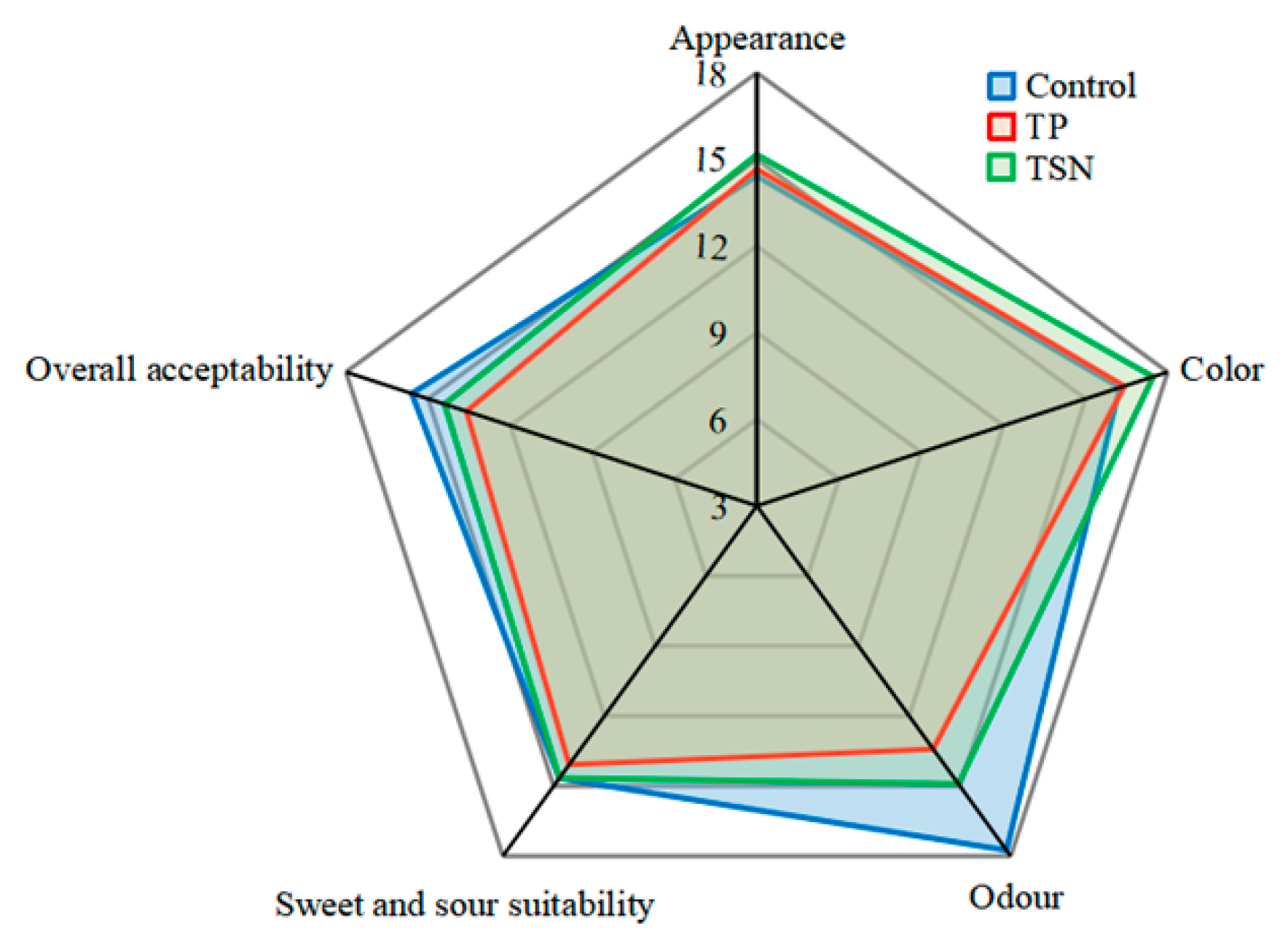
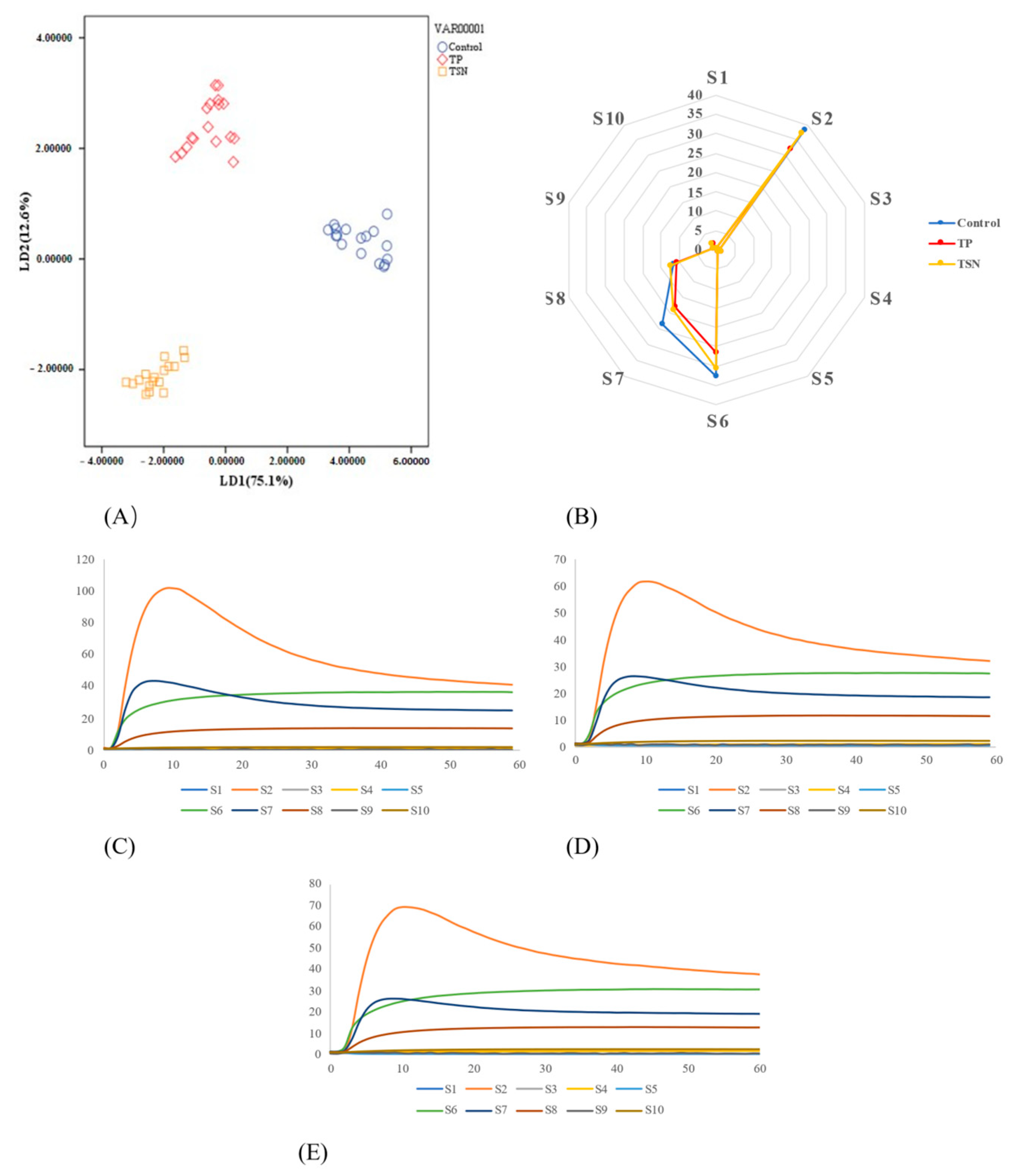
| Processing Method | Color | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | H° | C* | |
| Control | 51.60 ± 0.03 c | −2.02 ± 0.01 a | 29.96 ± 0.04 b | 0.00 ± 0.00 c | 93.86 ± 0.03 b | 30.03 ± 0.04 b |
| TP | 52.85 ± 0.45 b | −2.18 ± 0.17 a | 30.44 ± 0.77 b | 1.46 ± 0.55 b | 94.11 ± 0.43 b | 30.52 ± 0.75 b |
| TSN | 55.07 ± 0.16 a | −2.92 ± 0.03 b | 33.39 ± 0.12 a | 4.96 ± 0.18 a | 95.00 ± 0.06 a | 33.51 ± 0.12 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Yuan, Q.; Gao, C.; Wang, X.; Zhu, B.; Wang, J.; Sun, X.; Ma, T. Thermosonication Combined with Natural Antimicrobial Nisin: A Potential Technique Ensuring Microbiological Safety and Improving the Quality Parameters of Orange Juice. Foods 2021, 10, 1851. https://doi.org/10.3390/foods10081851
Zhao Q, Yuan Q, Gao C, Wang X, Zhu B, Wang J, Sun X, Ma T. Thermosonication Combined with Natural Antimicrobial Nisin: A Potential Technique Ensuring Microbiological Safety and Improving the Quality Parameters of Orange Juice. Foods. 2021; 10(8):1851. https://doi.org/10.3390/foods10081851
Chicago/Turabian StyleZhao, Qinyu, Quyu Yuan, Chenxu Gao, Xiaoyang Wang, Bihe Zhu, Jiaqi Wang, Xiangyu Sun, and Tingting Ma. 2021. "Thermosonication Combined with Natural Antimicrobial Nisin: A Potential Technique Ensuring Microbiological Safety and Improving the Quality Parameters of Orange Juice" Foods 10, no. 8: 1851. https://doi.org/10.3390/foods10081851
APA StyleZhao, Q., Yuan, Q., Gao, C., Wang, X., Zhu, B., Wang, J., Sun, X., & Ma, T. (2021). Thermosonication Combined with Natural Antimicrobial Nisin: A Potential Technique Ensuring Microbiological Safety and Improving the Quality Parameters of Orange Juice. Foods, 10(8), 1851. https://doi.org/10.3390/foods10081851








