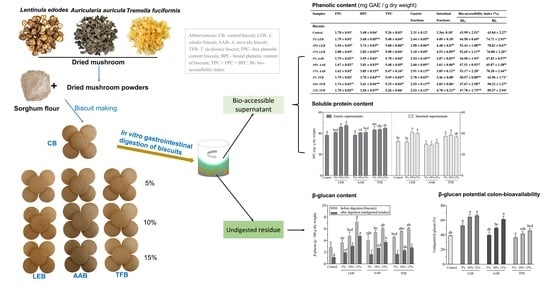
Delivery of Phenolic Compounds, Peptides and β-Glucan to the Gastrointestinal Tract by Incorporating Dietary Fibre-Rich Mushrooms into Sorghum Biscuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biscuits
2.3. In Vitro Gastrointestinal Digestion
2.4. BCA Assay and Protein Profile
2.5. Extraction of Phenolic Compounds
2.6. Determination of Phenolic Content and Antioxidant Activity
2.6.1. Phenolic Content Determination
2.6.2. Antioxidant Activity
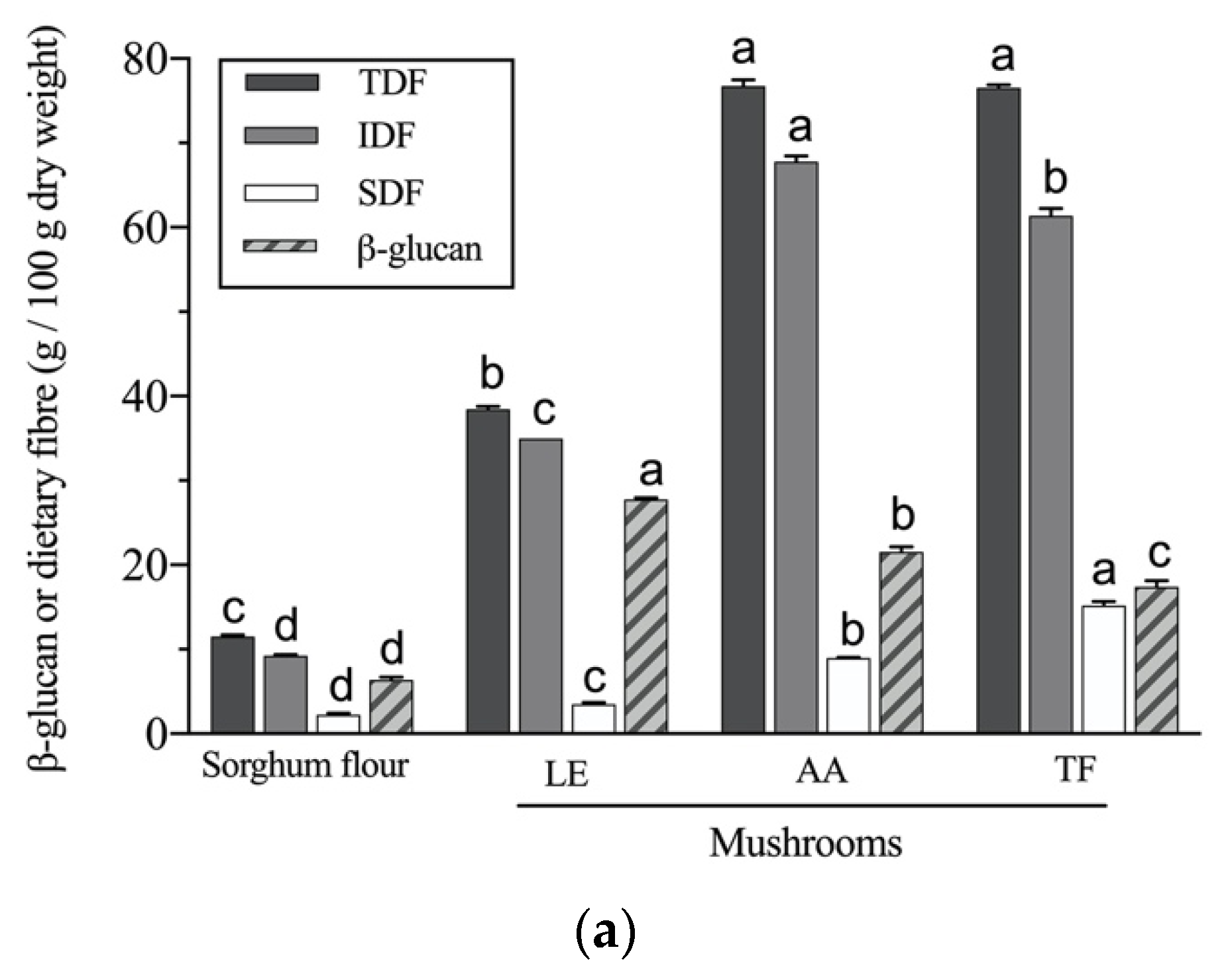
2.7. β-Glucan Determination
2.8. Nutritional Analysis
2.9. Statistical Analysis
3. Results and discussion
3.1. Phenolic Content
3.2. Protein Profile and Soluble Protein Content after Digestion
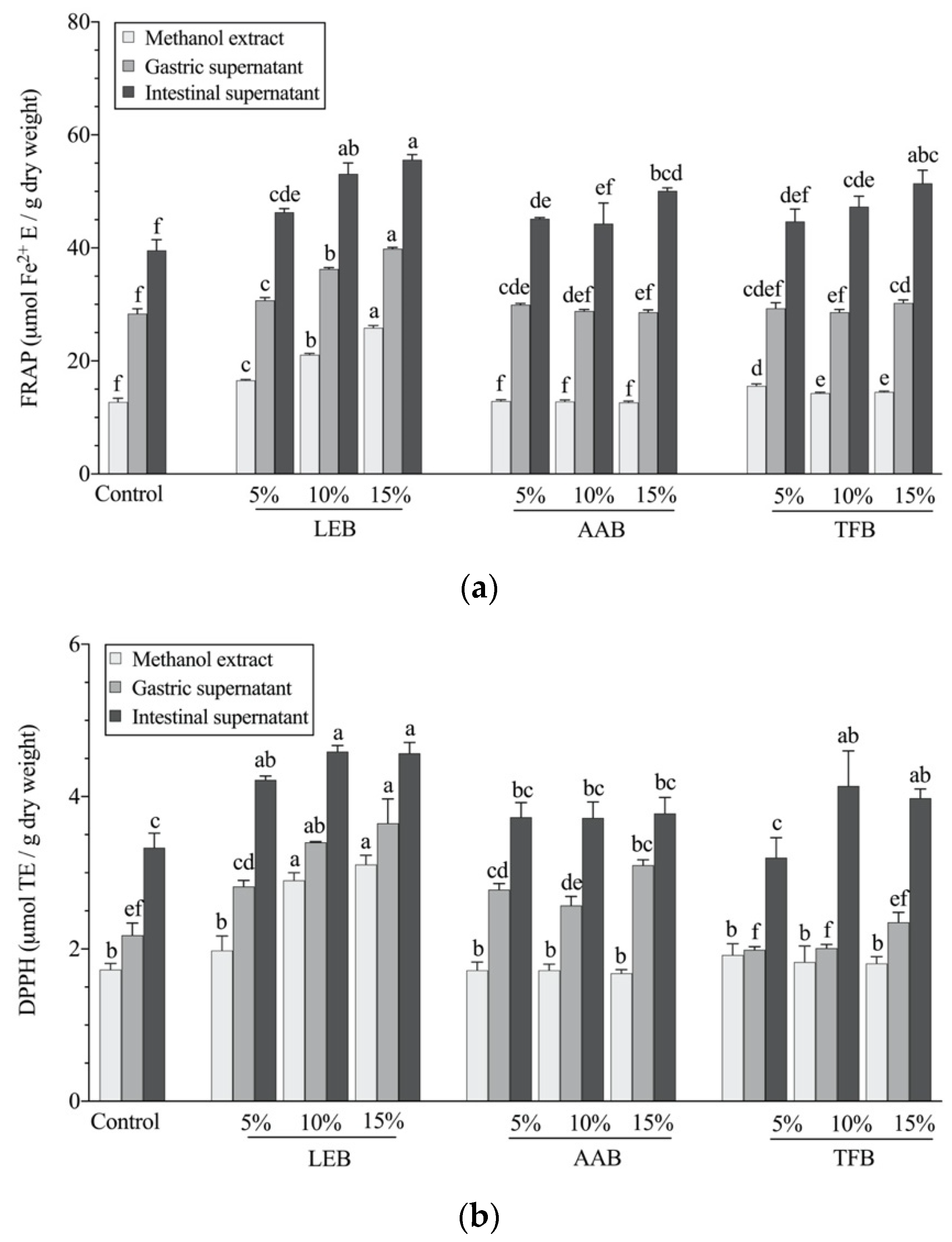
3.3. In Vitro Antioxidant Activity after Digestion
3.4. Phenolic and Antioxidants Content in Undigested Residue
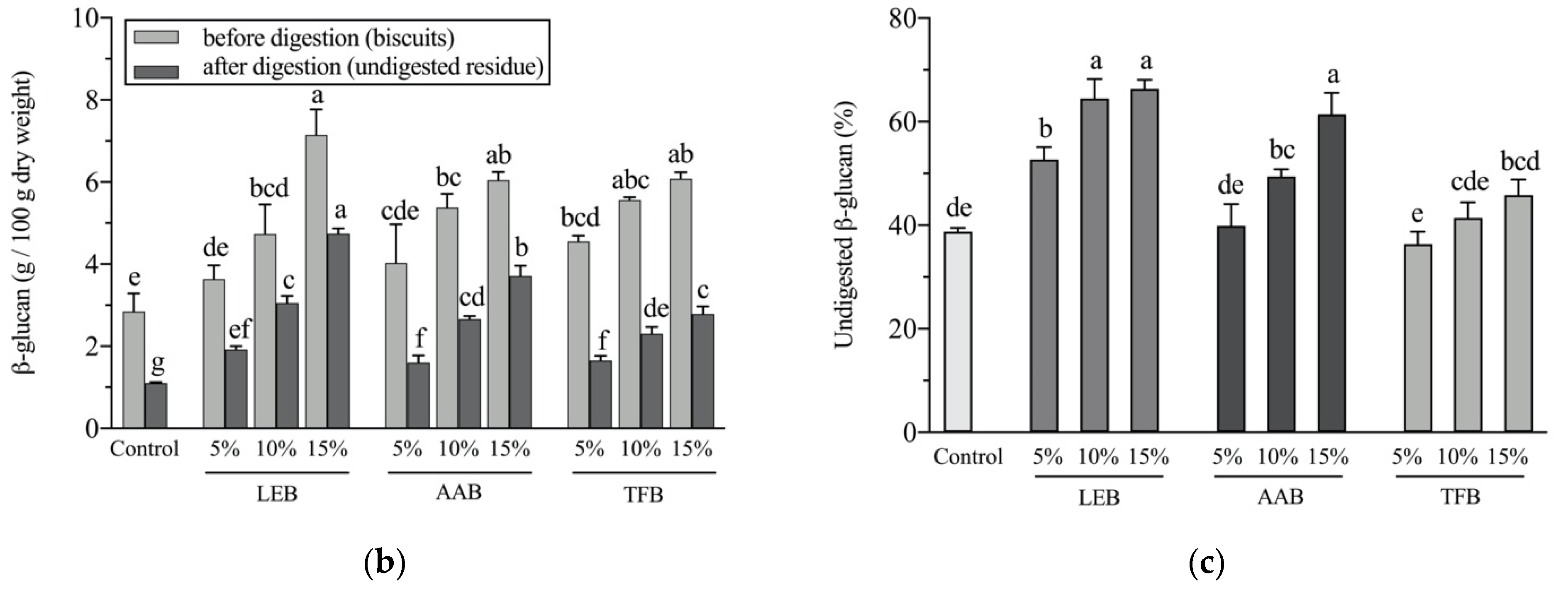
3.5. β-Glucan Potential Colon-Bioavailability
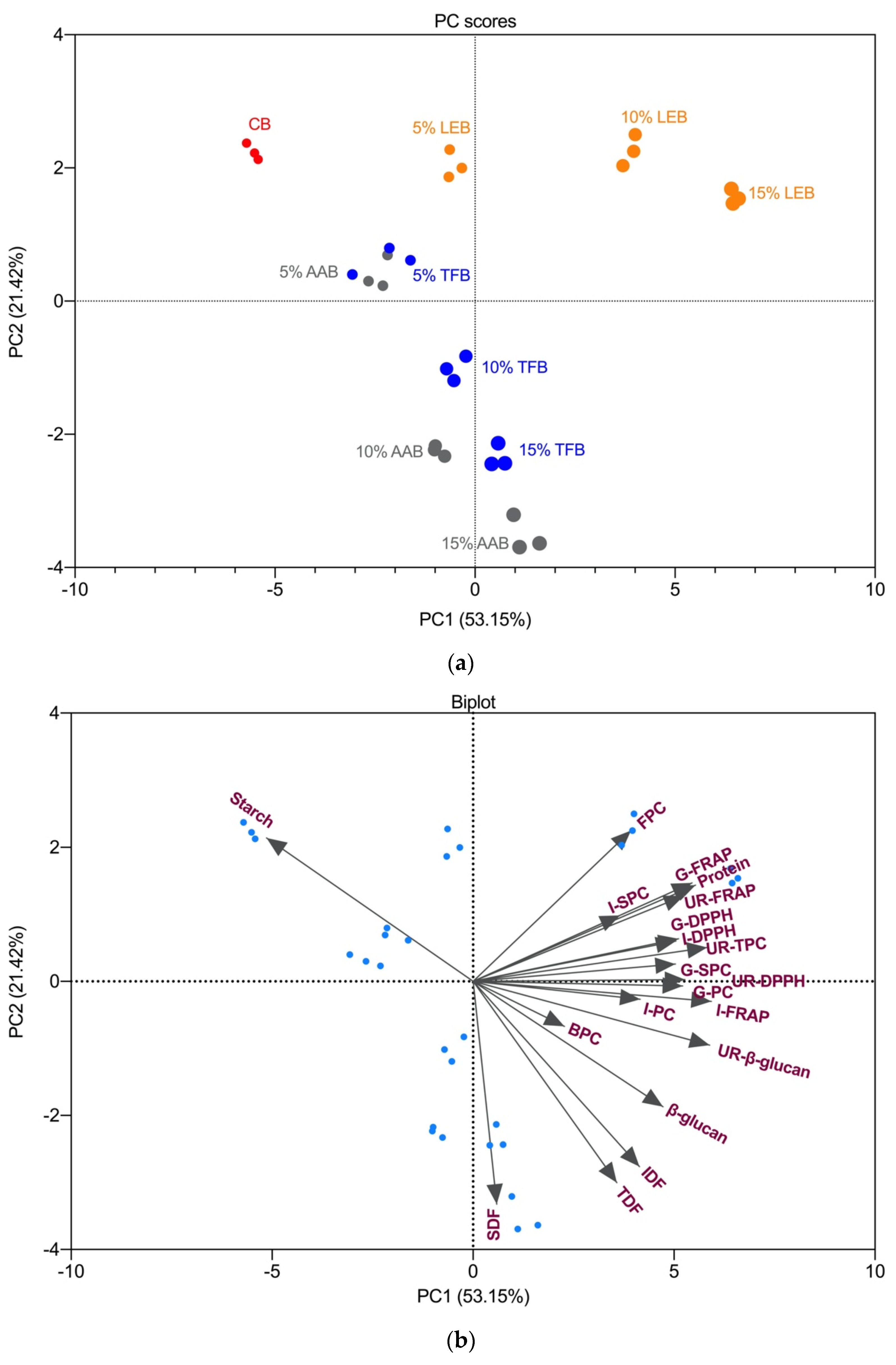
3.6. Principal Component Analysis and Correlations
3.7. Nutritional Value
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Espitia-Hernandez, P.; Chavez Gonzalez, M.L.; Ascacio-Valdes, J.A.; Davila-Medina, D.; Flores-Naveda, A.; Silva, T.; Ruelas Chacon, X.; Sepulveda, L. Sorghum (Sorghum bicolor L.) as a potential source of bioactive substances and their biological properties. Crit. Rev. Food Sci. Nutr. 2020, 1–12. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Acquisgrana, M.d.R.; Gomez Pamies, L.C.; Martinez Amezaga, N.M.J.; Quiroga, F.M.; Ribotta, P.D.; Benítez, E.I. Impact of moisture and grinding on yield, physical, chemical and thermal properties of wholegrain flour obtained from hydrothermally treated sorghum grains. Int. J. Food Sci. Technol. 2020, 55, 2901–2909. [Google Scholar] [CrossRef]
- Gosine, L.; McSweeney, M.B. Consumers’ attitudes towards alternative grains: A conjoint analysis study. Int. J. Food Sci. Technol. 2019, 54, 1588–1596. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Serna-Saldívar, S.O. Functionality and characterization of kafirin-rich protein extracts from different whole and decorticated sorghum genotypes. J. Cereal Sci. 2016, 70, 57–65. [Google Scholar] [CrossRef]
- Marengo, M.; Bonomi, F.; Marti, A.; Pagani, M.A.; Elkhalifa, A.E.O.; Iametti, S. Molecular features of fermented and sprouted sorghum flours relate to their suitability as components of enriched gluten-free pasta. LWT Food Sci. Technol. 2015, 63, 511–518. [Google Scholar] [CrossRef]
- Cabrera-Ramirez, A.H.; Luzardo-Ocampo, I.; Ramirez-Jimenez, A.K.; Morales-Sanchez, E.; Campos-Vega, R.; Gaytan-Martinez, M. Effect of the nixtamalization process on the protein bioaccessibility of white and red sorghum flours during in vitro gastrointestinal digestion. Food Res. Int. 2020, 134, 109234. [Google Scholar] [CrossRef] [PubMed]
- De Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Belton, P.S.; Beta, T.; Duodu, K.G. Increasing the utilisation of sorghum, millets and pseudocereals: Developments in the science of their phenolic phytochemicals, biofortification and protein functionality. J. Cereal Sci. 2014, 59, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Brennan, M.A.; Serventi, L.; Brennan, C.S. Incorporation of mushroom powder into bread dough-effects on dough rheology and bread properties. Cereal Chem. 2018, 95, 418–427. [Google Scholar] [CrossRef]
- Radzki, W.; Ziaja-Sołtys, M.; Nowak, J.; Topolska, J.; Bogucka-Kocka, A.; Sławińska, A.; Michalak-Majewska, M.; Jabłońska-Ryś, E.; Kuczumow, A. Impact of processing on polysaccharides obtained from button mushroom (Agaricus bisporus). Int. J. Food Sci. Technol. 2019, 54, 1405–1412. [Google Scholar] [CrossRef]
- Khongdetch, J.; Laohakunjit, N.; Kaprasob, R. King Boletus mushroom-derived bioactive protein hydrolysate: Characterisation, antioxidant, ACE inhibitory and cytotoxic activities. Int. J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Colosimo, R.; Warren, F.J.; Edwards, C.H.; Ryden, P.; Dyer, P.S.; Finnigan, T.J.A.; Wilde, P.J. Comparison of the behavior of fungal and plant cell wall during gastrointestinal digestion and resulting health effects: A review. Trends Food Sci. Technol. 2021, 110, 132–141. [Google Scholar] [CrossRef]
- Williams, B.A.; Mikkelsen, D.; le Paih, L.; Gidley, M.J. In vitro fermentation kinetics and end-products of cereal arabinoxylans and (1,3;1,4)-β-glucans by porcine faeces. J. Cereal Sci. 2011, 53, 53–58. [Google Scholar] [CrossRef]
- Wang, L.; Brennan, M.A.; Guan, W.; Liu, J.; Zhao, H.; Brennan, C.S. Edible mushrooms dietary fibre and antioxidants: Effects on glycaemic load manipulation and their correlations pre-and post-simulated in vitro digestion. Food Chem. 2021, 351, 129320. [Google Scholar] [CrossRef]
- Tu, J.; Brennan, M.; Brennan, C. An insight into the mechanism of interactions between mushroom polysaccharides and starch. Curr. Opin. Food Sci. 2021, 37, 17–25. [Google Scholar] [CrossRef]
- Biao, Y.; Chen, X.; Wang, S.; Chen, G.; McClements, D.J.; Zhao, L. Impact of mushroom (Pleurotus eryngii) flour upon quality attributes of wheat dough and functional cookies-baked products. Food Sci. Nutr. 2020, 8, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodriguez, R.; Contreras, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber-Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Barros, R.G.C.; Pereira, U.C.; Andrade, J.K.S.; de Oliveira, C.S.; Vasconcelos, S.V.; Narain, N. In vitro gastrointestinal digestion and probiotics fermentation impact on bioaccessbility of phenolics compounds and antioxidant capacity of some native and exotic fruit residues with potential antidiabetic effects. Food Res. Int. 2020, 136, 109614. [Google Scholar] [CrossRef]
- Blanco Canalis, M.S.; Baroni, M.V.; Leon, A.E.; Ribotta, P.D. Effect of peach puree incorportion on cookie quality and on simulated digestion of polyphenols and antioxidant properties. Food Chem. 2020, 333, 127464. [Google Scholar] [CrossRef]
- Wu, G.; Hui, X.; Mu, J.; Gong, X.; Stipkovits, L.; Brennan, M.A.; Brennan, C.S. Functionalization of sodium caseinate fortified with blackcurrant concentrate via spray-drying and freeze-drying techniques: The nutritional properties of the fortified particles. LWT Food Sci. Technol. 2021, 142, 111051. [Google Scholar] [CrossRef]
- Gong, X.; Morton, J.D.; Bhat, Z.F.; Mason, S.L.; Bekhit, A.E.D.A. Comparative efficacy of actinidin from green and gold kiwi fruit extract onin vitrosimulated protein digestion of beef Semitendinosusand its myofibrillar protein fraction. Int. J. Food Sci. Technol. 2019, 55, 742–750. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; Brennan, M.; Guan, W.; Liu, J.; Wang, M.; Wen, X.; He, J.; Brennan, C. In vitro gastric digestion antioxidant and cellular radical scavenging activities of wheat-shiitake noodles. Food Chem. 2020, 330, 127214. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Li, Y.; Huang, Y.; Zhang, J. Antioxidant activity of free and hydrolyzed phenolic compounds in soluble and insoluble dietary fibres derived from hulless barley. LWT Food Sci. Technol. 2019, 111, 534–540. [Google Scholar] [CrossRef]
- Polat, H.; Dursun Capar, T.; Inanir, C.; Ekici, L.; Yalcin, H. Formulation of functional crackers enriched with germinated lentil extract: A Response Surface Methodology Box-Behnken Design. LWT Food Sci. Technol. 2020, 123, 109065. [Google Scholar] [CrossRef]
- Wu, G.; Hui, X.; Stipkovits, L.; Rachman, A.; Tu, J.; Brennan, M.A.; Brennan, C.S. Whey protein-blackcurrant concentrate particles obtained by spray-drying and freeze-drying for delivering structural and health benefits of cookies. Innov. Food Sci. Emerg. Technol. 2021, 68, 102606. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of beta-glucan in mushrooms and mycelial products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Prosky, L.; Asp, N.-G.; Schweizer, T.F.; Devries, J.W.; Furda, I. Determination of insoluble and soluble dietary fiber in foods and food products: Collaborative study. J. AOAC Int. 1992, 75, 360–367. [Google Scholar] [CrossRef]
- Lang, G.H.; Lindemann, I.D.S.; Ferreira, C.D.; Hoffmann, J.F.; Vanier, N.L.; de Oliveira, M. Effects of drying temperature and long-term storage conditions on black rice phenolic compounds. Food Chem. 2019, 287, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Irondi, E.A.; Adegoke, B.M.; Effion, E.S.; Oyewo, S.O.; Alamu, E.O.; Boligon, A.A. Enzymes inhibitory property, antioxidant activity and phenolics profile of raw and roasted red sorghum grains in vitro. Food Sci. Hum. Wellness 2019, 8, 142–148. [Google Scholar] [CrossRef]
- Rocchetti, G.; Rizzi, C.; Pasini, G.; Lucini, L.; Giuberti, G.; Simonato, B. Effect of moringa oleifera l. leaf powder addition on the phenolic bioaccessibility and on in vitro starch digestibility of durum wheat fresh pasta. Foods 2020, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, H.; Szawara-Nowak, D.; Wronkowska, M. Bioaccessibility of anti-AGEs activity, antioxidant capacity and phenolics from water biscuits prepared from fermented buckwheat flours. LWT Food Sci. Technol. 2020, 123, 109051. [Google Scholar] [CrossRef]
- Liu, C.; Ge, S.; Yang, J.; Xu, Y.; Zhao, M.; Xiong, L.; Sun, Q. Adsorption mechanism of polyphenols onto starch nanoparticles and enhanced antioxidant activity under adverse conditions. J. Funct. Foods 2016, 26, 632–644. [Google Scholar] [CrossRef]
- Dantas, A.M.; Mafaldo, I.M.; Oliveira, P.M.L.; Lima, M.D.S.; Magnani, M.; Borges, G. Bioaccessibility of phenolic compounds in native and exotic frozen pulps explored in Brazil using a digestion model coupled with a simulated intestinal barrier. Food Chem. 2019, 274, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.j.; Tan, C.; Feng, Y. Solvent extraction and in vitro simulated gastrointestinal digestion of phenolic compounds from purple sweet potato. Int. J. Food Sci. Technol. 2019, 54, 2887–2896. [Google Scholar] [CrossRef]
- Quan, W.; Tao, Y.; Lu, M.; Yuan, B.; Chen, J.; Zeng, M.; Qin, F.; Guo, F.; He, Z. Stability of the phenolic compounds and antioxidant capacity of five fruit (apple, orange, grape, pomelo and kiwi) juices during in vitro-simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2018, 53, 1131–1139. [Google Scholar] [CrossRef]
- Wu, T.; Taylor, C.; Nebl, T.; Ng, K.; Bennett, L.E. Effects of chemical composition and baking on in vitro digestibility of proteins in breads made from selected gluten-containing and gluten-free flours. Food Chem. 2017, 233, 514–524. [Google Scholar] [CrossRef]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of different protein sources using the INFOGEST static digestion model. Food Res. Int. 2020, 130, 108996. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.R.; Liu, C.L.; Lee, H.P.; Chen, J.K. The identification and characterization of chitotriosidase activity in pancreatin from porcine pancreas. Molecules 2013, 18, 2978–2987. [Google Scholar] [CrossRef] [Green Version]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Joye, I. Protein digestibility of cereal products. Foods 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phongthai, S.; Rawdkuen, S. Fractionation and characterization of antioxidant peptides from rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Cereal Chem. 2020, 97, 316–325. [Google Scholar] [CrossRef]
- Morales, D.; Miguel, M.; Garces-Rimon, M. Pseudocereals: A novel source of biologically active peptides. Crit. Rev. Food Sci. Nutr. 2020, 9, 1537–1544. [Google Scholar] [CrossRef]
- Ashwath Kumar, K.; Sharma, G.K.; Anilakumar, K.R. Influence of multigrain premix on nutritional, in-vitro and in-vivo protein digestibility of multigrain biscuit. J. Food Sci. Technol. 2019, 56, 746–753. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Bustos, M.C.; Vignola, M.B.; Paesani, C.; Salinas, C.N.; Perez, G.T. A study on fibre addition to gluten free bread: Its effects on bread quality and in vitro digestibility. J. Food Sci. Technol. 2017, 54, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with alpha-amylase and alpha-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Baczek, N.; Jarmulowicz, A.; Wronkowska, M.; Haros, C.M. Assessment of the glycaemic index, content of bioactive compounds, and their in vitro bioaccessibility in oat-buckwheat breads. Food Chem. 2020, 330, 127199. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Suwannachot, J.; Ogawa, Y. In vitro gastrointestinal digestion of crisphead lettuce: Changes in bioactive compounds and antioxidant potential. Food Chem. 2020, 311, 125885. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Brennan, M.A.; Narciso, J.; Guan, W.; Zhang, J.; Yuan, L.; Serventi, L.; Brennan, C.S. Correlations between the phenolic and fibre composition of mushrooms and the glycaemic and textural characteristics of mushroom enriched extruded products. LWT Food Sci. Technol. 2020, 118, 108730. [Google Scholar] [CrossRef]
- Sanchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2017, 2, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Motilva, M.J.; Ludwig, I.A. Beta-glucan and phenolic compounds: Their concentration and behavior during in vitro gastrointestinal digestion and colonic fermentation of different barley-based food products. J. Agric. Food Chem. 2018, 66, 8966–8975. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Liu, S.; Zheng, Y.; Zhang, X.; He, Z.; Wang, Z.; Wang, Y.; Xie, J.; Chen, Y.; Yu, Q. Release and metabolism of bound polyphenols from carrot dietary fiber and their potential activity in in vitro digestion and colonic fermentation. Food Function 2020, 11, 6652–6665. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [Green Version]
- Alahmed, A.; Simsek, S. Pre-harvest glyphosate application effects on properties of β-glucan from oat groats. J. Cereal Sci. 2020, 96, 103119. [Google Scholar] [CrossRef]
- Morales, D.; Smiderle, F.R.; Piris, A.J.; Soler-Rivas, C.; Prodanov, M. Production of a β-d-glucan-rich extract from Shiitake mushrooms (Lentinula edodes) by an extraction/microfiltration/reverse osmosis (nanofiltration) process. Innov. Food Sci. Emerg. Technol. 2019, 51, 80–90. [Google Scholar] [CrossRef]
- Shoukat, M.; Sorrentino, A. Cereal β-glucan: A promising prebiotic polysaccharide and its impact on the gut health. Int. J. Food Sci. Technol. 2021, 56, 2088–2097. [Google Scholar] [CrossRef]
- Zhuang, H.; Chen, Z.; Feng, T.; Yang, Y.; Zhang, J.; Liu, G.; Li, Z.; Ye, R. Characterization of Lentinus edodes beta-glucan influencing the in vitro starch digestibility of wheat starch gel. Food Chem. 2017, 224, 294–301. [Google Scholar] [CrossRef]
- Vetvicka, V.; Gover, O.; Karpovsky, M.; Hayby, H.; Danay, O.; Ezov, N.; Hadar, Y.; Schwartz, B. Immune-modulating activities of glucans extracted from Pleurotus ostreatus and Pleurotus eryngii. J. Funct. Foods 2019, 54, 81–91. [Google Scholar] [CrossRef]
- Singh, A.; Dutta, P.K. Extraction of chitin-glucan complex from Agaricus bisporus: Characterization and antibacterial activity. J. Polym. Mater. 2017, 34, 1–9. [Google Scholar]
- Wang, X.; Wang, W.; Wang, L.; Yu, C.; Zhang, G.; Zhu, H.; Wang, C.; Zhao, S.; Hu, C.A.; Liu, Y. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ji, Y.; Chen, X.; Su, A.; Ma, G.; Chen, G.; Hu, Q.; Zhao, L. Effects of a beta-type glycosidic polysaccharide from Flammulina velutipes on anti-inflammation and gut microbiota modulation in colitis mice. Food Funct. 2020, 11, 4259–4274. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Luo, J.; Bai, T.; Chen, H. Effect of digestion on bound phenolic content, antioxidant activity and hypoglycemic ability of insoluble dietary fibre from four Triticeae crops. J. Food Biochem. 2021, 45, e13746. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello, L.A.; Álvarez-Parrilla, E.; de la Rosad, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef]
- Liu, H.; Wu, H.; Wang, Q. Health-promoting effects of dietary polysaccharide extracted from Dendrobium aphyllum on mice colon, including microbiota and immune modulation. Int. J. Food Sci. Technol. 2019, 54, 1684–1696. [Google Scholar] [CrossRef]
- FDA. Food labelling: Health claims; soluble fiber from whole oats and risk of coronary heart disease. Fed. Regist. 1997, 62, 5343–15344. [Google Scholar]

| Samples | FPC | BPC | TPC | Gastric Fractions | Intestinal Fractions | Bio-Accessibility Index (%) | |
|---|---|---|---|---|---|---|---|
| BIG | BII | ||||||
| Ingredients | |||||||
| Sorghum | 2.98 ± 0.18 B | 7.49 ± 0.14 A | 10.47 ± 0.30 B | 3.89 ± 0.01 C | 6.45 ± 0.06 C | 37.23 ± 1.11 C | 61.69 ± 2.14 C |
| L. edodes | 7.16 ± 0.11 A | 7.47 ± 0.22 A | 14.63 ± 0.24 A | 9.55 ± 0.08 A | 13.36 ± 0.38 A | 65.26 ± 0.64 A | 91.33 ± 1.30 A |
| A. auricula | 1.37 ± 0.07 C | 7.30 ± 0.17 A | 8.67 ± 0.10 C | 4.80 ± 0.22 B | 6.93 ± 0.28 BC | 55.29 ± 2.47 B | 79.87 ± 4.00 B |
| T. fuciformis | 1.23 ± 0.07 C | 6.65 ± 0.03 B | 7.89 ± 0.06 D | 4.58 ± 0.11 B | 7.50 ± 0.12 B | 58.08 ± 1.75 B | 95.08 ± 1.19 A |
| Biscuits | |||||||
| Control | 1.78 ± 0.01 c | 3.48 ± 0.04 e | 5.26 ± 0.03 e | 2.31 ± 0.12 e | 3.36± 0.10 e | 43.99 ± 2.51 d | 63.84 ± 2.27 e |
| 5% LEB | 1.79 ± 0.01 c | 3.68 ± 0.05 bcd | 5.48 ± 0.04 d | 2.44 ± 0.05 de | 4.09 ± 0.18 c | 44.58 ± 0.69 d | 74.71 ± 2.91 bc |
| 10% LEB | 1.94 ± 0.03 b | 3.74 ± 0.03 bc | 5.68 ± 0.06 bc | 2.98 ± 0.06 ab | 4.48 ± 0.07 b | 52.42 ± 1.08 abc | 78.82 ± 0.67 b |
| 15% LEB | 2.08 ± 0.03 a | 3.82 ± 0.02 ab | 5.90 ± 0.04 a | 3.10 ± 0.05 a | 4.53 ± 0.09 ab | 52.65 ± 1.11 ab | 76.88 ± 1.20 b |
| 5% AAB | 1.75 ± 0.02 cd | 3.95 ± 0.02 a | 5.70 ± 0.04 b | 2.54 ± 0.10 cde | 3.87 ± 0.03 cd | 44.50 ± 1.93 d | 67.83 ± 0.57 de |
| 10% AAB | 1.67 ± 0.02 ef | 3.81 ± 0.03 ab | 5.48 ± 0.05 d | 2.60 ± 0.05 cd | 3.61 ± 0.06 de | 47.51 ± 0.92 cd | 65.87 ± 1.58 de |
| 15% AAB | 1.62 ± 0.02 f | 3.85 ± 0.15 ab | 5.47 ± 0.16 d | 2.91 ± 0.13 ab | 3.85 ± 0.12 cd | 53.17 ± 2.28 a | 70.28 ± 2.44 cd |
| 5% TFB | 1.79 ± 0.02 c | 3.70 ± 0.04 bcd | 5.49 ± 0.04 cd | 2.78 ± 0.03 bc | 3.46 ± 0.08 e | 50.57 ± 0.80 abc | 62.96 ± 1.71 e |
| 10% TFB | 1.74 ± 0.03 cd | 3.61 ± 0.04 cde | 5.35 ± 0.04 de | 2.55 ± 0.13 cde | 4.83 ± 0.06 a | 47.67 ± 2.58 cd | 90.32 ± 1.27 a |
| 15% TFB | 1.70 ± 0.02 de | 3.56 ± 0.07 de | 5.26 ± 0.06 e | 2.52 ± 0.12 de | 4.70 ± 0.21 ab | 47.78 ± 1.73 bcd | 89.37 ± 2.94 a |
| Samples | Phenolic Content (mg GAE/g dw) | FRAP (μmol Fe2+ E/g dw) | DPPH (μmol TE/g dw) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Free | Bound | Total | Free | Bound | Total | Free | Bound | Total | |
| Control biscuit | 0.58 ± 0.01 e | 0.56 ± 0.01 f | 1.15 ± 0.01 f | 6.34 ± 0.41 d | 8.41 ± 0.17 d | 14.75 ± 0.46 e | 0.95 ± 0.00 e | 0.91 ± 0.06 c | 1.85 ± 0.06 e |
| 5% LEB | 0.78 ± 0.01 b | 0.90 ± 0.02 ab | 1.67 ± 0.03 b | 7.04 ± 0.17 ab | 12.59 ± 0.38 a | 19.62 ± 0.45 a | 1.07± 0.01 ab | 1.05 ± 0.03 ab | 2.12 ± 0.03 abcd |
| 10% LEB | 0.84 ± 0.01 a | 0.94 ± 0.05 a | 1.78 ± 0.05 a | 7.05 ± 0.20 ab | 13.19 ± 0.41 a | 20.24 ± 0.55 a | 1.09 ± 0.02 a | 1.14 ± 0.06 a | 2.23 ± 0.06 ab |
| 15% LEB | 0.85 ± 0.02 a | 0.96 ± 0.03 a | 1.77 ± 0.06 a | 7.31 ± 0.07 a | 12.91 ± 0.10 a | 20.22 ± 0.04 a | 1.09 ± 0.00 a | 1.15 ± 0.02 a | 2.24 ± 0.02 a |
| 5% AAB | 0.60 ± 0.01 e | 0.77 ± 0.02 cde | 1.37 ± 0.02 de | 5.27 ± 0.13 e | 9.92 ± 0.26 c | 15.20 ± 0.37 de | 0.93 ± 0.02 e | 1.06 ± 0.01 ab | 1.99 ± 0.02 de |
| 10% AAB | 0.65 ± 0.01 d | 0.79 ± 0.02 cd | 1.45 ± 0.01 d | 5.30 ± 0.09 e | 10.18 ± 0.15 bc | 15.49 ± 0.24 cde | 1.04 ± 0.01 bc | 1.10 ± 0.04 ab | 2.13 ± 0.04 abc |
| 15% AAB | 0.72 ± 0.02 c | 0.84 ± 0.03 bc | 1.56 ± 0.03 c | 5.64 ± 0.08 e | 10.99 ± 0.16 b | 16.63 ± 0.24 bc | 1.04 ± 0.03 bc | 1.10 ± 0.05 ab | 2.14± 0.07 abc |
| 5% TFB | 0.59 ± 0.01 e | 0.71 ± 0.01 e | 1.30 ± 0.01 e | 6.41 ± 0.06 cd | 9.61 ± 0.69 c | 16.02 ± 0.75 bcd | 1.00 ± 0.01 cd | 1.10 ± 0.02 ab | 2.10 ± 0.03 bcd |
| 10% TFB | 0.67 ± 0.01 d | 0.72 ± 0.03 de | 1.39 ± 0.02 de | 6.70 ± 0.07 bcd | 10.00 ± 0.17 bc | 16.70 ± 0.24 b | 0.99 ± 0.01 d | 1.00 ± 0.01 bc | 2.00 ± 0.01 d |
| 15% TFB | 0.70 ± 0.04 cd | 0.75 ± 0.03 de | 1.45 ± 0.04 d | 6.85 ± 0.12 abc | 10.07 ± 0.47 bc | 16.91 ± 0.39 b | 0.99 ± 0.01 d | 1.04 ± 0.06 ab | 2.02 ± 0.07 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, J.; Brennan, M.A.; Wu, G.; Bai, W.; Cheng, P.; Tian, B.; Brennan, C.S. Delivery of Phenolic Compounds, Peptides and β-Glucan to the Gastrointestinal Tract by Incorporating Dietary Fibre-Rich Mushrooms into Sorghum Biscuits. Foods 2021, 10, 1812. https://doi.org/10.3390/foods10081812
Tu J, Brennan MA, Wu G, Bai W, Cheng P, Tian B, Brennan CS. Delivery of Phenolic Compounds, Peptides and β-Glucan to the Gastrointestinal Tract by Incorporating Dietary Fibre-Rich Mushrooms into Sorghum Biscuits. Foods. 2021; 10(8):1812. https://doi.org/10.3390/foods10081812
Chicago/Turabian StyleTu, Juncai, Margaret Anne Brennan, Gang Wu, Weidong Bai, Ping Cheng, Bin Tian, and Charles Stephen Brennan. 2021. "Delivery of Phenolic Compounds, Peptides and β-Glucan to the Gastrointestinal Tract by Incorporating Dietary Fibre-Rich Mushrooms into Sorghum Biscuits" Foods 10, no. 8: 1812. https://doi.org/10.3390/foods10081812
APA StyleTu, J., Brennan, M. A., Wu, G., Bai, W., Cheng, P., Tian, B., & Brennan, C. S. (2021). Delivery of Phenolic Compounds, Peptides and β-Glucan to the Gastrointestinal Tract by Incorporating Dietary Fibre-Rich Mushrooms into Sorghum Biscuits. Foods, 10(8), 1812. https://doi.org/10.3390/foods10081812