Effect of UV-C Irradiation, Storage and Subsequent Cooking on Chemical Constituents of Fresh-Cut Potatoes
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Chemicals and Standards
2.3. Sample Preparation
2.4. UV-C Treatment
2.5. Cooking Treatments
2.6. Phenolics Analysis
2.6.1. Extraction of Phenolics
2.6.2. UPLC MS2 Analysis of Phenolics
2.7. Sugar Analyses
2.7.1. Extraction of Sugars
2.7.2. HPLC Analysis of Sugars
2.8. Acrylamide Analysis
2.8.1. Extraction of Acrylamide
2.8.2. UPLC MS2 Analysis of Acrylamide
2.9. Statistical Analysis
3. Results and Discussion
3.1. Phenolics Analysis
3.2. Sugars Analysis
3.3. Acrylamide Analysis
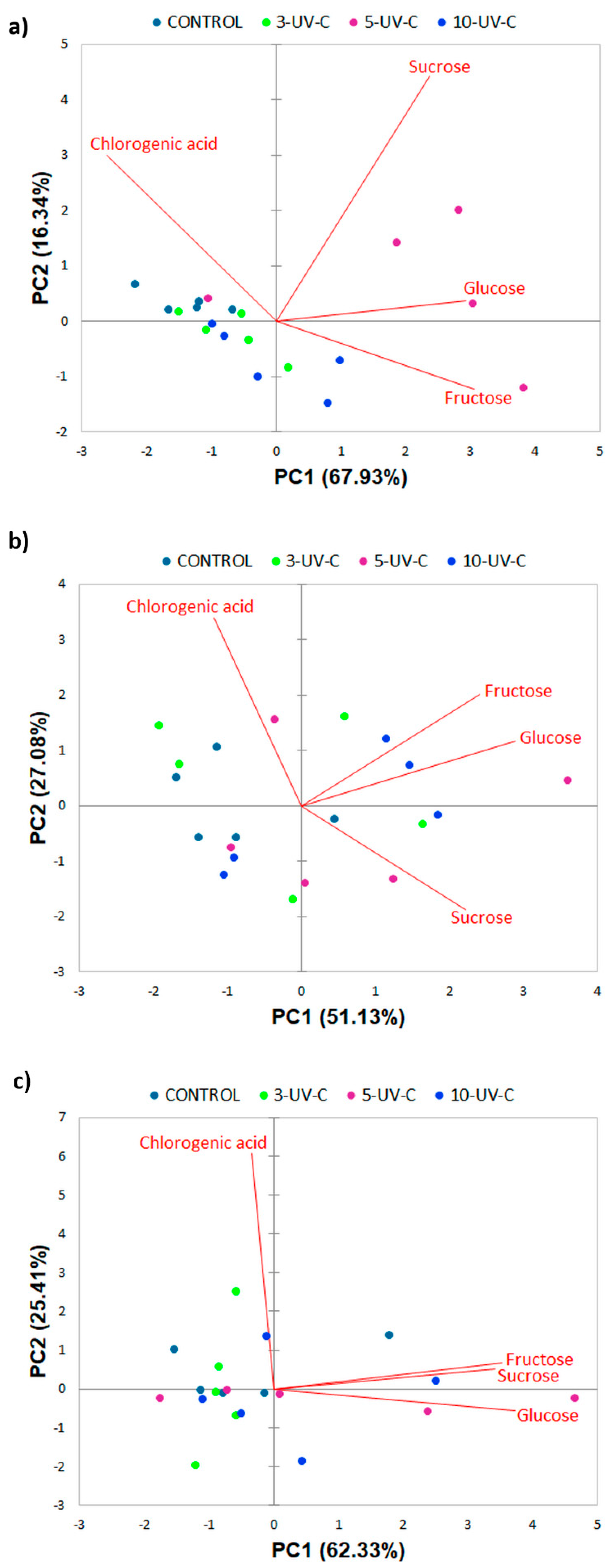
3.4. PCA Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baldwin, E.A.; Bai, J. Physiology of fresh-cut fruits and vegetables. In Advances in Fresh-Cut Fruits and Vegetables Processing; Martin-Belloso, O., Soliva-Fortuny, R., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 87–113. [Google Scholar]
- Rico, D.; Martin-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, J.M.; Rushing, J.W. Effect of ultraviolet-C light on quality and microbial population of fresh-cut watermelon. Postharvest Biol. Technol. 2006, 40, 256–261. [Google Scholar] [CrossRef]
- Lamikanra, O.; Kueneman, D.; Ukuku, D.; Bett-Garber, K.L. Effect of processing under ultraviolet light on the shelf life of fresh-cut cantaloupe melon. J. Food Sci. 2005, 70, C534–C539. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Brandão, T.R.S.; Silva, C.L.M. Emerging technologies to improve the safety and quality of fruits and vegetables. In Novel Technologies in Food Science. Integrating Food Science and Engineering Knowledge into the Food Chain; McElhatton, A., Sobral, P.J.A., Eds.; Springer: New York, NY, USA, 2012; Volume 7, pp. 261–297. [Google Scholar] [CrossRef]
- Manzocco, L.; Nicoli, M.C. Surface processing: Existing and potential applications of ultraviolet light. Crit. Rev. Food Sci. Nutr. 2015, 55, 469–484. [Google Scholar] [CrossRef]
- Teoh, L.S.; Lasekan, O.; Adzahan, N.M.; Hashim, N. The effect of ultraviolet treatment on enzymatic activity and total phenolic content of minimally processed potato slices. J. Food Sci. Technol. 2016, 53, 3035–3042. [Google Scholar] [CrossRef] [Green Version]
- Moreno, C.; Andrade-Cuvi, M.J.; Zaro, M.J.; Darre, M.; Vicente, A.R.; Concellon, A. Short UV-C Treatment Prevents Browning and Extends the Shelf-Life of Fresh-Cut Carambola. J. Food Qual. 2017. [Google Scholar] [CrossRef]
- Alegria, C.; Pinheiro, J.; Duthoit, M.; Goncalves, E.M.; Moldao-Martins, M.; Abreu, M. Fresh-cut carrot (cv. Nantes) quality as affected by abiotic stress (heat shock and UV-C irradiation) pre-treatments. LWT Food Sci. Technol. 2012, 48, 197–203. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velazquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC Light Enhances the Biosynthesis of Phenolic Antioxidants in Fresh-Cut Carrot through a Synergistic Effect with Wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Beltrán, J.A.; Barbosa-Cánovas, G.V. Advantages and Limitations on Processing Foods by UV Light. Food Sci. Technol. Int. 2004, 10, 137–147. [Google Scholar] [CrossRef]
- Pan, Y.-G.; Zu, H. Effect of UV-C Radiation on the Quality of Fresh-cut Pineapples. Procedia Eng. 2012, 37, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Gomez, P.L.; Alzamora, S.M.; Castro, M.A.; Salvatori, D.M. Effect of ultraviolet-C light dose on quality of cut-apple: Microorganism, color and compression behavior. J. Food Eng. 2010, 98, 60–70. [Google Scholar] [CrossRef]
- Artes-Hernandez, F.; Escalona, V.H.; Robles, P.A.; Martinez-Hernandez, G.B.; Artes, F. Effect of UV-C radiation on quality of minimally processed spinach leaves. J. Sci. Food Agric. 2009, 89, 414–421. [Google Scholar] [CrossRef]
- Lin, Q.; Xie, Y.J.; Liu, W.; Zhang, J.; Cheng, S.Z.; Xie, X.F.; Guan, W.Q.; Wang, Z.D. UV-C treatment on physiological response of potato (Solanum tuberosum L.) during low temperature storage. J. Food Sci. Technol. 2017, 54, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Jakubowski, T.; Krolczyk, J.B. Method for the reduction of natural losses of potato tubers during their long-term storage. Sustainability 2020, 12, 1048. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.B.O.; Honorio, S.L.; Messias, C.L.; Oton, M.; Gomez, P.A. Effect of UV-C radiation and fluorescent light to control postharvest soft rot in potato seed tubers. Sci. Hortic. 2015, 181, 174–181. [Google Scholar] [CrossRef]
- Sobol, Z.; Jakubowski, T.; Surma, M. Effect of Potato Tuber Exposure to UV-C Radiation and Semi-Product Soaking in Water on Acrylamide Content in French Fries Dry Matter. Sustainability 2020, 12, 3426. [Google Scholar] [CrossRef] [Green Version]
- Bethke, P.C.; Bussan, A.J. Acrylamide in processed potato products. Am. J. Potato Res. 2013, 90, 403–424. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Acrylamide, IARC monographs on the evaluation of carcinogenic risks to humans. In Some Industrial Chemicals; International Agency for Research on Cancer: Lyon, France, 1994; Volume 60, pp. 389–433. [Google Scholar]
- European Commission. Commission Regulation (EU), 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, 304, 24–44. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on Acrylamide in Food. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2015.4104 (accessed on 25 May 2021).
- Xie, Y.J.; Lin, Q.; Guan, W.Q.; Cheng, S.Z.; Wang, Z.D.; Sun, C.D. Comparison of Sodium Acid Sulfate and UV-C Treatment on Browning and Storage Quality of Fresh-Cut Potatoes. J. Food Qual. 2017. [Google Scholar] [CrossRef] [Green Version]
- Saltveit, M.E. Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Nicolaus, R.A.; Piattelli, M.; Fattorusso, E. Structure of Melanins + Melanogenesis.4. On Some Natural Melanins. Tetrahedron 1964, 20, 1163. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Dite Hunjek, D.; Pranjic, T.; Repajic, M.; Levaj, B. Fresh-cut potato quality and sensory: Effect of cultivar, age, processing, and cooking during storage. J. Food Sci. 2020, 85, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Dite Hunjek, D.; Pelaic, Z.; Cosic, Z.; Pedisic, S.; Repajic, M.; Levaj, B. Chemical constituents of fresh-cut potato as affected by cultivar, age, storage, and cooking. J. Food Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Elez Garofulic, I.; Zoric, Z.; Pedisic, S.; Brncic, M.; Dragovic-Uzelac, V. UPLC-MS(2) Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting. Supplement; Association of Official Analytical Chemists: Rockville, MD, USA, 1990; Volume 15. [Google Scholar]
- Al-Taher, F. Analysis of Acrylamide in French Fries Using Agilent SampliQ QuEChERS AOAC Kit and LC/MS/MS; Agilent Technologies: Santa Clara, CA, USA, 2010. [Google Scholar]
- Deußer, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Perla, V.; Holm, D.G.; Jayanty, S.S. Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT Food Sci. Technol. 2012, 45, 161–171. [Google Scholar] [CrossRef]
- Andre, C.M.; Schafleitner, R.; Guignard, C.; Oufir, M.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.F.; Evers, D.; Larondelle, Y. Modification of the health-promoting value of potato tubers field grown under drought stress: Emphasis on dietary antioxidant and glycoalkaloid contents in five native andean cultivars (Solanum tuberosum L.). J. Agric. Food Chem. 2009, 57, 599–609. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plazas, M.; Prohens, J.; Cunat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Andujar, I. Reducing Capacity, chlorogenic acid content and biological activity in a collection of scarlet (Solanum aethiopicum) and Gboma (S. macrocarpon) eggplants. Int. J. Mol. Sci. 2014, 15, 17221–17241. [Google Scholar] [CrossRef] [Green Version]
- Andre, C.M.; Legay, S.; Iammarino, C.; Ziebel, J.; Guignard, C.; Larondelle, Y.; Hausman, J.F.; Evers, D.; Miranda, L.M. The potato in the human diet: A complex matrix with potential health benefits. Potato Res. 2014, 57, 201–214. [Google Scholar] [CrossRef]
- Li, L.; Wu, M.; Zhao, M.; Guo, M.; Liu, H. Enzymatic properties on browning of fresh-cut potato. IOP Conf. Ser. Mater. Sci. Eng. 2018, 397, 012116. [Google Scholar] [CrossRef]
- Amaki, K.; Saito, E.; Taniguchi, K.; Joshita, K.; Murata, M. Role of chlorogenic acid quinone and interaction of chlorogenic acid quinone and catechins in the enzymatic browning of apple. Biosci. Biotechnol. Biochem. 2011, 75, 829–832. [Google Scholar] [CrossRef]
- Tudela, J.A.; Cantos, E.; Espin, J.C.; Tomas-Barberan, F.A.; Gil, M.I. Induction of antioxidant flavonol biosynthesis in fresh-cut potatoes. Effect of domestic cooking. J. Agric. Food Chem. 2002, 50, 5925–5931. [Google Scholar] [CrossRef] [PubMed]
- Blessington, T.; Nzaramba, M.N.; Scheuring, D.C.; Hale, A.L.; Reddivari, L.; Miller, J.C. Cooking Methods and Storage Treatments of Potato: Effects on Carotenoids, Antioxidant Activity, and Phenolics. Am. J. Potato Res. 2010, 87, 479–491. [Google Scholar] [CrossRef]
- Tian, J.H.; Chen, J.C.; Ye, X.Q.; Chen, S.G. Health benefits of the potato affected by domestic cooking: A review. Food Chem. 2016, 202, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.F.; Sethi, S.; Joshi, A.; Singh, A.M.; Raigond, P.; Singh, M.K.; Yadav, R.K. Biochemical and functional attributes of raw and boiled potato flesh and peel powders for suitability in food applications. J. Food Sci. Technol. 2020, 57, 3955–3965. [Google Scholar] [CrossRef]
- Knutsen, S.H.; Dimitrijevic, S.; Molteberg, E.L.; Segtnan, V.H.; Kaaber, L.; Wicklund, T. The influence of variety, agronomical factors and storage on the potential for acrylamide formation in potatoes grown in Norway. LWT Food Sci. Technol. 2009, 42, 550–556. [Google Scholar] [CrossRef]
- Davies, H.V.; Jefferies, R.A.; Scobie, L. Hexose Accumulation in Cold-Stored Tubers of Potato (Solanum-Tuberosum L.)—The Effects of Water-Stress. J. Plant Physiol. 1989, 134, 471–475. [Google Scholar] [CrossRef]
- Zhou, H.J.; Zhang, X.N.; Su, M.S.; Du, J.H.; Li, X.W.; Ye, Z.W. Effects of Ultraviolet-C Pretreatment on Sugar Metabolism in Yellow Peaches during Shelf Life. Hortscience 2020, 55, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Amrein, T.M.; Schonbachler, B.; Rohner, F.; Lukac, H.; Schneider, H.; Keiser, A.; Escher, F.; Amado, R. Potential for acrylamide formation in potatoes: Data from the 2003 harvest. Eur. Food Res. Technol. 2004, 219, 572–578. [Google Scholar] [CrossRef]
- De Wilde, T.; De Meulenaer, B.; Mestdagh, F.; Govaert, Y.; Vandeburie, S.; Ooghe, W.; Fraselle, S.; Demeulemeester, K.; Van Peteghem, C.; Calus, A.; et al. Influence of storage practices on acrylamide formation during potato frying. J. Agric. Food Chem. 2005, 53, 6550–6557. [Google Scholar] [CrossRef]
- Pedreschi, F.; Travisany, X.; Reyes, C.; Troncoso, E.; Pedreschi, R. Kinetics of extraction of reducing sugar during blanching of potato slices. J. Food Eng. 2009, 91, 443–447. [Google Scholar] [CrossRef]
- Zhang, Y.; Kahl, D.H.W.; Bizimungu, B.; Lu, Z.X. Effects of blanching treatments on acrylamide, asparagine, reducing sugars and colour in potato chips. J. Food Sci. Technol. 2018, 55, 4028–4041. [Google Scholar] [CrossRef] [PubMed]
- Marquez, G.; AÑOn, M.C. Influence of reducing sugars and amino acids in the color development of fried potatoes. J. Food Sci. 2006, 51, 157–160. [Google Scholar] [CrossRef]
- Dresow, J.F.; Bohm, H. The influence of volatile compounds of the flavour of raw, boiled and baked potatoes: Impact of agricultural measures on the volatile components. Landbauforsch. vTI Agric. For. Res. 2009, 59, 309–337. [Google Scholar]
- Singh, A.; Raigond, P.; Lal, M.K.; Singh, B.; Thakur, N.; Changan, S.S.; Kumar, D.; Dutt, S. Effect of cooking methods on glycemic index and in vitro bioaccessibility of potato (Solanum tuberosum L.) carbohydrates. LWT Food Sci. Technol. 2020, 127. [Google Scholar] [CrossRef]
- Elmore, J.; Briddon, A.; Dodson, A.; Muttucumaru, N.; Halford, N.G.; Mottram, D.J.F.C. Acrylamide in potato crisps prepared from 20 UK-grown varieties: Effects of variety and tuber storage time. Food Chem. 2015, 182, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Biedermann-Brem, S.; Noti, A.; Grob, K.; Imhof, D.; Bazzocco, D.; Pfefferle, A. How much reducing sugar may potatoes contain to avoid excessive acrylamide formation during roasting and baking? Eur. Food Res. Technol. 2003, 217, 369–373. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Ke, J.; Corke, H. Compositions of phenolic compounds, amino acids and reducing sugars in commercial potato varieties and their effects on acrylamide formation. J. Sci. Food Agric. 2010, 90, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Kalita, D.; Holm, D.G.; Jayanty, S.S. Role of polyphenols in acrylamide formation in the fried products of potato tubers with colored flesh. Food Res. Int. 2013, 54, 753–759. [Google Scholar] [CrossRef]

| Source of Variation | Chlorogenic Acid | Fructose | Glucose | Sucrose |
|---|---|---|---|---|
| UV-C treatment | p = 0.052 | p = 0.005 * | p = 0.078 | p < 0.001 * |
| Control | 11.32 ± 1.06 a | 0.179 ± 0.006 a | 0.259 ± 0.015 a | 0.212 ± 0.012 a |
| 3-UV-C | 10.00 ± 0.80 a | 0.191 ± 0.012 a | 0.259 ± 0.012 a | 0.226 ± 0.011 a |
| 5-UV-C | 8.82 ± 0.66 a | 0.267 ± 0.022 b | 0.364 ± 0.035 a | 0.435 ± 0.058 b |
| 10-UV-C | 8.55 ± 0.57 a | 0.210 ± 0.014 ab | 0.286 ± 0.013 a | 0.253 ± 0.014 ab |
| Storage days | p = 0.5968 | p < 0.001 * | p = 0.06 | p = 0.069 |
| 0 | 9.98 ± 1.14 a | 0.150 ± 0.005 a | 0.238 ± 0.011 a | 0.257 ± 0.013 a |
| 8 | 9.65 ± 0.95 a | 0.177 ± 0.011 ab | 0.296 ± 0.024 a | 0.307 ± 0.040 a |
| 11 | 9.57 ± 0.90 a | 0.196 ± 0.011 bc | 0.276 ± 0.022 a | 0.307 ± 0.063 a |
| 15 | 9.58 ± 0.77 a | 0.260 ± 0.021 c | 0.309 ± 0.023 a | 0.323 ± 0.037 a |
| 23 | 9.59 ± 0.81 a | 0.275 ± 0.020 c | 0.341 ± 0.035 a | 0.214 ± 0.017 a |
| Cooking method | p < 0.001 * | p = 0.001 * | p < 0.001 * | p < 0.001 * |
| Raw | 15.20 ± 0.52 b | 0.260 ± 0.018 b | 0.397 ± 0.023 b | 0.393 ± 0.044 b |
| Boiled | 6.77 ± 0.14 a | 0.194 ± 0.009 a | 0.244 ± 0.009 a | 0.231 ± 0.014 a |
| Fried | 7.05 ± 0.19 a | 0.181 ± 0.008 a | 0.235 ± 0.011 a | 0.220 ± 0.012 a |
| UV-C treatment x storage days | p = 0.360 | p = 0.766 | p = 0.603 | p = 0.293 |
| Control × 0 | 11.47 ± 3.00 a | 0.152 ± 0.003 a | 0.232 ± 0.009 a | 0.228 ± 0.010 a |
| 3-UV-C × 0 | 9.16 ± 2.60 a | 0.149 ± 0.006 a | 0.228 ± 0.017 a | 0.227 ± 0.020 a |
| 5-UV-C × 0 | 10.44 ± 2.31 a | 0.147 ± 0.016 a | 0.227 ± 0.031 a | 0.266 ± 0.030 a |
| 10-UV-C × 0 | 8.83 ± 1.33 a | 0.154 ± 0.014 a | 0.266 ± 0.027 a | 0.305 ± 0.030 a |
| p = 0.324 | p = 0.003 * | p = 0.240 | p = 0.020 * | |
| Control × 8 | 10.48 ± 2.29 a | 0.165 ± 0.008 ab | 0.234 ± 0.029 a | 0.235 ± 0.030 ab |
| 3-UV-C × 8 | 10.36 ± 1.74 a | 0.138 ± 0.010 a | 0.269 ± 0.019 a | 0.207 ± 0.017 a |
| 5-UV-C × 8 | 8.79 ± 1.81 a | 0.253 ± 0.022 b | 0.417 ± 0.072 a | 0.525 ± 0.125 b |
| 10-UV-C × 8 | 8.98 ± 2.10 a | 0.154 ± 0.010 ab | 0.265 ± 0.021 a | 0.261 ± 0.015 ab |
| p = 0.176 | p = 0.051 | p = 0.416 | p = 0.294 | |
| Control × 11 | 11.13 ± 2.64 a | 0.191 ± 0.006 a | 0.247 ± 0.019 a | 0.202 ± 0.034 a |
| 3-UV-C × 11 | 10.69 ± 1.84 a | 0.169 ± 0.009 a | 0.272 ± 0.047 a | 0.226 ± 0.030 a |
| 5-UV-C × 11 | 8.54 ± 1.30 a | 0.250 ± 0.036 a | 0.345 ± 0.064 a | 0.586 ± 0.223 a |
| 10-UV-C × 11 | 7.91 ± 1.16 a | 0.174 ± 0.011 a | 0.240 ± 0.028 a | 0.213 ± 0.009 a |
| p = 0.415 | p = 0.015 * | p = 0.120 | p = 0.020 * | |
| Control × 15 | 11.38 ± 2.04 a | 0.177 ± 0.017 a | 0.270 ± 0.052 a | 0.2140 ± 0.020 a |
| 3-UV-C × 15 | 9.54 ± 1.89 a | 0.241 ± 0.025 ab | 0.252 ± 0.019 a | 0.265 ± 0.031 ab |
| 5-UV-C × 15 | 8.40 ± 1.21 a | 0.360 ± 0.053 b | 0.407 ± 0.053 a | 0.530 ± 0.097 b |
| 10-UV-C × 15 | 8.98 ± 0.82 a | 0.261 ± 0.032 ab | 0.305 ± 0.029 a | 0.281 ± 0.051 ab |
| p = 0.329 | p = 0.104 | p = 0.528 | p = 0.575 | |
| Control × 23 | 12.12 ± 2.65 a | 0.210 ± 0.016 a | 0.313 ± 0.047 a | 0.179 ± 0.032 a |
| 3-UV-C × 23 | 10.27 ± 1.22 a | 0.260 ± 0.032 a | 0.274 ± 0.030 a | 0.206 ± 0.024 a |
| 5-UV-C × 23 | 7.91 ± 0.52 a | 0.324 ± 0.065 a | 0.423 ± 0.130 a | 0.267 ± 0.052 a |
| 10-UV-C × 23 | 8.06 ± 0.95 a | 0.306 ± 0.018 a | 0.353 ± 0.025 a | 0.203 ± 0.019 a |
| Cooking method × storage days | p < 0.001 * | p = 0.094 | p = 0.001 * | p = 0.003 * |
| Raw × 0 | 17.28 ± 1.07 b | 0.159 ± 0.008 a | 0.278 ± 0.011 b | 0.308 ± 0.020 b |
| Boiled × 0 | 6.32 ± 0.20 a | 0.158 ± 0.010 a | 0.248 ± 0.020 b | 0.256 ± 0.020 ab |
| Fried × 0 | 6.33 ± 0.35 a | 0.134 ± 0.008 a | 0.189 ± 0.011 a | 0.206 ± 0.012 a |
| p < 0.001 * | p = 0.235 | p = 0.001 * | p = 0.009 * | |
| Raw × 8 | 15.83 ± 0.50 b | 0.198 ± 0.021 a | 0.388 ± 0.050 b | 0.448 ± 0.100 b |
| Boiled × 8 | 6.93 ± 0.50 a | 0.149 ± 0.010 a | 0.205 ± 0.012 a | 0.220 ± 0.010 a |
| Fried × 8 | 6.20 ± 0.30 a | 0.185 ± 0.023 a | 0.296 ± 0.029 ab | 0.253 ± 0.040 a |
| p < 0.001 * | p = 0.018 * | p < 0.001 * | p = 0.027 * | |
| Raw × 11 | 15.02 ± 1.20 b | 0.239 ± 0.028 b | 0.395 ± 0.038 b | 0.503 ± 0.169 b |
| Boiled × 11 | 6.64 ± 0.31 a | 0.170 ± 0.007 a | 0.228 ± 0.013 a | 0.256 ± 0.031 ab |
| Fried × 11 | 7.04 ± 0.21 a | 0.178 ± 0.007 ab | 0.204 ± 0.007 a | 0.161 ± 0.025 a |
| p < 0.001 * | p = 0.006 * | p < 0.001 * | p = 0.031 * | |
| Raw × 15 | 14.21 ± 0.99 b | 0.348 ± 0.044 b | 0.414 ± 0.039 b | 0.459 ± 0.084 b |
| Boiled × 15 | 7.01 ± 0.25 a | 0.243 ± 0.016 ab | 0.291 ± 0.022 a | 0.285 ± 0.043 ab |
| Fried × 15 | 7.51 ± 0.40 a | 0.188 ± 0.019 a | 0.221 ± 0.018 a | 0.224 ± 0.022 a |
| p = 0.001 * | p = 0.014 * | p = 0.001 * | p = 0.002 * | |
| Raw × 23 | 13.65 ± 1.60 b | 0.357 ± 0.042 b | 0.512 ± 0.071 b | 0.247 ± 0.041 b |
| Boiled × 23 | 6.97 ± 0.22 a | 0.250 ± 0.019 ab | 0.247 ± 0.017 a | 0.140 ± 0.008 a |
| Fried × 23 | 8.15 ± 0.49 a | 0.219 ± 0.021 a | 0.264 ± 0.026 a | 0.255 ± 0.008 b |
| Cooking method × UV-C treatment | p < 0.001 * | p = 0.012 * | p = 0.003 * | p < 0.001 * |
| Raw × Control | 19.29 ± 0.45 c | 0.193 ± 0.009 a | 0.343 ± 0.026 a | 0.234 ± 0.023 a |
| Raw × 3-UV-C | 15.73 ± 0.43 b | 0.225 ± 0.025 ab | 0.333 ± 0.018 a | 0.278 ± 0.009 a |
| Raw × 5-UV-C | 13.27 ± 0.93 a | 0.381 ± 0.044 b | 0.575 ± 0.055 b | 0.754 ± 0.112 b |
| Raw × 10-UV-C | 12.50 ± 0.57 a | 0.242 ± 0.031 ab | 0.337 ± 0.018 a | 0.306 ± 0.031 a |
| p = 0.204 | p = 0.492 | p = 0.206 | p = 0.245 | |
| Boiled × Control | 7.07 ± 0.18 a | 0.169 ± 0.007 a | 0.210 ± 0.012 a | 0.201 ± 0.019 a |
| Boiled × 3-UV-C | 7.09 ± 0.42 a | 0.193 ± 0.023 a | 0.239 ± 0.009 a | 0.210 ± 0.023 a |
| Boiled × 5-UV-C | 6.53 ± 0.25 a | 0.209 ± 0.019 a | 0.257 ± 0.019 a | 0.288 ± 0.037 a |
| Boiled × 10-UV-C | 6.40 ± 0.14 a | 0.204 ± 0.019 a | 0.270 ± 0.022 a | 0.226 ± 0.021 a |
| p = 0.050 * | p = 0.136 | p = 0.219 | p = 0.052 | |
| Fried × Control | 7.59 ± 0.24 b | 0.174 ± 0.014 a | 0.225 ± 0.017 a | 0.200 ± 0.019 a |
| Fried × 3-UV-C | 7.19 ± 0.58 ab | 0.156 ± 0.006 a | 0.206 ± 0.011 a | 0.190 ± 0.009 a |
| Fried × 5-UV-C | 6.64 ± 0.13 a | 0.210 ± 0.021 a | 0.259 ± 0.030 a | 0.262 ± 0.038 a |
| Fried × 10-UV-C | 6.76 ± 0.41 ab | 0.184 ± 0.019 a | 0.250 ± 0.020 a | 0.227 ± 0.008 a |
| Grand mean | 9.67 | 0.212 | 0.292 | 0.281 |
| Source of Variation | Acrylamide |
|---|---|
| UV-C treatment | p < 0.001 * |
| Control | 480 ± 17.0 a |
| 3-UV-C | 558 ± 10.2 a |
| 5-UV-C | 763 ± 26.5 b |
| 10-UV-C | 590 ± 21.4 ab |
| Storage days | p = 0.187 |
| 0 | 530 ± 35.6 a |
| 8 | 573 ± 30.5 a |
| 11 | 586 ± 47.7 a |
| 15 | 628 ± 45.1 a |
| 23 | 670 ± 44.9 a |
| UV-C treatment × storage days | p = 0.003 * |
| Control × 0 | 390 ± 15.9 a |
| 3-UV-C × 0 | 544 ± 25.1 bc |
| 5-UV-C × 0 | 649 ± 20.5 c |
| 10-UV-C × 0 | 539 ± 4.8 b |
| p = 0.006 * | |
| Control × 8 | 490 ± 11.9 a |
| 3-UV-C × 8 | 558 ± 7.4 a |
| 5-UV-C × 8 | 701 ± 28.1 b |
| 10-UV-C × 8 | 544 ± 23.2 a |
| p = 0.001 * | |
| Control × 11 | 481± 15.3 a |
| 3-UV-C × 11 | 518 ± 15.2 a |
| 5-UV-C × 11 | 798 ± 29.6 b |
| 10-UV-C × 11 | 547 ± 7.3 a |
| p = 0.001 * | |
| Control × 15 | 502 ± 14.4 a |
| 3-UV-C × 15 | 572 ± 9.3 ab |
| 5-UV-C × 15 | 818 ± 32.6 c |
| 10-UV-C × 15 | 620 ± 10.7 b |
| p = 0.001 * | |
| Control × 23 | 537 ± 12.5 a |
| 3-UV-C × 23 | 597 ± 11.2 a |
| 5-UV-C × 23 | 847 ± 15.5 c |
| 10-UV-C × 23 | 699 ± 25.1 b |
| Grand mean | 670 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelaić, Z.; Čošić, Z.; Pedisić, S.; Repajić, M.; Zorić, Z.; Levaj, B. Effect of UV-C Irradiation, Storage and Subsequent Cooking on Chemical Constituents of Fresh-Cut Potatoes. Foods 2021, 10, 1698. https://doi.org/10.3390/foods10081698
Pelaić Z, Čošić Z, Pedisić S, Repajić M, Zorić Z, Levaj B. Effect of UV-C Irradiation, Storage and Subsequent Cooking on Chemical Constituents of Fresh-Cut Potatoes. Foods. 2021; 10(8):1698. https://doi.org/10.3390/foods10081698
Chicago/Turabian StylePelaić, Zdenka, Zrinka Čošić, Sandra Pedisić, Maja Repajić, Zoran Zorić, and Branka Levaj. 2021. "Effect of UV-C Irradiation, Storage and Subsequent Cooking on Chemical Constituents of Fresh-Cut Potatoes" Foods 10, no. 8: 1698. https://doi.org/10.3390/foods10081698
APA StylePelaić, Z., Čošić, Z., Pedisić, S., Repajić, M., Zorić, Z., & Levaj, B. (2021). Effect of UV-C Irradiation, Storage and Subsequent Cooking on Chemical Constituents of Fresh-Cut Potatoes. Foods, 10(8), 1698. https://doi.org/10.3390/foods10081698









