Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from Opuntia stricta var. Dillenii Fruits and Products of Their Industrialization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents, Reagents, and Standards
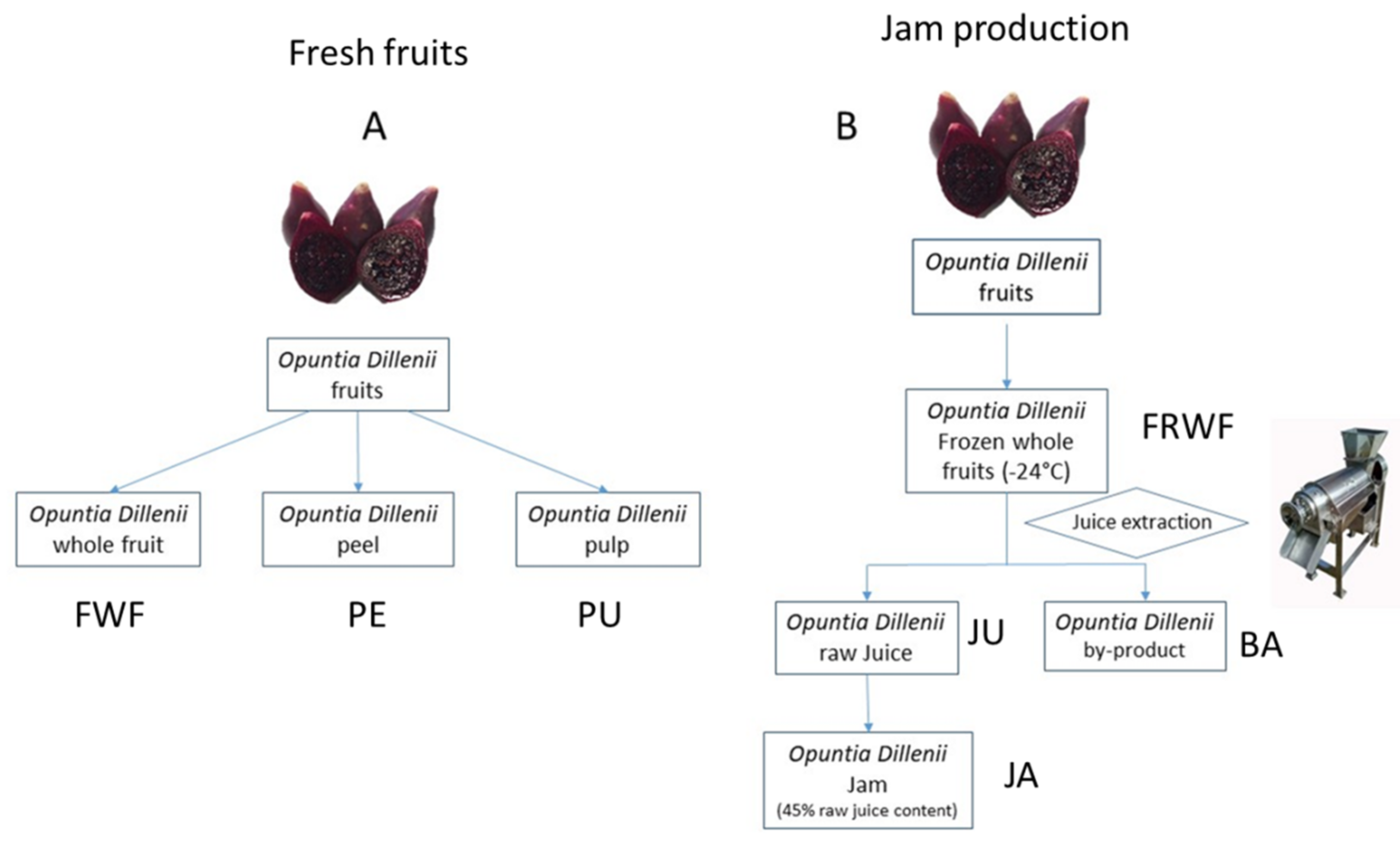
2.2. Plant Material
2.3. Physicochemical Analysis
2.4. Betalain and Phenolic Compounds Extraction for Characterization
2.5. In Vitro Digestion Assay
2.6. Betalain and Phenolic Characterization and Quantification by High-Performance Liquid Chromatography
2.7. Statistical Analyses
3. Results
3.1. Physicochemical Characteristics of Opuntia stricta, var. Dillenii Whole Fruit
3.2. Identification of Betalains and Phenolic Compounds
3.2.1. Betalains
3.2.2. Phenolic Compounds
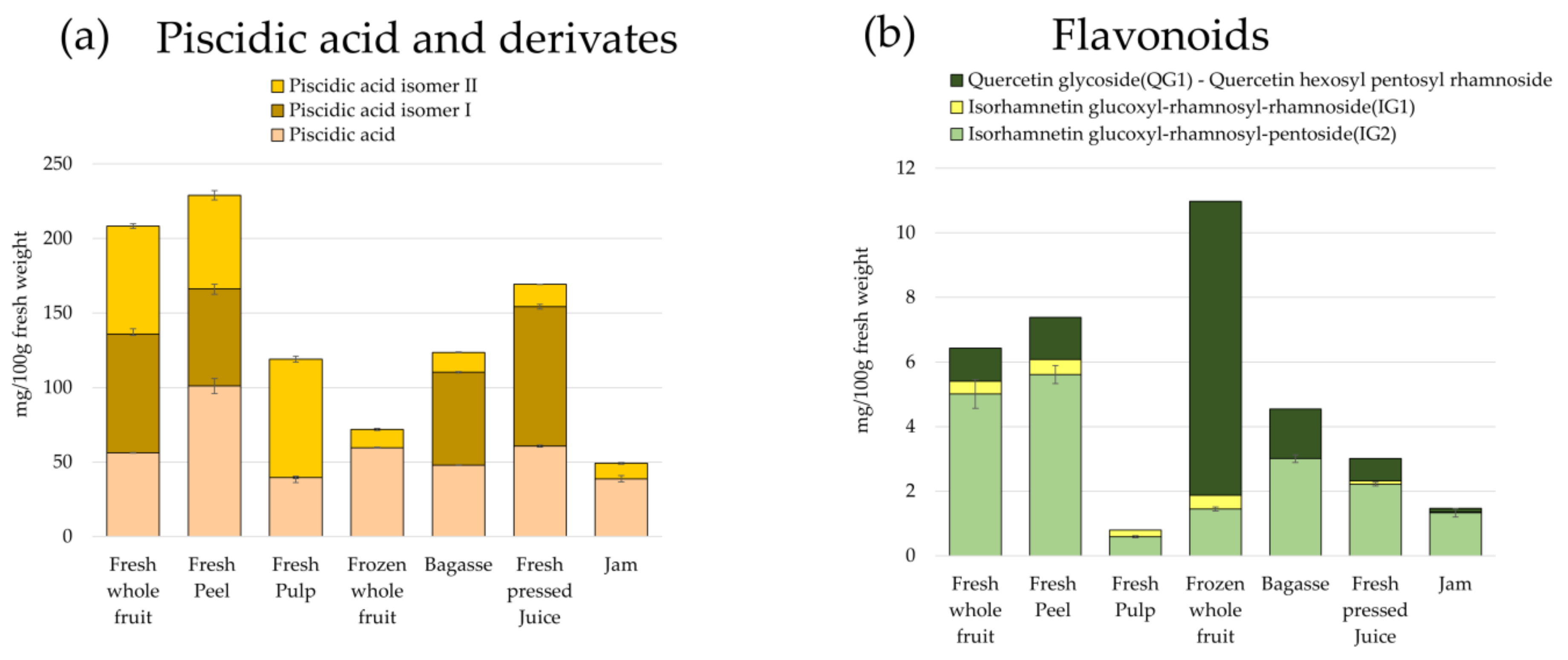
3.3. Quantification of Betalains and Phenolic Compounds
3.3.1. Quantification of Betalains
3.3.2. Quantification of Phenolic Compounds
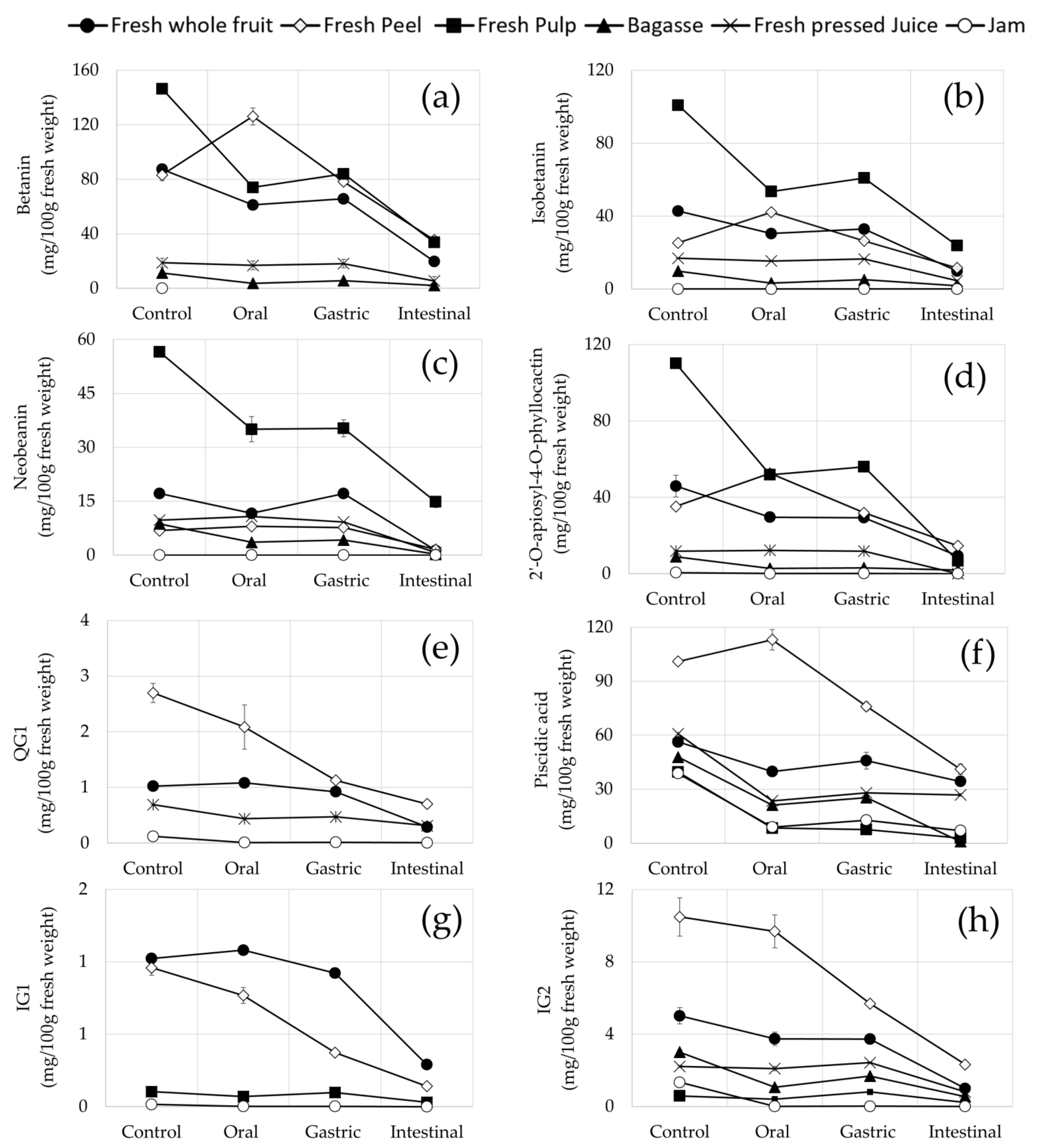
3.4. Stability and Recovery of Opuntia stricta var. Dillenii betalains and Phenolic Compounds during In Vitro Gastrointestinal Digestion
3.4.1. Stability and Recovery of Betalains and Phenolic Compounds in Fresh Fruit Pulp and Jam (Edible Samples)
3.4.2. Stability and Recovery of Betalains and Phenolic Compounds in Non-Edible Opuntia stricta var. Dillenii Samples
3.5. Bioaccessibility of Opuntia stricta var. Dillenii betalains and Phenolic Compounds during In Vitro Gastrointestinal Digestion
3.5.1. Bioaccessibility in Opuntia stricta var. Dillenii Edible Samples (Pulp and Jam)
3.5.2. Bioaccessibility of Betalains and Phenolic Compounds in Opuntia stricta var. Dillenii Inedible Samples
4. Discussion
4.1. Betalain and Phenolic Compounds in Opuntia stricta var. Dillenii Fruit Samples
4.1.1. Betalains in Opuntia stricta var. Dillenii Samples
4.1.2. Phenolic Compounds in Opuntia stricta var. Dillenii Samples
4.2. Stability and Recovery of Opuntia stricta var. Dillenii betalains and Phenolic Compounds during In Vitro Gastrointestinal Digestion
4.3. Bioaccessibility of Opuntia stricta var. Dillenii betalains and Phenolic Compounds during In Vitro Gastrointestinal Digestion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Betancourt, C.; Cejudo-Bastante, M.J.; Heredia, F.J.; Hurtado, N. Pigment composition and antioxidant capacity of betacyanins and betaxanthins fractions of Opuntia dillenii (Ker Gawl) Haw cactus fruit. Food Res. Int. 2017, 101, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Noguera-Artiaga, L.; Lipan, L.; Carbonell-Barrachina, Á.A.; Rocamora-Montiel, B.; Legua, P.; Hernández, F.; López-Lluch, D. Economic estimation of cactus pear production and its feasibility in Spain. Trends Food Sci. Technol. 2020, 103, 379–385. [Google Scholar] [CrossRef]
- Cano, M.; Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J. Characterization of carotenoid profile of Spanish Sanguinos and Verdal prickly pear (Opuntia ficus-indica, spp.) tissues. Food Chem. 2017, 237, 612–622. [Google Scholar] [CrossRef] [PubMed]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Tran, T.M.T.; Thanh, B.N.; Moussa-Ayoub, T.E.; Rohn, S.; Jerz, G. Profiling of polar metabolites in fruits of Opuntia stricta var. dillenii by ion-pair high-performance countercurrent chromatography and off-line electrospray mass-spectrometry injection. J. Chromatogr. A 2019, 1601, 274–287. [Google Scholar] [CrossRef]
- Missaoui, M.; D’Antuono, I.; D’Imperio, M.; Linsalata, V.; Boukhchina, S.; Logrieco, A.F.; Cardinali, A. Characterization of micronutrients, bioaccessibility and antioxidant activity of prickly pear cladodes as functional ingredient. Molecules 2020, 25, 2176. [Google Scholar] [CrossRef]
- Zhao, L.; Lan, Q.; Huang, Z.; Ouyang, L.; Zeng, F. Antidiabetic effect of a newly identified component of Opuntia dillenii polysaccharides. Phytomedicine 2011, 18, 661–668. [Google Scholar] [CrossRef]
- Medina, E.D.; Rodríguez-Rodríguez, E.M.; Romero, C.D. Chemical characterization of Opuntia dillenii and Opuntia ficus indica fruits. Food Chem. 2007, 103, 38–45. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación. Análisis Provincial de Superficie, Arboles Diseminados, Rendimiento y Producción, 2018 (Chumbera); MAPA: Madrid, Spain, 2019; Available online: https://www.mapa.gob.es/estadistica/pags/anuario/2019-Avance/CAPITULOSPDF/CAPITULO07/pdfc07_9.19.1.pdf (accessed on 23 June 2021).
- Gómez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive stability and bioaccessibility of antioxidants in prickly pear fruits from the Canary Islands: Healthy foods and ingredients. Antioxidants 2020, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Moussa-Ayoub, T.E.; Jaeger, H.; Youssef, K.; Knorr, D.; El-Samahy, S.; Kroh, L.W.; Rohn, S. Technological characteristics and selected bioactive compounds of Opuntia dillenii cactus fruit juice following the impact of pulsed electric field pre-treatment. Food Chem. 2016, 210, 249–261. [Google Scholar] [CrossRef]
- Castellar, R.; Obón, J.M.; Alacid, M.; Fernández-López, J.A. Color properties and stability of Betacyanins from Opuntia fruits. J. Agric. Food Chem. 2003, 51, 2772–2776. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Xu, D.; Xu, Y.; Zheng, X. Purified Betacyanins from Hylocereus undatus peel ameliorate obesity and insulin resistance in high-fat-diet-fed mice. J. Agric. Food Chem. 2015, 64, 236–244. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Rodríguez-Rodríguez, C.; Gutiérrez-Uribe, J.A.; Cepeda-Cañedo, E.; Serna-Saldívar, S.O. Bioaccessibility, intestinal permeability and plasma stability of Isorhamnetin glycosides from Opuntia ficus-indica (L.). Int. J. Mol. Sci. 2017, 18, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirazinia, R.; Rahimi, V.B.; Kehkhaie, A.R.; Sahebkar, A.; Rakhshandeh, H.; Askari, V.R. Opuntia dillenii: A forgotten plant with promising pharmacological properties. J. Pharmacopunct. 2019, 22, 16–27. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Montiel-Sánchez, M.; García-Cayuela, T.; Gómez-Maqueo, A.; García, H.S.; Cano, M.P. In vitro gastrointestinal stability, bioaccessibility and potential biological activities of betalains and phenolic compounds in cactus berry fruits (Myrtillocactus geometrizans). Food Chem. 2020, 342, 128087. [Google Scholar] [CrossRef]
- Castellanos-Santiago, E.; Yahia, E.M. Identification and quantification of betalains from the fruits of 10 Mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef]
- Vieira Teixeira da Silva, D.; Dos Santos Baião, D.; De Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.; Flosi Paschoalin, M.V. Betanin, a natural food additive: Stability, bioavailability, antioxidant and preservative ability assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, T.M.; El-Samahy, S.K.; Rohn, S.; Kroh, L.W. Flavonols, betacyanins content and antioxidant activity of cactus Opuntia macrorhiza fruits. Food Res. Int. 2011, 44, 2169–2174. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Castellar, R.; Obón, J.M.; Almela, L. Monitoring by liquid chromatography coupled to mass spectrometry the impact of pH and temperature on the pigment pattern of cactus pear fruit extracts. J. Chromatogr. Sci. 2007, 45, 120–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinwari, K.J.; Rao, P.S. Stability of bioactive compounds in fruit jam and jelly during processing and storage: A review. Trends Food Sci. Technol. 2018, 75, 181–193. [Google Scholar] [CrossRef]
- Farag, M.A.; Sallam, I.E.; Fekry, M.I.; Zaghloul, S.S.; El-Dine, R.S. Metabolite profiling of three Opuntia ficus-indica fruit cultivars using UPLC-QTOF-MS in relation to their antioxidant potential. Food Biosci. 2020, 36, 100673. [Google Scholar] [CrossRef]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.-W.; De Leon-Rodriguez, A.; Fomsgaard, I.S.; De La Rosa, A.P.B. Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J. Food Compos. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.M.; Bronze, M.R. Contribution to the characterization of Opuntia spp. juices by LC–DAD–ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Kolniak-Ostek, J.; Kita, A.; Miedzianka, J.; Andreu-Coll, L.; Legua, P.; Hernandez, F. Characterization of Bioactive Compounds of Opuntia ficus-indica (L.) Mill. Seeds from Spanish Cultivars. Molecules 2020, 25, 5734. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Lamine, M.; Damergi, B.; Ezahra, F.; Feres, N.; Jallouli, S.; Hammami, M.; Khammassi, S.; Selmi, S.; Elkahoui, S.; et al. Natural colourants analysis and biological activities. Association to molecular markers to explore the biodiversity of Opuntia species. Phytochem. Anal. 2020, 31, 892–904. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, M.A.; Rao, G.S.; Arai, T.; Iida, A.; Tokuda, H. Cytotoxic effect of the red beetroot (Beta vulgaris L.) Extract compared to doxorubicin (adriamycin) in the human prostate (pc-3) and breast (mcf-7) cancer cell lines. Anti-Cancer Agents Med. Chem. 2011, 11, 280–284. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef]
- Han, J.; Tan, C.; Wang, Y.; Yang, S.; Tan, D. Betanin reduces the accumulation and cross-links of collagen in high-fructose-fed rat heart through inhibiting non-enzymatic glycation. Chem. Interact. 2015, 227, 37–44. [Google Scholar] [CrossRef]
- Ressaissi, A.; Attia, N.; Falé, P.L.; Pacheco, L.; Victor, B.L.; Machuqueiro, M.; Serralheiro, M.L.M. Isorhamnetin de-rivatives and piscidic acid for hypercholesterolemia: Cholesterol permeability, HMG-CoA reductase inhibition, and docking studies. Arch. Pharm. Res. 2017, 40, 1278–1286. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Fernández-López, R.; Welti-Chanes, J.; Cano, M.P. Inhibitory potential of prickly pears and their isolated bioactives against digestive enzymes linked to type 2 diabetes and inflammatory response. J. Sci. Food Agric. 2019, 99, 6380–6391. [Google Scholar] [CrossRef] [PubMed]
- González-Ponce, H.A.; Martínez-Saldaña, M.C.; Rincón-Sánchez, A.R.; Sumaya-Martínez, M.T.; Buist-Homan, M.; Faber, K.N.; Moshage, H.; Jaramillo-Juárez, F. Hepatoprotective effect of Opuntia robusta and Opuntia streptacantha fruits against acetaminophen-induced acute liver damage. Nutrients 2016, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahdoura, H.; Adouni, K.; Khlifi, A.; Dridi, I.; Haouas, Z.; Neffati, F.; Flamini, G.; Mosbah, H.; Achour, L. Hepatoprotective effect of Opuntia microdasys (Lehm.) Pfeiff flowers against diabetes type II induced in rats. Biomed. Pharmacother. 2017, 94, 79–87. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Vasto, S.; Pintaudi, A.M.; Livrea, M.A.; Allegra, M. Short-term cactus pear [Opuntia ficus-indica (L.) Mill] fruit supplementation ameliorates the inflammatory profile and is associated with improved antioxidant status among healthy humans. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In Vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef]
- Choo, K.Y.; Ong, Y.Y.; Lim, R.L.H.; Tan, C.P.; Ho, C.W. Study on bioaccessibility of betacyanins from red dragon fruit (Hylocereus polyrhizus). Food Sci. Biotechnol. 2019, 28, 1163–1169. [Google Scholar] [CrossRef]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I. Plant Betalains: Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef] [Green Version]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.; Livrea, M.A. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef] [Green Version]

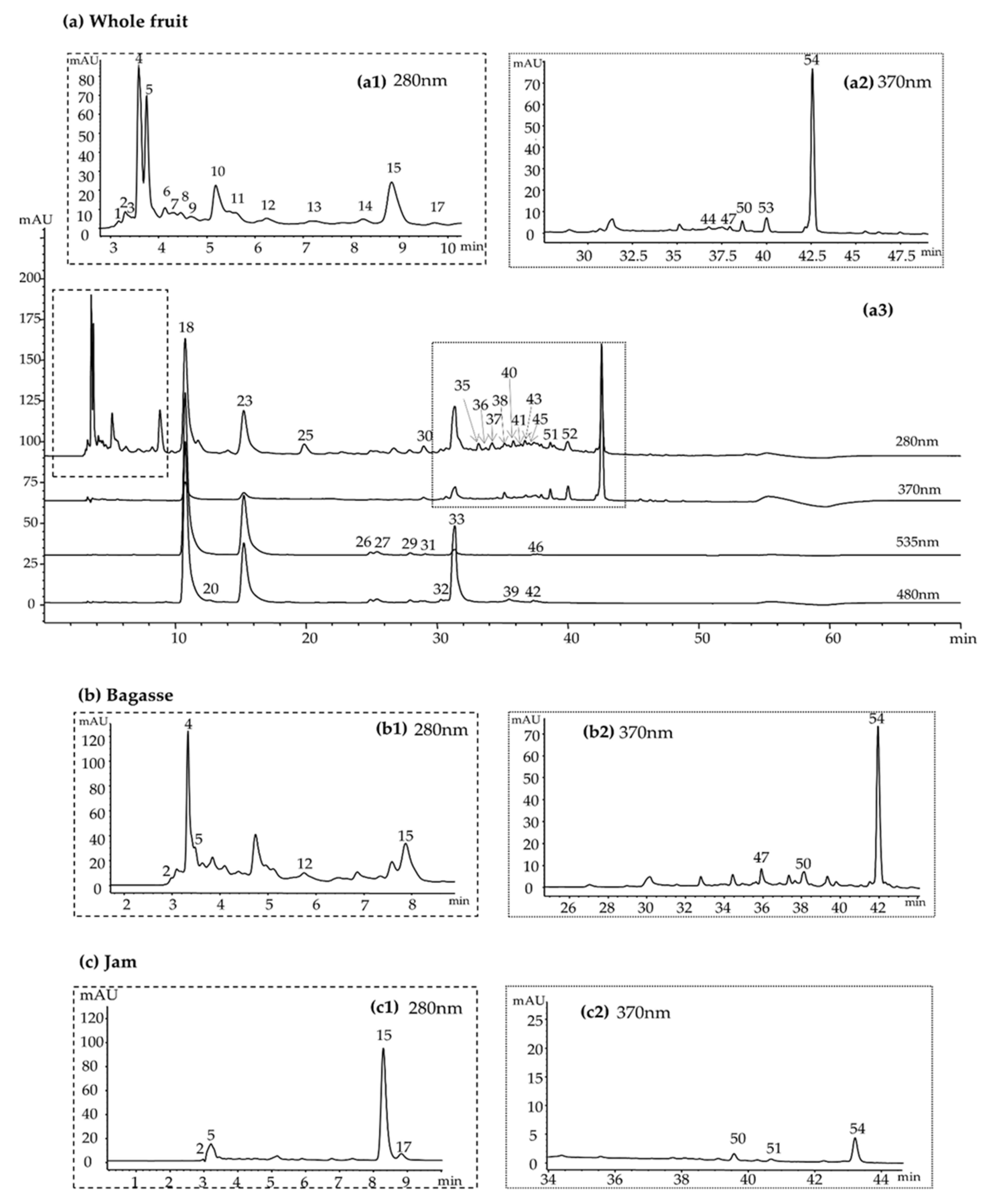

| Peak * | tR (min) | Compounds | UV λmax (nm) | [M-H]+ | [M-H]− | MS/MS (m/z) | Samples 1 |
|---|---|---|---|---|---|---|---|
| 1 | 3.17 | Pyruvic acid | 230 | 87 | 59.01 | FWF, JU, BA | |
| 2 | 3.31 | Gallic acid derivative | 232, 271 | 331 | 271, 211, 169, 151, 125, 113, 89, 71, 59 | FWF, JU, BA, JA | |
| 3 | 3.47 | Ascorbic acid | 285 | 175 | 115, 89 | FWF, PE, PU, FRFW, JU, BA, JA | |
| 4 | 3.59 | Piscidic acid isomer I | 272 | 255 | 193, 165, 135, 119 107 | FWF, PE, PU, JU, BA, JA | |
| 5 | 3.75 | Piscidic acid isomer II | 278 | 255 | 193, 165, 135, 119 107 | FWF, PE, PU, JU, BA; JA | |
| 6 | 4.13 | Citric acid | 233 | 191 | 111, 87, 67 | FWF, JU | |
| 7 | 4.31 | Ferulic acid derivative I | 233 | 489 | 295,235,193,175,149 | FWF, | |
| 8 | 4.49 | Unknown | 241 | -- | -- | FWF, | |
| 9 | 4.70 | Ferulic acid derivative II | 231 | 489 | 235,193,175,149 | FWF, | |
| 10 | 5.18 | Protocatechuic acid derivative | 230 (sh), 284 | 297 | 286, 153, 86 | FWF, PU | |
| 11 | 5.46 | Quinic acid | 230(sh), 272 | 191 | 111, 85, 67 | FWF, JU, | |
| 12 | 6.24 | Gallic acid | 274 | 169 | 125, 107, 97, 79, 69, 51, 41 | FWF, PE, JU, BA | |
| 13 | 7.17 | p-hydroxybenzoic acid | 262 | 301 | 139, 121 | FWF, | |
| 14 | 8.24 | Eucomic acid derivative I | 280 | 525 | 295, 235, 239, 195, 179 | FWF, PU, | |
| 15 | 8.83 | Piscidic acid | 272 | 255 | 193, 165, 135, 119 107 | FWF, FRWF, PE, PU, JU, BA, JA | |
| 16 | 9.42 | 15,17-bidecarboxy-betanin | 480 | 463 | 301 | FRWF, JA | |
| 17 | 9.73 | Eucomic acid isomer I | 280 | 239 | 195,179,149,133 | FWF, JA | |
| 18 | 10.76 | Betanin | 535 | 551 | 390, 389 | FWF, FRWF, PE, PU, JU, BA | |
| 19 | 11.67 | Piscidic acid derivative I | 275 | 487 | 255, 193, 165, 135, 107 | FRWF, PE, JA | |
| 20 | 11.73 | 17-decarboxy-betanin | 504 | 507 | 390, 389 | FWF, FRWF, PE, PU, JU, BA | |
| 21 | 12.97 | Cyclo-dopa-5-O-β-glucoside | 230, 275 | 358 | 196 | JA | |
| 22 | 14.42 | Cyclo-dopa-5-O-α-glucoside (isomer) | 282 | 358 | 196 | JA | |
| 23 | 15.23 | Isobetanin | 535 | 551 | 390, 389 | FWF, FRWF, PE, PU, JU, BA | |
| 24 | 15.48 | 17-decarboxy-isobetanin | 504 | 505 | 461 | JU, BA | |
| 25 | 19.85 | Eucomic acid | 278 | 239 | 195, 179, 149, 133 | FWF, PE, PU | |
| 26 | 24.91 | Betanidin | 538 | 389 | 345, 150 | FWF, PU, JU, BA | |
| 27 | 25.42 | 6′-O-sinapoyl-O-gompherin | 539 | 755 | 225 | FWF, PE, PU, JU, BA | |
| 28 | 26.70 | Eucomic acid isomer II | 280 | 239 | 195, 179, 149, 133 | FWF, | |
| 29 | 27.90 | 2′-O-apiosyl-4-O-phyllocactin | 537 | 767 | 551 | FWF, PE, PU, JU, BA, JA | |
| 30 | 28.97 | Unknown | 331 | FWF, JU, BA | |||
| 31 | 29.08 | 5″-O-E-sinapoyl-2′-apyosil-phyllocactin | 248,330,540 | 975 | --- | FWF, PE, PU, JU, BA, JA | |
| 32 | 30.27 | 17-Descarboxy-neobetanin | 451 | 505 | 343 | FWF, | |
| 33 | 31.34 | Neobetanin | 467 | 549 | 387 | FWF, FRWF, PE, PU, JU, BA | |
| 34 | 31.84 | Neobetanin isomer I | 465 | 549 | 387 | PU | |
| 35 | 33.16 | 4-Hydroxybenzoic acid 4-O-glucoside c | 270 | 299 | 137, 119, 93 | FWF, JA, | |
| 36 | 33.72 | Ferulic acid | 297, 328 | 193.1 | 177, 161, 133 | FWF, PE, | |
| 37 | 34.20 | 4-Hydroxybenzoic acid 4-O-glucoside isomer c | 267 | 299 | 137, 119, 93 | FWF, PE, | |
| 38 | 35.14 | Quercetin-3-O-rhamnosyl-rutinoside (QG3) | 358 | 757 | 611, 303 | FWF, | |
| 39 | 35.49 | Neobetanin isomer II | 449 | 549 | 387 | FWF, JU, BA | |
| 40 | 35.82 | Ellagic acid derivative I | 256, 296(sh) 352 | 1085 | 479, 300, 273 | FWF, | |
| 41 | 36.15 | Ferulic acid derivative c | 245, 327 | 355 | 239,193,175 | FWF, FWF, | |
| 42 | 36.40 | Neobetanin isomer III | 444 | 549 | 387 | FWF, JU, BA | |
| 43 | 36.71 | p-coumaric acid | 280, 312 | 165 | 166, 187 | FWF, | |
| 44 | 36.78 | Protocatechuic acid derivative | 356 | 498 | 137, 111, 109, 97 | FWF, | |
| 45 | 37.00 | p-coumaric acid derivative c | 237, 318 | 191 | 145, 119, 45, 27 | FWF, | |
| 46 | 37.39 | 15R/15S-Betanidin | 530 | 389 | 371, 342, 297, 194, 150, 132 | FWF, FRWF, PE, JU, BA | |
| 47 | 37.95 | Ellagic acid | 366, 255 | 303 | 301 | 285, 283, 257, 229, 184, 134 | FWF, PE; JU, BA |
| 48 | 38.32 | Ellagic acid rhamnoside | 258, 352 | 447 | 352, 262, 160, 146 | FWF | |
| 49 | 38.54 | Myricetin | 255, 372 | 319 | 153, 113 | FWF | |
| 50 | 38.65 | Quercetin hexosyl pentosyl rhamnoside (QG1) | 255,358 | 426 | 303, 191, 120 | FWF, FRWF, PE, JU, BA, JA | |
| 51 | 38.72 | Myricitrin (myricetin 3-rhamnoside) | 255, 374 | 465 | 319, 147 | FWF | |
| 52 | 39.56 | Quercetin glycoside (QG2)—Quercetin hexose pentoside | 255, 353 | 653 | 303, 177 | FWF, FRWF | |
| 53 | 40.00 | Isorhamnetin glucoxyl-rhamnosyl-rhamnoside (IG1) | 256, 356 | 771 | 625; 317, 85 | FWF, FRWF, PE, PU, JU, JA | |
| 54 | 42.57 | Isorhamnetin glucoxyl-rhamnosyl-pentoside (IG2) | 254, 356 | 757 | 317, 167, 86 | FWF, FRWF, PE, PU, JU, BA, JA | |
| 55 | 42.85 | Isorhamnetin hexosyl-hexosyl-pentoside (IG3) | 353 | 757 | 317 | FWF | |
| 56 | 43.08 | Rutin (quercetin-3-rutinoside) a | 352, 299(sh) | 611 | 303, 229, 137 | FWF | |
| 57 | 43.32 | Isorhamnetin glucosyl-pentoside (IG4) a | 352, 293(sh) | 611 | 479, 317, 177 | FWF | |
| 58 | 45.54 | Kaempferol-glucosyl-rhamnoside (KG1) | 356 | 595 | 597 | 287 | FW, |
| 59 | 45.82 | Isorhamnetin glucosyl-rhamnoside (IG5) | 353 | 625 | 317, 85 | FW, | |
| 60 | 46.19 | Isorhamentin glucoside b | 330, 299(sh) | 814 | 641, 317, 169 | FW, |
| Recovery (%) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | In Vitro phase | Fresh Whole Fruit (FWF) | Fresh Peel (PE) | Fresh Pulp (PU) | Bagasse By-Product (BA) | Fresh Pressed Juice (JU) | Jam (JA) |
| BETALAINS | |||||||
| Betanin | Oral | 80.10 ± 2.79 Bc | 101.71 ± 7.59 Ce | 60.58 ± 1.90 Bb | 52.25 ± 1.06 Ba | 99.43 ± 1.52 Bd | n.d. |
| Gastric | 75.16 ± 0.88 Bc | 94.00 ± 1.69 Bd | 57.31 ± 0.27 Cb | 49.87 ± 1.60 Ca | 96.34 ± 2.03 Bd | n.d. | |
| Intestinal | 22.42 ± 1.29 Aab | 42.58 ± 3.35 Ac | 22.95 ± 0.01 Aab | 19.03 ± 1.14 Aa | 28.53 ± 2.19 Ab | n.d. | |
| Isobetanin | Oral | 71.07 ± 3.63 Bc | 166.33± 8.32 Ce | 53.03 ± 2.01 Bb | 33.54 ± 1.27 Ba | 90.98 ± 1.61 Bd | n.d. |
| Gastric | 76.78 ± 0.25 Bc | 104.11 ± 3.27 Be | 60.38 ± 0.18 ab | 52.11 ± 1.99 Ca | 97.22 ± 2.11 Bd | n.d. | |
| Intestinal | 22.85 ± 1.72 Ab | 45.67 ± 1.66 Ac | 23.60 ± 0.06 Ab | 18.52 ± 1.20 Aa | 26.44 ± 2.42 Ab | n.d. | |
| Betanidin | Oral | 155.11 ± 0.02 Be | 78.52 ± 3.93 Cb | 84.72 ± 2.75 Bc | 30.90 ± 0.18 Ba | 109.82 ± 2.50 Bd | n.d. |
| Gastric | 157.49 ± 10.63 Be | 46.49 ± 2.32 Ba | 91.85 ± 1.55 Cb | 45.78 ± 2.22 Ca | 112.93 ± 1.42 Bc | n.d. | |
| Intestinal | 47.06 ± 0.77 Ae | 21.74 ± 1.09 Ab | 36.37 ± 0.35 Ad | 10.38 ± 0.28 Aa | 25.95 ± 3.14 Ac | n.d. | |
| 2′-O-apiosyl-4-O-phyllocactin | Oral | 65.09 ± 4.59 Bd | 149.40 ± 7.49 Cf | 47.00 ± 1.61 Bc | 31.01 ± 0.34 Bb | 103.51 ± 2.11 Ae | 10.38 ± 1.35 Ba |
| Gastric | 65.09± 4.05 Bc | 90.58 ± 0.48 Bd | 50.85 ± 0.01 Cc | 34.61 ± 1.73 Cb | 100.74 ± 2.85 Ad | 16.12 ± 0.88 Ca | |
| Intestinal | 20.42 ± 4.04 Ab | 41.32 ± 0.78 Ac | 5.97 ± 0.30 Aa | 21.94 ± 0.71 Ab | 0 | 0 | |
| Neobetanin | Oral | 67.96 ± 9.33 Bb | 116.94 ± 5.85 Cc | 61.92 ± 6.48 Bb | 41.14 ± 1.63 Ba | 110.47 ± 2.57 Cc | n.d. |
| Gastric | 60.08 ± 9.70 Cbc | 112.27 ± 5.61 Bc | 62.41 ± 4.45 Ba | 48.74 ± 3.13 Ca | 94.78 ± 3.16 Bb | n.d. | |
| Intestinal | 7.63 ± 2.96 Ab | 23.26 ± 1.16 Ac | 26.22 ± 2.90 Ac | 3.08 ± 0.15 Aab | 7.20 ± 0.52 Ab | n.d. | |
| PHENOLICS ACIDS | |||||||
| Piscidic acid | Oral | 70.82 ± 2.74 Bd | 111.92 ± 5.60 Ce | 21.39 ± 0.96 Ba | 44.39 ± 0.87 Bc | 33.80 ± 1.19 Ab | 23.16 ± 2.02 Aa |
| Gastric | 81.49 ± 7.81A Bc | 75.26 ± 2.39 Bc | 19.34 ± 0.74 Ba | 53.09 ± 1.72 Cb | 46.11 ± 0.19 Ab | 33.20 ± 0.79 Bb | |
| Intestinal | 61.35 ± 3.07 Ae | 40.71 ± 2.78 Ad | 7.43 ± 0.35 Ab | 2.03 ± 0.08 Aa | 44.17 ± 1.78 Ad | 18.13 ± 0.97 Ac | |
| FLAVONOIDS | |||||||
| Quercetin glycoside(QG1)—Quercetin hexosyl pentosyl rhamnoside | Oral | 105.65 ± 0.07 Cd | 137.98 ± 6.90 Ce | n.d. | 0 | 63.53 ± 0.44 Bc | 9.86 ± 0.92 Bb |
| Gastric | 90.21 ± 0.84 Bd | 86.35 ± 1.72 Bd | n.d. | 0 | 68.36 ± 2.54 Bc | 14.76 ± 0.80 Cb | |
| Intestinal | 28.33 ± 2.18 Ab | 53.72 ± 5.19 Ad | n.d. | 0 | 45.38 ± 1.78 Ac | 7.15 ± 0.36Aa | |
| Isorhamnetin glucoxyl-rhamnosyl-rhamnoside (IG1) | Oral | 81.74 ± 2.07 Cc | 158.05 ± 7.54 Cd | 0 | n.d. | 68.15 ± 8.04 Bb | 11.39 ± 2.81 Ba |
| Gastric | 64.82 ± 2.28 Bc | 80.77 ± 1.00 Bd | 0 | n.d. | 94.36 ± 0.91 Ce | 16.36 ± 3.49 Bb | |
| Intestinal | 15.27 ± 0.88 Ab | 30.62 ± 0.98 Ac | 0 | n.d. | 29.43 ± 3.51 Ac | 0.00 ± 0.00 Aa | |
| Isorhamnetin glucoxyl-rhamnosyl-pentoside (IG2) | Oral | 75.43 ± 14.17 Bc | 148.28 ± 7.41 Cd | 68.56 ± 12.80 Ac | 35.45 ± 1.44 Bb | 94.44 ± 2.76 Bc | 0.94 ± 0.20 Aa |
| Gastric | 74.74 ± 4.14 Bc | 101.38 ± 0.97 Bd | 136.88 ± 4.33 Be | 56.20 ± 2.75 Cb | 109.25 ± 0.90 Cd | 1.33 ± 0.20 Aa | |
| Intestinal | 20.02 ± 2.95 Ab | 41.40 ± 1.09 Ac | 37.77 ± 1.85 Ac | 18.49 ± 1.85 Ab | 36.33 ± 1.34 Ac | 0.81 ± 0.24 Aa | |
| Bioaccessibility (%) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Fresh Whole Fruit (FWF) | Fresh Peel (PE) | Fresh Pulp (PU) | Bagasse By-Product (BA) | Fresh Pressed Juice (JU) | Jam (JA) | |
| BETALAINS | |||||||
| Betanin | 22.42 ± 1.29 ab | 42.58 ± 3.35 c | 22.95 ± 0.01 ab | 19.03 ± 1.14 a | 28.53 ± 2.29 b | 0 | |
| Isobetanin | 22.85 ± 1.72 ab | 45.67 ± 1.66 c | 23.60 ± 0.06 ab | 18.52 ± 1.20 a | 26.44 ± 2.42 b | 0 | |
| Betanidin | 47.06 ± 0.77 d | 21.74 ± 1.09 b | 36.37 ± 0.35 c | 10.38 ± 0.28 a | 25.95 ± 3.14 b | 0 | |
| 2′-O-apiosyl-4-O-phyllocactin | 20.42 ± 4.04 c | 41.32 ± 0.78 d | 5.97 ± 0.30 b | 21.94 ± 0.71 c | 0 | 0 | |
| Neobetanin | 7.63 ± 2.96 a | 23.26 ± 1.16 b | 26.22 ± 2.90 b | 3.08 ± 0.15 a | 7.20 ± 0.52 a | 0 | |
| PHENOLICS ACIDS | |||||||
| Piscidic acid | 61.35 ± 3.07 e | 40.71 ± 2.78 d | 7.43 ± 0.35 b | 2.03 ± 0.08 a | 44.17 ± 1.78 d | 18.13 ± 0.97 c | |
| FLAVONOIDS | |||||||
| Quercetin glycoside(QG1)—Quercetin hexosyl pentosyl rhamnoside | 28.33 ± 2.18 b | 53.72 ± 5.19 d | 0 | 0 | 45.38 ± 1.78 c | 7.15 ± 0.36 a | |
| Isorhamnetin glucoxyl-rhamnosyl-rhamnoside(IG1) | 15.27 ± 0.88 b | 30.62 ± 0.98 c | 0 | 0 | 29.43 ± 3.51 c | 0 | |
| Isorhamnetin glucoxyl-rhamnosyl-pentoside(IG2) | 20.02 ± 2.95 b | 41.40 ± 1.09 c | 37.77 ± 1.85 c | 18.49 ± 1.85 b | 36.33 ± 1.34 c | 0.81 ± 0.24 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from Opuntia stricta var. Dillenii Fruits and Products of Their Industrialization. Foods 2021, 10, 1593. https://doi.org/10.3390/foods10071593
Gómez-López I, Lobo-Rodrigo G, Portillo MP, Cano MP. Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from Opuntia stricta var. Dillenii Fruits and Products of Their Industrialization. Foods. 2021; 10(7):1593. https://doi.org/10.3390/foods10071593
Chicago/Turabian StyleGómez-López, Iván, Gloria Lobo-Rodrigo, María P. Portillo, and M. Pilar Cano. 2021. "Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from Opuntia stricta var. Dillenii Fruits and Products of Their Industrialization" Foods 10, no. 7: 1593. https://doi.org/10.3390/foods10071593
APA StyleGómez-López, I., Lobo-Rodrigo, G., Portillo, M. P., & Cano, M. P. (2021). Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from Opuntia stricta var. Dillenii Fruits and Products of Their Industrialization. Foods, 10(7), 1593. https://doi.org/10.3390/foods10071593








