Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns
Abstract
:1. Introduction
2. Natural Bioactive Compounds
2.1. Antioxidants
2.2. Preservatives
2.3. Anti-Browning
2.4. Colorants
2.5. Thickeners
3. Food Waste Bioactive Compounds Regulatory and Legislative Issues
3.1. European Union
3.2. Non EU Contries
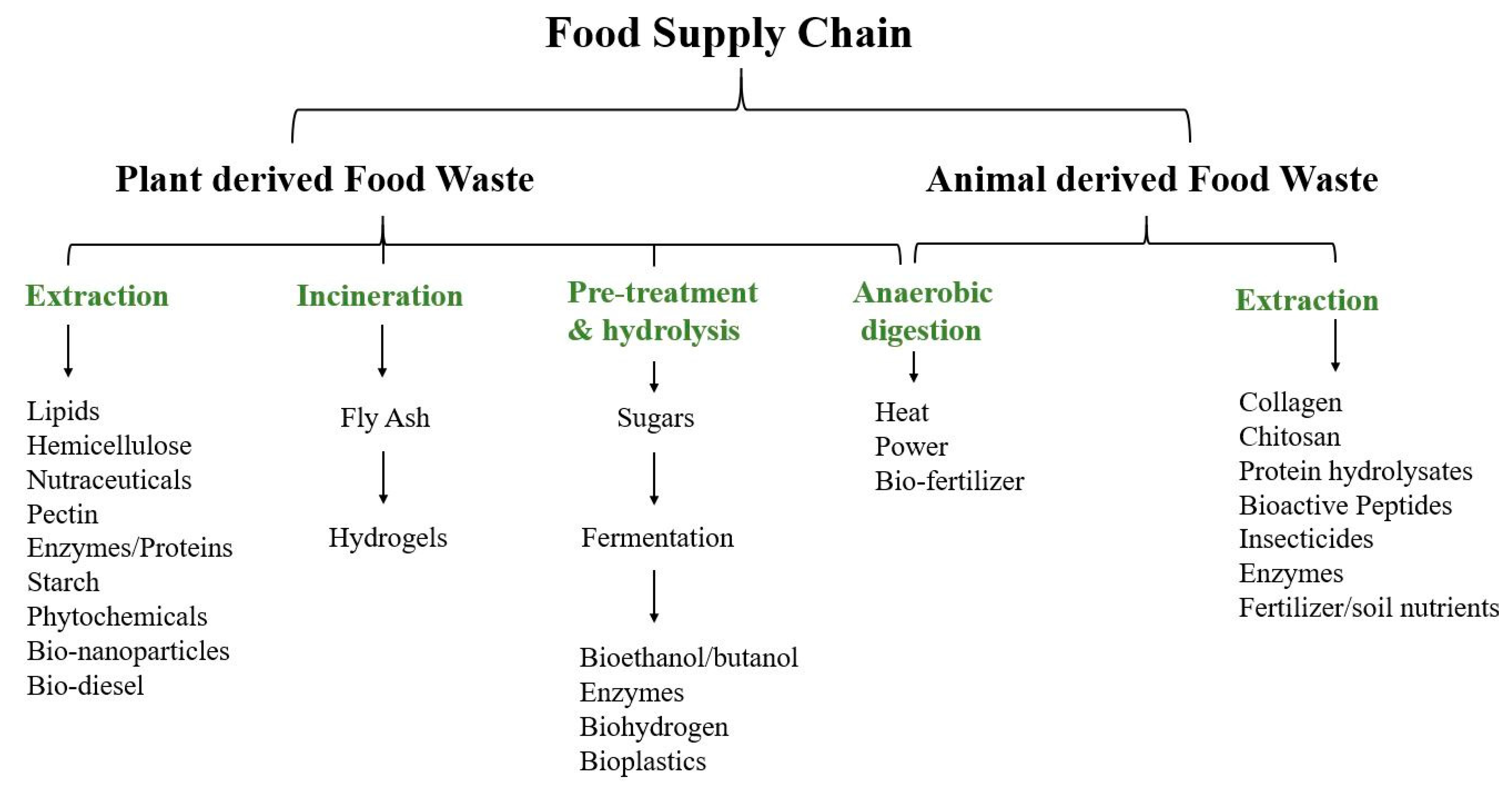
4. Safety Issues Related with Food Waste Valorization
| Contaminants | Type | By-products | Reference |
|---|---|---|---|
| Pesticides | Cyprodinil Dimethomorph Famoxadone | Grape skin extract | [77] |
| Dimetoathe Diazinon Fenitrothion Chlorpyrifos Methidathion | Tomato carotenoid extract | [89] | |
| Mycotoxins | Aflatoxin B1 Fumonisin B1 Ochratoxin A (OTA) | Coffee husk and silverskin | [90] |
| Orange peel extracts | [80] | ||
| Grape skin extract | [77] | ||
| Bacteria’s and molds | Norovirus Salmonella Campylobacter Bacillus E. coli | Meat, poultry, dairy, fruits, vegetables, seafood, grains, and nuts | [91] |
| Heavy metals | Cadmium (Cd) Lead (Pb) | Grape skin extract | [77] |
| Green tea extract | [92] | ||
| Biogenic amines | Cadaverine Putrescine Ethanolamine Ethylamine | Grape skin extract | [77] |
| Phenylethylamine | Rice, soy, almond, coconut and oat press cake | [93] |
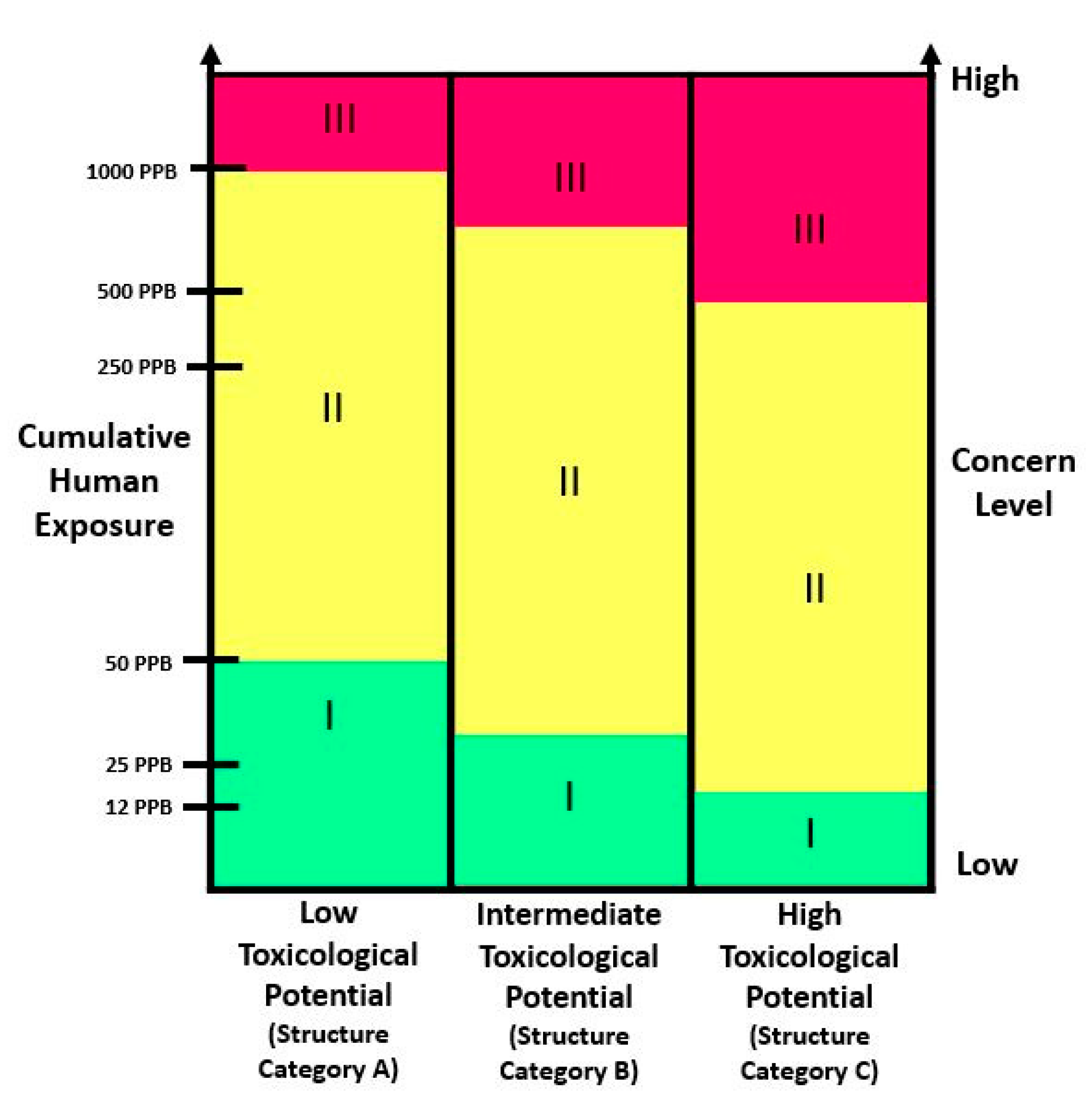
5. Toxicological Evaluation
| Toxicity Tests | Concern Level I | Concern Level II | Concern Level III |
| Genetic Toxicity Tests | If the cumulative estimated daily intake of a food ingredient is 50 ppb or less but greater than 0.5 ppb:
| ||
| Short-term toxicity tests with rodents | 28 Day oral repeated dose study in Rodents (OECD 407). Screening for neurotoxicity and immunotoxicity. | ||
| Subchronic toxicity studies with nonrodents | Study in rodents at least 90 days of repeated dose (OECD TG 408). Screening for neurotoxicity and immunotoxicity. | ||
| One-Year Toxicity Studies with Non-Rodents | Long-term toxicity tests with non-rodents (usually dogs) should be conducted for a minimum of 12 months (OECD TG 452). Screening for neurotoxicity and immunotoxicity. | ||
| Chronic toxicity and carcinogenicity |
| ||
| Reproduction and Development toxicity studies |
|
| |
| Metabolism and pharmacokinetic studies | If indicated by available data or information. | ||
| Clinical studies (in Humans) | If indicated by available data or information. There is no requirement for obtaining clinical safety data for food additives. However, could be necessary if indicated by available data. | ||
6. Challenges for New “Smart-Foods” for Health
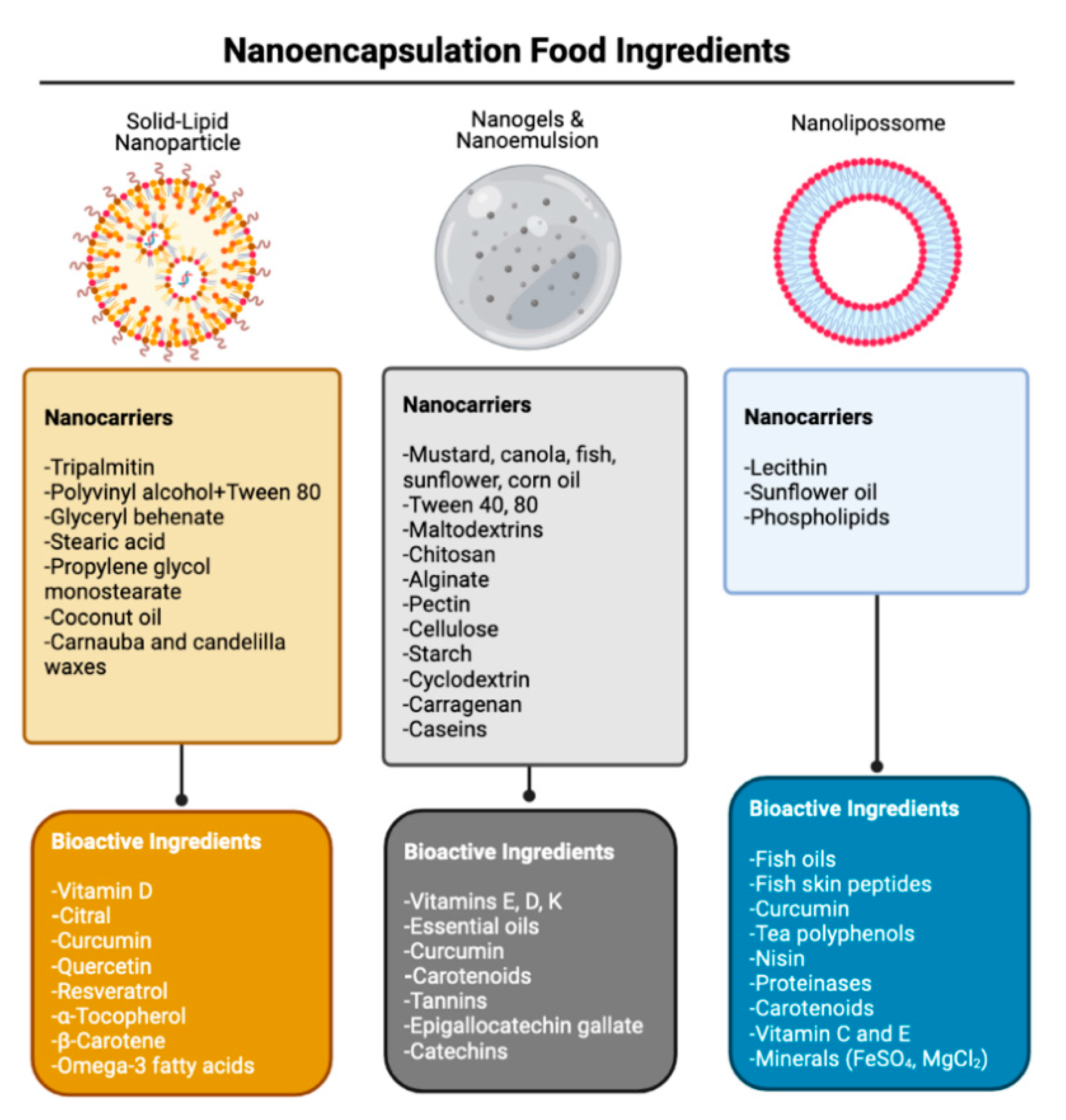
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Global Food Losses and Food Waste; FAO: Rome, Italy, 2011. [Google Scholar]
- Cattaneo, A.; Federighi, G.; Vaz, S. The Environmental Impact of Reducing Food Loss and Waste: A Critical Assessment. Food Policy 2021, 98, 101890. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [Green Version]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds from Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Faustino, A.M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [Green Version]
- Osorio, L.; Flórez-López, E.; Grande-Tovar, C. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Brenes Peralta, L.; Jiménez Morales, M.F.; Freire, M., Jr.; Belik, W.; Basso, N.; Polenta, G.A.; Giraldo, C.; Granados, S. Challenges and Initiatives in Reducing Food Losses and Waste: Latin America and the Caribbean. In Achieving Sustainable Production of Poultry Meat Volume 1: Safety, Quality and Sustainability; Elhadi, M.Y., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 787–802. [Google Scholar]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive Potential of Fruit and Vegetable Wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [CrossRef] [PubMed]
- Amicarelli, V.; Tricase, C.; Spada, A.; Bux, C. Households’ Food Waste Behavior at Local Scale: A Cluster Analysis after the COVID-19 Lockdown. Sustainability 2021, 13, 3283. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Florença, S.G.; Barroca, M.J.; Anjos, O. The Link between the Consumer and the Innovations in Food Product Development. Foods 2020, 9, 1317. [Google Scholar] [CrossRef] [PubMed]
- Dietary Supplements Market Size, Share, Growth, Trends & Forecasts to 2026|COVID-19 Impact Analysis|MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/dietary-supplements-market-973.html (accessed on 23 April 2021).
- Moldes, A.; Vecino, X.; Cruz, J. Nutraceuticals and Food Additives; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 143–164. [Google Scholar]
- Augustin, M.; Sanguansri, L. Challenges in Developing Delivery Systems for Food Additives, Nutraceuticals and Dietary Supplements; Elsevier: Amsterdam, The Netherlands, 2012; pp. 19–48. [Google Scholar]
- Devkota, L.; Montet, D.; Anal, A.K. Regulatory and Legislative Issues for Food Waste Utilization. Food Process. Prod. Their Util. 2017, 535–548. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Matias, A. Chapter 9—Food Industry Processing By-Products in the Role of Alternative and Innovative Food Ingredients and Products in Consumers Wellness; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 239–281. ISBN 978-0-12-816453-2. [Google Scholar]
- Zarrabi, A.; Alipoor Amro Abadi, M.; Khorasani, S.; Mohammadabadi, M.-R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essien, S.O.; Young, B.; Baroutian, S. Recent Advances in Subcritical Water and Supercritical Carbon Dioxide Extraction of Bioactive Compounds from Plant Materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Vettorazzi, A.; De Cerain, A.L.; Sanz-Serrano, J.; Gil, A.G.; Azqueta, A. European Regulatory Framework and Safety Assessment of Food-Related Bioactive Compounds. Nutrients 2020, 12, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the Debate for a Regulatory Framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronson, J.K. Defining ‘Nutraceuticals’: Neither Nutritious nor Pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Ferreira, I.C. Wastes and By-Products: Upcoming Sources of Carotenoids for Biotechnological Purposes and Health-Related Applications. Trends Food Sci. Technol. 2017, 62, 33–48. [Google Scholar] [CrossRef]
- Télessy, I.G. Nutraceuticals. In The Role of Functional Food Security in Global Health; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 409–421. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 27 February 2021).
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]
- Corrêa, A.P.F.; Daroit, D.J.; Fontoura, R.; Meira, S.M.M.; Segalin, J.; Brandelli, A. Hydrolysates of Sheep Cheese Whey as a Source of Bioactive Peptides with Antioxidant and Angiotensin-Converting Enzyme Inhibitory Activities. Peptides 2014, 61, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Delgado, C.O.; Fleuri, L.F. Orange and Mango By-Products: Agro-Industrial Waste as Source of Bioactive Compounds and Botanical Versus Commercial Description—A Review. Food Rev. Int. 2015, 32, 1–14. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart Advanced Solvents for Bioactive Compounds Recovery from Agri-Food By-Products: A Review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the Chemistry, Food Applications, Legislation and Role as Preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.A.; Albuquerque, T.G.; Alves, R.C.; Oliveira, B.; Costa, H.S. Melon (Cucumis melo L.) By-Products: Potential Food Ingredients for Novel Functional Foods? Trends Food Sci. Technol. 2020, 98, 181–189. [Google Scholar] [CrossRef]
- Muíño, I.; Díaz, M.T.; Apeleo, E.; Perez-Santaescolastica, C.; Rivas-Cañedo, A.; Pérez, C.; Cañeque, V.; Lauzurica, S.; de la Fuente, J. Valorisation of an Extract from Olive Oil Waste as a Natural Antioxidant for Reducing Meat Waste Resulting from Oxidative Processes. J. Clean. Prod. 2017, 140, 924–932. [Google Scholar] [CrossRef]
- Bitalebi, S.; Nikoo, M.; Rahmanifarah, K.; Noori, F.; Gavlighi, H.A. Effect of Apple Peel Extract as Natural Antioxidant on Lipid and Protein Oxidation of Rainbow Trout (Oncorhynchus Mykiss) Mince. Int. Aquat. Res. 2019, 11, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.L.; Patel, J.; Jaroni, D.; Friedman, M.; Ravishankar, S. Antimicrobial Activity of Apple, Hibiscus, Olive, and Hydrogen Peroxide Formulations against Salmonella enterica on Organic Leafy Greens. J. Food Prot. 2011, 74, 1676–1683. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial Effect of Olive (Olea europaea L.) Leaves Extract in Raw Peeled Undeveined Shrimp (Penaeus semisulcatus). Int. J. Veter Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.; Stepanyan, V.; Allen, P.; O’Grady, M.; Kerry, J. Effect of Lutein, Sesamol, Ellagic Acid and Olive Leaf Extract on the Quality and Shelf-Life Stability of Packaged Raw Minced Beef Patties. Meat Sci. 2010, 84, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and Antimicrobial Activity of Rosemary, Pomegranate and Olive Extracts in Fish Patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and Antimicrobial Activity of Pomegranate Peel Extract Improves the Shelf Life of Chicken Products. Int. J. Food Sci. Technol. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of Antioxidant Properties of Pomegranate Peel Extract in Comparison with Pomegranate Pulp Extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Zavala, J.F.A.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.; González-Aguilar, G. Agro-Industrial Potential of Exotic Fruit Byproducts as a Source of Food Additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Dias, C.; Fonseca, A.; Amaro, A.L.; Vilas-Boas, A.A.; Oliveira, A.; Santos, S.A.O.; Silvestre, A.J.D.; Rocha, S.M.; Isidoro, N.; Pintado, M. Natural-Based Antioxidant Extracts as Potential Mitigators of Fruit Browning. Antioxidants 2020, 9, 715. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of Fruits and Vegetable Wastes and By-Products to Produce Natural Pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Rezende, C.A.; Rodrigues, R.A.; Barbero, G.F.; Rosa, P.D.T.V.E.; Martínez, J. Encapsulation of Anthocyanin-Rich Extract from Blackberry Residues by Spray-Drying, Freeze-Drying and Supercritical Antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; De Castro, M.D.L. Towards a Comprehensive Exploitation of Citrus. Trends Food Sci. Technol. 2014, 39, 63–75. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- de Boer, A.; Krul, L.; Fehr, M.; Geurts, L.; Kramer, N.; Urbieta, M.T.; van der Harst, J.; van de Water, B.; Venema, K.; Schütte, K.; et al. Animal-Free Strategies in Food Safety & Nutrition: What are We Waiting for? Part I: Food Safety. Trends Food Sci. Technol. 2020, 106, 469–484. [Google Scholar] [CrossRef]
- European Commission. General Health and Food Safety: Guidance on Submissions for Safety Evaluation of Substances Added for Specific Nutritional Purposes in the Manufacture of Foods; European Commission, Ed.; 2020; Available online: https://ec.europa.eu/food/system/files/2020-02/labelling_nutrition-supplements-adm_guidance_safety_substances_en.pdf (accessed on 15 February 2021).
- Badolati, N.; Masselli, R.; Maisto, M.; Di Minno, A.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. Genotoxicity Assessment of Three Nutraceuticals Containing Natural Antioxidants Extracted from Agri-Food Waste Biomasses. Foods 2020, 9, 1461. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Boesten, J.; Bragard, C.; Halldorsson, T.; Hernandez-Jerez, A.; Hougaard-Bennekou, S.; Koutsoumanis, K.; et al. Genotoxicity Assessment of Chemical Mixtures. EFSA J. 2019, 17, e05519. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Chen, S.; Li, J.; Xie, X. Decreasing Consumers’ Risk Perception of Food Additives by Knowledge Enhancement in China. Food Qual. Prefer. 2020, 79, 103781. [Google Scholar] [CrossRef]
- Iriondo-DeHond, M.; Miguel, E.; Del Castillo, M.D. Food Byproducts as Sustainable Ingredients for Innovative and Healthy Dairy Foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef] [Green Version]
- European Commission Questions and Answers on Food Additives. Available online: https://ec.europa.eu/commission/presscorner/detail/en/MEMO_11_783 (accessed on 15 February 2021).
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA) Dietary Supplements|FDA. Available online: https://www.fda.gov/food/dietary-supplements (accessed on 17 March 2021).
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, J.A. Functional Foods and Health: A US Perspective. Br. J. Nutr. 2002, 88, S152–S158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, L.D.; Fernández-Ruiz, V.; Cámara, M. The Frontier between Nutrition and Pharma: The International Regulatory Framework of Functional Foods, Food Supplements and Nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020, 60, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Dietary Supplement Health and Education Act (DSHEA) of Public Law 103-417. 103rd Congress. Available online: https://ods.od.nih.gov/About/DSHEA_Wording.aspx (accessed on 20 September 2019).
- Low, T.Y.; Wong, K.O.; Yap, A.L.L.; De Haan, L.H.J.; Rietjens, I.M.C.M. The Regulatory Framework Across International Jurisdictions for Risks Associated with Consumption of Botanical Food Supplements. Compr. Rev. Food Sci. Food Saf. 2017, 16, 821–834. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health, Labour and Welfare: Food with Health Claims, Food for Special Dietary Uses, and Nutrition Labeling. Available online: https://www.mhlw.go.jp/english/topics/foodsafety/fhc/02.html (accessed on 18 March 2021).
- State Administration for Market Regulation|National Institute of Metrology of China|NIM. Available online: https://en.nim.ac.cn/node/647 (accessed on 18 March 2021).
- Chemical Inspection & Regulation Service Introduction to Food Additive Laws and Food Safety Standards in China. Available online: http://www.cirs-reach.com/China_Chemical_Regulation/Food_additives_law_in_China.html (accessed on 18 March 2021).
- Food Safety and Standards Authority of India (FSSAI) Health Supplements. Available online: https://fssai.gov.in/cms/health-supplements.php (accessed on 18 March 2021).
- Lai, W.T.; Khong, N.M.; Lim, S.S.; Hee, Y.Y.; Sim, B.I.; Lau, K.Y.; Lai, O.M. A Review: Modified Agricultural By-Products for the Development and Fortification of Food Products and Nutraceuticals. Trends Food Sci. Technol. 2017, 59, 148–160. [Google Scholar] [CrossRef]
- Medina-Pastor, P.; Triacchini, G. The 2018 European Union Report on Pesticide Residues in Food. EFSA J. 2020, 18, e06057. [Google Scholar] [CrossRef] [Green Version]
- Zayats, M.; Leschev, S.; Petrashkevich, N.; Zayats, M.; Kadenczki, L.; Szitás, R.; Dobrik, H.S.; Keresztény, N. Distribution of Pesticides in N-Hexane/Water and N-Hexane/Acetonitrile Systems and Estimation of Possibilities of Their Extraction Isolation and Preconcentration from Various Matrices. Anal. Chim. Acta 2013, 774, 33–43. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. Citrinin in Foods and Supplements: A Review of Occurrence and Analytical Methodologies. Foods 2020, 10, 14. [Google Scholar] [CrossRef]
- Fibigr, J.; Šatínský, D.; Solich, P. Current Trends in the Analysis and Quality Control of Food Supplements Based on Plant Extracts. Anal. Chim. Acta 2018, 1036, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Steyn, P.S. Mycotoxins, General View, Chemistry and Structure. Toxicol. Lett. 1995, 82–83, 843–851. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Foglia, P.; Samperi, R.; Laganà, A. Multiclass Mycotoxin Analysis in Food, Environmental and Biological Matrices with Chromatography/Mass Spectrometry. Mass Spectrom. Rev. 2012, 31, 466–503. [Google Scholar] [CrossRef]
- European Commision Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006R1881 (accessed on 16 February 2021).
- European Commission Commission Regulation (EU) No 105/2010 of 5 February 2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Ochratoxin AT. Available online: moz-extension://fd8a28c2-b860-e94b-8024-74ebc1a9d350/enhanced-reader.html?openApp&pdf=https%3A%2F%2Feur-lex.europa.eu%2FLexUriServ%2FLexUriServ.do%3Furi%3DOJ%3AL%3A2010%3A035%3A0007%3A0008%3AEN%3APDF (accessed on 16 February 2021).
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Presence of Mycotoxins in Milk Thistle (Silybum marianum) Food Supplements: A Review. Toxins 2020, 12, 782. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of Byproducts and Waste Materials from Meat, Poultry and Fish Processing Industries: A Review. J. Food Sci. Technol. 2011, 49, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Maciorowski, K.; Herrera, P.; Jones, F.; Pillai, S.; Ricke, S. Effects on Poultry and Livestock of Feed Contamination with Bacteria and Fungi. Anim. Feed. Sci. Technol. 2007, 133, 109–136. [Google Scholar] [CrossRef]
- Costa, J.G.; Vidovic, B.; Saraiva, N.; Costa, M.D.C.; Del Favero, G.; Marko, D.; Oliveira, N.G.; Fernandes, A.S. Contaminants: A Dark Side of Food Supplements? Free. Radic. Res. 2019, 53, 1113–1135. [Google Scholar] [CrossRef]
- Sun, G.-X.; Williams, P.N.; Carey, A.-M.; Zhu, Y.-G.; Deacon, C.; Raab, A.; Feldmann, J.; Islam, R.M.; Meharg, A.A. Inorganic Arsenic in Rice Bran and Its Products Are an Order of Magnitude Higher than in Bulk Grain. Environ. Sci. Technol. 2008, 42, 7542–7546. [Google Scholar] [CrossRef] [PubMed]
- Moncalvo, A.; Marinoni, L.; Dordoni, R.; Garrido, G.D.; Lavelli, V.; Spigno, G. Waste Grape Skins: Evaluation of Safety Aspects for the Production of Functional Powders and Extracts for the Food Sector. Food Addit. Contam. Part A 2016, 33, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Gama, M.R.; Rocha, F.R. Solventless Separation of Underivatized Biogenic Amines by Sequential Injection Chromatography. Microchem. J. 2020, 156, 104839. [Google Scholar] [CrossRef]
- Rebitzer, G.; Ekvall, T.; Frischknecht, R.; Hunkeler, D.; Norris, G.; Rydberg, T.; Schmidt, W.-P.; Suh, S.; Weidema, B.; Pennington, D. Life Cycle Assessment: Part 1: Framework, Goal and Scope Definition, Inventory Analysis, and Applications. Environ. Int. 2004, 30, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, U.; Ain, Q.U.; Yaseen, M.; Fan, X.; Yao, X.; Tong, Z.; Liu, B. Modification of Bentonite with Orange Peels Extract and Its Application as Mycotoxins’ Binder in Buffered Solutions and Simulated Gastrointestinal Fluids. J. Clean. Prod. 2020, 267, 122105. [Google Scholar] [CrossRef]
- Kang, F.; Ge, Y.; Hu, X.; Goikavi, C.; Waigi, M.G.; Gao, Y.; Ling, W. Understanding the Sorption Mechanisms of Aflatoxin B1 to Kaolinite, Illite, and Smectite Clays via a Comparative Computational Study. J. Hazard. Mater. 2016, 320, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.-H.; Wang, P.; Yang, H.-J.; Chen, Y. The Efficacy of Bamboo Charcoal in Comparison with Smectite to Reduce the Detrimental Effect of Aflatoxin B1 on In Vitro Rumen Fermentation of a Hay-Rich Feed Mixture. Toxins 2014, 6, 2008–2023. [Google Scholar] [CrossRef] [PubMed]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A. A Review of the Mycotoxin Adsorbing Agents, with an Emphasis on Their Multi-Binding Capacity, for Animal Feed Decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic Compounds Recovered from Agro-Food By-Products Using Membrane Technologies: An Overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Garcia-Castello, E.M. Valorization of Artichoke Wastewaters by Integrated Membrane Process. Water Res. 2014, 48, 363–374. [Google Scholar] [CrossRef]
- Figueroa, R.A.R.; Cassano, A.; Drioli, E. Ultrafiltration of Orange Press Liquor: Optimization for Permeate Flux and Fouling Index by Response Surface Methodology. Sep. Purif. Technol. 2011, 80, 1–10. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. Comparison of the Performance of UF Membranes in Olive Mill Wastewaters Treatment. Water Res. 2011, 45, 3197–3204. [Google Scholar] [CrossRef]
- Cinar, A.; Onbaşı, E. Mycotoxins: The Hidden Danger in Foods. In Mycotoxins and Food Safety; IntechOpen: London, UK, 2020. [Google Scholar]
- Cortes, J.M.; Vazquez, A.; Santa-María, G.; Blanch, G.P.; Villén, J. Pesticide Residue Analysis by RPLC–GC in Lycopene and Other Carotenoids Obtained from Tomatoes by Supercritical Fluid Extraction. Food Chem. 2009, 113, 280–284. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; García, N.A.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Escobar, F.V.; Blanch, G.P.; Andres, M.I.S.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of Coffee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Thakali, A.; MacRae, J.D. A Review of Chemical and Microbial Contamination in Food: What are the Threats to a Circular Food System? Environ. Res. 2021, 194, 110635. [Google Scholar] [CrossRef]
- Sullivan, J.; Greenfield, J.; Cumberford, G.; Grant, J.; Stewart, J. Extraction Efficiencies of Heavy Metals in Hydroethanolic Solvent from Herbs of Commerce. J. AOAC Int. 2010, 93, 496–498. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Bartkevics, V.; Pugajeva, I.; Borisova, A.; Zokaityte, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Klupsaite, D.; Zadeike, D.; et al. Challenges Associated with Byproducts Valorization—Comparison Study of Safety Parameters of Ultrasonicated and Fermented Plant-Based Byproducts. Foods 2020, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Coppens, P.; da Silva, M.F.; Pettman, S. European Regulations on Nutraceuticals, Dietary Supplements and Functional Foods: A Framework Based on Safety. Toxicology 2006, 221, 59–74. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Guidance for Submission for Food Additive Evaluations. EFSA J. 2012, 10, 2760. [Google Scholar] [CrossRef]
- European Food Safety Authority; EFSA Scientific Committee. Guidance on Safety Assessment of Botanicals and Botanical Preparations Intended for Use as Ingredients in Food Supplements. EFSA J. 2009, 7. [Google Scholar] [CrossRef]
- FDA. Redbook 2000: III Recommended Toxicity Studies|FDA; FDA: Silver Spring, MA, USA, 2007. [Google Scholar]
- The European Parlament Directive 2010/63/EU. 2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 15 February 2021).
- European Parliament and of the Council on the Registration. No 440/2008 of 30 May 2008 Laying Down Test Methods Pursuant to Regulation (EC) No 1907/2006 of the European parliament and of the Council on the Registration, Evaluation, authorization and restriction of Chemicals (REACH). 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R0440 (accessed on 15 February 2021).
- EFSA Directive 2004/10/EC of the European Parliament and of the Council of 11 February 2004 on the Harmonization of Laws, Regulations and Administrative Provisions Relating to the Application of the Principles of Good Laboratory Practice and the Verification; Official Journal of the European Union: Luxembourg, 2004.
- Creton, S.; Billington, R.; Davies, W.; Dent, M.P.; Hawksworth, G.M.; Parry, S.; Travis, K.Z. Application of Toxicokinetics to Improve Chemical Risk Assessment: Implications for the Use of Animals. Regul. Toxicol. Pharmacol. 2009, 55, 291–299. [Google Scholar] [CrossRef]
- Coecke, S.; Pelkonen, O.; Leite, S.B.; Bernauer, U.; Bessems, J.G.; Bois, F.; Gundert-Remy, U.; Loizou, G.; Testai, E.; Zaldívar, J.-M. Toxicokinetics as a Key to the Integrated Toxicity Risk Assessment Based Primarily on Non-Animal Approaches. Toxicol. Vitr. 2013, 27, 1570–1577. [Google Scholar] [CrossRef]
- Gooderham, N.J.; Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Rosol, T.J.; Bastaki, M.; Linman, M.J.; et al. The Safety Evaluation of Food Flavoring Substances: The Role of Genotoxicity Studies. Crit. Rev. Toxicol. 2020, 50, 1–27. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are Olive Pomace Powders a Safe Source of Bioactives and Nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef]
- Medrano-Padial, C.; Puerto, M.; Prieto, A.I.; Ayala, N.; Beaumont, P.; Rouger, C.; Krisa, S.; Pichardo, S. In Vivo Genotoxicity Evaluation of a Stilbene Extract Prior to Its Use as a Natural Additive: A Combination of The Micronucleus Test and The Comet Assay. Foods 2021, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Pressman, P.; Clemens, R.; Hayes, W.; Reddy, C. Food Additive Safety. Toxicol. Res. Appl. 2017, 1. [Google Scholar] [CrossRef] [Green Version]
- Assadpour, E.; Jafari, S.M. A Systematic Review on Nanoencapsulation of Food Bioactive Ingredients and Nutraceuticals by Various Nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Botelho, F.; Brandhoff, M.P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; Quiros, L.; et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Morris, V. Emerging Roles of Engineered Nanomaterials in the Food Industry. Trends Biotechnol. 2011, 29, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid Nano Scale Cargos for the Protection and Delivery of Food Bioactive Ingredients and Nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An Overview of Liposomal Nano-Encapsulation Techniques and Its Applications in Food and Nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Walia, N.; Dasgupta, N.; Ranjan, S.; Chen, L.; Ramalingam, C. Fish Oil Based Vitamin D Nanoencapsulation by Ultrasonication and Bioaccessibility Analysis in Simulated Gastro-Intestinal Tract. Ultrason. Sonochem. 2017, 39, 623–635. [Google Scholar] [CrossRef]
- Wang, X.; Yu, D.-G.; Li, X.-Y.; Bligh, S.A.; Williams, G.R. Electrospun Medicated Shellac Nanofibers for Colon-Targeted Drug Delivery. Int. J. Pharm. 2015, 490, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Raei, M.; Rajabzadeh, G.; Zibaei, S.; Jafari, S.M.; Sani, A.M. Nano-Encapsulation of Isolated Lactoferrin from Camel Milk by Calcium Alginate and Evaluation of Its Release. Int. J. Biol. Macromol. 2015, 79, 669–673. [Google Scholar] [CrossRef]
- Ahmad, M.; Mudgil, P.; Gani, A.; Hamed, F.; Masoodi, F.A.; Maqsood, S. Nano-Encapsulation of Catechin in Starch Nanoparticles: Characterization, Release Behavior and Bioactivity Retention during Simulated In-Vitro Digestion. Food Chem. 2019, 270, 95–104. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of Hydrophobic and Low-Soluble Food Bioactive Compounds within Different Nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Khorasani, S.; Danaei, M.; Mozafari, M. Nanoliposome Technology for the Food and Nutraceutical Industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Santos, V.D.S.; Ribeiro, A.P.B.; Santana, M.H.A. Solid Lipid Nanoparticles as Carriers for Lipophilic Compounds for Applications in Foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Hemar, Y. Nano- and Micro-Structured Assemblies for Encapsulation of Food Ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rajakumar, G.; Chung, I.-M. Nanotechnology: Current Uses and Future Applications in the Food Industry. 3 Biotech 2018, 8, 74. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef] [PubMed]
- Center for Food Safety Center for Food Safety. Available online: https://www.centerforfoodsafety.org/nanotechnology-in-food (accessed on 30 March 2021).
- Altin, G.; Gültekin-Özgüven, M.; Ozcelik, B. Liposomal Dispersion and Powder Systems for Delivery of Cocoa Hull Waste Phenolics via Ayran (Drinking Yoghurt): Comparative Studies on In-Vitro Bioaccessibility and Antioxidant Capacity. Food Hydrocoll. 2018, 81, 364–370. [Google Scholar] [CrossRef]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G.; Youssef, M.M. Encapsulated Bioactive Ingredients of grape by-products applicate in fresh-cut fruit and juices diminished the ochratoxins. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Utpott, M.; Assis, R.Q.; Pagno, C.H.; Krigger, S.P.; Rodrigues, E.; Rios, A.D.O.; Flôres, S.H. Evaluation of the Use of Industrial Wastes on the Encapsulation of Betalains Extracted from Red Pitaya Pulp (Hylocereus polyrhizus) by Spray Drying: Powder Stability and Application. Food Bioprocess Technol. 2020, 13, 1940–1953. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Ramaswamy, H.S. Effect of Microencapsulation on Antioxidant and Antifungal Properties of Aqueous Extract of Pomegranate Peel. J. Food Sci. Technol. 2020, 57, 723–733. [Google Scholar] [CrossRef]
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of Pomegranate Peel Nanoparticles on Quality Attributes of Meatballs during Refrigerated Storage. LWT 2018, 89, 489–495. [Google Scholar] [CrossRef]
- Dehalu, V.; Weigel, S.; Rebe, S.; Grombe, R.; Löbenberg, R.; Delahaut, P. Production and Characterization of Antibodies against Crosslinked Gelatin Nanoparticles and First Steps toward Developing an ELISA Screening Kit. Anal. Bioanal. Chem. 2012, 403, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Katouzian, I.; Jafari, S.M. Nano-Encapsulation as a Promising Approach for Targeted Delivery and Controlled Release of Vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Jafari, S.M.; McClements, D.J. Chapter One—Nanotechnology Approaches for Increasing Nutrient Bioavailability; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 81, pp. 1–30. ISBN 1043-4526. [Google Scholar]
- McClements, D.J.; Xiao, H. Is Nano Safe in Foods? Establishing the Factors Impacting the Gastrointestinal Fate and Toxicity of Organic and Inorganic Food-Grade Nanoparticles. NPJ Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef]
- Wu, H.; Yin, J.-J.; Wamer, W.G.; Zeng, M.; Lo, Y.M. Reactive Oxygen Species-Related Activities of Nano-Iron Metal and Nano-Iron Oxides. J. Food Drug Anal. 2014, 22, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Design of Nano-Laminated Coatings to Control Bioavailability of Lipophilic Food Components. J. Food Sci. 2010, 75, R30–R42. [Google Scholar] [CrossRef] [Green Version]
- Salvia-Trujillo, L.; Martín-Belloso, O.; McClements, D.J. Excipient Nanoemulsions for Improving Oral Bioavailability of Bioactives. Nanomaterials 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, E.E.; Fröhlich, E. Cytotoxicity of Nanoparticles Contained in Food on Intestinal Cells and the Gut Microbiota. Int. J. Mol. Sci. 2016, 17, 509. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Guidance on Risk Assessment of the Application of Nanoscience and Nanotechnologies in the Food and Feed Chain: Part 1, Human and Animal Health. EFSA Journal. 2018, 16, e05327. [Google Scholar] [CrossRef] [Green Version]
- Duvall, M.N. FDA Regulation of Nanotechnology; Beveridge and Diamond, PG: Washington, DC, USA, 2012. [Google Scholar]

| Toxicity Tests | Tier 1 | Tier 2 | Tier 3 |
|---|---|---|---|
| Toxicokinetic | Studies of in vitro gastrointestinal metabolism to establish whether compounds or metabolites are absorbed. | Studies to define ADME and other basic toxicokinetic parameters (T1/2, AUC, bioavailability, Cmax and Tmax) following a single dose (OECD TG 147) together with in vivo assessment of ADME for identification and quantification of metabolites. | Animal studies with repeated administration doses involving studies to steady state which would be approximately five terminal half-lives. Human Clinical Trials. |
| Genotoxicity | Basic testing battery:
| Follow-up of a positive result in basic test battery.
| |
| Toxicity Tests (subchronic, chronic and carcinogenicity) | Subchronic toxicity study
|
| Short-term tests with transgenic mouse models (p53 +/−, rasH2, Tg.AC, Xpa−/− and Xpa−/−p53+/−) (OECD 488) Neurotoxicity, immunotoxicity or endocrine-mediated studies |
| Reproductive and Developmental toxicity |
| Studies for endocrine, developmental neurotoxicity (OECD TG 426), and mode of action studies. | |
| Triggers for next tier… |
|
| |
| Additional Studies |
| ||
| Food Products | Application | Bioactive Components | Source | Nanoparticles Technique | Reference |
|---|---|---|---|---|---|
| Drinking Yogurt | Antioxidant ingredient | Catechin, epicatechin, quercetin, ferulic acid, gallic acid, p-coumaric acid, syringic acid, trans-cinnamic acid, vanillic acid, and vanillin | Cocoa hull waste | Liposomal systems | [124] |
| Juices and fruit salads | Reduce mycotoxins | p-Coumaric and ferulic acids, epicatechin, quercetin | Grape stem and leaf extracts | Microencapsulation | [125] |
| Yogurt | Colorant | Betalains | Red pitaya peel | Microencapsulation | [126] |
| Cupcakes | Antimicrobial | Polyphenols | Pomegranate peel | Microencapsulation | [127] |
| Beef meatballs | Antimicrobial and antioxidant | Polyphenols (Punicalagin) | Pomegranate peel | Lyophilized pomegranate peel nanoparticles | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. https://doi.org/10.3390/foods10071564
Vilas-Boas AA, Pintado M, Oliveira ALS. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods. 2021; 10(7):1564. https://doi.org/10.3390/foods10071564
Chicago/Turabian StyleVilas-Boas, Ana A., Manuela Pintado, and Ana L. S. Oliveira. 2021. "Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns" Foods 10, no. 7: 1564. https://doi.org/10.3390/foods10071564
APA StyleVilas-Boas, A. A., Pintado, M., & Oliveira, A. L. S. (2021). Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods, 10(7), 1564. https://doi.org/10.3390/foods10071564








