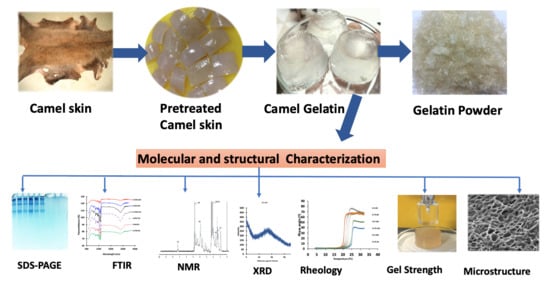
Molecular, Structural, and Rheological Characterization of Camel Skin Gelatin Extracted Using Different Pretreatment Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Camel Skin Preparation
2.2. Alkaline Pretreatment of the Camel Skin and Extraction of Gelatin
2.3. Analyses
2.3.1. Yield of Extraction
2.3.2. Determination of Gel Strength
2.3.3. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Structural Characterization of the Camel Skin Gelatin
2.4.1. Fourier Transform Infrared Spectroscopy Analysis (FTIR)
2.4.2. Proton Nuclear Magnetic Resonance (1H NMR)
2.4.3. X-ray Diffraction Determination (XRD)
2.4.4. Microstructural Features by Scanning Electron Microscopy
2.5. Determination of the Gelling and Melting Temperature
2.6. Statistical Analysis
3. Results and Discussion
3.1. Yield of Extraction
3.2. Gel Strength
3.3. Protein Pattern of the Camel Skin Gelatin as Affected by the Alkaline Pretreatment
3.4. Structural Elucidation of the Camel Skin Gelatin via Fourier Transform Infrared (FTIR) Spectroscopy
3.5. Structural Elucidation of the Camel Skin Gelatin by Nuclear Magnetic Resonance (NMR) Spectroscopy
3.6. X-ray Diffraction (XRD) Analysis of Camel Skin Gelatin
3.7. Microstructure of Gelatin
3.8. Gelling and Melting Temperatures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morrison, N.A.; Clark, R.C.; Chen, Y.L.; Talashek, T.; Sworn, G. Gelatin alternatives for the food industry. In Physical Chemistry and Industrial Application of Gellan Gum; Nishinari, K., Kremer, F., Lagaly, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 127–131. [Google Scholar]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Effect of extraction temperature on functional properties and antioxidative activities of gelatin from shark skin. Food Bioprocess Technol. 2012, 5, 2646–2654. [Google Scholar] [CrossRef]
- Sheela, A.K. Gelatin Market—Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2012–2018. Available online: http://www.transparencymarketresearch.com/gelatin.html (accessed on 23 October 2019).
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nation (FAO, STAT). Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 23 October 2019).
- Al-Hassan, A.A. Gelatin from camel skin: Extraction ad Characterization. Food Hydrocoll. 2020, 101, 105457. [Google Scholar] [CrossRef]
- Ahmed, M.; Benjakul, S. Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocoll. 2011, 25, 381–388. [Google Scholar] [CrossRef]
- Lassoued, I.; Jridi, M.; Nasri, R.; Dammak, A.; Hajji, M.; Nasri, M. Characteristics and functional properties of gelatin from thornback ray skin obtained by pepsin-aided process in comparison with commercial halal bovine gelatin. Food Hydrocoll. 2014, 41, 3019–3318. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Kishimura, H. Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocoll. 2012, 29, 389–397. [Google Scholar] [CrossRef]
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and Gel Properties of Gelatin from Goat Skin as Influenced by Alkaline-pretreatment Conditions. Asian Aust. J. Animal Sci. 2016, 29, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanmartín-Masiá, E.; Poveda-Reyes, S.; Gallego Ferrer, G. Extracellular matrix–inspired gelatin/hyaluronic acid injectable hydrogels. Int. J. Poly Mater. Poly Biomater. 2017, 66, 280–288. [Google Scholar] [CrossRef]
- Boran, G.; Mulvaney, S.J.; Regenstein, J.M. Rheological properties of gelatin from silver carp skin compared to commercially available gelatins from different sources. J. Food Sci. 2010, 75, 565–571. [Google Scholar] [CrossRef]
- Benjakul, S.; Oungbho, K.; Visessanguan, W.; Thiansilakul, Y.; Roytrakul, S. Characteristics of gelatin from the skins of bigeye snapper, Priacanthus tayenus and Priacanthus macracanthus. Food Chem. 2009, 116, 445–451. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Sinthusamran, S.; Sae-leaw, T.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of Asian Bullfrog (Rana tigerina). Food Biophys. 2017, 12, 289. [Google Scholar] [CrossRef] [Green Version]
- Tumerkan, E.T.; Cansu, U.; Boran, G.; Regenstein, J.M.; Ozogul, F. Physicochemical and functional properties of gelatin obtained from tuna, frog and chicken skins. Food Chem. 2019, 287, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Al-Kahtani, H.A.; Jaswir, I.; Ismail, E.A.; Ahmed, M.A.; Hammed, A.M.; Olorunnisola, S.; Octavianti, F. Structural characteristics of camel-bone gelatin by demineralization and extraction. Int. J. Food Prop. 2017, 20, 2559–2568. [Google Scholar] [CrossRef]
- Benjakul, S.; Kittiphattanabawon, P.; Regenstein, J.M. Fish gelatin. In Food Biochemistry and Food; Simpson, B.K., Nollet, L.M.L., Toldrae, F., Eds.; John Wiley & Sons Inc.: Ames, IA, USA, 2012; pp. 388–405. [Google Scholar]
- Badii, F.; Howell, N.K. Fish gelatin: Structure, gelling properties and interaction with egg albumen proteins. Food Hydrocoll. 2006, 20, 630–640. [Google Scholar] [CrossRef]
- Liu, J.; Xu, G.; Yuan, S.; Jiang, P. The effect of macromolecules on foam stability in sodium dodecyl sulfate/cetylpyridinium bromide mixtures. J. Dispers. Sci. Technol. 2003, 24, 779–787. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Comparative study on characteristics of gelatin from the skins of brown banded bamboo shark and blacktip shark as affected by extraction conditions. Food Hydrocoll. 2010, 24, 164–171. [Google Scholar] [CrossRef]
- Friess, W.; Lee, G. Basic thermo analytical studies of insoluble collagen matrices. Biomaterials 1996, 17, 2289–2294. [Google Scholar] [CrossRef]
- Fullerton, G.D.; Nes, E.; Amurao, M.; Rahal, A.; Krasnosselskaia, L.; Cameron, I. An NMR method to characterize multiple water compartments on mammalian collagen. Cell Biol. Int. 2006, 30, 66–73. [Google Scholar] [CrossRef]
- Uriarte-Montoya, M.H.; Arias-Moscoso, J.L.; Plascencia-Jatomea, M.; Santacruz-Ortega, H.; Rouzaud-Sández, O.; Cardenas-Lopez, J.L. Jumbo squid (Dosidicus gigas) mantle collagen: Extraction, characterization, and potential application in the preparation of chitosan–collagen biofilms. BioTechnology 2010, 101, 4212–4219. [Google Scholar] [CrossRef]
- Constantine, K.L.; Goldfarb, V.; Wittekind, M.; Anthony, J.; Ng, L.; Muller, L. Sequential 1H and 15N NMR assignments and secondary structure of a recombinant anti-digoxin antibody VL domain. J. Biomol. NMR 1992, 31, 5033–5043. [Google Scholar]
- Mazaki, T.; Shiozaki, Y.; Yamane, K.; Yoshida, A.; Nakamura, M.; Yoshida, Y.; Zhou, D.; Kitajima, T.; Tanaka, M.; Ito, Y.; et al. A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Sci. Rep. 2014, 4, 4457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teramoto, N.; Hayashi, A.; Yamanaka, K.; Sakiyama, A.; Nakano, A.; Shibata, M. Preparation and mechanical properties of photo-crosslinked fish gelatin/imogolite nanofiber composite hydrogel. Materials 2012, 5, 2573–2585. [Google Scholar] [CrossRef] [Green Version]
- Badii, F.; MacNaughtan, W.; Mitchell, J.R.; Farhat, I. The effect of drying temperature on physical properties of thin gelatin films. Drying Tech. 2014, 32, 30–38. [Google Scholar] [CrossRef]
- Diaz-Calderon, P.; Flores, E.; Gonzalez-Munoz, A.; Pepczynska, M.; Quero, F.; Enrione, J. Influence of extraction variables on the structure and physical properties of salmon gelatin. Food Hydrocoll. 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Benjakul, S.; O’brien, N.M. Effect of pretreatments and defatting of seabass skins on properties and fishy odor of gelatin. J. Food Biochem. 2016, 40, 741–753. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014, 152, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–333. [Google Scholar] [CrossRef]






| Parameters | ||
|---|---|---|
| Samples | Yield (%) | Gel Strength (g) |
| 0.5 M-6 h | 3.2 ± 0.11 g | 175.0 ± 11.31 f |
| 0.75 M-6 h | 4.2 ± 0.10 g | 196.5 ± 14.14 ef |
| 0.5 M-12 h | 3.2 ± 0.55 g | 200.8 ± 13.08 e |
| 0.75 M-12 h | 4.1 ± 0.88 g | 247.8 ± 20.15 d |
| 0.5 M-24 h | 6.6 ± 0.26 ef | 247.0 ± 12.72 d |
| 0.75 M-24 h | 8.2 ± 0.44 d | 322.8 ± 29.69 b |
| 0.5 M-30 h | 5.9 ± 0.66 f | 268.8 ± 15.20 c |
| 0.75 M-30 h | 7.8 ± 0.15 de | 306.8 ± 13.08 b |
| 0.5 M-36 h | 9.4 ± 1.22 d | 254.3 ± 13.08 d |
| 0.75 M-36 h | 16.1 ± 0.89 c | 319.5 ± 18.38 b |
| 0.5 M-42 h | 18.9 ± 0.76 b | 365.5 ± 7.07 a |
| 0.75 M-42 h | 22.6 ± 1.65 a | 256.3 ± 8.13 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawale, O.S.; Abuibaid, A.; Hamed, F.; Kittiphattanabawon, P.; Maqsood, S. Molecular, Structural, and Rheological Characterization of Camel Skin Gelatin Extracted Using Different Pretreatment Conditions. Foods 2021, 10, 1563. https://doi.org/10.3390/foods10071563
Fawale OS, Abuibaid A, Hamed F, Kittiphattanabawon P, Maqsood S. Molecular, Structural, and Rheological Characterization of Camel Skin Gelatin Extracted Using Different Pretreatment Conditions. Foods. 2021; 10(7):1563. https://doi.org/10.3390/foods10071563
Chicago/Turabian StyleFawale, Olumide Samson, Ahlam Abuibaid, Fathalla Hamed, Phanat Kittiphattanabawon, and Sajid Maqsood. 2021. "Molecular, Structural, and Rheological Characterization of Camel Skin Gelatin Extracted Using Different Pretreatment Conditions" Foods 10, no. 7: 1563. https://doi.org/10.3390/foods10071563
APA StyleFawale, O. S., Abuibaid, A., Hamed, F., Kittiphattanabawon, P., & Maqsood, S. (2021). Molecular, Structural, and Rheological Characterization of Camel Skin Gelatin Extracted Using Different Pretreatment Conditions. Foods, 10(7), 1563. https://doi.org/10.3390/foods10071563