Occurrence of Chemical Contaminants in Peruvian Produce: A Food-Safety Perspective
Abstract
1. Introduction
2. Peru Food Laws and Regulations for Chemical Contaminants
3. Inclusion Criteria for Review
4. Chemical Contaminants
4.1. Pesticide Residues in Produce
4.2. Heavy Metals in Produce
4.3. Mycotoxin Contamination in Produce
5. Water and Chemical Contaminants
6. Chemical Contaminants and Public Health
7. Opportunities to Reduce Chemical Hazards in Produce
- Strengthen the education pipeline: academic partnerships could help develop and lead training programs that combine research-based knowledge and practical agricultural experience to disseminate information on food-safety practices to the food producers. Training opportunities should be designed and promoted in ways that are accessible to and that engage farmers to adopt and adapt practices that support both agriculture sustainability and food safety.
- Supportive regional agricultural services: investment from the public and private sectors in infrastructure to support small-scale farmers with facilities and equipment, such as refrigerators for storage, and regular calibrations and technical checks of pesticide sprayers for more precise and effective pesticide application and protection of crops.
- Research partnerships: promote research that would develop methods to establish baseline levels for chemical contaminants in soils, irrigation water, and fruits and vegetables. Such baseline data can help locate soil fields, water sources, and crops that are at higher risk of chemical contamination and that require corrective interventions, thus promoting a stronger connection between the food industry and public health.
- Harmonizing food systems and public health: increased coordination and cooperation with the Peruvian healthcare system to implement surveillance systems that will be able to conduct traceback investigations to identify sources of disease outbreaks and stop current foodborne illnesses. Additionally, effective surveillance systems should also be able to estimate the long-term burden of foodborne diseases, evaluate priorities, and devise control and preventive measures.
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ministerio de Agricultura y Riego (MINAGRI). Boletin Estadistico Mensual “el agro en cifras”, Mes: Junio. 2019. Available online: https://www.minagri.gob.pe/portal/download/pdf/herramientas/boletines/agro-cifras/boletin-estadistico-mensual-el-agro-en-cifras-junio19-050919.pdf (accessed on 15 March 2021).
- World Bank. International Bank for Reconstruction and Development International Finance Corporation and Multilateral Investment Guarantee Agency Country Partnership Framework for the Republic of Peru for the Period FY17-FY21. Available online: http://documents.worldbank.org/curated/en/522711493949637279/pdf/Peru-CPF-112299-PE-04102017.pdf (accessed on 15 March 2021).
- Penny, M.E.; Meza, K.S.; Creed-Kanashiro, H.M.; Marin, R.M.; Donovan, J. Fruits and vegetables are incorporated into home cuisine in different ways that are relevant to promoting increased consumption. Matern. Child Nutr. 2017, 13, e12356. [Google Scholar] [CrossRef]
- Schwarz, J.; Mathijs, E. Globalization and the sustainable exploitation of scarce groundwater in coastal Peru. J. Clean. Prod. 2017, 147, 231–241. [Google Scholar] [CrossRef]
- Borgoño, N. Reporte de Enfermedades Transmitidas Por Alimentos (ETA) en el Perú. Bol. Epidemiol. Peru 2019, 28, 381–383. Available online: https://www.dge.gob.pe/portal/docs/vigilancia/boletines/2019/15.pdf (accessed on 1 May 2021).
- Jaffee, S.; Henson, S.; Unnevehr, L.; Grace, D.; Cassou, E. The Safe Food Imperative: Accelerating Progress in Low- and Middle-Income Countries; World Bank Group: Washington, DC, USA; Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/30568/9781464813450.pdf?sequence=6&isAllowed=y (accessed on 15 March 2021).
- World Health Organization (WHO). WHO Estimates of the Global Burden of Foodborne Disease: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/199350/9789241565165_eng.pdf;jsessionid=36557E6AEC6E64773156483CEFCC5663 (accessed on 15 March 2021).
- Kher, S.V.; De Jonge, J.; Wentholt, M.T.A.; Deliza, R.; De Andrade, J.C.; Cnossen, H.J.; Luijckx, N.B.L.; Frewer, L.J. Consumer perceptions of risks of chemical and microbiological contaminants associated with food chains: A cross-national study. Int. J. Consum. Stud. 2013, 37, 73–83. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=20533 (accessed on 15 March 2021).
- Food and Agriculture Organization and World Health Organization (FAO and WHO). Codex Alimentarius Commission–Procedural Manual Twenty-Seventh Edition. Available online: http://www.fao.org/3/ca2329en/CA2329EN.pdf (accessed on 15 March 2021).
- George, C.M.; Sima, L.; Arias, M.H.; Mihalic, J.; Cabrera, L.Z.; Danz, D.; Checkley, W.; Gilman, R.H. Arsenic exposure in drinking water: An unrecognized health threat in Peru. Bull. World Health Organ. 2014, 92, 565–572. [Google Scholar] [CrossRef]
- McCabe-Sellers, B.J.; Beattie, S.E. Food safety: Emerging trends in foodborne illness surveillance and prevention. J. Am. Diet. Assoc. 2004, 104, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Loza, A. Peru: Food and Agricultural Import Regulations and Standards Report. FAIRS Annual Country Report 2018. United States Department of Agriculture Foreign Agricultural Service. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Food%20and%20Agricultural%20Import%20Regulations%20and%20Standards%20Report_Lima_Peru_3-22-2019.pdf (accessed on 15 March 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Aprueban el Plan Anual de Monitoreo de Residuos Químicos y Otros Contaminantes en Alimentos Agropecuarios Primarios y Piensos, Para el Período Julio a Diciembre del 2019. Directoral Resolution N°0056-2019-MINAGRI-SENASA-DIAIA. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2019/09/RD-N%C2%B0-0056-2019-MINAGRI-SENASA-DIAIA.pdf (accessed on 15 March 2021).
- Ministerio del Ambiente (MINAM). Aprueban los Estándares Nacionales de Calidad Ambiental Para el Agua. Supreme Decree N°004-2017-MINAM. Available online: http://www.minam.gob.pe/wp-content/uploads/2017/06/DS-004-2017-MINAM.pdf (accessed on 15 March 2021).
- Ministerio del Ambiente (MINAM). Aprueban los Estándares Nacionales de Calidad Ambiental Para Suelo. Supreme Decree N°011-2017-MINAM. Available online: http://www.minam.gob.pe/wp-content/uploads/2017/12/DS_011-2017-MINAM.pdf (accessed on 15 March 2021).
- PROM PERU. Guia de Requisitos de Acceso de Alimentos a los Estados Unidos. Available online: https://www.siicex.gob.pe/siicex/documentosportal/1025163015radB52B3.pdf (accessed on 15 March 2021).
- United States Food and Drug Administration (US FDA). FSMA Final Rule on Foreign Supplier Verification Programs (FSVP) for Importers of Food for Humans and Animals. Available online: https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-foreign-supplier-verification-programs-fsvp-importers-food-humans-and-animals (accessed on 15 March 2021).
- European Commission, Health and Consumers Directorate-General. Final Report of an Audit Carried Out in Peru from 19 to 27 November 2014 in Order to Evaluate Controls of Pesticides in Foods of Plant Origin Intended for Export to the European Union. Available online: http://ec.europa.eu/food/fvo/act_getPDFannx.cfm?ANX_ID=7974 (accessed on 15 March 2021).
- World Bank. Gaining Momentum in Peruvian Agriculture: Opportunities to Increase Productivity and Enhance Competitiveness. Available online: http://documents1.worldbank.org/curated/pt/107451498513689693/pdf/P162084-06-26-2017-1498513685623.pdf (accessed on 15 March 2021).
- World Food Programme. Peru Annual Country Report. 2019. Available online: https://docs.wfp.org/api/documents/WFP-0000113855/download/ (accessed on 15 March 2021).
- Chung, B. Control de los contaminantes químicos en el Perú. Rev. Peru. Med. Exp. Salud Pública 2008, 25, 413–418. [Google Scholar]
- Ministerio de Agricultura y Riego (MINAGRI). Anuario Estadístico: Medios de Producción Agropecuarios. 2016. Available online: http://siea.minagri.gob.pe/siea/sites/default/files/anuario-medios-produccion-agropecuarios-2016_281217_1.pdf (accessed on 15 March 2021).
- Cruz-Escalon, A. Situacion Actual del Consumo de Pesticidas en el Peru. Bachelor's Thesis, Facultad de Agronomia, Universidad Nacional Agraria La Molina, Lima, Peru, 2017. Available online: http://repositorio.lamolina.edu.pe/bitstream/handle/UNALM/2976/E71-C7-T.pdf?sequence=1&isAllowed=y (accessed on 15 March 2021).
- Aquino-Anchirayco, M.S.; Castro-Mere, C.C. Análisis de Residuo de Plaguicida Organofosforado (Methamidophos) en Muestras de Papa de Mercados de Lima Metropolitana. Bachelor's Thesis, Facultad de Farmacia y Quimica, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2008. Available online: http://cybertesis.unmsm.edu.pe/bitstream/handle/cybertesis/1613/Aquino_am.pdf (accessed on 15 May 2021).
- Campos-Chávez, C.; Palacios-Alcántara, A. Determinación por HPLC de Residuos de Insecticida Órganofosforado (Methamidophos) en Tomates Comercializados en Lima-Perú. Bachelor's Thesis, Facultad de Farmacia y Bioquímica, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2010. Available online: https://cybertesis.unmsm.edu.pe/handle/20.500.12672/1611 (accessed on 15 May 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Aprueban Norma Sanitaria Que Establece Los Limites Maximos de Residuos (LMR) de Plaguicidas de Uso Agricola en Alimentos de Consumo Humano. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2014/11/RM-1006-2016-MINSA-con-NTS-128-MINSA-2016-DIGESA-LMR-Plaguicidas.pdf (accessed on 15 March 2021).
- Hjorth, K.; Johansen, K.; Holen, B.; Andersson, A.; Christensen, H.B.; Siivinen, K.; Toome, M. Pesticide residues in fruits and vegetables from South America–A Nordic project. Food Control. 2011, 22, 1701–1706. [Google Scholar] [CrossRef]
- Servicio Nacional de Sanidad Agraria (SENASA). Informe del Monitoreo de Contaminantes Químicos en Alimentos Agropecuarios Primarios, Año 2011; SENASA: Lima, Peru, 2011. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2016/12/Informe-M-2011.pdf (accessed on 15 March 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Informe del Monitoreo de Contaminantes Químicos en Alimentos Agropecuarios Primarios, Año 2012; SENASA: Lima, Peru, 2012. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2016/12/Informe-del-Monitoreo-de-Residuos-Qu%C3%ADmicos-y-otros-Contaminantes-en-Alimentos-Agropecuarios-Primarios-a%C3%B1o-2012.pdf (accessed on 15 March 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Informe del monitoreo de Contaminantes Químicos en Alimentos Agropecuarios Primarios, Año 2013; SENASA: Lima, Peru, 2013. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2016/12/INFORME_-2013.pdf (accessed on 15 March 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Informe del Monitoreo de Contaminantes Químicos en Alimentos Agropecuarios Primarios, Año 2014; SENASA: Lima, Peru, 2014. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2016/08/Informe-de-monitoreo-2014-1.pdf (accessed on 15 March 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Informe del Monitoreo de Contaminantes Químicos en Alimentos Agropecuarios Primarios, Año 2015; SENASA: Lima, Peru, 2015. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2016/08/Informe-de-monitoreo.pdf (accessed on 15 March 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Informe del Monitoreo de Contaminantes Químicos en Alimentos Agropecuarios Primarios, Año 2016; SENASA: Lima, Peru, 2016. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2016/08/Informe-de-monitoreo-2016.pdf (accessed on 15 March 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Informe del Monitoreo de Contaminantes Químicos en Alimentos Agropecuarios Primarios, Año 2017; SENASA: Lima, Peru, 2017. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2018/12/INFORME-FINAL-DEL-PLAN-DE-MONITOREO-2017-1.pdf (accessed on 15 March 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Informe del Monitoreo de Contaminantes Químicos en Alimentos Agropecuarios Primarios, Año 2018; SENASA: Lima, Peru, 2018. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2020/06/INFORME-FINAL-DEL-PLAN-DE-MONITOREO-2018-revisi%C3%B3n-31.03.docx (accessed on 15 March 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Informe del Monitoreo de Contaminantes Químicos en Alimentos Agropecuarios Primarios, Año 2019; SENASA: Lima, Peru, 2019. Available online: https://cdn.www.gob.pe/uploads/document/file/1516765/Informe%20del%20plan%20anual%20de%20monitoreo%20de%20contaminantes%202019.pdf (accessed on 15 March 2021).
- Delgado-Zegarra, J.; Alvarez-Risco, A.; Yáñez, J.A. Indiscriminate use of pesticides and lack of sanitary control in the domestic market in Peru. Pan Am. J. Public Health 2018, 42, e3. [Google Scholar] [CrossRef]
- United States Food and Drug Administration (US FDA). Import Alerts for a Country/Area: Peru. Available online: https://www.accessdata.fda.gov/cms_ia/country_PE.html (accessed on 15 March 2021).
- Zhang, Z.Y.; Liu, X.J.; Yu, X.Y.; Zhang, C.Z.; Hong, X.Y. Pesticide residues in the spring cabbage (Brassica oleracea L. var. capitata) grown in open field. Food Control. 2007, 18, 723–730. [Google Scholar] [CrossRef]
- Elgueta, S.; Moyano, S.; Sepúlveda, P.; Quiroz, C.; Correa, A. Pesticide residues in leafy vegetables and human health risk assessment in North Central agricultural areas of Chile. Food Addit. Contam. Part. B Surveill. 2017, 10, 105–112. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef]
- Morgan, J.B.; Connolly, E.L. Plant-soil interactions: Nutrient uptake. Nat. Educ. Knowl. 2013, 4, 2. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human, 1st ed.; Springer: New York, NY, USA, 2007; pp. 3–85. [Google Scholar]
- De la Flor, P.; Mining and Economic Development in Peru. Article in Revista Harvard Review of Latin America. Available online: http://revista.drclas.harvard.edu/files/revista/files/37165_pandr_revista_proof.pdf?m=1410442834 (accessed on 15 March 2021).
- Bebbington, A.; Williams, M. Water and mining conflicts in Peru. Mt. Res. Dev. 2008, 28, 190–195. [Google Scholar] [CrossRef]
- Tasrina, R.C.; Rowshon, A.; Mustafizur, A.M.R.; Rafiqul, I.; Ali, M.P. Heavy metals contamination in vegetables and its growing soil. J. Environ. Anal. Chem. 2015, 2, 142. [Google Scholar] [CrossRef]
- van Geen, A.; Bravo, C.; Gil, V.; Sherpa, S.; Jack, D. Lead exposure from soil in Peruvian mining towns: A national assessment supported by two contrasting examples. Bull. World Health Organ. 2012, 90, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Barenys, M.; Boix, N.; Farran-Codina, A.; Palma-Linares, I.; Montserrat, R.; Curto, A.; Gomez-Catalan, J.; Ortiz, P.; Deza, N.; Llobet, J.M. Heavy metal and metalloids intake risk assessment in the diet of a rural population living near a gold mine in the Peruvian Andes (Cajamarca). Food Chem. Toxicol. 2014, 71, 254–263. [Google Scholar] [CrossRef]
- Jassir, M.; Shaker, A.; Khaliq, M.A. Deposition of heavy metals on green leafy vegetables sold on roadsides of Riyadh City, Saudi Arabia. Bull. Environ. Contam. Toxicol. 2005, 75, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yan, X.; Zeng, C.; Zhang, M.; Shrestha, S.; Devkota, L.P.; Yao, T. Influence of traffic activity on heavy metal concentrations of roadside farmland soil in mountainous areas. Int. J. Environ. Res. Public Health 2012, 9, 1715–1731. [Google Scholar] [CrossRef]
- Calderon, E.L.; Calderon, P.D. Concentración de Metales Pesados en Hortalizas que se Comercializan en el Consumo Modelo de Piura. Revista en Internet, Departamento Académico de Ingeniería Química, Universidad Nacional de Piura, Perú. 2002. Available online: http://www.unp.edu.pe/institutos/iipd/trabajosinvestigacion/NUEVOS%20TRABAJOS%20A%20PUBLICAR/MINAS/Trabajo%20de%20Investigacion-Esther%20calderon2.docx (accessed on 15 May 2021).
- Madueño, F.; García, M. Determinación de metales pesados (Plomo y Cadmio) en lechuga (Lactuca sativa) de mercados de Lima metropolitana. Cienc. Investig. 2018, 21, 19–23. [Google Scholar] [CrossRef]
- Calderon, E.L.; Concha, R. Evaluación de las Concentraciones de Metales Pesados Para Determinar la Calidad de frutas de Consumo Masivo en la Ciudad de Piura. Online Article, Departamento Académico de Ingeniería Química, Universidad Nacional de Piura, Peru. 2012. Available online: http://www.unp.edu.pe/institutos/iipd/trabajosinvestigacion/facultadminasquimica-esthercalderon.pdf (accessed on 15 May 2021).
- Custodio, M.; Peñaloza, R.; Orellana, E.; Aguilar-Cáceres, M.A.; Maldonado-Oré, E.M. Heavy metals and arsenic in soil and cereal grains and potential human risk in the central region of Peru. J. Ecol. Eng. 2021, 22, 206–220. [Google Scholar] [CrossRef]
- Zug, K.L.M.; Huamaní-Yupanqui, H.A.; Meyberg, F.; Cierjacks, J.S.; Cierjacks, A. Cadmium accumulation in Peruvian cacao (Theobroma cacao L.) and opportunities for mitigation. Water Air Soil Pollut. 2019, 230, 72. [Google Scholar] [CrossRef]
- Arévalo-Gardini, E.; Arévalo-Hernández, C.O.; Baligar, V.C.; He, Z.L. Heavy metal accumulation in leaves and beans of cacao (Theobroma cacao L.) in major cacao growing regions in Peru. Sci. Total Environ. 2017, 605–606, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Huamani, J.A.; Breña-Ore, J.L.; Sespedes-Varkarsel, S.; Huamanchumo de la Cuba, L.; Centeno-Rojas, L.; Otiniano-Zavala, A.; Andrade-Choque, J.; Valverde-Espinoza, S.; Castillo-Sequera, J.L. Study to determine levels of cadmium in cocoa crops applied to inland areas of Peru: “The case of the Campo Verde-Honoria Tournavista corridor”. Agronomy 2020, 10, 1576. [Google Scholar] [CrossRef]
- Soto-Benavente, M.; Rodriguez-Achata, L.; Olivera, M.; Arostegui-Sanchez, V.; Colina-Nano, C.; Garate-Quispe, J. Riesgos para la salud por metales pesados en productos agrícolas cultivados en áreas abandonadas por la minería aurífera en la Amazonía peruana. Sci. Agropecu. 2020, 11, 49–59. [Google Scholar] [CrossRef]
- De la O-Valenzuela, Z.O.; Quispe-Aguirre, J.; Cardenas-Orihuela, R.A. Determinación Cuantitativa de Plomo y Cadmio en Diez Tipos de Yuca (Manihot Esculenta) Comercializadas en el Mercado del Distrito de San Martin de Pangoa, de la Ciudad de Satipo, Departamento de Junin, de Enero a Marzo de 2017. Bachelor's Thesis, Facultad de Farmacia y Bioquimica, Universidad Wiener, Arequipa, Peru, 2017. Available online: http://repositorio.uwiener.edu.pe/handle/123456789/1187 (accessed on 15 March 2021).
- Quispe-Cruz, N.M.; Paredez de Gómez, T.B. Evaluacion del Riesgo de Toxicidad a Traves de Contaminantes en Cultivos Agricolas de Tallo Corto en la Cuenca Media Quilca-Vitor-Chili, Los Tunales Tiabaya. Bachelor's Thesis, Facultad Ingenieria de Procesos, Universidad Nacional de San Agustin de Arequipa, Peru. Available online: http://repositorio.unsa.edu.pe/handle/UNSA/10804 (accessed on 15 March 2021).
- Román-Ochoa, Y.; Delgado, G.; Tejada, T.; Yucra, H.; Durand, A.; Hamaker, B. Heavy metal contamination and health risk assessment in grains and grain-based processed food in Arequipa region of Peru. Chemosphere 2021, 274, 129792. [Google Scholar] [CrossRef]
- Juan de Dios-Miranda, M.K. Niveles de Arsénico y Cadmio en Muestras de Cebolla (Allium cepa) Expendidas en la Ciudad de Lima. Bachelor's Thesis, Facultad de Farmacia y Bioquimica, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2018. Available online: https://cybertesis.unmsm.edu.pe/handle/20.500.12672/8873 (accessed on 15 March 2021).
- López, D.L.; García, O.M.; Madueño, V.F.; Bautista, C.N.; Marín, V.G.; Olórtegui, C.D. Metales pesados en tres variedades de Solanum tuberosum L. (papa) expendidos en el mercado mayorista de Santa Anita (Lima-Peru). Cienc. Investig. 2020, 23, 25–30. [Google Scholar] [CrossRef]
- Luna-Arenas, R.N.; Rodríguez-Lozada, V.A.; Lizano-Gutiérrez, J.V. Determinación de las Concentraciones de Cadmio y Plomo en Papa (Solanum Tuberosum) Cosechada en las Cuencas de los Ríos Mashcón y Chonta–Cajamarca. Bachelor's Thesis, Facultad de Farmacia y Bioquimica, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2016. Available online: https://cybertesis.unmsm.edu.pe/handle/20.500.12672/4678 (accessed on 15 March 2021).
- Orellana, E.; Bastos, M.C.; Cuadrado, W.; Zárate, R.; Sarapura, V.; Yallico, L.; Tabra, F.; Bao, D. Heavy metals in native potato and health risk assessment in highland Andean zones of Junin, Peru. J. Environ. Prot. 2020, 11, 921–937. [Google Scholar] [CrossRef]
- Mondal, D.; Periche, R.; Tineo, B.; Bermejo, L.A.; Rahman, M.M.; Siddique, A.B.; Rahman, M.A.; Solis, J.L.; Cruz, G. Arsenic in Peruvian rice cultivated in the major rice growing region of Tumbes river basin. Chemosphere 2020, 241, 125070. [Google Scholar] [CrossRef]
- Aguilar, M.; Mondaca, P.; Ginocchio, R.; Vidal, K.; Sauvé, S.; Neaman, A. Comparison of exposure to trace elements through vegetable consumption between a mining area and an agricultural area in central Chile. Environ. Sci. Pollut. Res. Int. 2018, 25, 19114–19121. [Google Scholar] [CrossRef]
- Musilova, J.; Bystricka, J.; Vollmannova, A.; Janotova, B.; Orsak, M.; Harangozo, L.; Hegedusova, A. Safety of potato consumption in slovak region contaminated by heavy metals due to previous mining activity. J. Food Qual. 2017, 1–11. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, R.; Jin, T.; Chen, J.; Zhang, J. Risk assessment for potentially toxic metal(loid)s in potatoes in the indigenous zinc smelting area of northwestern Guizhou Province, China. Food Chem. Toxicol. 2018, 120, 328–339. [Google Scholar] [CrossRef]
- Servicio Nacional de Sanidad Agraria (SENASA). Plan Anual de Monitoreo de Residous Quimicos y Otros Contaminantes en Alimentos Aagropecuarios Primarios y Piensos Para el año. 2020. Available online: https://www.senasa.gob.pe/senasa/descargasarchivos/2020/08/PLAN-ANUAL-DE-MONITOREO-2020-27.07.pdf (accessed on 15 March 2021).
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Almela, L.; Rabe, V.; Sánchez, B.; Torrella, F.; López-Pérez, J.P.; Gabaldón, J.A.; Guardiola, L. Ochratoxin A in red paprika: Relationship with the origin of the raw material. Food Microbiol. 2007, 24, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, T.; Tsuchiya, Y.; Asai, T.; Okano, K.; Ito, N.; Endoh, K.; Yamamoto, M.; Nakamura, K. Ochratoxin A contamination of red chili peppers from Chile, Bolivia and Peru, Countries with a high incidence of gallbladder cancer. Asian Pac. J. Cancer Prev. 2015, 16, 5987–5991. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tsuchiya, Y.; Okano, K.; Piscoya, A.; Nishi, C.Y.; Ikoma, T.; Oyama, T.; Ikegami, K.; Yamamoto, M. Aflatoxin contamination of red chili pepper from Bolivia and Peru, countries with high gallbladder cancer incidence rates. Asian Pac. J. Cancer Prev. 2012, 13, 5167–5170. [Google Scholar] [CrossRef] [PubMed]
- Coloma, Z.N.; Oliveira, M.S.; Dilkin, P.; Mallmann, A.O.; Almeida, C.A.A.; Mallmann, C.A. Mycotoxin occurrence in Peruvian purple maize. World Mycotoxin J. 2019, 12, 307–315. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Rocha, A.; Sulyok, M.; Krska, R.; Mallmann, C.A. Natural mycotoxin contamination of maize (Zea mays L.) in the south region of Brazil. Food Control. 2017, 73, 127–132. [Google Scholar] [CrossRef]
- Ashiq, S. Natural occurrence of mycotoxins in food and feed: Pakistan perspective. Compr. Rev. Food Sci. Food Saf. 2015, 14, 159–175. [Google Scholar] [CrossRef]
- Soriano, M.; Bejar, V.; Bonilla, P. Frecuencia de hongos anemófilos productores de micotoxinas en algunos mercados de Lima. Detección de patulinas en manzanas en descomposición. Cienc. Investig. 2002, 5, 36–45. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Code of Practice for the Prevention and Reduction of Patulin Contamination in Apple Juice and Apple Juice Ingredients in Other Beverages; Food and Agriculture Organization: Rome, Italy, 2003; Volume 50, pp. 1–8. Available online: http://www.fao.org/input/download/standards/405/CXP_050e.pdf (accessed on 15 March 2021).
- García-Díaz, M.; Gil-Serna, J.; Vázquez, C.; Botia, M.N.; Patiño, B. A comprehensive study on the occurrence of mycotoxins and their producing fungi during the maize production cycle in Spain. Microorganisms 2020, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Granados-Chinchilla, F.; Molina, A.; Chavarría, G.; Alfaro-Cascante, M.; Bogantes-Ledezma, D.; Murillo-Williams, A. Aflatoxins occurrence through the foodchain in Costa Rica: Applying the one health approach to mycotoxin surveillance. Food Control. 2017, 82, 217–226. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Riego (MINAGRI). Contenidos Minimos del Programa Presupuestal: Aprovechamientos de Los Recursos Hidricos Para Uso Agrario. Available online: http://minagri.gob.pe/portal/download/programas-presupuestales/inf-programa/anexo2-pp42-2017.pdf (accessed on 15 March 2021).
- Ministerio de Agricultura y Riego (MINAGRI). Contaminacion del Agua. Available online: https://www.minagri.gob.pe/portal/54-sector-agrario/cuencas-e-hidrografia/374-problematica (accessed on 15 March 2021).
- Palm, B.; Kylic, H.; Chappa-Santa Maria, C. Pesticide Use in Rice Cultivation in Tarapoto, Peru. Usage Patterns and Pesticide Residues in Water Sources. Master’s Thesis, Department of Environmental Assessment, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2007. Available online: https://stud.epsilon.slu.se/12968/1/palm_b_171117.pdf (accessed on 15 March 2021).
- Bergmann, A.J.; North, P.E.; Vasquez, L.; Bello, H.; Gastañaga Ruiz, M.D.C.; Anderson, K.A. Multi-class chemical exposure in rural Peru using silicone wristbands. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 560–568. [Google Scholar] [CrossRef]
- Pacherres-Pinto, M.L.; Ramos-Gorbeña, J.C. Determinación de la Calidad de Agua de las Cuencas de Los Ríos Chillón, Rímac y Lurín Mediante Indicadores Químicos y Biológicos. Bachelor's Thesis, Facultad de Ciencias Biologicas, Universidad Ricardo Palma, Santiago de Surco, Peru, 2019. Available online: http://repositorio.urp.edu.pe/handle/URP/2838 (accessed on 15 March 2021).
- Custodio, M.; Álvarez, D.; Cuadrado, W.; Montalvo, R.; Ochoa, S. Potentially toxic metals and metalloids in surface water intended for human consumption and other uses in the Mantaro River watershed, Peru. Soil Water Res. 2020, 15, 237–245. [Google Scholar] [CrossRef]
- Quispe-Zuñiga, M.R.; Santos, F.; Callo-Concha, D.; Greve, K. Impact of heavy metals on community farming activities in the Central Peruvian Andes. Minerals 2019, 9, 647. [Google Scholar] [CrossRef]
- Huaranga-Moreno, F.; Méndez-García, E.; Quilcat-León, V.; Huaranga-Arévalo, F. Contaminacion por Metales Pesados en la Cuenca del rio Moche, 1980–2010, La Libertad-Peru. Sci. Agropecu. 2012, 3, 235–247. Available online: https://revistas.unitru.edu.pe/index.php/scientiaagrop/article/download/86/96 (accessed on 15 March 2021).
- Autoridad Nacional del Agua (ANA). Plan Maestro del Proyecto de Restauración del río Rímac: Informe Final. Available online: https://hdl.handle.net/20.500.12543/637 (accessed on 15 May 2021).
- Flores-Lozano, H.H.; Lara-Ascorbe, D. Evaluación de la Concentración de Metales Pesados en las Aguas del río Grande y Su Relación con la Actividad Minera. Master’s Thesis, Escuela de Posgrado, Universidad Nacional de Cajamarca, Cajamarca, Peru, 2016. Available online: http://repositorio.unc.edu.pe/handle/UNC/1299 (accessed on 15 March 2021).
- Sotero-Solis, V.; Alva-Astudillo, M. Contenido de metales pesados en agua y sedimento en el bajo Nanay. Cienc. Amaz. 2013, 3, 24–32. [Google Scholar] [CrossRef][Green Version]
- Salas-Urviola, F.B. Determinación de Metales Pesados en las Aguas del río Ananea Debido a la Actividad Minera Aurífera, Puno-Perú. Rev. Investig. Esc. Posgrado-Univ. Nac. Altiplano Puno-Perú 2014, 5, 47–53. Available online: http://revistas.unap.edu.pe/epg/index.php/investigaciones/article/view/14/11 (accessed on 15 March 2021).
- Garcia-Flores de Nieto, B.V.; Diaz-Rodriguez, J. Contaminación del Agua por Metales Pesados As, B, Cu, Pb, Cd y CN- en las Cuencas de los Ríos Tambo, Quilca, Camaná y Ocoña de la Región Arequipa. Ph.D. Thesis, Escuela de Posgrado, Universidad Nacional de San Agustin de Arequipa, Arequipa, Peru, 2019. Available online: http://repositorio.unsa.edu.pe/handle/UNSA/10764 (accessed on 15 March 2021).
- Peña-Quispe, G.; Yabarrena-Uscamayta, L.A. Estudio del Efecto de Contaminación de los Productos Agrícolas Irrigados con Aguas de la Subcuenca del rio Huatanay, Cusco–Perú. Bachelor's Thesis, Facultad de Ciencias Agrarias, 2019. Available online: http://repositorio.unsaac.edu.pe/handle/UNSAAC/4259 (accessed on 15 March 2021).
- Chung-Tong, B. La Minería Aurífera en el Perú y la Contaminación del Ambiente. Rev. Acad. Peru Salud 2011, 18. Available online: https://sisbib.unmsm.edu.pe/BVRevistas/rev_academia/2011_n2/pdf/a14v18n2.pdf (accessed on 15 March 2021).
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and unknowns on burden of disease due to chemicals: A systematic review. Environ. Health 2011, 10, 9. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Consorcio Agroecologico Peruano. Propuesta de Lista de Plaguicidas a ser Prohibidos o Restringidos. Available online: http://consorcioagroecologico.pe/wp-content/uploads/2020/08/LISTA-DE-PLAGUICIDAS-impreso-2019.pdf (accessed on 3 May 2021).
- Osorio, L.G.; Murray, D.; Rosenthal, E. Corporate impunity in Taucamarca: 19 years on, still no justice. Bus. Hum. Rights J. 2019, 4, 329–336. [Google Scholar] [CrossRef]
- El Comercio. Ayacucho: Revelan en Qué Alimentos se Halló Plaguicida Que Causó Intoxicación Masiva. Available online: https://elcomercio.pe/peru/ayacucho/ayacucho-revelan-producto-hallo-plaguicida-causo-intoxicacion-masiva-noticia-559012-noticia/ (accessed on 2 May 2021).
- Ministerio de Salud (MINSA). Sala de Situación de Salud: Vigilancia Epidemiológica del Riesgo de Exposición e Intoxicación por Plaguicidas. Available online: https://www.dge.gob.pe/portal/docs/vigilancia/sala/2019/SE29/plaguicidas.pdf (accessed on 15 March 2021).
- Cataño, H.C.; Carranza, E.; Huamaní, C.; Hernández, A.F. Plasma cholinesterase levels and health symptoms in Peruvian farm workers exposed to organophosphate pesticides. Arch. Environ. Contam. Toxicol. 2008, 55, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Montoro, Y.; Moreno, R.; Gomero, L.; Reyes, M. Características de uso de plaguicidas quimicos y riesgos para la salud en agricultores de la Sierra Central del Perú. Rev. Peru. Med. Exp. Salud Publica 2009, 26, 466–472. [Google Scholar]
- Yucra, S.; Steenland, K.; Chung, A.; Choque, F.; Gonzales, G.F. Dialkyl phosphate metabolites of organophosphorus in applicators of agricultural pesticides in Majes-Arequipa (Peru). J. Occup. Med. Toxicol. 2006, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud (MINSA). Vigilancia Epidemiologica Exposicion a Metales Pesados. Available online: http://www.dge.gob.pe/portal/docs/tools/teleconferencia/2019/SE082019/04.pdf (accessed on 15 March 2021).
- Castro-Bedriñana, J.; Chirinos-Peinado, D.; Ríos-Ríos, E. Niveles de plomo en gestantes y consumo en la ciudad de la Oroya, Perú. Rev. Peru. Med. Exp. Salud Publica 2013, 30, 393–398. [Google Scholar] [CrossRef]
- Borja-Aburto, V.; Hertz-Picciotto, I.; Lopez, M.; Farias, P.; Rios, C.; Blanco, J. Blood lead levels measured prospectively and risk of spontaneous abortion. Am. J. Epidemiol. 1999, 150, 590–597. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I. The evidence that lead increases the risk for spontaneous abortion. Am. J. Ind. Med. 2000, 38, 300–309. [Google Scholar] [CrossRef]
- Cederstav, A.; Barandiarán, A. La Oroya Cannot Wait. Sociedad Peruana de Derecho Ambiental. Available online: https://aida-americas.org/sites/default/files/publication/La_Oroya_Cannot_Wait_1_0.pdf (accessed on 15 March 2021).
- UNICEF. The Toxic Truth: Children’s Exposure to Lead Pollution Undermines a Generation of Future Potential. Available online: https://www.unicef.org/media/73246/file/The-toxic-truth-children%E2%80%99s-exposure-to-lead-pollution-2020.pdf (accessed on 30 May 2021).
- Vega-Dienstmaier, J.M.; Salinas-Piélago, J.E.; Gutiérrez-Campos, M.D.R.; Mandamiento-Ayquipa, R.D.; Yara-Hokama, M.D.C.; Ponce-Canchihuamán, J.; Castro-Morales, J. Lead levels and cognitive abilities in Peruvian children. Rev. Bras Psiquiatr. 2006, 28, 33–39. [Google Scholar] [CrossRef]
- Tchernitchin, A.N.; Lapin, N.; Molina, L.; Molina, G.; Tchernitchin, N.A.; Acevedo, C.; Alonso, P. Human exposure to lead in Chile. In Reviews of Environmental Contamination and Toxicology; George, W.W., Herbert, N.N., Daniel, R.D., Eds.; Springer: New York, NY, USA, 2006; pp. 93–139. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Terao, M.; Okano, K.; Nakamura, K.; Oyama, M.; Ikegami, K.; Yamamoto, M. Mutagenicity and mutagens of the red chili pepper as gallbladder cancer risk factor in Chilean women. Asian Pac. J. Cancer Prev. 2011, 12, 471–476. [Google Scholar]
- Costa, J.; Rodríguez, R.; Garcia-Cela, E.; Medina, A.; Magan, N.; Lima, N.; Battilani, P.; Santos, C. Overview of fungi and mycotoxin contamination in Capsicum pepper and in its derivatives. Toxins 2019, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Gao, Y.T.; Dean, M.; Egner, P.; Nepal, C.; Jones, K.; Wang, B.; Rashid, A.; Luo, W.; Van Dyke, A.L.; et al. Association of Aflatoxin and Gallbladder Cancer. Gastroenterology 2017, 153, 488–494.e1. [Google Scholar] [CrossRef]
- Eskola, M.; Elliott, C.T.; Hajšlová, J.; Steiner, D.; Krska, R. Towards a dietary-exposome assessment of chemicals in food: An update on the chronic health risks for the European consumer. Crit. Rev. Food Sci. Nutr. 2020, 60, 1890–1911. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Tanaka, J.H.; Tenorio-Mucha, J.; Villarreal-Zegarra, D.; Carrillo-Larco, R.; Bernabe-Ortiz, A. Cancer-related mortality in Peru: Trends from 2003 to 2016. PLoS ONE 2020, 15, e0228867. [Google Scholar] [CrossRef]
- Anyanwu, B.O.; Ezejiofor, A.N.; Igweze, Z.N.; Orisakwe, O.E. Heavy metal mixture exposure and effects in developing nations: An update. Toxics 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- OJEU. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve Thesustainable Use of Pesticides (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02009L0128-20190726&from=EN (accessed on 18 June 2021).
- Servicio Nacional de Sanidad Agraria (SENASA). Miles de Agricultores ya Aplican Control Biológico Para Manejo de Plagas. Available online: https://www.senasa.gob.pe/senasacontigo/miles-de-agricultores-ya-aplican-control-biologico-para-manejo-de-plagas/ (accessed on 15 March 2021).
- International Maize and Wheat Improvement Center. Training on Mycotoxins Supports Peruvian Maize Exports. Available online: https://www.cimmyt.org/news/training-on-mycotoxins-supports-peruvian-maize-exports/ (accessed on 15 March 2021).
- Garcia-Chevesich, P.; García, V.; Martínez, G.; Zea, J.; Ticona, J.; Alejo, F.; Vanneste, J.; Acker, S.; Vanzin, G.; Malone, A.; et al. Inexpensive organic materials and their applications towards heavy metal attenuation in waters from southern Peru. Water 2020, 12, 2948. [Google Scholar] [CrossRef]
- Memon, G.Z.; Bhanger, M.I.; Akhtar, M.; Talpur, F.N.; Memon, J.R. Adsorption of methyl parathion pesticide from water using watermelon peels as a low cost adsorbent. Chem. Eng. Sci. 2008, 138, 616–621. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, X.; Domene, X.; Alcañiz, J.; Liao, X.; Palet, C. Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci. Rep. 2019, 9, 9869. [Google Scholar] [CrossRef]
- Syed, J.H.; Alamdar, A.; Mohammad, A.; Ahad, K.; Shabir, Z.; Ahmed, H.; Ali, S.M.; Sani, S.G.; Bokhari, H.; Gallagher, K.D.; et al. Pesticide residues in fruits and vegetables from Pakistan: A review of the occurrence and associated human health risks. Environ. Sci. Pollut. Res. 2014, 21, 13367–13393. [Google Scholar] [CrossRef]
- Chung, S.W. How effective are common household preparations on removing pesticide residues from fruit and vegetables? A review. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Wang, H.; Yong, W.; Zhang, F.; Sun, L.; Yang, M.L.; Wu, Y.N.; Chu, X.G. The effects of washing and cooking on chlorpyrifos and its toxic metabolites in vegetables. Food Control. 2011, 22, 54–58. [Google Scholar] [CrossRef]
| Heavy Metal | Maximum Contaminant Limits | ||||
|---|---|---|---|---|---|
| Irrigation Water (mg/L) | Fruits (mg/kg) | Vegetables (mg/kg) | Grains (mg/kg) | ||
| WHO Standards | Peru Standards | ||||
| As | 0.1 | 0.1 | NE | 0.1 | 0.2 |
| Cd | 0.01 | 0.01 | 0.2 | 0.1, except for | 0.1 in cereals |
| 0.2 in leafy greens | 0.2 in rice | ||||
| and wheat | |||||
| Pb | 5 | 0.05 | 0.1 | 0.1, except for 0.3 in leafy greens, and 0.2 in pulses | 0.2 |
| Cr | 0.1 | 0.1 | NE * | ||
| Mn | 0.2 | 0.2 | 5 * | ||
| Zn | 2 | 2 | 100 * | ||
| Cu | 0.2 | 0.2 | 30 * | ||
| Hg | NE | 0.001 | 0.02 * | ||
| Region | Chemical Insecticides | Herbicides | Fungicides | |||
|---|---|---|---|---|---|---|
| Farms that Apply | Farms that Do Not Apply | Farms that Apply | Farms that Do Not Apply | Farms that Apply | Farms that Do Not Apply | |
| Coast | 67 | 33 | 55 | 45 | 52 | 48 |
| Sierra | 37 | 63 | 14 | 86 | 25 | 75 |
| Amazon | 16 | 84 | 29 | 71 | 14 | 86 |
| Produce | Pesticide Residue | Mean Concentration (mg/kg) | MRL 1 | Reference |
|---|---|---|---|---|
| Potatoes | Methamidophos | 2.7 | 0.05 | [27] |
| Mandarin | Thiabendazole | 5.1 | 5 | [30] |
| Tomato | Methamidophos | 1 | 1 | [28] |
| Peas | Fenhexamid | 0.16 | 0.05 | [30] |
| Table grapes | Methomyl | 0.12 | 0.05 |
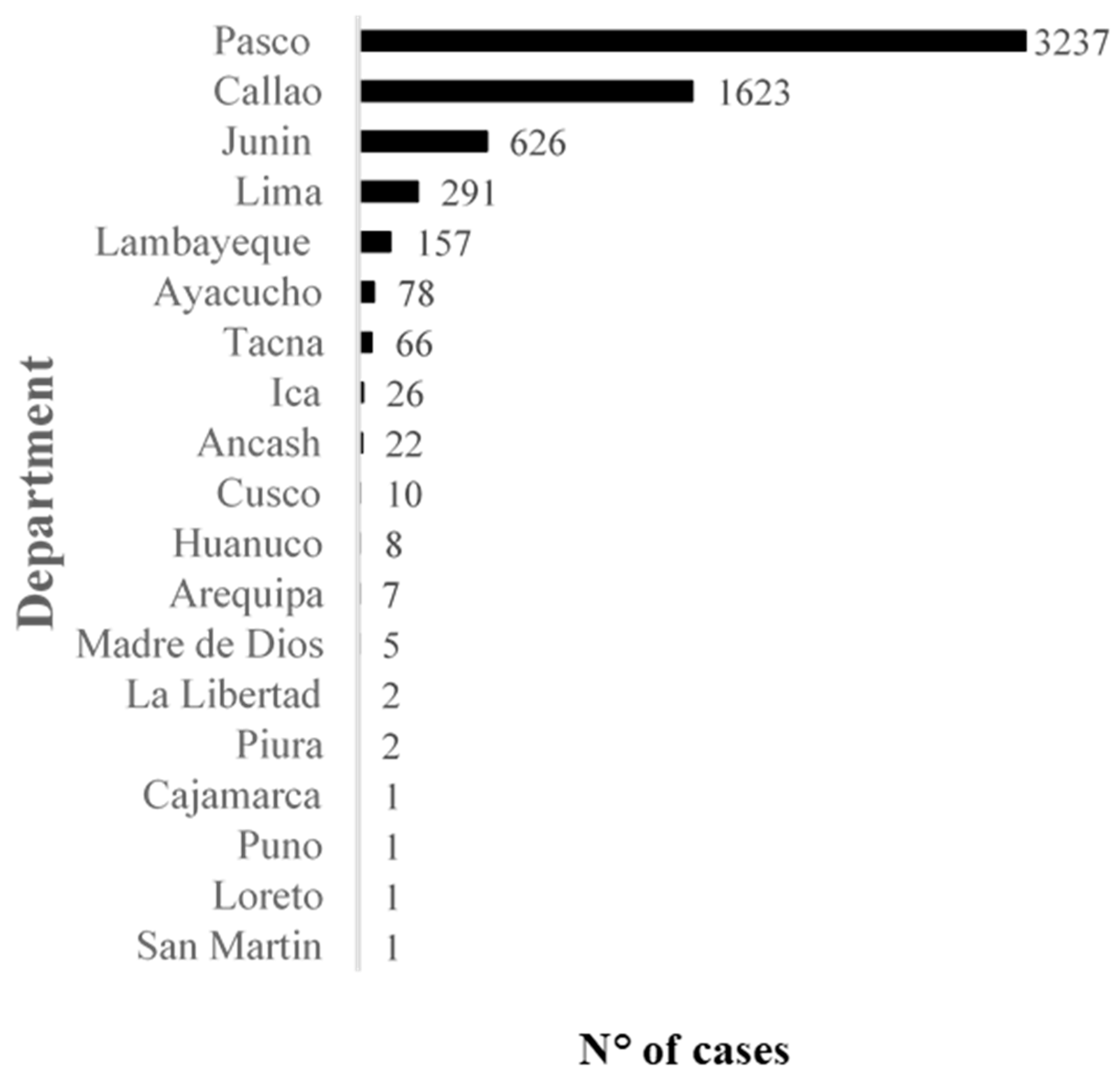
| Year | Total | N° of Noncompliant Samples (%) | Departments with >40% Noncompliant Samples | |
|---|---|---|---|---|
| Pesticide Residues | Mycotoxins 1 | |||
| 2011 | 252 | 73 (34.5) | 14/42 (33.3) | Cajamarca, Ica, Piura, Tacna. |
| 2012 | 725 | 193 (26.7) | 8/62 (12.9) | Tacna, La Libertad |
| 2013 | 705 | 175 (24.8) | 19/74 (25.7) | La Libertad, Tacna |
| 2014 | 756 | 228 (30.2) | 6/84 (7.14) | Arequipa |
| 2015 | 733 | 216 (29.5) | 11/84 (13.1) | Piura |
| 2016 | 738 | 199 (27.0) | 2/43 (4.65) | La Libertad |
| 2017 | 761 | 76 (10.0) | 6/77 (7.79) | None |
| 2018 | 793 | 105 (13.2) | 2/79 (2.53) | None |
| 2019 | 1779 | 373 (21) 2 | Amazonas, Arequipa, Apurimac, Ayacucho, Junin, Ica, La Libertad, Lambayeque, Lima, Piura, Tacna | |
| Produce | Mean Concentration (mg/kg) | Origin | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| Pb | Zn | Cu | Cd | As | Cr | |||
| Apple | 0.10 | 2.4 | 0.6 | BDL | 0.05 | NM | Lima | [56] |
| Avocado | 0.18 a | 2.9 | 0.32 | BDL | BDL | NM | Morropon, Chulucanas | [54] |
| Barley | BDL | 60 | 19 | NM | 0.14 | NM | Junin | [57] |
| Cabbage | 9.3 a | 1.1 | BDL | BDL | BDL | NM | Trujillo | [54] |
| Cacao bean | NM | NM | NM | 2.5 | NM | NM | Huanuco | [58] |
| 2.2 a | 52 | 30 | 0.97 | NM | 4.83 | Amazonas | [59] | |
| 1.0 | 44 | 26 | 0.77 | NM | 1.0 | Cajamarca | ||
| 1.0 | 40 | 19 | 0.17 | NM | BDL | Cuzco | ||
| 1.5 a | 37 | 25 | 0.64 | NM | 1.0 | Huanuco | ||
| 2.7 a | 47 | 28 | 0.41 | NM | BDL | Junin | ||
| 3.8 a | 59 | 27 | 1.6 | NM | 1.0 | Piura | ||
| 1.7 a | 44 | 27 | 0.79 | NM | 1.0 | San Martin | ||
| 2.8 a | 52 | 26 | 1.8 | NM | 1.0 | Tumbes | ||
| 1.7 a | 74 | NM | 0.96 | NM | NM | Ucayali, Huanuco | [60] | |
| Carrot | 17 a | 6.6 | BDL | BDL | BDL | NM | Trujillo | [54] |
| Cassava root | 5.8 a | NM | NM | 0.039 | 3.0 a | NM | Hualgayoc | [61] |
| 0.13 a | NM | NM | 0.05 | NM | NM | Junin | [62] | |
| Cauliflower | 4.4 a | 2.4 | BDL | BDL | BDL | NM | Trujillo | [54] |
| Chili pepper | 11 a | 3.0 | 0.32 | BDL | BDL | NM | Trujillo | [54] |
| Cilantro | 3.2 a | 4.0 | 0.89 | BDL | 2.050 a | NM | Trujillo | [54] |
| 0.050 | NM | NM | 0.025 | 0.05 | 0.031 | Arequipa | [63] | |
| Corn | BDL | 37 | 2.1 | NM | 0.0785 | NM | Junin | [57] |
| Green pea | 2.0 a | 5.5 | 1.7 | BDL | BDL | NM | Trujillo | [54] |
| Lettuce | 4.2 a | 2.5 | 0.63 | BDL | 0.900 a | NM | Trujillo | [54] |
| 2.1 a | NM | NM | 0.1 | NM | NM | Sierra | [55] | |
| 0.37 a | NM | NM | 0.066 | NM | NM | Costa | ||
| Lime | 0.12 a | 1.5 | BDL | 0.065 | BDL | NM | Trujillo | [56] |
| Mango | 0.087 | 1.5 | BDL | BDL | BDL | NM | Chulucanas | [56] |
| Corn | 0.010 | NM | NM | BDL | 0.030 | NM | Arequipa | [64] |
| Onion | 1.5 a | 0.70 | 0.23 | 0.023 | BDL | NM | Trujillo | [54] |
| NM | NM | NM | 0.060 a | 0.042 | NM | Arequipa | [65] | |
| NM | NM | NM | 0.040 | 0.040 | NM | Trujillo, Huaral | [65] | |
| Orange | 0.10 | 2.4 | BDL | BDL | BDL | NM | Sullana, Lima | [56] |
| Papaya | 0.090 | 2.0 | 0.63 | BDL | 0.20 | NM | Sullana | [56] |
| Plantain | 0.090 | 1.0 | BDL | BDL | BDL | NM | Chulucanas | [56] |
| Plum | 0.20 a | 2.5 | 1.1 | 0.023 | BDL | NM | Piura | [56] |
| Potato | 0.10 | NM | NM | 0.029 | 0.00040 | NM | Lima | [66] |
| BDL | NM | NM | 0.31 a | NM | NM | Cajamarca | [67] | |
| 0.41 a | 18 | NM | 0.042 | 0.13 | NM | Junin | [68] | |
| Quinoa | 0.040 | NM | NM | 0.020 | 0.060 | NM | Arequipa | [64] |
| Rice | 0.086 | 13 | 4.0 | 0.33 | >0.20 a | 0.151 | Tumbes | [69] |
| 0.040 | NM | NM | 0.11 | 0.17 | NM | Arequipa | [64] | |
| Strawberry | 0.20 a | 3.5 | 0.30 | BDL | BDL | NM | Trujillo | [56] |
| Tomato | 1.9 a | 1.4 | 1.1 | 0.065 | BDL | NM | Trujillo | [54] |
| Turnip | 5.3 a | 2.7 | 0.59 | BDL | BDL | NM | Trujillo | [54] |
| Watermelon | 0.090 | 0.70 | BDL | BDL | BDL | NM | Trujillo | [56] |
| Source | Location | Heavy-Metal Levels | Reference |
|---|---|---|---|
| Tumbes River | Tumbes | 0.016 mg/L As | [69] |
| Cunas sub-basin | Junin | 0.60 mg/L As a 19 mg/L Mn a 0.023 mg/L Cd a 12 mg/L Zn a | [91] |
| Aimaraes sub-basin | Junin | 0.010 mg/L Cd 51 mg/L Fe 2.1 mg/L Mn a | |
| (Irrigation canals of) Mantaro River | Junin | 0.18 mg/L As a | [90] |
| Rimac River | Lima | 0.47 mg/L As a 0.029 mg/L Cd a 21 mg/L Mn a | [93] |
| 0.27 mg/L Pb a 0.016 mg/L Cd a 0.38 mg/L Cu a | [89] | ||
| Chillon River | Callao | 0.15 mg/L Pb a 0.012 mg/L Cd a 0.31 mg/L Cu a | [89] |
| Lurin River | Lima | 0.076 mg/L Pb a 0.018 mg/L Cd a 0.18 mg/L Cu | [89] |
| Grande River | Cajamarca | 0.25 mg/L Pb a | [94] |
| Nanay River | Loreto | 0.11 mg/L Pb a | [95] |
| Ananea River | Puno | 0.16 mg/L Cd a 6.3 mg/L As a 1.4 mg/L Pb a 0.99 mg/L Cr a | [96] |
| Tambo River | Arequipa | 0.20 mg/L As a | [97] |
| Quilca River | 0.11 mg/L Pb a | ||
| Camana River | 0.050 mg/L Pb | ||
| Huatanay River | Cusco | 0.0012 mg/L Pb 0.0036 mg/L As 0.0010 mg/L Cr | [98] |
| Caychihue River | Madre de Dios | 0.20 mg/L Pb a 0.20 mg/L As a | [99] |
| Moche River | La Libertad | 0.0050–0.91 mg/L Pb a 0.0010–0.11 mg/L A sa 0.0010–0.20 mg/L Cd a 0.045–1.2 mg/L Cu a | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galagarza, O.A.; Ramirez-Hernandez, A.; Oliver, H.F.; Álvarez Rodríguez, M.V.; Valdez Ortiz, M.d.C.; Pachari Vera, E.; Cereceda, Y.; Diaz-Valencia, Y.K.; Deering, A.J. Occurrence of Chemical Contaminants in Peruvian Produce: A Food-Safety Perspective. Foods 2021, 10, 1461. https://doi.org/10.3390/foods10071461
Galagarza OA, Ramirez-Hernandez A, Oliver HF, Álvarez Rodríguez MV, Valdez Ortiz MdC, Pachari Vera E, Cereceda Y, Diaz-Valencia YK, Deering AJ. Occurrence of Chemical Contaminants in Peruvian Produce: A Food-Safety Perspective. Foods. 2021; 10(7):1461. https://doi.org/10.3390/foods10071461
Chicago/Turabian StyleGalagarza, Oscar A., Alejandra Ramirez-Hernandez, Haley F. Oliver, Mariel V. Álvarez Rodríguez, María del Carmen Valdez Ortiz, Erika Pachari Vera, Yakelin Cereceda, Yemina K. Diaz-Valencia, and Amanda J. Deering. 2021. "Occurrence of Chemical Contaminants in Peruvian Produce: A Food-Safety Perspective" Foods 10, no. 7: 1461. https://doi.org/10.3390/foods10071461
APA StyleGalagarza, O. A., Ramirez-Hernandez, A., Oliver, H. F., Álvarez Rodríguez, M. V., Valdez Ortiz, M. d. C., Pachari Vera, E., Cereceda, Y., Diaz-Valencia, Y. K., & Deering, A. J. (2021). Occurrence of Chemical Contaminants in Peruvian Produce: A Food-Safety Perspective. Foods, 10(7), 1461. https://doi.org/10.3390/foods10071461