Effect of Ginger on Chemical Composition, Physical and Sensory Characteristics of Chicken Soup
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Soup
2.3. Physical Characterization of Soup Properties
2.3.1. Zeta Potential and Particle Size Estimation
2.3.2. Rheological Behavior and Dynamic Oscillatory Rheological Measurements
2.3.3. Color Parameters Analysis
2.4. Total Sugar Analysis
2.5. Free Amino Acids Analysis
2.6. Organic Acids Analysis
2.7. 5′-Nucleotides Analysis
2.8. Equivalent Umami Concentration
2.9. Mineral Analysis
2.10. Electronic Tongue
2.11. Statistical Analysis
3. Results and Discussion
3.1. Color Analysis, Particle Size, and Zeta Potential
3.2. Viscosity Analysis
3.3. Total Sugar Analysis
3.4. Free Amino Acids Analysis
3.5. Organic Acids Analysis
3.6. Nucleotides Analysis
3.7. Equivalent Umami Concentration
3.8. Mineral Analysis
3.9. Electronic Tongue Analysis
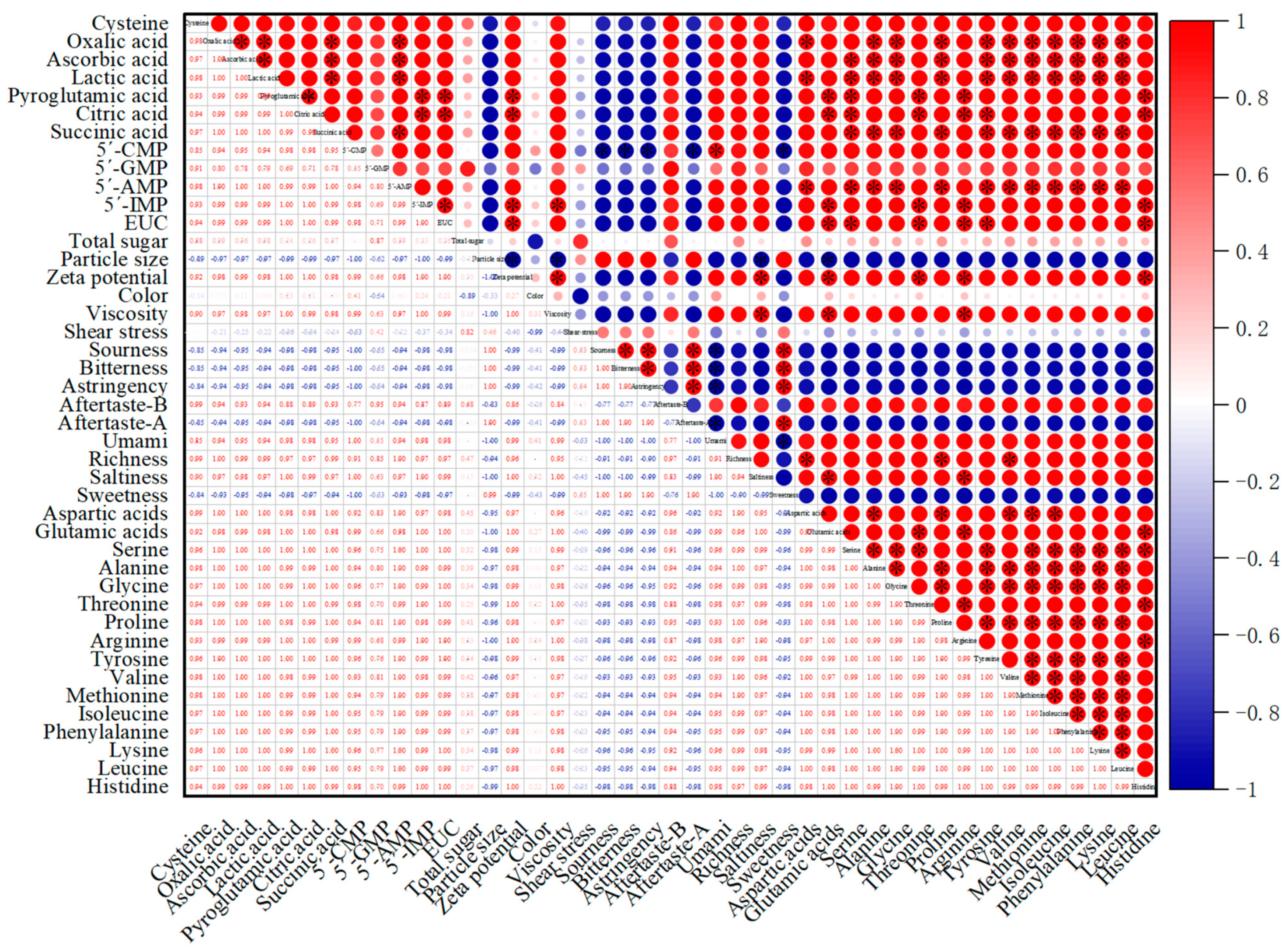
3.10. Correlation Analysis
4. Conclusions
Supplementary Materials
 represents ginger soup,
represents ginger soup,  represents chicken soup, and
represents chicken soup, and  represents ginger chicken soup. Table S1: The color of different soups. Table S2: EUC values of different soups.
represents ginger chicken soup. Table S1: The color of different soups. Table S2: EUC values of different soups.Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishimura, T.; Goto, S.; Miura, K.; Takakura, Y.; Egusa, A.S.; Wakabayashi, H. Umami compounds enhance the intensity of retronasal sensation of aromas from model chicken soups. Food Chem. 2016, 196, 577–583. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Lin, D.; Gao, G.; Wang, H.; Guo, J.; Ping, R.; Ke, L. Boiling-induced nanoparticles and their constitutive proteins from Isatis indigotica Fort. root decoction: Purification and identification. J. Tradit. Complement. Med. 2017, 7, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Ke, L.; Zhou, J.; Lu, W.; Gao, G.; Rao, P. The power of soups: Super-hero or team-work? Trends Food Sci. Technol. 2011, 22, 492–497. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, W.; Yang, H.; Yan, Z.; Ge, C.; Liao, G.; Su, H. 1H NMR-based water-soluble lower molecule characterization and fatty acid composition of Chinese native chickens and commercial broiler. Food Res. Int. 2021, 140, 110008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jin, C.; Wang, X.; Yan, H.; Tan, H.; Gao, C. Dietary supplementation with pioglitazone hydrochloride and L-carnosine improves the growth performance, muscle fatty acid profiles and shelf life of yellow-feathered broiler chickens. Anim. Nutr. 2020, 7, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Hayat, K.; Cui, H.; Hussain, S.; Ho, C.; Zhang, X. Characterization of flavor active non-volatile compounds in chicken broth and correlated contributing constituent compounds in muscle through sensory evaluation and partial least square regression analysis. LWT Food Sci. Technol. 2020, 118, 108768. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, X.; Ding, Q.; Zhang, Y.; Sun, B.; Chen, H.; Sun, Y. Comparison of non-volatile umami components in chicken soup and chicken enzymatic hydrolysate. Food Res. Int. 2017, 102, 559–566. [Google Scholar] [CrossRef]
- Zou, J.; Xu, M.; Zou, Y.; Yang, B. Chemical compositions and sensory characteristics of pork rib and Silkie chicken soups prepared by various cooking techniques. Food Chem. 2021, 345, 128755. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L.; Bode, O. Maillard reaction products as encapsulants for fish oil powders. J. Food Sci. 2006, 71, 25–32. [Google Scholar] [CrossRef]
- Abdullahi, A.; Khairulmazmi, A.; Yasmeen, S.; Ismail, I.S.; Norhayu, A.; Sulaiman, M.R.; Ahmed, O.H.; Ismail, M.R. Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arab J. Chem. 2020, 13, 8012–8025. [Google Scholar] [CrossRef]
- Yan, H.; Li, P.; Zhou, G.; Wang, Y.; Bao, B.; Wu, Q.; Huang, S. Rapid and practical qualitative and quantitative evaluation of non-fumigated ginger and sulfur-fumigated ginger via Fourier-transform infrared spectroscopy and chemometric methods. Food Chem. 2021, 341, 128241. [Google Scholar] [CrossRef] [PubMed]
- Muhialdin, B.J.; Kadum, H.; Fathallah, S.; Hussin, A.S.M. Metabolomics profiling and antibacterial activity of fermented ginger paste extends the shelf life of chicken meat. LWT Food Sci. Technol. 2020, 132, 109897. [Google Scholar] [CrossRef]
- Fan, M.; Xiao, Q.; Xie, J.; Cheng, J.; Sun, B.; Du, W.; Wang, Y.; Wang, T. Aroma compounds in chicken broths of Beijing Youji and commercial broilers. J. Agric. Food Chem. 2018, 66, 10242–10251. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.; Wu, N.; Wu, F.; Yu, D.; Elfalleh, W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2020, 98, 105306. [Google Scholar] [CrossRef]
- Zhu, Y.; Bhandari, B.; Prakash, S. Relating the tribo-rheological properties of chocolate flavoured milk to temporal aspects of texture. Int. Dairy J. 2020, 110, 104794. [Google Scholar] [CrossRef]
- Sugumar, J.K.; Guha, P. Study on the formulation and optimization of functional soup mix of Solanum nigrum leaves. Int. J. Gastron. Food Sci. 2020, 20, 100208. [Google Scholar] [CrossRef]
- Rover, M.R.; Johnston, P.A.; Lamsal, B.P.; Brown, R.C. Total water-soluble sugars quantification in bio-oil using the phenol–sulfuric acid assay. J. Anal. Appl. Pyrol. 2013, 104, 194–201. [Google Scholar] [CrossRef]
- Duan, W.; Wang, L.; Zhang, Y.; Huang, Y.; Xiao, J.; Pu, D. Optimization of the cooking methods of stir-fried beef by instrumental analysis. J. Food Process. Preserv. 2020, 44, e14425. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Huang, Y.; Xiao, J.; Zhang, Y.; Zhang, H. Comparison of nonvolatile taste components in 18 strong fragrance spices. Int. J. Food Prop. 2020, 23, 340–353. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Huang, Y.; Xiao, J.; Zhang, Y.; Tang, Y. Determination of free amino acids, organic acids, and nucleotides in 29 elegant spices. Food Sci. Nutr. 2020, 8, 3777–3792. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Gong, X.; Xu, H.; Xin, G. Evaluation of umami taste components of mushroom (Suillus granulatus) of different grades prepared by different drying methods. Food Sci. Hum. Well 2020, 9, 192–198. [Google Scholar] [CrossRef]
- Wang, A.C.; Lu, R.; Xie, L. Improved algorithm for estimating the optical properties of food products using spatially-resolved diffuse reflectance. J. Food Eng. 2017, 212, 1–11. [Google Scholar] [CrossRef]
- Qi, J.; Wang, H.; Zhang, W.; Deng, S.; Zhou, G.; Xu, X. Identification and characterization of the proteins in broth of stewed traditional Chinese yellow-feathered chickens. Poult. Sci. 2018, 97, 1852–1860. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Fu, X.; Zheng, L.; Xiao, Z.; Zhao, M. Comparison of aroma-active compounds in broiler broth and native chicken broth by aroma extract dilution analysis (AEDA), odor activity value (OAV) and omission experiment. Food Chem. 2018, 265, 274–280. [Google Scholar] [CrossRef]
- Ravindran, G.; Matia-Merino, L. Starch–fenugreek (Trigonella foenum-graecum L.) polysaccharide interactions in pure and soup systems. Food Hydrocoll. 2009, 23, 1047–1053. [Google Scholar] [CrossRef]
- Li, Y.; Fan, D.; Zhao, Y.; Wang, M. Effects of quercetin and cinnamaldehyde on the nutrient release from beef into soup during stewing process. LWT Food Sci. Technol. 2020, 131, 109712. [Google Scholar] [CrossRef]
- Verheyen, D.; Altin, O.; Skipnes, D.; Erdogdu, F.; Skara, T.; Van Impe, J.F. Thermal inactivation of Listeria monocytogenes in the Shaka agitated reciprocal retort: Influence of food matrix rheology and fat content. Food Bioprod. Process. 2020, 125, 22–36. [Google Scholar] [CrossRef]
- Boehm, M.W.; Warren, F.J.; Baier, S.K.; Gidley, M.J.; Stokes, J.R. A method for developing structure-rheology relationships in comminuted plant-based food and non-ideal soft particle suspensions. Food Hydrocoll. 2019, 96, 475–480. [Google Scholar] [CrossRef]
- Zhu, D.; Shen, Y.; Wei, L.; Xu, L.; Cao, X.; Liu, H.; Li, J. Effect of particle size on the stability and flavor of cloudy apple juice. Food Chem. 2020, 328, 126967. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Terashima, M.; Hozan, D.; Shirai, K. Preparation and dynamic viscoelasticity characterization of alkali-solubilized collagen from shark skin. J. Agric. Food Chem. 2000, 48, 685–690. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Pimentel, T.C.; Freitas, M.Q.; Moraes, J.; Fernandes, L.M.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; Borges, F.O.; et al. Chocolate milk drink processed by cold plasma technology: Physical characteristics, thermal behavior and microstructure. LWT Food Sci. Technol. 2019, 102, 324–329. [Google Scholar] [CrossRef]
- Costa, M.F.; Pimentel, T.C.; Guimaraes, J.T.; Balthazar, C.F.; Rocha, R.S.; Cavalcanti, R.N.; Esmerino, E.A.; Freitas, M.Q.; Raices, R.S.L.; Silva, M.C.; et al. Impact of prebiotics on the rheological characteristics and volatile compounds of Greek yogurt. LWT Food Sci. Technol. 2019, 105, 371–376. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.; Zou, Z.; Shang, X.; Wang, H.; Bansal, N. Glycosylated fish gelatin emulsion: Rheological, tribological properties and its application as model coffee creamers. Food Hydrocoll. 2019, 102, 105552. [Google Scholar] [CrossRef]
- Pal, R. Effect of droplet size on the rheology of emulsions. AIChE J. 1996, 42, 3181–3190. [Google Scholar]
- Qian, X.; Fan, X.; Su, H.; Zhang, J.; Tao, N.; Zhong, J.; Wang, X.; Han, B. Migration of lipid and other components and formation of micro/nano-sized colloidal structure in Tuna (Thunnus obesus) head soup. LWT Food Sci. Technol. 2019, 111, 69–76. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, W.; Xiao, J.; Liu, H.; Zhou, C.; Zhang, Y.; Tang, Y.; Sun, B.; Li, Z. Characterization of the taste compounds in 20 pungent spices by high-performance liquid chromatography. J. Food Meas Charact. 2021, 15, 1680–1692. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Y.; Yu, H.; Zhang, H.; Yu, Z.; Ding, L.; Liu, J.; Li, J.; Chen, F. The enrichment and characterization of ginger-derived glycoprotein using magnetic particles. Food Chem. 2018, 244, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Duan, W.; Huang, Y.; Zhang, Y.; Sun, B.; Pu, D.; Tang, Y.; Liu, C. Sensory taste properties of chicken (Hy-Line brown) soup as prepared with five different parts of the chicken. Int. J. Food Prop. 2020, 23, 1804–1824. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, W.; Wang, L.; Xiao, J.; Zhang, Y. Orthogonal optimization of beef stir-fried process followed by isolation and identification of the umami peptides by consecutive chromatography and LC-Q-TOF/MS. Int. J. Food Prop. 2020, 22, 1773–1785. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, K.; Ding, Q.; Zhang, Y.; Sun, B.; Chen, H. Effects of two cooking methods on the taste components of Sanhuang chicken and Black-bone silky fowl meat. J. Food Process. Preserv. 2018, 42, e13772. [Google Scholar] [CrossRef]
- Zou, Y.H.; Kang, D.; Liu, R.; Qi, J.; Zhou, G.; Zhang, W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultraso Sonochem. 2018, 46, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.D.; Yen, C.T.; Mau, J.L. Non-volatile taste components of various broth cubes. Food Chem. 2007, 101, 932–937. [Google Scholar] [CrossRef]
- Trinta, V.D.; Padilha, P.D.; Petronilho, S.; Santelli, R.E.; Braz, B.F.; Freire, A.S.; Saunders, C.; da Rocha, H.F.; Sanz-Medel, A.; Fernandez-Sanchez, M.L. Total metal content and chemical speciation analysis of iron, copper, zinc and iodine in human breast milk using high-performance liquid chromatography separation and inductively coupled plasma mass spectrometry detection. Food Chem. 2020, 326, 126978. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors-a systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Goudoulas, T.B.; Germann, N. Phase transition kinetics and rheology of gelatin-alginate mixtures. Food Hydrocoll. 2017, 66, 49–60. [Google Scholar] [CrossRef]
- Pu, D.; Duan, W.; Huang, Y.; Zhang, L.; Zhang, Y.; Sun, B.; Ren, F.; Zhang, H.Y.; Chen, H.; He, J.; et al. Characterization of the dynamic texture perception and the impact factors on the bolus texture changes during oral processing. Food Chem. 2021, 339, 128078. [Google Scholar] [CrossRef]
 represent ginger soup,
represent ginger soup,  represent chicken soup,
represent chicken soup,  represent ginger chicken soup.
represent ginger chicken soup.
 represent ginger soup,
represent ginger soup,  represent chicken soup,
represent chicken soup,  represent ginger chicken soup.
represent ginger chicken soup.
| Samples | Particle Size (nm) | Zeta Potential (mV) | Total Sugar (mg/mL) |
|---|---|---|---|
| Ginger soup | 969.87 ± 100.02 b | −20.80 ± 1.80 a | 49.98 ± 0.00 b |
| Chicken soup | 150.53 ± 1.91 a | −6.00 ± 1.28 b | 36.97 ± 0.25 a |
| Ginger chicken soup | 136.43 ± 1.80 a | −4.70 ± 1.16 b | 70.58 ± 2.66 c |
| Compounds | Contents (μg/mL) | |||
|---|---|---|---|---|
| Ginger Soup | Chicken Soup | Ginger Chicken Soup | ||
| Free amino acids | ||||
| Umami | Asp (Aspartic acid) | 3.86 ± 1.35 a | 34.88 ± 2.47 b | 46.23 ± 0.23 c |
| Glu (Glutamic acid) | n.d. | 112.90 ± 0.36 a | 122.74 ± 0.35 b | |
| Total | 3.86 ± 1.35 a | 147.77 ± 2.83 b | 168.97 ± 0.58 c | |
| Sweetness | Ser (Serine) | 4.85 ± 0.02 a | 50.17 ± 0.96 b | 60.60 ± 0.36 c |
| Ala (Alanine) | 1.83 ± 0.01 a | 60.38 ± 0.18 b | 82.29 ± 0.12 c | |
| Gly (Glycine) | n.d. | 49.04 ± 0.47 a | 63.05 ± 0.29 b | |
| Thr (Threonine) | n.d. | 33.27 ± 0.20 a | 38.33 ± 0.13 b | |
| Pro (Proline) | n.d. | 25.90 ± 0.23 a | 37.58 ± 0.51 b | |
| Total | 6.68 ± 0.02 a | 218.77 ± 2.03 b | 281.85 ± 1.41 c | |
| Bitterness | Arg (Arginine) | n.d. | 52.92 ± 1.15 a | 58.39 ± 0.18 b |
| Tyr (Tyrosine) | n.d. | 18.99 ± 0.04 a | 24.42 ± 0.00 b | |
| Val (Valine) | n.d. | 25.81 ± 1.32 a | 34.83 ± 0.09 b | |
| Met (Methionine) | 9.14 ± 0.22 a | 47.35 ± 3.31 b | 59.19 ± 2.41 c | |
| Ile (Isoleucine) | n.d. | 17.67 ± 0.05 a | 24.06 ± 0.04 b | |
| Phe (Phenylalanine) | n.d. | 25.81 ± 1.19 a | 33.06 ± 0.37 b | |
| Lys (Lysine) | n.d. | 36.85 ± 0.41 a | 48.53 ± 0.22 b | |
| Leu (Leucine) | n.d. | 32.69 ± 0.48 a | 44.15 ± 0.59 b | |
| His (Histidine) | n.d. | 23.68 ± 0.08 a | 27.71 ± 0.21 b | |
| Total | 9.14 ± 0.22 a | 281.76 ± 8.03 b | 354.34 ± 4.10 c | |
| Tasteless | Cys-Cys (Cysteine) | n.d. | 4.94 ± 0.25 a | 8.81 ± 0.05 b |
| Total free amino acids | 19.68 ± 1.59 a | 653.24 ± 13.14 b | 813.97 ± 6.14 c | |
| Organic acids | ||||
| Oxalic acid | 5.92 ± 0.03 a | 105.68 ± 1.93 b | 144.35 ± 5.32 c | |
| Ascorbic acid | n.d. | 35.83 ± 0.91 a | 47.57 ± 0.87 b | |
| Lactic acid | n.d. | 1112.98 ± 18.36 a | 1523.58 ± 33.13 b | |
| Pyroglutamic acid | n.d. | 156.58 ± 3.34 a | 179.40 ± 3.13 b | |
| Citric acid | 7.72 ± 0.24 a | 3999.96 ± 85.46 b | 4692.41 ± 80.44 c | |
| Succinic acid | n.d. | 689.40 ± 13.24 a | 927.30 ± 17.77 b | |
| Total organic acids | 13.64 ± 0.27 a | 6100.43 ± 123.24 b | 7514.61 ± 140.66 c | |
| Nucleotides | ||||
| 5′-CMP | 16.62 ± 2.06 a | 80.47 ± 2.27 b | 76.27 ± 7.84 b | |
| 5′-GMP | 28.75 ± 1.00 a | 39.25 ± 0.53 b | 42.38 ± 2.82 b | |
| 5′-AMP | 10.44 ± 0.97 a | 36.76 ± 1.97 b | 40.14 ± 8.34 b | |
| 5′-IMP | 15.81 ± 0.73 a | 20.50 ± 0.16 b | 21.52 ± 0.54 b | |
| Total nucleotides | 71.62 ± 4.76 a | 176.98 ± 4.93 b | 180.31 ± 19.54 b | |
| Elements | Contents (mg/L) | ||
|---|---|---|---|
| Ginger Soup | Chicken Soup | Ginger Chicken Soup | |
| Ca | 18.39 ± 0.24 a | 88.50 ± 1.22 c | 66.83 ± 0.29 b |
| Cr | 0.27 ± 0.00 a | 0.84 ± 0.01 c | 0.78 ± 0.02 b |
| Cu | n.d. | 0.11 ± 0.00 a | n.d. |
| Fe | 0.94 ± 0.00 a | 2.06 ± 0.02 b | n.d. |
| K | 184 ± 2.20 a | 1082.50 ± 13.23 c | 818.33 ± 2.89 b |
| Mg | 49.45 ± 0.09 a | 37.04 ± 0.11 b | 28.90 ± 0.09 c |
| Na | 99.63 ± 2.78 a | 393.38 ± 6.17 c | 290.67 ± 2.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, W.; Liang, L.; Huang, Y.; Zhang, Y.; Sun, B.; Li, L. Effect of Ginger on Chemical Composition, Physical and Sensory Characteristics of Chicken Soup. Foods 2021, 10, 1456. https://doi.org/10.3390/foods10071456
Duan W, Liang L, Huang Y, Zhang Y, Sun B, Li L. Effect of Ginger on Chemical Composition, Physical and Sensory Characteristics of Chicken Soup. Foods. 2021; 10(7):1456. https://doi.org/10.3390/foods10071456
Chicago/Turabian StyleDuan, Wen, Li Liang, Yan Huang, Yuyu Zhang, Baoguo Sun, and Lina Li. 2021. "Effect of Ginger on Chemical Composition, Physical and Sensory Characteristics of Chicken Soup" Foods 10, no. 7: 1456. https://doi.org/10.3390/foods10071456
APA StyleDuan, W., Liang, L., Huang, Y., Zhang, Y., Sun, B., & Li, L. (2021). Effect of Ginger on Chemical Composition, Physical and Sensory Characteristics of Chicken Soup. Foods, 10(7), 1456. https://doi.org/10.3390/foods10071456






