Investigation of the Effectiveness of Disinfectants Used in Meat-Processing Facilities to Control Clostridium sporogenes and Clostridioides difficile Spores
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Harvesting of Spores
2.1.1. Clostridium Sporogenes
2.1.2. Clostridioides Difficile
2.2. Preparation of Disinfectant Agents
2.3. Suspension Tests
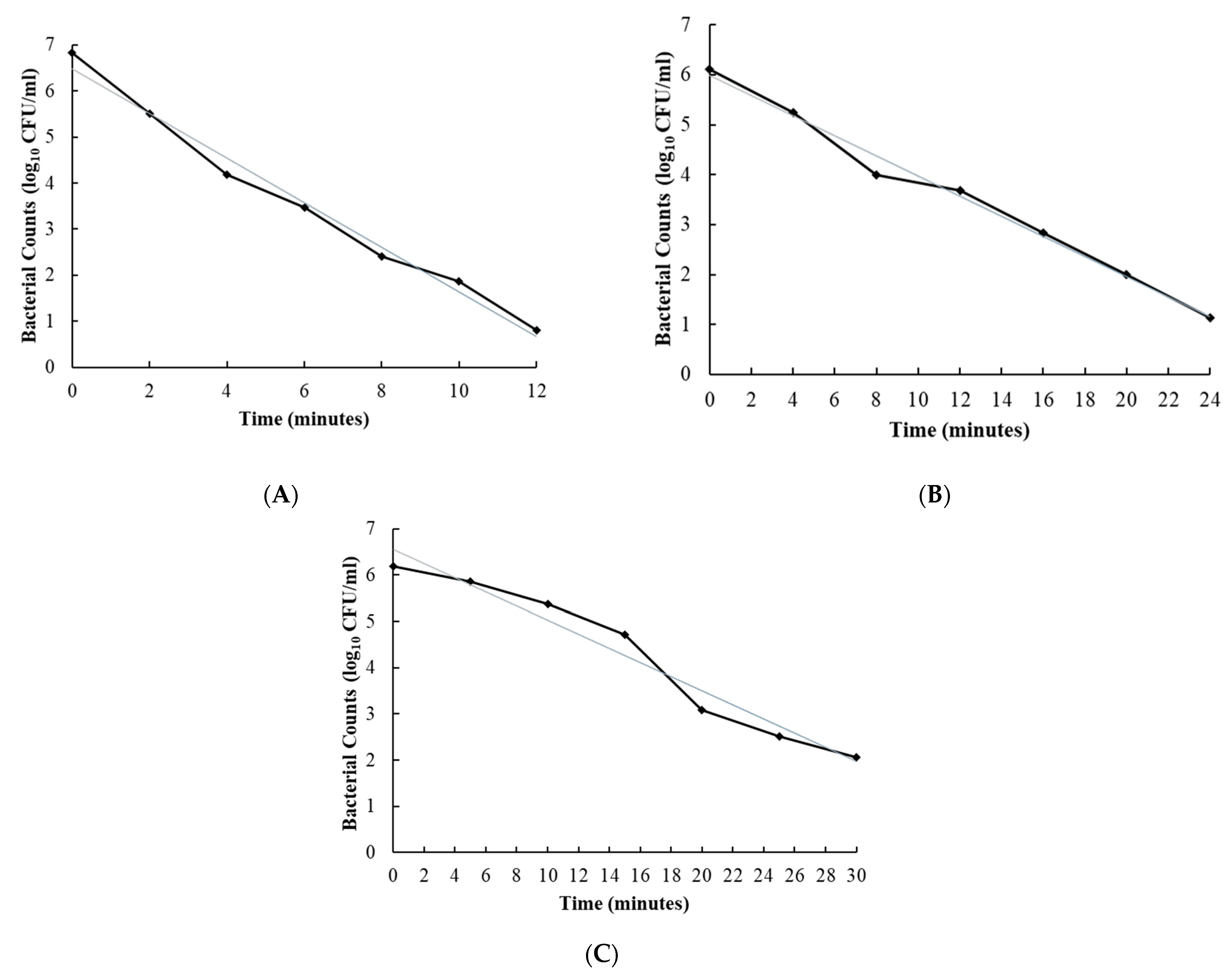
2.4. Calculating Decimal Reduction Times (D-Values) for Most Effective Disinfectants
2.5. Microbial Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvat, G.; Colin, P. Cleaning and disinfection practice in the meat industries of Europe. Rev. Sci. Tech. 1995, 14, 313–341. [Google Scholar] [CrossRef] [PubMed]
- Wirtanen, G.; Salo, S. Disinfection in Food Processing—Efficacy Testing of Disinfectants. Rev. Environ. Sci. Biotechnol. 2003, 2, 293–306. [Google Scholar] [CrossRef]
- Van Asselt, A.; Giffel, M.T. Pathogen resistance and adaptation to disinfectants and sanitisers. In Understanding Pathogen Behaviour; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 484–506. [Google Scholar]
- Sansebastiano, G.; Zoni, R.; Bigliardi, L. Cleaning and Disinfection Procedures in the Food Industry General Aspects and Practical Applications. In Food Safety; McElhatton, A., Marshall, R.J., Eds.; Springer: Boston, MA, USA, 2007; pp. 253–280. [Google Scholar] [CrossRef]
- Penna, T.C.V.; Mazzola, P.G.; Martins, A.M.S. The efficacy of chemical agents in cleaning and disinfection programs. BMC Infect. Dis. 2001, 1, 16. [Google Scholar] [CrossRef][Green Version]
- Rodriguez, C.; Avesani, V.; Van Broeck, J.; Taminiau, B.; Delmée, M.; Daube, G. Presence of Clostridium difficile in pigs and cattle intestinal contents and carcass contamination at the slaughterhouse in Belgium. Int. J. Food Microbiol. 2013, 166, 256–262. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Public Health Risk Associated with Botulism as Foodborne Zoonoses. Toxins 2020, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Bolton, D.J.; Doherty, A.M.; Sheridan, J.J. Beef HACCP: Intervention and non-intervention systems. Int. J. Food Microbiol. 2001, 66, 119–129. [Google Scholar] [CrossRef]
- Wang, S.; Brunt, J.; Peck, M.; Setlow, P.; Li, Y.-Q. Analysis of the Germination of Individual Clostridium sporogenes Spores with and without Germinant Receptors and Cortex-Lytic Enzymes. Front. Microbiol. 2017, 8, 2047. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Sorg, J.A.; Sun, X. Clostridioides difficile Biology: Sporulation, Germination, and Corresponding Therapies for C. difficile Infection. Front. Cell. Infect. Microbiol. 2018, 8, 29. [Google Scholar] [CrossRef]
- Schill, K.M.; Wang, Y.; Butler, R.R.; Pombert, J.-F.; Reddy, N.R.; Skinner, G.E.; Larkin, J.W. Genetic Diversity of Clostridium sporogenes PA 3679 Isolates Obtained from Different Sources as Resolved by Pulsed-Field Gel Electrophoresis and High-Throughput Sequencing. Appl. Environ. Microbiol. 2016, 82, 384–393. [Google Scholar] [CrossRef][Green Version]
- Peck, M.W. Biology and Genomic Analysis of Clostridium botulinum. Adv. Microb. Physiol. 2009, 55, 183–320. [Google Scholar] [CrossRef]
- Brown, J.L.; Tran-Dinh, N.; Chapman, B. Clostridium sporogenes PA 3679 and Its Uses in the Derivation of Thermal Processing Schedules for Low-Acid Shelf-Stable Foods and as a Research Model for Proteolytic Clostridium botulinum. J. Food Prot. 2012, 75, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Dunn, M.; Ogden, L.; Jefferies, L.; Eggett, D.; Steele, F. Conditions associated with Clostridium sporogenes growth as a surrogate for Clostridium botulinum in nonthermally processed canned butter. J. Dairy Sci. 2013, 96, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Weigand, M.R.; Pena-Gonzalez, A.; Shirey, T.B.; Broeker, R.G.; Ishaq, M.K.; Konstantinidis, K.T.; Raphael, B.H. Implications of genome-based discrimination between Clostridium botulinum group I and Clostridium sporogenes strains for bacterial tax-onomy. Appl. Environ. Microbiol. 2015, 81, 5420–5429. [Google Scholar] [CrossRef]
- Lawson, P.A.; Citron, D.M.; Tyrrell, K.L.; Finegold, S.M. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe 2016, 40, 95–99. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Notification of changes in taxonomic opinion previously published outside the IJSEM. Int. J. Syst. Evol. Microbiol. 2016, 66, 2469–2470. [Google Scholar] [CrossRef] [PubMed]
- Lessa, F.C.; Gould, C.V.; McDonald, L.C. Current Status of Clostridium difficile Infection Epidemiology. Clin. Infect. Dis. 2012, 55, S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Warriner, K.; Xu, C.; Habash, M.; Sultan, S.; Weese, S. Dissemination of Clostridium difficilein food and the environment: Significant sources ofC. difficilecommunity-acquired infection? J. Appl. Microbiol. 2016, 122, 542–553. [Google Scholar] [CrossRef]
- Weese, J.S.; Avery, B.P.; Rousseau, J.; Reid-Smith, R.J. Detection and Enumeration of Clostridium difficile Spores in Retail Beef and Pork. Appl. Environ. Microbiol. 2009, 75, 5009–5011. [Google Scholar] [CrossRef]
- de Boer, E.; Zwartkruis-Nahuis, A.; Heuvelink, A.E.; Harmanus, C.; Kuijper, E. Prevalence of Clostridium difficile in retailed meat in The Netherlands. Int. J. Food Microbiol. 2011, 144, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, Z.; Weese, S.; Ezzatpanah, H.; Jalali, M.; Chamani, M. Occurrence of Clostridium difficile in seasoned hamburgers and seven processing plants in Iran. BMC Microbiol. 2014, 14, 283. [Google Scholar] [CrossRef]
- Bouttier, S.; Barc, M.-C.; Félix, B.; Lambert, S.; Collignon, A.; Barbut, F. Clostridium difficilein Ground Meat, France. Emerg. Infect. Dis. 2010, 16, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Bacterial Spores and Chemical Sporicidal Agents. Clin. Microbiol. Rev. 1990, 3, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Omidbakhsh, N. Evaluation of sporicidal activities of selected environmental surface disinfectants: Carrier tests with the spores of Clostridium difficile and its surrogates. Am. J. Infect. Control. 2010, 38, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Casadei, M.; Ingram, R.; Skinner, R.; Gaze, J. Heat resistance of Paenibacillus polymyxa in relation to pH and acidulants. J. Appl. Microbiol. 2000, 89, 801–806. [Google Scholar] [CrossRef]
- Schaeffer, A.B.; Fulton, M.D. A simplified method of staining endospores. Science 1933, 77, 194. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, S.; Blanco, J.L.; Astorga, R.J.; Gómez-Laguna, J.; Barrero-Domínguez, B.; Galán-Relaño, A.; Harmanus, C.; Kuijper, E.; García, M.E. Distribution and tracking of Clostridium difficile and Clostridium perfringens in a free-range pig abattoir and processing plant. Food Res. Int. 2018, 113, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.A.; Heap, J.T.; Minton, N.P. The diverse sporulation characteristics of Clostridium difficile clinical isolates are not associated with type. Anaerobe 2010, 16, 618–622. [Google Scholar] [CrossRef]
- McSharry, S.; Koolman, L.; Whyte, P.; Bolton, D. An investigation of the survival and/or growth of Clostridioides (Clostridium) difficile in beef stored under aerobic, anaerobic and commercial vacuum packaging conditions at 2 °C and 20 °C. Food Control. 2021, 119, 107475. [Google Scholar] [CrossRef]
- Rutala, W.A.; Gergen, M.F.; Weber, D.J. Sporicidal Activity of Chemical Sterilants Used in Hospitals. Infect. Control. Hosp. Epidemiol. 1993, 14, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Block, S.S. Peroxygen compounds. In Disinfection, Sterilisation and Preservation; Block, S.S., Ed.; Lippincott Williams & Wil-kins: Philadelphia, PA, USA, 2001; pp. 185–204. [Google Scholar]
- Rutala, W.A.; Weber, D.J.; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities; Centers for Disease Control: Atlanta, GA, USA, 2008; pp. 1–163. [Google Scholar]
- Leggett, M.J.; Schwarz, J.S.; Burke, P.A.; McDonnell, G.; Denyer, S.P.; Maillard, J.-Y. Mechanism of Sporicidal Activity for the Synergistic Combination of Peracetic Acid and Hydrogen Peroxide. Appl. Environ. Microbiol. 2016, 82, 1035–1039. [Google Scholar] [CrossRef]
- Dvorak, G. Disinfection; Center for Food Security and Public Health, Iowa State University: Ames, IA, USA, 2005; pp. 1–22. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.361.3783&rep=rep1&type=pdf (accessed on 8 April 2021).
- Sattar, S.A.; Springthorpe, V.S.; Rochon, M. A product based on accelerated & stabilized hydrogen peroxide: Evidence for broad-spectrum germicidal activity. Can. J. Infect. Control 1998, 13, 123–130. [Google Scholar]
- Wardle, M.D.; Renninger, G.M. Bactericidal effect of hydrogen peroxide on spacecraft isolates. Appl. Microbiol. 1975, 30, 710–711. [Google Scholar] [CrossRef]
- Bayliss, C.E.; Waites, W.M. The Effect of Hydrogen Peroxide on Spores of Clostridium bifermentans. J. Gen. Microbiol. 1976, 96, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Baldry, M. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J. Appl. Bacteriol. 1983, 54, 417–423. [Google Scholar] [CrossRef]
- Baldry, M.G.C.; Fraser, J.A.L. Disinfection with peroxygens. In Industrial Biocides: Critical Reports on Applied Chemistry; Payne, K.R., Ed.; John Wiley & Sons: Chichester, UK, 1988; Volume 23, pp. 91–116. [Google Scholar]
- Schoeb, T.R.; Rahija, R.J. Gnotobiotics. In American College of Laboratory Animal Medicine, Laboratory Animal Medicine; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; Academic Press: Cambridge, MA, USA, 2015; Chapter 26; pp. 1263–1296. [Google Scholar] [CrossRef]
- Sagripanti, J.-L.; Bonifacino, A. Comparative sporicidal effect of liquid chemical germicides on three medical devices contaminated with spores of Bacillus subtilis. Am. J. Infect. Control 1996, 24, 364–371. [Google Scholar] [CrossRef]
- Vizcaino-Alcaide, M.J.; Herruzo-Cabrera, R.; Fernandez-Aceñero, M.J. Comparison of the disinfectant efficacy of Perasafe® and 2% glutaraldehyde in in vitro tests. J. Hosp. Infect. 2003, 53, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Blakistone, B.; Chuyate, R.; Kautter, D.; Charbonneau, J.; Suit, K. Efficacy of Oxonia Active Against Selected Spore Formers†. J. Food Prot. 1999, 62, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Srinivasan, S.; Holton, J.; Ridgway, G. Evaluation of microbicidal activity of a new disinfectant: Sterilox® 2500 against Clostridium difficile spores, Helicobacter pylori, vancomycin resistant Enterococcus species, Candida albicans and several Mycobacterium species. J. Hosp. Infect. 1999, 41, 101–105. [Google Scholar] [CrossRef]
- Dawson, L.F.; Valiente, E.; Donahue, E.H.; Birchenough, G.; Wren, B.W. Hypervirulent Clostridium difficile PCR-Ribotypes Exhibit Resistance to Widely Used Disinfectants. PLoS ONE 2011, 6, e25754. [Google Scholar] [CrossRef]
- Cho, W.-I.; Chung, M.-S. Sporicidal activities and mechanism of surfactant components against Clostridium sporogenes spores. J. Food Sci. Technol. 2018, 55, 4675–4680. [Google Scholar] [CrossRef]
- Cortesia, C.; Vilchèze, C.; Bernut, A.; Contreras, W.; Gómez, K.; de Waard, J.; Jacobs, W.R.; Kremer, L.; Takiff, H. Acetic Acid, the Active Component of Vinegar, Is an Effective Tuberculocidal Disinfectant. mBio 2014, 5, e00013-14. [Google Scholar] [CrossRef] [PubMed]
- Kunanusont, N.; Punyadarsaniya, D.; Jantafong, T.; Pojprasath, T.; Takehara, K.; Ruenphet, S. Bactericidal efficacy of potassium peroxymonosulfate under various concentrations, organic material conditions, exposure timing and its application on various surface carriers. J. Vet. Med. Sci. 2020, 82, 320–324. [Google Scholar] [CrossRef]
- Bolder, N.M. The Efficacy of Virkon S against 5 Salmonella Serovars, Campylobacter Jejuni and Staphylococcus Aureus (MRSA); Report 09/CV10353/NBO/sn; Central Veterinary Institute of Wagenningen UR: Lelystad, The Netherlands, 2009; pp. 1–10. Available online: https://virkons.dk/wp-content/uploads/2017/09/2009-Virkon-S-Wageningen-EN-1656-salmonella-campylobacter-MRSA-NO.227.pdf (accessed on 16 February 2021).
- Rashid, T.; Haghighi, F.; Hasan, I.; Bassères, E.; Alam, M.J.; Sharma, S.V.; Lai, D.; DuPont, H.L.; Garey, K.W. Activity of Hospital Disinfectants against Vegetative Cells and Spores of Clostridioides difficile Embedded in Biofilms. Antimicrob. Agents Chemother. 2019, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kopper, G.; Mirecki, S.; Kljujev, I.S.; Raičević, V.B.; Lalevic, B.T.; Jovičić-Petrović, J.; Stojanovski, S.; Blazekovic-Dimovska, D. Hygiene in Primary Production. In Food Safety Management; Motarjemi, Y., Lelieveld, H., Eds.; Academic Press: Cambridge, MA, USA, 2014; Chapter 23; pp. 559–621. [Google Scholar] [CrossRef]
- Van Eijk, H.; Majoor, F. Cleaning Procedures in the Factory: Types of Detergents. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 1379–1381. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Sodium Dichloroisocyanurate in Drinking-Water. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/sodium_dichloroisocyanurate_2add_feb2008.pdf (accessed on 10 April 2021).
- Massicotte, R.; Mbeh, D.A.; Mafu, A.A.; Toulouse, M.; Jacobs, D.; Yahia, L.; Pichette, G. Disinfection effect of sodium di-chloroisocyanurate (NaDCC) on various surfaces in medical facilities using different techniques. Interdiscip. J. Chem. 2019, 3, 1–5. [Google Scholar] [CrossRef]
- Heling, I.; Rotstein, I.; Dinur, T.; Szwec-Levine, Y.; Steinberg, D. Bactericidal and Cytotoxic Effects of Sodium Hypochlorite and Sodium Dichloroisocyanurate Solutions In Vitro. J. Endod. 2001, 27, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, P.G.; Penna, T.C.V.; Martins, A.M.D.S. Determination of decimal reduction time (D value) of chemical agents used in hospitals for disinfection purposes. BMC Infect. Dis. 2003, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Tennen, R.; Setlow, B.; Davis, K.; Loshon, C.; Setlow, P. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J. Appl. Microbiol. 2000, 89, 330–338. [Google Scholar] [CrossRef] [PubMed]
- March, J.K.; Pratt, M.D.; Lowe, C.; Cohen, M.N.; Satterfield, B.A.; Schaalje, B.; O’Neill, K.L.; Robison, R.A. The differential effects of heat-shocking on the viability of spores from Bacillus anthracis, Bacillus subtilis, and Clostridium sporogenes after treatment with peracetic acid- and glutaraldehyde-based disinfectants. Microbiologyopen 2015, 4, 764–773. [Google Scholar] [CrossRef]
- Pereira, B.M.P.; Tagkopoulos, I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019, 85, 1–13. [Google Scholar] [CrossRef]
- Marple, B.; Roland, P.; Benninger, M. Safety Review of Benzalkonium Chloride Used as a Preservative in Intranasal Solutions: An Overview of Conflicting Data and Opinions. Otolaryngol. Neck Surg. 2004, 130, 131–141. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlostathis, S. The role of quaternary ammonium compounds on antimicrobial resistance in the environment. In Antimicrobial Resistance in the Environment; Keen, P.L., Montforts, M.H.M.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 349–386. [Google Scholar]
- Condell, O.; Iversen, C.; Cooney, S.; Power, K.A.; Walsh, C.; Burgess, C.; Fanning, S. Efficacy of Biocides Used in the Modern Food Industry to Control Salmonella enterica, and Links between Biocide Tolerance and Resistance to Clinically Relevant Antimicrobial Compounds. Appl. Environ. Microbiol. 2012, 78, 3087–3097. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Girotti, N. Guidelines for Using Sodium Hypochlorite as a disinfectant for Biological Waste; City-Wide Oc-cupational Health & Safety and Workplace Health; Western University: London, ON, Canada, 2015; Available online: https://www.uwo.ca/hr/form_doc/health_safety/doc/procedures/bleach_sop.pdf (accessed on 12 April 2021).
- Vargová, M.; Laktičová, K.; Hromada, R.; Cimboláková, I.; Uher, I.; Papajová, I.; Peter, K. Sanitation and the Environment. Environmental Factors Affecting Human Health. Intechopen 2020. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Operational Guidelines Cleaning and Disinfection. Available online: http://www.dem.ri.gov/topics/erp/nahems_cleaning_and_disinfection.pdf (accessed on 7 April 2021).
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of Disinfectant Quaternary Ammonium Compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-H.; Chung, Y.-H. Effects of Didecyldimethylammonium Chloride on Sprague-Dawley Rats after Two Weeks of Inhalation Exposure. Toxicol. Res. 2014, 30, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wei, Q.; Tie, J.; Wang, C.; Rao, L.; Zhang, W. Synergistic sporicidal effect of ethanol on a combination of orthophthalaldehyde and Didecyldimethylammonium chloride. Lett. Appl. Microbiol. 2014, 59, 272–277. [Google Scholar] [CrossRef]
- Azanza, P.V. Hydrogen peroxide, Peroxyacetic acid, Octanoic acid, Peroxyoctanoic acid, and 1- hydroxyethyli-dene-1,1-Diphosphonic acid (HEDP) as components of antimicrobial washing solution. Chemical Technical Assessment. FAO (63rd JECFA). 2004, pp. 1–7. Available online: https://fao.org/fileadmin/templates/agns/pdf/jecfa/cta/63/antimicrobials.pdf (accessed on 13 March 2021).
- HERA. Human & Environmental Risk Assessment on ingredients of European Household Cleaning Products Alcohol Ethox-ysulphates (AES) Environmental Risk Assessment. 2004, pp. 1–36. Available online: https://www.heraproject.com/files/1-E-04-HERA%20AES%20ENV%20%20web%20wd.pdf (accessed on 31 March 2021).
- Man, V.F.; Magnuson, J.P.; Lentsch, S.E. Medium Chain Perosycarboxylic Acid Compositions. U.S. Patent 9,888,684, 13 February 2017. Available online: https://patentimages.storage.googleapis.com/50/22/94/3263e9ce6cecd8/US10568322.pdf (accessed on 13 March 2021).
- Friman, M.J.; Eklund, M.H.; Pitkälä, A.H.; Rajala-Schultz, P.J.; Rantala, M.H.J. Description of two Serratia marcescens associated mastitis outbreaks in Finnish dairy farms and a review of literature. Acta Vet. Scand. 2019, 61, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.J. Formulation of Carpet Cleaners. In Handbook for Cleaning/Decontamination of Surfaces; Elsevier Science: Amsterdam, The Netherlands, 2007; Volume 1, pp. 103–123. [Google Scholar] [CrossRef]
- Charcosset, C. Principles on membrane and membrane processes. In Membrane Processes in Biotechnology and Pharmaceutics; Elsevier: Amsterdam, The Netherlands, 2012; Chapter 1; pp. 1–41. [Google Scholar] [CrossRef]
- European Communities. EUR 21315 EN, European Union Risk Assessment Report: Tetrasodium Ethylenediaminetetraace-Tate (Na4EDTA). Available online: https://echa.europa.eu/documents/10162/415c121b-12cd-40a2-bd56-812c57c303ce (accessed on 18 March 2021).
- Maris, P. Modes of action of disinfectants. Rev. Sci. Tech. 1995, 14, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Vipond, I.; Bloomfield, S. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J. Hosp. Infect. 2004, 58, 42–49. [Google Scholar] [CrossRef]
- Wheeldon, L.; Worthington, T.; Hilton, A.; Lambert, P.; Elliott, T. Sporicidal activity of two disinfectants against Clostridium difficile spores. Br. J. Nurs. 2008, 17, 316–320. [Google Scholar] [CrossRef]
- Pottage, T.; Richardson, C.; Parks, S.; Walker, J.; Bennett, A. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J. Hosp. Infect. 2010, 74, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Speight, S.; Moy, A.; Macken, S.; Chitnis, R.; Hoffman, P.; Davies, A.; Bennett, A.; Walker, J. Evaluation of the sporicidal activity of different chemical disinfectants used in hospitals against Clostridium difficile. J. Hosp. Infect. 2011, 79, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Leggett, M.J.; McDonnell, G.; Denyer, S.; Setlow, S.; Maillard, J.-Y. Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 2012, 113, 485–498. [Google Scholar] [CrossRef]
- Barra-Carrasco, J.; Paredes-Sabja, D. Clostridium difficile spores: A major threat to the hospital environment. Futur. Microbiol. 2014, 9, 475–486. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Guidance on the Biocidal Products Regulation Volume I: Identity of the Active Sub-Stance/Physioco-Chemical Properties/Analytical Methodology—Information Requirements, Evaluation and Assessment. Parts A+B+C, Ver 2.0; European Chemicals Agency: Helsinki, Finland, 2018. [Google Scholar] [CrossRef]


| Disinfectant | Chemical Composition 1 | Recommended Concentration |
|---|---|---|
| 1 | N-(3-Aminopropyl)-N-Dodecylpropane-1,3-Diamine (1–10%), Alcohols, C9-11, Ethoxylated (1–10%), Tetrasodium Ethylene Diamine Tetraacetate (1–10%), Propan-2-OL (1–10%) | 1% |
| 2 | Hydrogen Peroxide (10–30%), Acetic acid (1–10%), peracetic acid (1–10%) | 10% |
| 3 | Hydrogen peroxide (≥ 25 ≤ 30%), Acetic acid (≥ 5 ≤ 10%), peracetic acid (≥ 2.5 ≤ 5%) | 2% |
| 4 | Acetic acid (10 ≤ 25%), Hydrogen Peroxide (5 ≤ 8%), Alkylethersulphates (<10%), Peracetic acid (1 ≤ 5%), Octanoic acid (1 ≤ 5%), Peroxyoctanoic acid (<1%) | 1.10% |
| 5 | Sodium hypochlorite (≥ 5.2 ≤ 10%), Sodium hydroxide (≥ 5 ≤ 10%), Alkylamine oxide (≥ 3 ≤ 5 %) | 5% |
| 6 | Ethylenediamine tetraacetate (≥ 10 ≤ 20%), Benzalkonium chloride (≥ 5 ≤ 10%), Isotridecanol, ethoxylated (≥ 1 ≤ 2.5%), Didecyl Dimethyl Ammonium Chloride (≥ 1 ≤ 2.5%), sodium hydroxide (≥ 0.25 ≤ 0.5%), propan-2-ol (≥ 0.1 ≤ 0.25%) | 1% |
| 7 | Peroxymonosulphate (30–50%), Sulphamic Acid (1–10%), Troclosene Sodium (1–10%) | 4% |
| 8 | Alkyl dimethyl benzyl ammonium chloride (15–30%), Didecyldimethylammonium chloride (5–15%), Glutaraldehyde (5–15%), Propan-2-ol (5–15%) | 1% |
| 9 | Alcohols, C9-11, Ethoxylated (10–30%), Orthophosphoric acid (10–30%), Sulphuric acid (1–10%), Iodine (1–10%) | 2% |
| 10 | Glutaraldehyde (10–30%), Benzalkonium Chloride (1–10%) | 2% |
| C. sporogenes Spores | C. difficile Spores | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDW | RCM Broth | SDW | BHI Broth | |||||||||||||
| 20 Min | 60 Min | 20 Min | 60 Min | 20 Min | 60 Min | 20 Min | 60 Min | |||||||||
| C 1 | SE 2 | C | SE | C | SE | C | SE | C | SE | C | SE | C | SE | C | SE | |
| Control 3 | 7.0 a | 0.10 | 7.0 a | 0.10 | 6.2 a | 0.25 | 6.2 a | 0.25 | 6.4 a | 0.07 | 6.4 a | 0.07 | 6.0 a | 0.05 | 6.0 a | 0.05 |
| 1 | 2.3 ce | 0.13 | 2.4 cd | 0.30 | 2.5 cd | 0.10 | 2.2 cd | 0.08 | 5.3 ab | 0.08 | 5.3 ab | 0.12 | 4.7 ab | 0.34 | 4.6 bc | 0.40 |
| 2 | 0.5 e | 0.43 | 0.1 d | 0.00 | 0.6 f | 0.28 | 0.2 e | 0.10 | 0.1 e | 0.00 | 0.1 e | 0.00 | 1.1 e | 0.14 | 0.1 d | 0.00 |
| 3 | 0.7 e | 0.68 | 0.5 d | 0.41 | 4.8 ab | 0.32 | 3.3 bc | 0.14 | 4.1 bc | 0.27 | 3.6 c | 0.45 | 5.9 a | 0.16 | 5.8 ab | 0.23 |
| 4 | 0.6 e | 0.55 | 0.3 d | 0.24 | 3.9 bc | 0.23 | 3.1 c | 0.81 | 0.5 e | 0.28 | 0.3 e | 0.16 | 5.8 a | 0.22 | 5.9 ab | 0.18 |
| 5 | 0.5 e | 0.34 | 0.6 cd | 0.33 | 6.1 a | 0.41 | 5.9 a | 0.37 | 0.1 e | 0.00 | 0.1 e | 0.00 | 6.0 a | 0.17 | 5.9 ab | 0.24 |
| 6 | 2.5 ce | 0.69 | 2.0 cd | 0.45 | 1.4 df | 0.33 | 0.6 de | 0.39 | 3.2 cd | 0.64 | 3.9 c | 0.15 | 4.4 bc | 0.22 | 4.1 c | 0.43 |
| 7 | 1.2 e | 0.81 | 0.4 d | 0.38 | 1.7 dfe | 0.21 | 0.1 e | 0.00 | 0.2 e | 0.10 | 0.1 e | 0.00 | 2.4 de | 0.21 | 0.2 d | 0.10 |
| 8 | 4.9 ab | 0.40 | 5.0 ab | 0.37 | 4.8 ab | 0.15 | 4.9 ab | 0.19 | 4.5 b | 0.06 | 4.6 bc | 0.19 | 4.2 bf | 0.42 | 4.2 c | 0.51 |
| 9 | 1.5 de | 0.73 | 0.5 d | 0.27 | 3.0 bce | 0.61 | 2.7 c | 0.81 | 0.3 e | 0.27 | 0.2 e | 0.10 | 3.5 bd | 0.26 | 3.8 c | 0.56 |
| 10 | 3.8 bcd | 0.86 | 2.8 bc | 0.69 | 2.8 ce | 0.23 | 0.8 de | 0.49 | 2.4 d | 0.40 | 2.2 d | 0.49 | 3.1 cdf | 0.11 | 0.3 d | 0.27 |
| Disinfectant | Spores | D Value (Minutes) | SE 1 |
|---|---|---|---|
| 2 | C. sporogenes | 2.1 | ±0.03 |
| C. difficile | 5.3 | ±0.02 | |
| 7 | C. sporogenes | 5.0 | ±0.02 |
| C. difficile | 5.5 | ±0.01 | |
| 10 | C. sporogenes | 6.6 | ±0.01 |
| C. difficile | 8.4 | ±0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McSharry, S.; Koolman, L.; Whyte, P.; Bolton, D. Investigation of the Effectiveness of Disinfectants Used in Meat-Processing Facilities to Control Clostridium sporogenes and Clostridioides difficile Spores. Foods 2021, 10, 1436. https://doi.org/10.3390/foods10061436
McSharry S, Koolman L, Whyte P, Bolton D. Investigation of the Effectiveness of Disinfectants Used in Meat-Processing Facilities to Control Clostridium sporogenes and Clostridioides difficile Spores. Foods. 2021; 10(6):1436. https://doi.org/10.3390/foods10061436
Chicago/Turabian StyleMcSharry, Siobhán, Leonard Koolman, Paul Whyte, and Declan Bolton. 2021. "Investigation of the Effectiveness of Disinfectants Used in Meat-Processing Facilities to Control Clostridium sporogenes and Clostridioides difficile Spores" Foods 10, no. 6: 1436. https://doi.org/10.3390/foods10061436
APA StyleMcSharry, S., Koolman, L., Whyte, P., & Bolton, D. (2021). Investigation of the Effectiveness of Disinfectants Used in Meat-Processing Facilities to Control Clostridium sporogenes and Clostridioides difficile Spores. Foods, 10(6), 1436. https://doi.org/10.3390/foods10061436








