Revisiting Non-Thermal Food Processing and Preservation Methods—Action Mechanisms, Pros and Cons: A Technological Update (2016–2021)
Abstract
1. Introduction
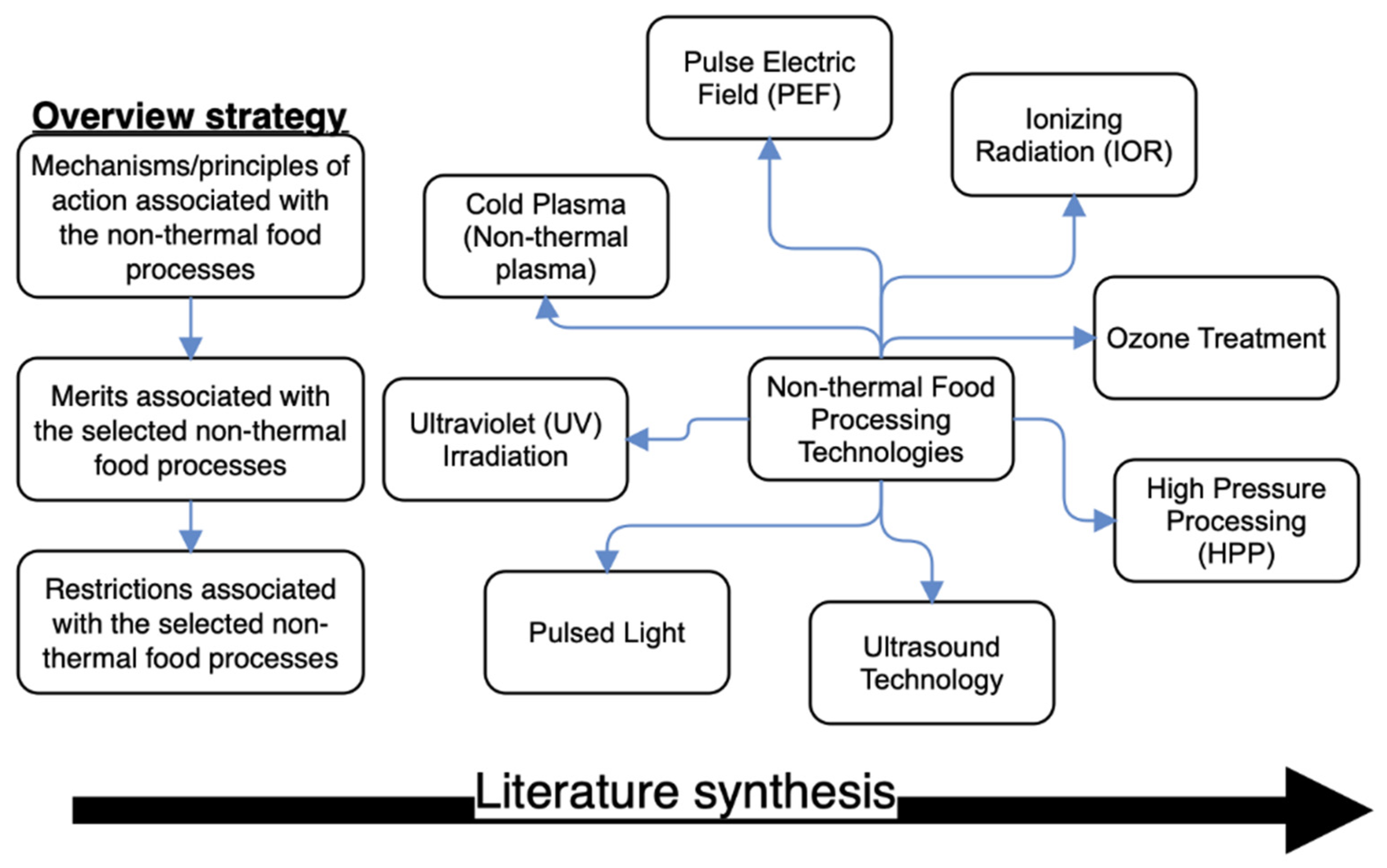
2. Action Mechanisms/Principles Associated with the Selected Non-Thermal Food Processing Technologies
2.1. Physical Treatments
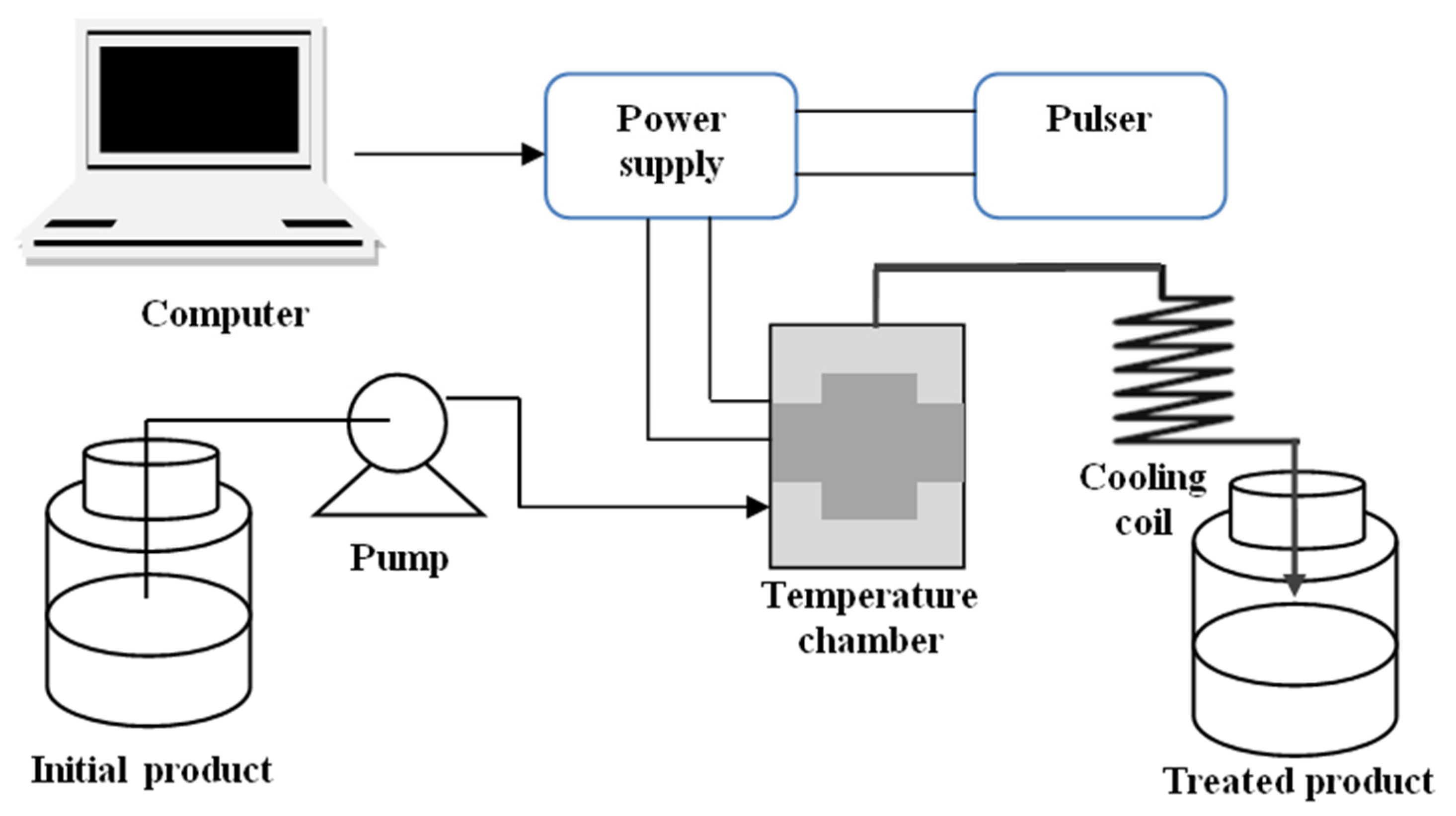
2.1.1. Pulsed Electric Field (PEF)
2.1.2. High-Pressure Processing (HPP)
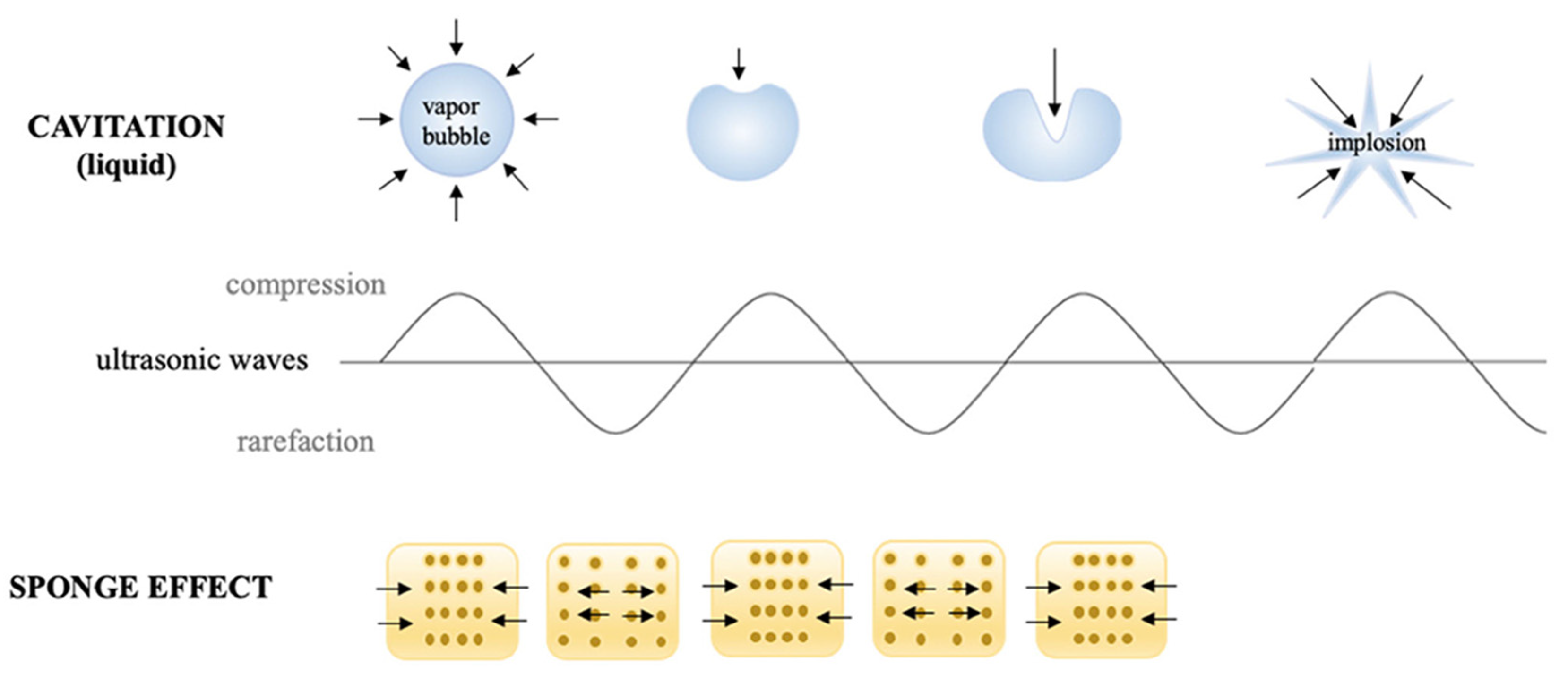
2.1.3. Ultrasound Technology
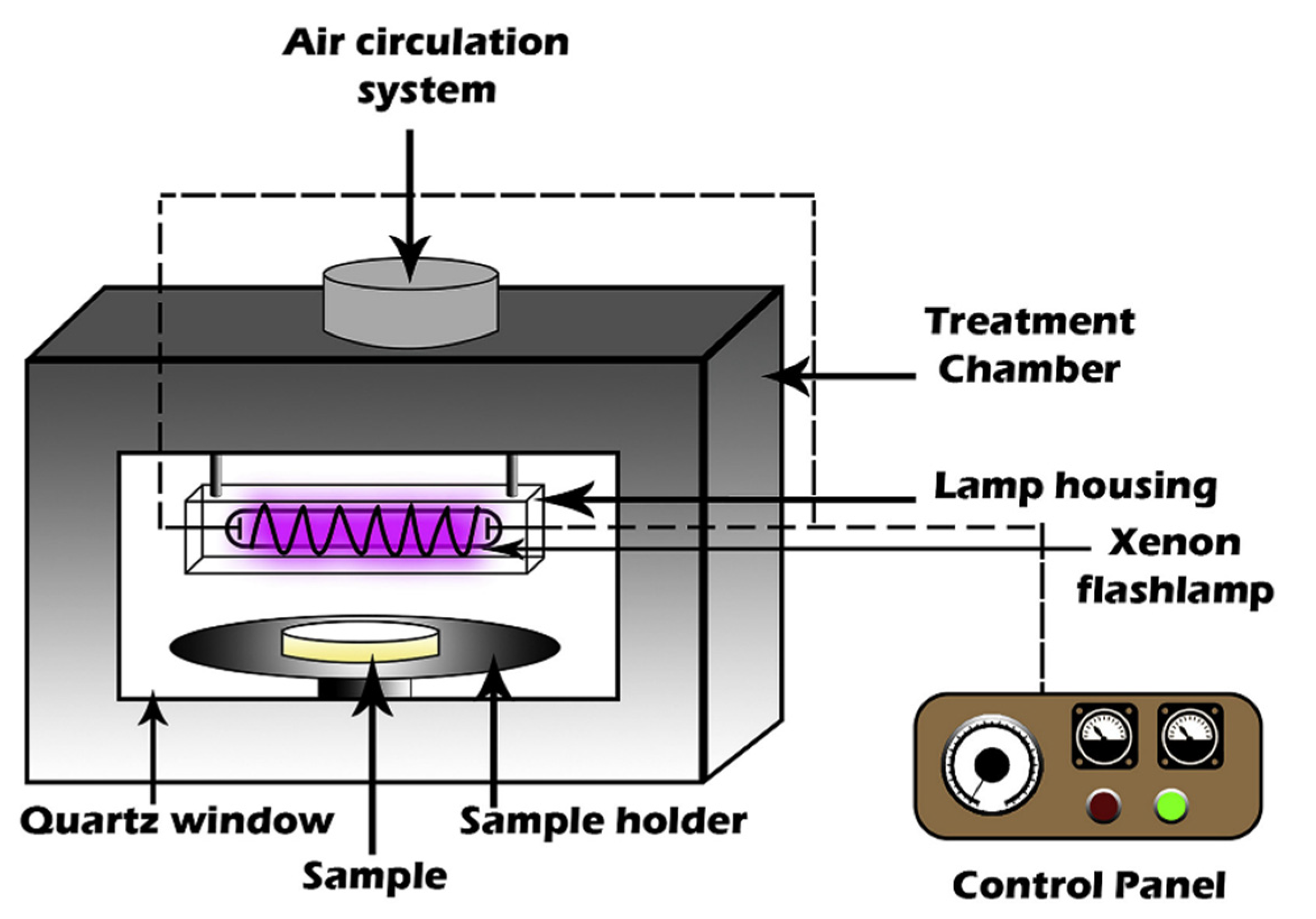
2.1.4. Pulsed Light (PL)
2.1.5. Ultraviolet (UV) Radiation
2.1.6. Ionizing Radiation (IOR)
2.2. Chemical Treatments
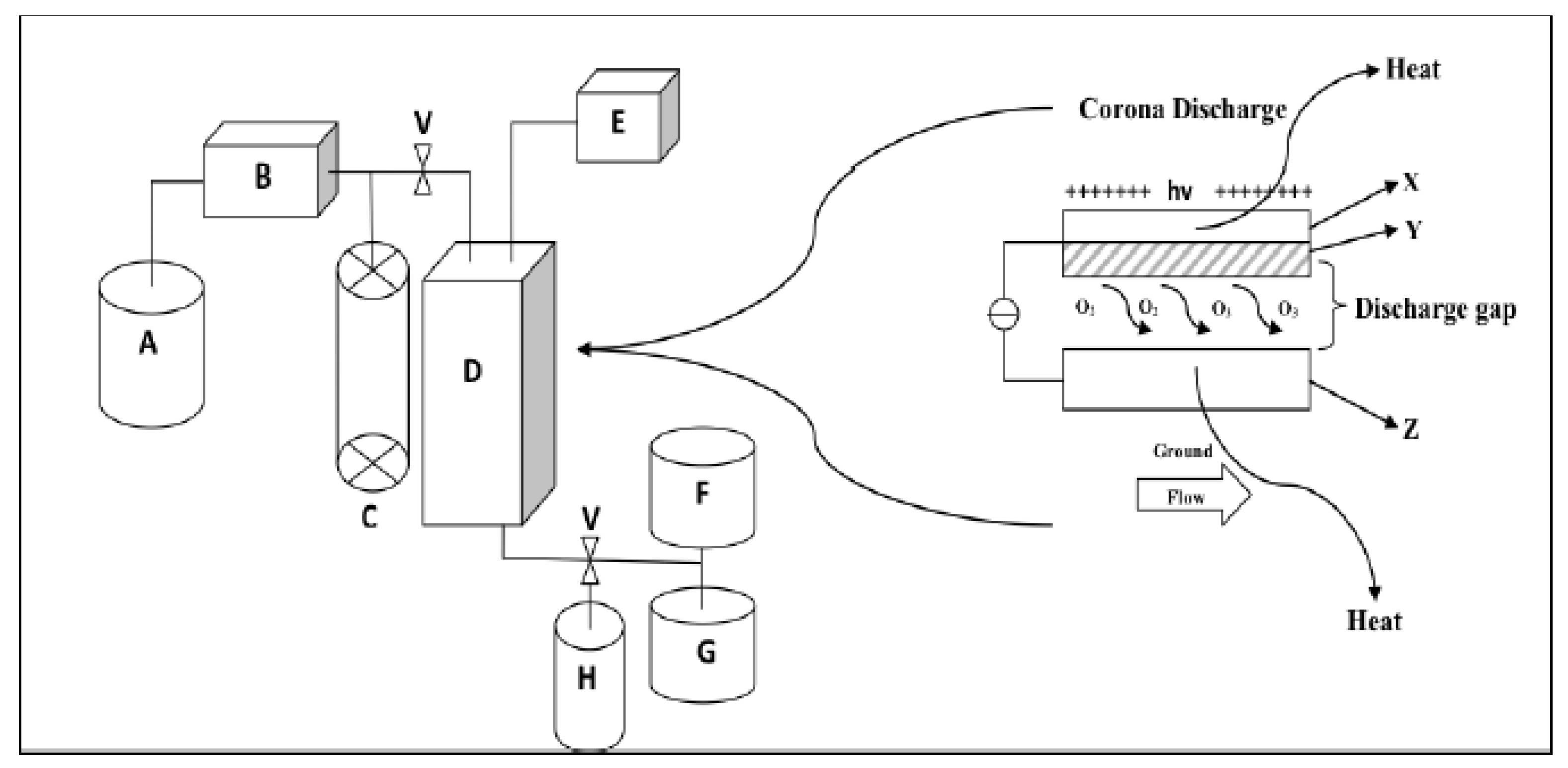
2.2.1. Ozone Treatment
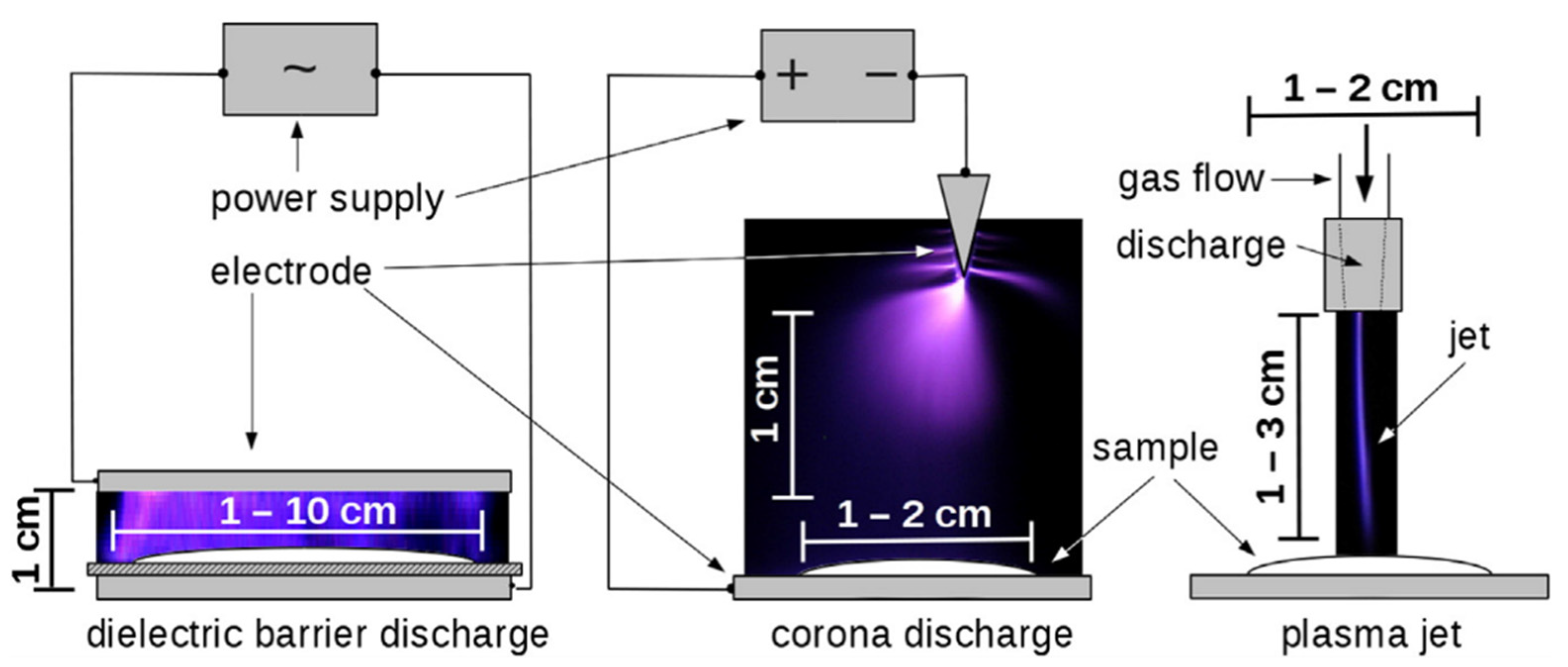
2.2.2. Cold Plasma (Non-Thermal Plasma)
3. Merits Associated with the Selected Non-Thermal Food Processing Techniques
3.1. Physical Treatments
3.1.1. Pulsed Electric Field (PEF)
3.1.2. High-Pressure Processing (HPP)
3.1.3. Ultrasound Technology
3.1.4. Pulsed Light (PL)
3.1.5. Ultraviolet (UV) Radiation
3.1.6. Ionizing Irradiation (IOR)
3.2. Chemical Treatments
3.2.1. Ozone Treatment
3.2.2. Cold Plasma (Non-Thermal Plasma)
4. Demerits Associated with the Selected Non-Thermal Food Processing Techniques
4.1. Physical Treatments
4.1.1. Pulsed Electric Field (PEF)
4.1.2. High-Pressure Processing (HPP)
4.1.3. Ultrasound Technology
4.1.4. Pulsed Light (PL)
4.1.5. Ultraviolet (UV) Radiation
4.1.6. Ionizing Irradiation (IOR)
4.2. Chemical Treatments
4.2.1. Ozone Treatment
4.2.2. Cold plasma (Non-Thermal Plasma)
5. Final Considerations
6. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E.-D. The Impact of Nonthermal Technologies on the Microbiological Quality of Juices: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 437–457. [Google Scholar] [CrossRef]
- La Peña, M.M.-D.; Welti-Chanes, J.; Martín-Belloso, O. Novel technologies to improve food safety and quality. Curr. Opin. Food Sci. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Troy, D.J.; Ojha, K.S.; Kerry, J.P.; Tiwari, B.K. Sustainable and consumer-friendly emerging technologies for application within the meat industry: An overview. Meat Sci. 2016, 120, 2–9. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Moreno-Vilet, L.; Villanueva-Rodríguez, S. Current status of emerging food processing technologies in Latin America: Novel non-thermal processing. Innov. Food Sci. Emerg. Technol. 2019, 58, 102233. [Google Scholar] [CrossRef]
- Knockaert, G.; Pulissery, S.K.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Carrot β-Carotene Degradation and Isomerization Kinetics during Thermal Processing in the Presence of Oil. J. Agric. Food Chem. 2012, 60, 10312–10319. [Google Scholar] [CrossRef]
- Zhong, S.; Vendrell-Pacheco, M.; Heskitt, B.; Chitchumroonchokchai, C.; Failla, M.L.; Sastry, S.K.; Francis, D.M.; Martin-Belloso, O.; Elez-Martinez, P.; Kopec, R.E. Novel Processing Technologies as Compared to Thermal Treatment on the Bioaccessibility and Caco-2 Cell Uptake of Carotenoids from Tomato and Kale-Based Juices. J. Agric. Food Chem. 2019, 67, 10185–10194. [Google Scholar] [CrossRef] [PubMed]
- Valdramidis, V.P.; Koutsoumanis, K.P. Challenges and perspectives of advanced technologies in processing, distribution and storage for improving food safety. Curr. Opin. Food Sci. 2016, 12, 63–69. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.; Dutta, S. Novel thermal and non-thermal processing of watermelon juice. Trends Food Sci. Technol. 2019, 93, 234–243. [Google Scholar] [CrossRef]
- Bahrami, A.; Baboli, Z.M.; Schimmel, K.; Jafari, S.M.; Williams, L. Efficiency of novel processing technologies for the control of Listeria monocytogenes in food products. Trends Food Sci. Technol. 2020, 96, 61–78. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of ozone in the food industry. LWT 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Komanapalli, I.R.; Lau, B.H.S. Ozone-induced damage of Escherichia coli K-12. Appl. Microbiol. Biotechnol. 1996, 46, 610–614. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.P.; Tiwari, B.K.; Cullen, P.J.; Rice, R.G. Ozone in Food Processing; John Wiley & Sons: Chichester, UK, 2012; p. 308. ISBN 978-1-4443-3442-5. [Google Scholar]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Critical reviews and recent advances of novel non-thermal processing techniques on the modification of food allergens. Crit. Rev. Food Sci. Nutr. 2021, 61, 196–210. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Zhang, M. Effects of high hydrostatic pressure processing and subsequent storage on phenolic contents and antioxidant activity in fruit and vegetable products. Int. J. Food Sci. Technol. 2017, 52, 3–12. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Eissa, A.H.A. Pulsed Electric Fields for Food Processing Technology. Struct. Funct. Food Eng. 2012, 11, 275–306. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Arroyo, C.; Cebrián, G.; Condon, S.; Pagán, R.; Auré, G.C. Development of resistance in Cronobacter sakazakii ATCC 29544 to thermal and nonthermal processes after exposure to stressing environmental conditions. J. Appl. Microbiol. 2012, 112, 561–570. [Google Scholar] [CrossRef]
- Zhang, Q.; Barbosa-Cánovas, G.V.; Swanson, B.G. Engineering aspects of pulsed electric field pasteurization. J. Food Eng. 1995, 25, 261–281. [Google Scholar] [CrossRef]
- Pal, M. Pulsed Electric Field Processing: An Emerging Technology for Food Preservation. J. Exp. Food Chem. 2017, 3, 2–3. [Google Scholar] [CrossRef]
- Sale, A.; Hamilton, W. Effects of high electric fields on microorganisms: I. Killing of bacteria and yeasts. Biochim. Biophys. Acta BBA Gen. Subj. 1967, 148, 781–788. [Google Scholar] [CrossRef]
- Santhirasegaram, V.; Razali, Z.; Somasundram, C. Safety Improvement of Fruit Juices by Novel Thermal and Nonthermal Processing. In Food Hygiene and Toxicology in Ready-to-Eat Foods; Kotzekidou, P., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 209–223. [Google Scholar]
- Kumar, S.; Agarwal, N.; Raghav, P.K. Pulsed electric field processing: A review. Int. J. Eng. Res. Modern Edu. 2016, 1, 111–118. [Google Scholar]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Alirezalu, K.; Munekata, P.E.S.; Parniakov, O.; Barba, F.J.; Witt, J.; Toepfl, S.; Wiktor, A.; Lorenzo, J.M. Pulsed electric field and mild heating for milk processing: A review on recent advances. J. Sci. Food Agric. 2020, 100, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F. Physico-chemical properties of fruit and vegetable juices as affected by pulsed electric field: A review. Int. J. Food Prop. 2020, 23, 1036–1050. [Google Scholar] [CrossRef]
- McAuley, C.M.; Singh, T.K.; Haro-Maza, J.F.; Williams, R.; Buckow, R. Microbiological and physicochemical stability of raw, pasteurised or pulsed electric field-treated milk. Innov. Food Sci. Emerg. Technol. 2016, 38, 365–373. [Google Scholar] [CrossRef]
- Timmermans, R.; Mastwijk, H.; Berendsen, L.; Nederhoff, A.; Matser, A.; Van Boekel, M.; Groot, M.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. Int. J. Food Microbiol. 2019, 298, 63–73. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Portu, J.; López, N.; Santamaría, P.; Gutiérrez, A.R.; López, R.; López-Alfaro, I. Pulsed Electric Field treatment after malolactic fermentation of Tempranillo Rioja wines: Influence on microbial, physicochemical and sensorial quality. Innov. Food Sci. Emerg. Technol. 2019, 51, 57–63. [Google Scholar] [CrossRef]
- Pallarés, N.; Berrada, H.; Tolosa, J.; Ferrer, E. Effect of high hydrostatic pressure (HPP) and pulsed electric field (PEF) technologies on reduction of aflatoxins in fruit juices. LWT 2021, 142, 111000. [Google Scholar] [CrossRef]
- Okpala, C.O.R.; Piggott, J.R.; Schaschke, C.J. Influence of high-pressure processing (HPP) on physico-chemical properties of fresh cheese. Innov. Food Sci. Emerg. Technol. 2010, 11, 61–67. [Google Scholar] [CrossRef]
- Heinz, V.; Buckow, R. Food preservation by high pressure. J. Verbrauch. Leb. 2010, 5, 73–78. [Google Scholar] [CrossRef]
- Mújica-Paz, H.; Valdez-Fragoso, A.; Samson, C.T.; Welti-Chanes, J.; Antonio Torres, J. High-Pressure Processing Technologies for Pasteurization and Sterilisation of Foods. Food Bioprocess. Technol. 2011, 4, 969. [Google Scholar] [CrossRef]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Bisconsin-Junior, A.; Rosenthal, A.; Monteiro, M. Optimisation of High Hydrostatic Pressure Processing of Pêra Rio Orange Juice. Food Bioprocess Technol. 2013, 7, 1670–1677. [Google Scholar] [CrossRef]
- Cheng, C.-X.; Jia, M.; Gui, Y.; Ma, Y. Comparison of the effects of novel processing technologies and conventional thermal pasteurisation on the nutritional quality and aroma of Mandarin (Citrus unshiu) juice. Innov. Food Sci. Emerg. Technol. 2020, 64, 102425. [Google Scholar] [CrossRef]
- Valdez-Fragoso, A.; Mújica-Paz, H.; Welti-Chanes, J.; Torres, J.A. Reaction Kinetics at High Pressure and Temperature: Effects on Milk Flavor Volatiles and on Chemical Compounds with Nutritional and Safety Importance in Several Foods. Food Bioprocess Technol. 2011, 4, 986–995. [Google Scholar] [CrossRef]
- Zacconi, C.; Giosuè, S.; Marudelli, M.; Scolari, G. Microbiological quality and safety of smoothies treated in different pressure–temperature domains: Effects on indigenous fruit microbiota and Listeria monocytogenes and their survival during storage. Eur. Food Res. Technol. 2015, 241, 317–328. [Google Scholar] [CrossRef]
- Boukil, A.; Perreault, V.; Chamberland, J.; Mezdour, S.; Pouliot, Y.; Doyen, A. High Hydrostatic Pressure-Assisted Enzymatic Hydrolysis Affect Mealworm Allergenic Proteins. Molecules 2020, 25, 2685. [Google Scholar] [CrossRef]
- Muntean, M.-V.; Marian, O.; Barbieru, V.; Cătunescu, G.M.; Ranta, O.; Drocas, I.; Terhes, S. High Pressure Processing in Food Industry—Characteristics and Applications. Agric. Agric. Sci. Procedia 2016, 10, 377–383. [Google Scholar] [CrossRef]
- Kurpiewska, K.; Biela, A.; Loch, J.I.; Lipowska, J.; Siuda, M.; Lewiński, K. Towards understanding the effect of high pressure on food protein allergenicity: β-lactoglobulin structural studies. Food Chem. 2019, 270, 315–321. [Google Scholar] [CrossRef]
- Önür, I.; Misra, N.; Barba, F.J.; Putnik, P.; Lorenzo, J.M.; Gökmen, V.; Alpas, H. Effects of ultrasound and high pressure on physicochemical properties and HMF formation in Turkish honey types. J. Food Eng. 2018, 219, 129–136. [Google Scholar] [CrossRef]
- Niakousari, M.; Gahruie, H.H.; Razmjooei, M.; Roohinejad, S.; Greiner, R. Effects of Innovative Processing Technologies on Microbial Targets Based on Food Categories: Comparing Traditional and Emerging Technologies for Food Preservation. In Innovative Technologies for Food Preservation; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubba, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 133–185. [Google Scholar]
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current status and future trends of high-pressure processing in food industry. Food Control. 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Jung, S.; Tonello-Samson, C. High Hydrostatic Pressure Food Processing: Potential and Limitations. In Alternatives to Conventional food Processing; Royal Society of Chemistry: London, UK, 2018; pp. 251–315. [Google Scholar]
- Verma, D.K.; Thakur, M.; Kumar, J.; Srivastav, P.P.; Al-Hilphy AR, S.; Patel, A.; Suleria HA, R. High Pressure Processing (HPP): Fundamental Concepts, Emerging Scope, and Food Application. In Emerging Thermal and Nonthermal Technologies in Food Processing; Apple Academic Press: Palm Bay, FL, USA, 2020; pp. 225–257. [Google Scholar]
- Bolumar, T.; Georget, E.; Mathys, A. High Pressure Processing (HPP) of Foods and its Combination with Electron Beam Processing. In Electron Beam Pasteurization and Complementary Food Processing Technologies; Woodhead Publishing: Sawston, UK, 2015; pp. 127–155. [Google Scholar]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Barba, F.J.; Skąpska, S.; Lorenzo, J.M.; Zambon, A.; Spilimbergo, S. Enzymatic, physicochemical, nutritional and phytochemical profile changes of apple (Golden Delicious L.) juice under supercritical carbon dioxide and long-term cold storage. Food Chem. 2018, 268, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Usaga, J.; Acosta, Ó.; Churey, J.J.; Padilla-Zakour, O.I.; Worobo, R.W. Evaluation of high pressure processing (HPP) inactivation of Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes in acid and acidified juices and beverages. Int. J. Food Microbiol. 2021, 339, 109034. [Google Scholar] [CrossRef]
- Pérez-Baltar, A.; Serrano, A.; Montiel, R.; Medina, M. Listeria monocytogenes inactivation in deboned dry-cured hams by high pressure processing. Meat Sci. 2020, 160, 107960. [Google Scholar] [CrossRef]
- Rocha-Pimienta, J.; Martillanes, S.; Ramirez, R.; Garcia-Parra, J.; Delgado-Adamez, J. Bacillus cereus spores and Staphylococcus aureus sub. aureus vegetative cells inactivation in human milk by high-pressure processing. Food Control. 2020, 113, 107212. [Google Scholar] [CrossRef]
- Fernandez, M.; Denoya, G.; Jagus, R.; Vaudagna, S.; Agüero, M. Microbiological, antioxidant and physicochemical stability of a fruit and vegetable smoothie treated by high pressure processing and stored at room temperature. LWT 2019, 105, 206–210. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Llera-Oyola, J.; Gil, M.V.; Ayuso-Yuste, M.C.; García-Parra, J.; Delgado-Adámez, J. Control of Listeria monocytogenes in sliced dry-cured Iberian ham by high pressure processing in combination with an eco-friendly packaging based on chitosan, nisin and phytochemicals from rice bran. Food Control. 2021, 124, 107933. [Google Scholar] [CrossRef]
- Chai, H.-E.; Sheen, S. Effect of high pressure processing, allyl isothiocyanate, and acetic acid stresses on Salmonella survivals, storage, and appearance color in raw ground chicken meat. Food Control. 2021, 123, 107784. [Google Scholar] [CrossRef]
- Jermann, C.; Koutchma, T.; Margas, E.; Leadley, C.; Ros-Polski, V. Mapping trends in novel and emerging food processing technologies around the world. Innov. Food Sci. Emerg. Technol. 2015, 31, 14–27. [Google Scholar] [CrossRef]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing. Ultrason. Sonochem. 2012, 19, 975–983. [Google Scholar] [CrossRef]
- Luo, K.; Oh, D.-H. Inactivation kinetics of Listeria monocytogenes and Salmonella enterica serovar Typhimurium on fresh-cut bell pepper treated with slightly acidic electrolyzed water combined with ultrasound and mild heat. Food Microbiol. 2016, 53 Pt B, 165–171. [Google Scholar] [CrossRef]
- Hamann, D.; Tonkiel, K.F.; Matthiensen, A.; Zeni, J.; Valduga, E.; Paroul, N.; Steffens, C.; Toniazzo, G.; Cansian, R. Ultrasound Use for Listeria Monocytogenes Attached Cells Removal from Industrial Brine Injection Needles. Ital. J. Food Sci. 2018, 30, 662–672. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Galanakis, C.M.; Brnčić, M.; Grimi, N.; Boussetta, N.; Mota, M.J.; Saraiva, J.A.; Patras, A.; Tiwari, B.; Barba, F.J. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015, 77, 743–752. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Gabriel, A.A. Inactivation behaviors of foodborne microorganisms in multi-frequency power ultrasound-treated orange juice. Food Control. 2014, 46, 189–196. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Mason, T.J. Chapter 6–Ultrasound Processing of Fluid Foods BT—Novel Thermal and Non-Thermal Technologies for Fluid Foods; Academic Press: San Diego, CA, USA, 2012; pp. 135–165. [Google Scholar]
- Astráin-Redín, L.; Alejandre, M.; Raso, J.; Cebrián, G.; Álvarez, I. Direct Contact Ultrasound in Food Processing: Impact on Food Quality. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Santillo, A.; Guimarães, J.T.; Bevilacqua, A.; Corbo, M.R.; Caroprese, M.; Marino, R.; Esmerino, E.A.; Silva, M.C.; Raices, R.S.; et al. Ultrasound processing of fresh and frozen semi-skimmed sheep milk and its effects on microbiological and physical-chemical quality. Ultrason. Sonochem. 2019, 51, 241–248. [Google Scholar] [CrossRef]
- Monteiro, S.H.; Silva, E.K.; Alvarenga, V.O.; Moraes, J.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.; Sant’Ana, A.S.; Meireles, M.A.A.; Cruz, A.G. Effects of ultrasound energy density on the non-thermal pasteurization of chocolate milk beverage. Ultrason. Sonochem. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Liao, X.; Li, J.; Muhammad, A.I.; Suo, Y.; Ahn, J.; Liu, D.; Chen, S.; Hu, Y.; Ye, X.; Ding, T. Preceding treatment of non-thermal plasma (NTP) assisted the bactericidal effect of ultrasound on Staphylococcus aureus. Food Control. 2018, 90, 241–248. [Google Scholar] [CrossRef]
- Shriver, S.K.; Yang, W.W. Thermal and Nonthermal Methods for Food Allergen Control. Food Eng. Rev. 2011, 3, 26–43. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2016, 57, 501–523. [Google Scholar] [CrossRef]
- Chung, Y.H.; Kwon, J.H.; Jung, J.A.; Lee, J.O.; Lee, K.S.; Kim, J.Y.; Ahn, K.M.; Lee, S.I.; Ryu, C.H. Evaluating the allergenicity of soybean by the fermentation. Pediatr. Allergy Respir. Dis. 2008, 18, 37–45. [Google Scholar]
- Mahendran, R.; Ramanan, K.R.; Barba, F.J.; Lorenzo, J.M.; López-Fernández, O.; Munekata, P.E.S.; Roohinejad, S.; Sant’Ana, A.S.; Tiwari, B.K. Recent advances in the application of pulsed light processing for improving food safety and increasing shelf life. Trends Food Sci. Technol. 2019, 88, 67–79. [Google Scholar] [CrossRef]
- Kaya, Z.; Unluturk, S. Processing of clear and turbid grape juice by a continuous flow UV system. Innov. Food Sci. Emerg. Technol. 2016, 33, 282–288. [Google Scholar] [CrossRef]
- Ramos-Villarroel, A.Y.; Aron-Maftei, N.; Martín-Belloso, O.; Soliva-Fortuny, R. Influence of spectral distribution on bacterial inactivation and quality changes of fresh-cut watermelon treated with intense light pulses. Postharvest Biol. Technol. 2012, 69, 32–39. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Sokorai, K.; Ukuku, D.O.; Jin, T.; Fan, X.; Olanya, O.M.; Leng, J.; Juneja, V. Effects of direct and in-package pulsed light treatment on inactivation of E. coli O157:H7 and reduction of microbial loads in Romaine lettuce. LWT 2021, 139, 110710. [Google Scholar] [CrossRef]
- Tao, T.; Ding, C.; Han, N.; Cui, Y.; Liu, X.; Zhang, C. Evaluation of pulsed light for inactivation of foodborne pathogens on fresh-cut lettuce: Effects on quality attributes during storage. Food Packag. Shelf Life 2019, 21, 100358. [Google Scholar] [CrossRef]
- Kramer, B.; Wunderlich, J.; Muranyi, P. Inactivation of Listeria innocua on packaged meat products by pulsed light. Food Packag. Shelf Life 2019, 21, 100353. [Google Scholar] [CrossRef]
- Abuagela, M.O.; Iqdiam, B.M.; Mostafa, H.; Marshall, S.M.; Yagiz, Y.; Marshall, M.R.; Gu, L.; Sarnoski, P. Combined effects of citric acid and pulsed light treatments to degrade B-aflatoxins in peanut. Food Bioprod. Process. 2019, 117, 396–403. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; Feng, L.X.J.; Bang, W.S.; Yuk, H.-G. Inactivation of Listeria monocytogenes and natural microbiota on raw salmon fillets using acidic electrolyzed water, ultraviolet light or/and ultrasounds. Food Control. 2017, 74, 54–60. [Google Scholar] [CrossRef]
- Vasuja, S.; Kumar, V. Ultra Violet Irradiation and its applications in Food Processing Industries: A Review. Int. J. Trend Res. Dev. 2018, 5, 343–346. [Google Scholar]
- Xuan, X.-T.; Ding, T.; Li, J.; Ahn, J.-H.; Zhao, Y.; Chen, S.-G.; Ye, X.-Q.; Liu, D.-H. Estimation of growth parameters of Listeria monocytogenes after sublethal heat and slightly acidic electrolyzed water (SAEW) treatment. Food Control. 2017, 71, 17–25. [Google Scholar] [CrossRef]
- Unluturk, S.; Atılgan, M.R.; Baysal, A.H.; Unluturk, M.S. Modeling inactivation kinetics of liquid egg white exposed to UV-C irradiation. Int. J. Food Microbiol. 2010, 142, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q.; et al. Ultraviolet radiation: An interesting technology to preserve quality and safety of milk and dairy foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Izmirlioglu, G.; Ouyang, B.; Demirci, A. Utilization of pulsed UV light for inactivation of Salmonella Enteritidis on shelled walnuts. LWT 2020, 134, 110023. [Google Scholar] [CrossRef]
- Atik, A.; Gümüş, T. The Effect of Different Doses of UV-C Treatment on Microbiological Quality of Bovine Milk. LWT 2021, 136, 110322. [Google Scholar] [CrossRef]
- Pedrós-Garrido, S.; Condón-Abanto, S.; Clemente, I.; Beltrán, J.; Lyng, J.; Bolton, D.; Brunton, N.; Whyte, P. Efficacy of ultraviolet light (UV-C) and pulsed light (PL) for the microbiological decontamination of raw salmon (Salmo salar) and food contact surface materials. Innov. Food Sci. Emerg. Technol. 2018, 50, 124–131. [Google Scholar] [CrossRef]
- Khouryieh, H.A. Novel and emerging technologies used by the U.S. food processing industry. Innov. Food Sci. Emerg. Technol. 2020, 67, 102559. [Google Scholar] [CrossRef]
- Zanardi, E.; Caligiani, A.; Novelli, E. New Insights to Detect Irradiated Food: An Overview. Food Anal. Methods 2017, 11, 224–235. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, D.-W.; Han, Z. Applications of electromagnetic fields for nonthermal inactivation of microorganisms in foods: An overview. Trends Food Sci. Technol. 2017, 64, 13–22. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Wholesomeness and safety aspects of irradiated foods. Food Chem. 2019, 285, 363–368. [Google Scholar] [CrossRef]
- Graham, D.M.; Pariza, M.W.; Glaze, W.H.; Erdman, J.W.; Newell, G.W.; Borzelleca, J.F. Use of ozone for food processing. Food Technol. 1997, 51, 72–76. [Google Scholar]
- FDA. Hazard analysis and critical control point (HACCP): Procedures for the safe and sanitary processing and importing of juice; final rule. Fed. Regist. 2001, 66, 6137–6202. [Google Scholar]
- Mustafa, M.G. Biochemical basis of ozone toxicity. Free. Radic. Biol. Med. 1990, 9, 245–265. [Google Scholar] [CrossRef]
- Beltran, F.J. Ozone Reaction Kinetics for Water and Wastewater System; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Gonçalves, A.A. Ozone: An emerging technology for the seafood industry. Brazilian Arch. Biol. Technol. 2009, 52, 1527–1539. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water. Part, I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Hill, A.G.; Rice, R.G. Historical Background, Properties and Applications. In Handbook of Ozone Technology and Applications; Rice, R.G., Netzer, A., Eds.; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1982; Volume 1, pp. 1–37. [Google Scholar]
- Hamelin, C. Production of single- and double-strand breaks in plasmid dna by ozone. Int. J. Radiat. Oncol. 1985, 11, 253–257. [Google Scholar] [CrossRef]
- Kim, J.-G.; Yousef, A.E.; Dave, S. Application of Ozone for Enhancing the Microbiological Safety and Quality of Foods: A Review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Okpala, C.O.R. Ozone delivery on food materials incorporating some bio-based processes: A succinct synopsis. Adv. Mater. Proc. 2017, 2, 469–478. [Google Scholar] [CrossRef]
- Pinto, L.; Baruzzi, F.; Ippolitto, A. Recent advances to control spoilage microorganisms in washing water of fruits and vegetables: The use of electrolyzed water. In III International Symposium on Postharvest Pathology: Using Science to Increase Food Availability, Bari, Italy; Ippolito, A., Sanzani, S.M., Wisniewski, M., Droby, S., Eds.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2016. [Google Scholar]
- Okpala, C.O.R. Fish Processing by Ozone Treatment—Is Further Investigation of Domestic Applications Needful? Chem. Eng. Trans. 2017, 57, 1813–1818. [Google Scholar]
- Karaca, H.; Velioglu, Y.S. Ozone Applications in Fruit and Vegetable Processing. Food Rev. Int. 2007, 23, 91–106. [Google Scholar] [CrossRef]
- Okpala, C.O.R. Investigation of quality attributes of ice-stored Pacific white shrimp (Litopenaeus vannamei) as affected by sequential minimal ozone treatment. LWT 2014, 57, 538–547. [Google Scholar] [CrossRef]
- Okpala, C.O.R. Quality evaluation and shelf life of minimal ozone-treated Pacific white shrimp (Litopenaeus vannameii) stored on ice. J.Verbrauch. Lebensm. 2015, 10, 49–57. [Google Scholar] [CrossRef]
- Okpala, C.O.R. Changes in some biochemical and microbiological properties of ozone-processed shrimp: Effects of increased ozone discharge combined with iced storage. J. Food Nutr. Res. 2018, 57, 48–56. [Google Scholar]
- Beltrán, D.; Selma, M.V.; Marín, A.; Gil Muñoz, M.I. Ozonated Water Extends the Shelf Life of Fresh-Cut Lettuce. J. Agric. Food Chem. 2005, 53, 5654–5663. [Google Scholar] [CrossRef]
- Tiwari, B.; O’Donnell, C.; Patras, A.; Brunton, N.; Cullen, P. Effect of ozone processing on anthocyanins and ascorbic acid degradation of strawberry juice. Food Chem. 2009, 113, 1119–1126. [Google Scholar] [CrossRef]
- Mendez, F.; Maier, D.; Mason, L.; Woloshuk, C. Penetration of ozone into columns of stored grains and effects on chemical composition and processing performance. J. Stored Prod. Res. 2003, 39, 33–44. [Google Scholar] [CrossRef]
- Tiwari, B.; Brennan, C.; Curran, T.; Gallagher, E.; Cullen, P.; Donnell, C.O. Application of ozone in grain processing. J. Cereal Sci. 2010, 51, 248–255. [Google Scholar] [CrossRef]
- Misra, N.; Kaur, S.; Tiwari, B.K.; Kaur, A.; Singh, N.; Cullen, P. Atmospheric pressure cold plasma (ACP) treatment of wheat flour. Food Hydrocoll. 2015, 44, 115–121. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Patil, S. Non-thermal plasma: An advanced technology for food industry. Food Sci. Technol. Int. 2020, 26, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Kawasaki, Y.; Izawa, S. Ferrous chloride and ferrous sulfate improve the fungicidal efficacy of cold atmospheric argon plasma on melanized Aureobasidium pullulans. J. Biosci. Bioeng. 2019, 128, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Farid, M. A review on recent development in non-conventional food sterilization technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Deng, X.; Shi, J.; Kong, M.G. Physical Mechanisms of Inactivation of Bacillus subtilis Spores Using Cold Atmospheric Plasmas. IEEE Trans. Plasma Sci. 2006, 34, 1310–1316. [Google Scholar] [CrossRef]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Timmons, C.; Pai, K.; Jacob, J.; Zhang, G.; Ma, L.M. Inactivation of Salmonella enterica, Shiga toxin-producing Escherichia coli, and Listeria monocytogenes by a novel surface discharge cold plasma design. Food Control. 2018, 84, 455–462. [Google Scholar] [CrossRef]
- Ma, R.; Feng, H.; Guo, J.; Liang, Y.; Zhang, Q.; Tian, Y.; Zhang, J.; Fang, J. An Efficient and Specific Protection of Non-Thermal Plasma-Induced Live Yeast Cell Derivative (LYCD) for Cells against Plasma Damage. Plasma Process. Polym. 2014, 11, 822–832. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.-U.; Min, S.C. Microbial decontamination of red pepper powder by cold plasma. Food Microbiol. 2014, 38, 128–136. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Hashemi, M. Cold atmospheric plasma manipulation of proteins in food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 2583–2597. [Google Scholar] [CrossRef] [PubMed]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores. J. Food Eng. 2017, 196, 139–149. [Google Scholar] [CrossRef]
- Jiang, Y.; Sokorai, K.; Pyrgiotakis, G.; Demokritou, P.; Li, X.; Mukhopadhyay, S.; Jin, T.; Fan, X. Cold plasma-activated hydrogen peroxide aerosol inactivates Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria innocua and maintains quality of grape tomato, spinach and cantaloupe. Int. J. Food Microbiol. 2017, 249, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Thirumdas, R.; Sarangapani, C.; Annapure, U. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Phan, K.T.K.; Phan, H.T.; Brennan, C.S.; Phimolsiripol, Y. Nonthermal plasma for pesticide and microbial elimination on fruits and vegetables: An overview. Int. J. Food Sci. Technol. 2017, 52, 2127–2137. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, S.C. Moisture vaporization-combined helium dielectric barrier discharge-cold plasma treatment for microbial decontamination of onion flakes. Food Control. 2018, 84, 321–329. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of Cold Plasma on Food Quality: A Review. Foods 2018, 7, 4. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Puligundla, P.; Mok, C. Cold plasma decontamination of brown rice grains: Impact on biochemical and sensory qualities of their corresponding seedlings and aqueous tea infusions. LWT 2020, 131, 109508. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Bourke, P. Effects of cold plasma on wheat grain microbiome and antimicrobial efficacy against challenge pathogens and their resistance. Int. J. Food Microbiol. 2020, 335, 108889. [Google Scholar] [CrossRef]
- Roh, S.H.; Oh, Y.J.; Lee, S.Y.; Kang, J.H.; Min, S.C. Inactivation of Escherichia coli O157:H7, Salmonella, Listeria monocytogenes, and Tulane virus in processed chicken breast via atmospheric in-package cold plasma treatment. LWT 2020, 127, 109429. [Google Scholar] [CrossRef]
- Bauer, A.; Ni, Y.; Bauer, S.; Paulsen, P.; Modic, M.; Walsh, J.; Smulders, F. The effects of atmospheric pressure cold plasma treatment on microbiological, physical-chemical and sensory characteristics of vacuum packaged beef loin. Meat Sci. 2017, 128, 77–87. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Kumar, Y.; Patel, K.K.; Kumar, K. Pulse electric field processing in food technology. Int. J. Eng. Stud. Tech. Approach 2015, 1, 6–17. [Google Scholar]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. The application of pulsed electric field as a sodium reducing strategy for meat products. Food Chem. 2020, 306, 125622. [Google Scholar] [CrossRef]
- Alles, M.C.; Smetana, S.; Parniakov, O.; Shorstkii, I.; Toepfl, S.; Aganovic, K.; Heinz, V. Bio-refinery of insects with Pulsed electric field pre-treatment. Innov. Food Sci. Emerg. Technol. 2020, 64, 102403. [Google Scholar] [CrossRef]
- Shorstkii, I.; Alles, M.C.; Parniakov, O.; Smetana, S.; Aganovic, K.; Sosnin, M.; Toepfl, S.; Heinz, V. Optimization of pulsed electric field assisted drying process of black soldier fly (Hermetia illucens) larvae. Dry. Technol. 2020, 1–9. [Google Scholar] [CrossRef]
- Ozkan, G.; Stübler, A.-S.; Aganovic, K.; Draeger, G.; Esatbeyoglu, T.; Capanoglu, E. Retention of polyphenols and vitamin C in cranberrybush purée (Viburnum opulus) by means of non-thermal treatments. Food Chem. 2021, 360, 129918. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Patil, G.R.; Singh, A.K. High hydrostatic pressure technology in dairy processing: A review. J. Food Sci. Technol. 2011, 48, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.I.; Arcand, Y. Current trends in green technologies in food productionand processing. Food Eng. Rev. 2013, 5, 1–17. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, O.; Buckow, R.; Koutchma, T.; Balasubramaniam, V. Energy Requirements for Alternative Food Processing Technologies-Principles, Assumptions, and Evaluation of Efficiency. Compr. Rev. Food Sci. Food Saf. 2015, 14, 536–554. [Google Scholar] [CrossRef]
- Cacace, F.; Bottani, E.; Rizzi, A.; Vignali, G. Evaluation of the economic and environmental sustainability of high pressure processing of foods. Innov. Food Sci. Emerg. Technol. 2020, 60, 102281. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Wang, C.-Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Analy 2020, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Júnior, L.M.; Cristianini, M.; Padula, M.; Anjos, C.A.R. Effect of high-pressure processing on characteristics of flexible packaging for foods and beverages. Food Res. Int. 2019, 119, 920–930. [Google Scholar] [CrossRef]
- Rode, T.M.; Rotabakk, B.T. Extending shelf life of desalted cod by high pressure processing. Innov. Food Sci. Emerg. Technol. 2021, 69, 102476. [Google Scholar] [CrossRef]
- Campus, M. High Pressure Processing of Meat, Meat Products and Seafood. Food Eng. Rev. 2010, 2, 256–273. [Google Scholar] [CrossRef]
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.-P.; Marchesseau, S. Potentialities and Limits of Some Non-thermal Technologies to Improve Sustainability of Food Processing. Front. Nutr. 2019, 5, 130. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Tiwari, B.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Gabriel, A.A. Inactivation of L isteria monocytogenes in Milk by Multifrequency Power Ultrasound. J. Food Process. Preserv. 2015, 39, 846–853. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; Ashokkumar, M.; Freitas, M.Q.; Cruz, A.G. High-intensity ultrasound: A novel technology for the development of probiotic and prebiotic dairy products. Ultrason. Sonochem. 2019, 57, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Hebbar, H.U. Pulsed light processing of foods for microbial safety. Food Qual. Saf. 2017, 1, 187–202. [Google Scholar] [CrossRef]
- Chaine, A.; Levy, C.; Lacour, B.; Riedel, C.; Carlin, F. Decontamination of Sugar Syrup by Pulsed Light. J. Food Prot. 2012, 75, 913–917. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed Light Treatments for Food Preservation. A Review. Food Bioprocess Technol. 2010, 3, 13–23. [Google Scholar] [CrossRef]
- Ozer, N.P.; Demirci, A. Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes inoculated on raw salmon fillets by pulsed UV-light treatment. Int. J. Food Sci. Technol. 2006, 41, 354–360. [Google Scholar] [CrossRef]
- Júnior, L.M.; Cristianini, M.; Anjos, C.A.R. Packaging aspects for processing and quality of foods treated by pulsed light. J. Food Process. Preserv. 2020, 44, 14902. [Google Scholar] [CrossRef]
- Gayán, E.; Serrano, M.J.; Raso, J.; Álvarez, I.; Condón, S. Inactivation of Salmonella enterica by UV-C Light Alone and in Combination with Mild Temperatures. Appl. Environ. Microbiol. 2012, 78, 8353–8361. [Google Scholar] [CrossRef] [PubMed]
- Fellows, P.J. Food Processing Technologies: Principles and Practices, 4th ed.; Woodhead Publishing: Sawston, UK; Elsevier: Cambridge, MA, USA, 2017; ISBN 978-0-08-101907-8. [Google Scholar]
- Indiarto, R.; Qonit, M.A.H. A review of irradiation technologies on food and agricultural products. Int. J. Sci. Technol. Res. 2020, 9, 4411–4414. [Google Scholar]
- Prakash, A. Particular applications of food irradiation fresh produce. Radiat. Phys. Chem. 2016, 129, 50–52. [Google Scholar] [CrossRef][Green Version]
- Handayani, M.; Permawati, H. Gamma irradiation technology to preservation of foodstuffs as an effort to maintain quality and acquaint the significant role of nuclear on food production to Indonesia society: A Review. Energy Procedia 2017, 127, 302–309. [Google Scholar] [CrossRef]
- Ricciardi, E.F.; Lacivita, V.; Conte, A.; Chiaravalle, E.; Zambrini, A.V.; Del Nobile, M.A. X-ray irradiation as a valid technique to prolong food shelf life: The case of ricotta cheese. Int. Dairy J. 2019, 99, 104547. [Google Scholar] [CrossRef]
- Indiarto, R.; Pratama, A.W.; Sari, T.I.; Theodora, H.C. Food Irradiation Technology: A Review of The Uses and Their Capabilities. Int. J. Eng. Trends Technol. 2020, 68, 91–98. [Google Scholar] [CrossRef]
- Casani, S.; Leth, T.; Knøchel, S. Water reuse in a shrimp processing line: Safety considerations using a HACCP approach. Food Control. 2006, 17, 540–550. [Google Scholar] [CrossRef]
- Cullen, P.; Tiwari, B.; O’Donnell, C.; Muthukumarappan, K. Modelling approaches to ozone processing of liquid foods. Trends Food Sci. Technol. 2009, 20, 125–136. [Google Scholar] [CrossRef]
- Charoux, C.M.G.; Patange, A.; Lamba, S.; O’Donnell, C.P.; Toward, B.K.; Scannell, A.G.M. Application of non thermal plasma technology on safety and quality of dried food ingredients. J. Appl. Microbiol. 2021, 130, 325–340. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Cheng, J.-H.; Sun, D.-W. Effects of Mild Oxidative and Structural Modifications Induced by Argon Plasma on Physicochemical Properties of Actomyosin from King Prawn (Litopenaeus vannamei). J. Agric. Food Chem. 2018, 66, 13285–13294. [Google Scholar] [CrossRef] [PubMed]
- Ziuzina, D.; Han, L.; Cullen, P.; Bourke, P. Cold plasma inactivation of internalised bacteria and biofilms for Salmonella enterica serovar Typhimurium, Listeria monocytogenes and Escherichia coli. Int. J. Food Microbiol. 2015, 210, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.; Ranadheera, C.S.; et al. Cold plasma processing of milk and dairy products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal Plasma Inactivation of Food-Borne Pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Mishra, R.; Bhatia, S.; Pal, R.; Visen, A.; Trivedi, H. Cold plasma: Emerging as the new standard in food safety. Res. Inv. Int. J. Eng. Sci. 2016, 6, 15–20. Available online: http://www.researchinventy.com/ (accessed on 15 March 2021).
- Ucar, Y.; Ceylan, Z.; Durmus, M.; Tomar, O.; Cetinkaya, T. Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends Food Sci. Technol. 2021, 114, 355–371. [Google Scholar] [CrossRef]
- Shahbaz, H.M.; Kim, J.U.; Kim, S.H.; Park, J. Chapter 7—Advances in Nonthermal Processing Technologies for Enhanced Microbiological Safety and Quality of Fresh Fruit and Juice Products. In Food Processing for Increased Quality and Consumption, Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 179–217. [Google Scholar]
- Priyadarshini, A.; Rajauria, G.; O’Donnell, C.P.; Tiwari, B.K. Emerging food processing technologies and factors impacting their industrial adoption. Crit. Rev. Food Sci. Nutr. 2019, 59, 3082–3101. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.; Bekhit, A.E.-D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Liepa, M.; Zagorska, J.; Galoburda, R. High-Pressure processing as novel technology in dairy industry: A Review. Res. Rural Dev. 2016, 1, 76–83. Available online: https://llufb.llu.lv/conference/Research-for-Rural-Development/2016/LatviaResRuralDev_22nd_vol1-76-83.pdf (accessed on 15 March 2021).
- Pingret, D.; Fabiano-Tixier, A.-S.; Chemat, F. Degradation during application of ultrasound in food processing: A review. Food Control. 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Harder, M.N.C.; Arthur, V.; Arthur, P.B. Irradiation of Foods: Processing Technology and Effects on Nutrients: Effect of Ionizing Radiation on Food Components. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 476–481. [Google Scholar]
- Magnavita, N.; Fileni, A. Occupational risk caused by ultrasound in medicine. Radiol. Med. 1994, 88, 107–111. [Google Scholar]
- Huang, Y.; Chen, H. A novel water-assisted pulsed light processing for decontamination of blueberries. Food Microbiol. 2014, 40, 1–8. [Google Scholar] [CrossRef]
- US FDA. US Food and Drug Administration: “UV Lights and Lamps: Ultraviolet-C Radiation, Disinfection, and Coronavirus”. 2021. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/uv-lights-and-lamps-ultraviolet-c-radiation-disinfection-and-coronavirus (accessed on 20 May 2021).
- Syaza, S.; Umar, R.; Hazmin, S.; Kamarudin, M.; Hassan, A.; Juahir, H. Non-ionizing radiation as threat in daily life. J. Fundam. Appl. Sci. 2018, 9, 308. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.-J.; Park, S.; Kim, K.; Choe, W.; Yoo, S.J.; Jo, C. Pathogen inactivation and quality changes in sliced cheddar cheese treated using flexible thin-layer dielectric barrier discharge plasma. Food Res. Int. 2015, 69, 57–63. [Google Scholar] [CrossRef]
- Gavahian, M.; Khaneghah, A.M. Cold plasma as a tool for the elimination of food contaminants: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2019, 60, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, F.; Stratakos, A.C.; Koidis, A.; Berardinelli, A.; Cevoli, C.; Ragni, L.; Mancusi, R.; Manfreda, G.; Trevisani, M. Atmospheric cold plasma process for vegetable leaf decontamination: A feasibility study on radicchio (red chicory, Cichorium intybus L.). Food Control. 2016, 60, 552–559. [Google Scholar] [CrossRef]
- Mandal, R.; Singh, A.; Singh, A.P. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Knorr, D.; Froehling, A.; Jaeger, H.; Reineke, K.; Schlueter, O.; Schoessler, K. Emerging Technologies for Targeted Food Processing. In Advances in Food Process Engineering Research and Applications; Yanniotis, S., Taoukis, P., Stoforos, N.G., Karathanos, V.T., Eds.; Springer: Boston, MA, USA, 2013; pp. 341–374. [Google Scholar]
- Song, H.P.; Kim, B.; Choe, J.H.; Jung, S.; Moon, S.Y.; Choe, W.; Jo, C. Evaluation of atmospheric pressure plasma to improve the safety of sliced cheese and ham inoculated by 3-strain cocktail Listeria monocytogenes. Food Microbiol. 2009, 26, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Kahar, S.P.; Ranveer, R.C.; Annapure, U.S. Cold plasma an emerging non thermal technology for milk and milk products: A review. Int. J. Dairy Technol. 2021. [Google Scholar] [CrossRef]
- Asaithambi, N.; Singh, S.K.; Singha, P. Current status of non-thermal processing of probiotic foods: A review. J. Food Eng. 2021, 303, 110567. [Google Scholar] [CrossRef]
- Okpala, C.O.R.; Korzeniowska, M. Understanding the relevance of quality management in agro-food product industry: From ethical considerations to assuring food hygiene quality safety standards and its associated processes. Food Rev. Int. 2021, (in press). [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting Non-Thermal Food Processing and Preservation Methods—Action Mechanisms, Pros and Cons: A Technological Update (2016–2021). Foods 2021, 10, 1430. https://doi.org/10.3390/foods10061430
Chacha JS, Zhang L, Ofoedu CE, Suleiman RA, Dotto JM, Roobab U, Agunbiade AO, Duguma HT, Mkojera BT, Hossaini SM, et al. Revisiting Non-Thermal Food Processing and Preservation Methods—Action Mechanisms, Pros and Cons: A Technological Update (2016–2021). Foods. 2021; 10(6):1430. https://doi.org/10.3390/foods10061430
Chicago/Turabian StyleChacha, James S., Liyan Zhang, Chigozie E. Ofoedu, Rashid A. Suleiman, Joachim M. Dotto, Ume Roobab, Adedoyin O. Agunbiade, Haile Tesfaye Duguma, Beatha T. Mkojera, Sayed Mahdi Hossaini, and et al. 2021. "Revisiting Non-Thermal Food Processing and Preservation Methods—Action Mechanisms, Pros and Cons: A Technological Update (2016–2021)" Foods 10, no. 6: 1430. https://doi.org/10.3390/foods10061430
APA StyleChacha, J. S., Zhang, L., Ofoedu, C. E., Suleiman, R. A., Dotto, J. M., Roobab, U., Agunbiade, A. O., Duguma, H. T., Mkojera, B. T., Hossaini, S. M., Rasaq, W. A., Shorstkii, I., Okpala, C. O. R., Korzeniowska, M., & Guiné, R. P. F. (2021). Revisiting Non-Thermal Food Processing and Preservation Methods—Action Mechanisms, Pros and Cons: A Technological Update (2016–2021). Foods, 10(6), 1430. https://doi.org/10.3390/foods10061430










