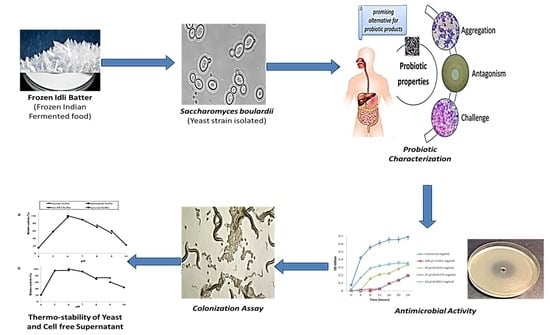
In Vitro Probiotic Evaluation of Saccharomyces boulardii with Antimicrobial Spectrum in a Caenorhabditis elegans Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenotypic and Genotypic Identification
2.2. Commercial Probiotics
2.3. Culture Technique
2.4. Acid and Alkaline Tolerance
2.5. Bile Tolerance
2.6. In Vitro Survival in Gastric Juice
2.7. Determination of Simulated Transit Tolerance
2.8. Cell Surface Hydrophobicity
2.9. C. elegans Gut Colonization Assay
2.10. Antimicrobial Activity
2.11. Aggregation and Co-Aggregation Assays
2.12. Susceptibility to Antibiotics
2.13. Carbohydrates Fermentation Assay
2.14. Stability of Cell-Free Supernatant
2.15. Statistical Analysis
3. Results and Discussion
3.1. Phenotypic and Genotypic Identification
3.2. Acid Tolerance
3.3. Effects of Bile Salt on the Viability
3.4. Effects of Low pH and Gastric Juice on the Viability (Pancreatin Tolerance Test)
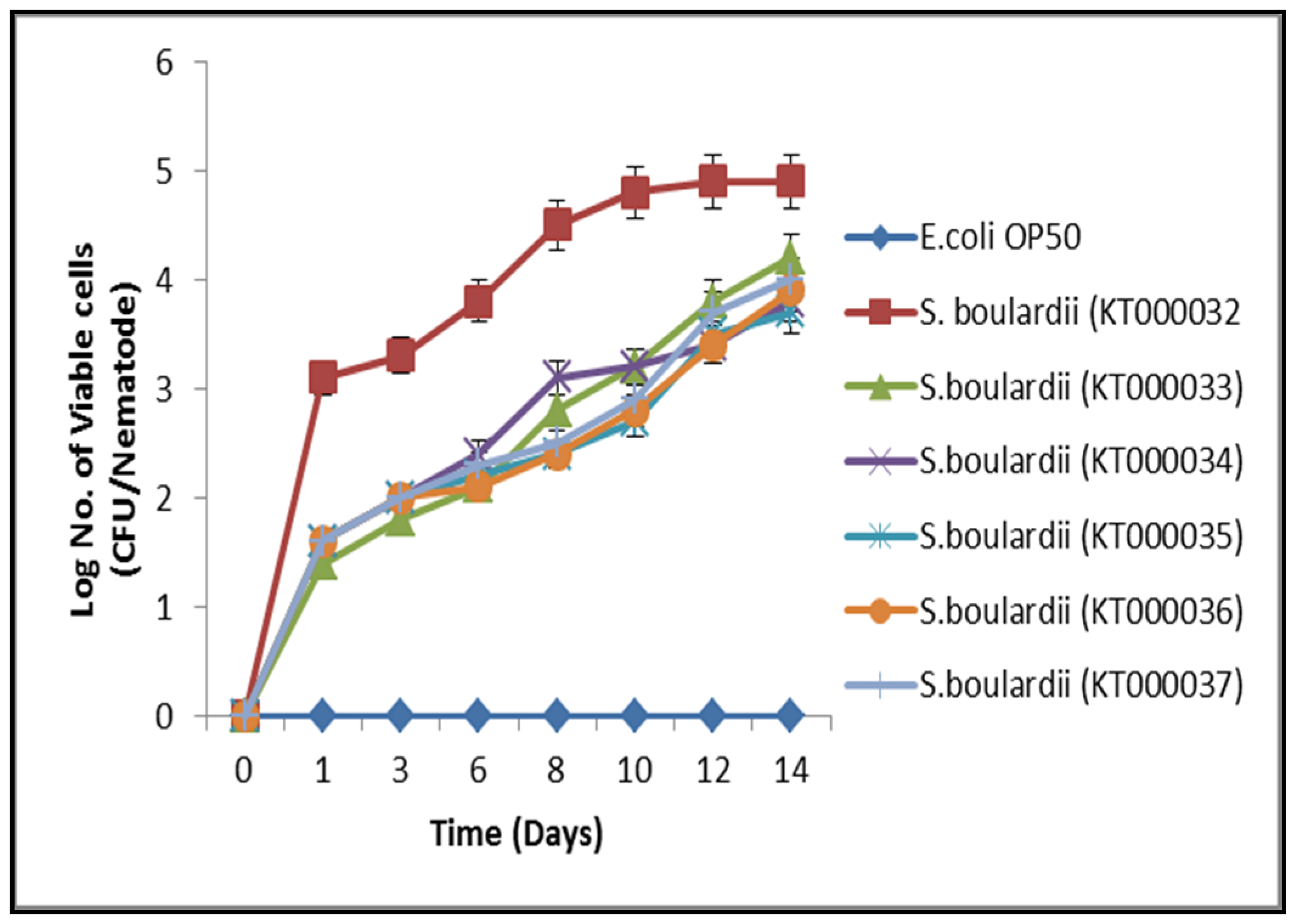
3.5. Gut Colonization Assay
3.5.1. Cell Surface Hydrophobicity—In Vitro
3.5.2. C. elegans Gut Colonization Ability—In Vivo Hydrophobicity Analysis
3.6. Antimicrobial Activity Determination
3.6.1. Disc Diffusion Method
3.6.2. Growth Curve—Minimum Growth Inhibitory Concentration Determination
3.7. Aggregation and Co-Aggregation
3.8. Susceptibility of Antibiotics
3.9. Sugar Fermentation Assay
3.10. Comparative Analysis of Antagonist Activity within Commercial and Isolated Probiotics
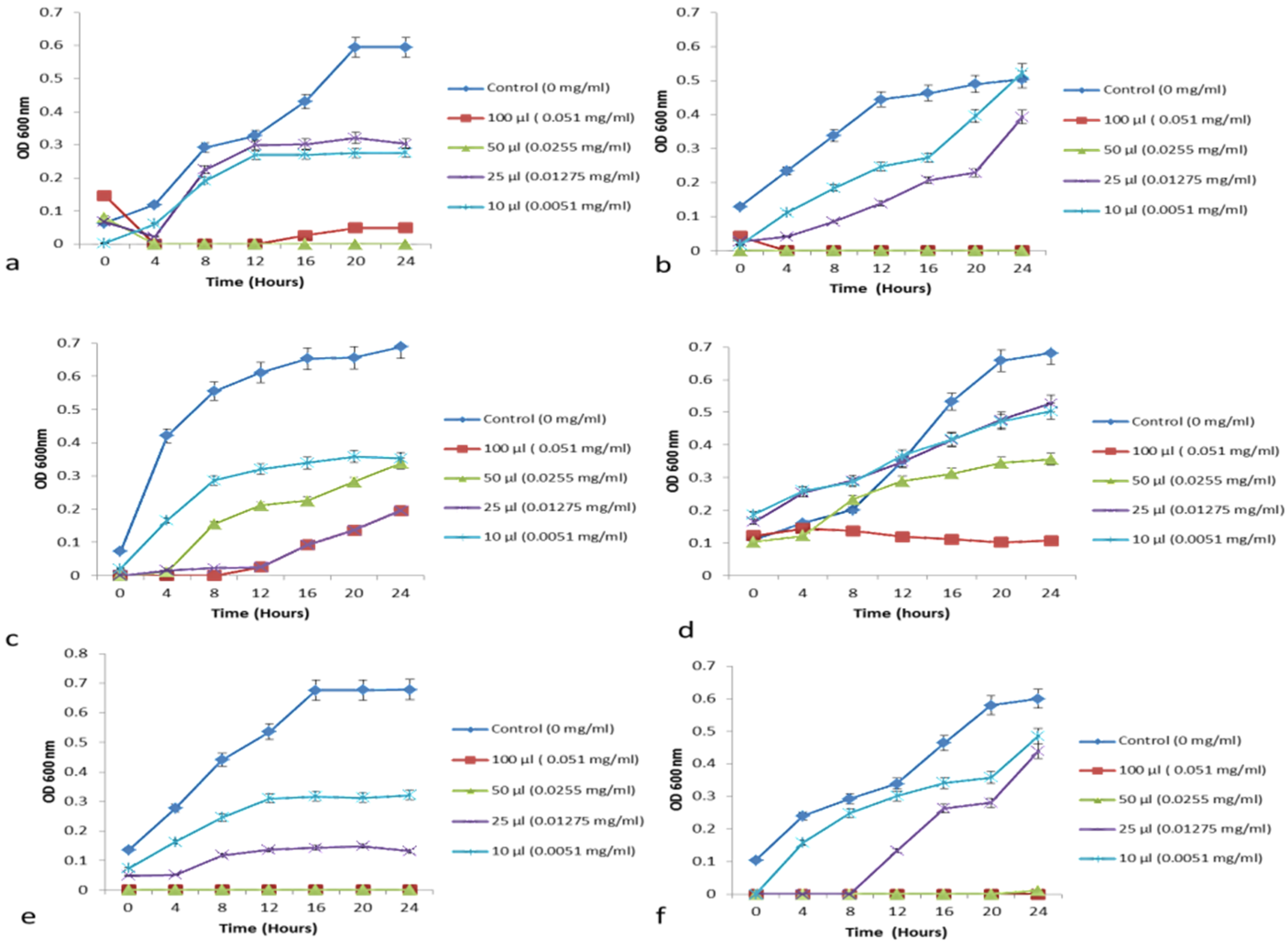
3.11. Thermo-Stability of the Yeasts
3.12. Stability of Cell-Free Supernatant (CFS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for isolation, phenotypic characterization, and maintenance of yeasts. In The Yeasts; Elsevier: Amsterdam, The Netherland, 2011; pp. 87–110. [Google Scholar]
- Rima, H.; Steve, L.; Ismail, F. Antimicrobial and probiotic properties of yeasts: From fundamental to novel applications. Front. Microbiol. 2012, 3, 421. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Saavedra, N.Y.; Romero-Geraldo, R. Cloning and sequencing the genomic encoding region of copper–zinc superoxide dismutase enzyme from several marine strains of the genus Debaryomyces (Lodder & Kreger-van Rij). Yeast 2011, 18, 1227–1238. [Google Scholar]
- Chelliah, R.; Ramakrishnan, S.R.; Prabhu, P.R.; Antony, U. Evaluation of antimicrobial activity and probiotic properties of wild-strain Pichia kudriavzevii isolated from frozen idli batter. Yeast 2016, 33, 385–401. [Google Scholar] [CrossRef]
- Hernandez-Saavedra, N.Y.; Ochoa, J.L.; Vázquez-Duhalt, R. Effect of salinity in the growth of the marine yeast Rhodotorula rubra. Microbios 1994, 80, 99–106. [Google Scholar]
- Henker, J.; Laass, M.; Blokhin, B.M.; Bolbot, Y.K.; Maydannik, V.G.; Elze, M.; Wolff, C.; Schulze, J. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur. J. Pediatrics 2007, 166, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cintas, L.M.; Casaus, M.P.; Herranz, C.; Nes, I.F.; Hernández, P.E. Bacteriocins of lactic acid bacteria. Food Sci. Technol. Int. 2001, 7, 281–305. [Google Scholar] [CrossRef]
- Merten, C.; Ferrari, P.; Bakker, M.; Boss, A.; Hearty, A.; Leclercq, C.; Lindtner, O.; Tlustos, C.; Verger, P.; Volatier, J.-L.; et al. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Addit. Contam. Part A 2011, 28, 975–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buts, J.P. Twenty-five years of research on Saccharomyces boulardii trophic effects: Updates and perspectives. Dig. Dis. Sci. 2009, 54, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Brunser, O.; Szajewska, H. Saccharomyces boulardii in childhood. Eur. J. Pediatrics 2009, 168, 253–265. [Google Scholar] [CrossRef]
- Czerucka, D.; Rampal, P. Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes Infect. 2002, 4, 733–739. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. yeast as probiotics–Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Foligné, B.; Dewulf, J.; Vandekerckove, P.; Pignède, G.; Pot, B. Probiotic yeasts: Anti-inflammatory potential of various non-pathogenic strains in experimental colitis in mice. World J. Gastroenterol. WJG 2010, 16, 2134. [Google Scholar] [CrossRef]
- Guslandi, M.; Giollo, P.; Testoni, P.A. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2003, 15, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Hyronimus, B.; Le Marrec, C.; Sassi, A.H.; Deschamps, A. Acid and bile tolerance of spore-forming lactic acid bacteria. Int. J. Food Microbiol. 2000, 61, 193–197. [Google Scholar] [CrossRef]
- Chen, C.-C.; Kong, M.-S.; Lai, M.-W.; Chao, H.-C.; Chang, K.-W.; Chen, S.-Y.; Huang, Y.-C.; Chiu, C.-H.; Li, W.-C.; Lin, P.-Y.; et al. Probiotics Have Clinical, Microbiologic, and Immunologic Efficacy in Acute Infectious Diarrhea. Pediatr. Infect. Dis. J. 2010, 29, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Psomas, E.-A.; Andrighetto, C.; Litopoulou-Tzanetaki, E.; Lombardi, A.; Tzanetakis, N. Some probiotic properties of yeast isolates from infant faeces and Feta cheese. Int. J. Food Microbiol. 2001, 69, 125–133. [Google Scholar] [CrossRef]
- Zago, M.; Lanza, B.; Rossetti, L.; Muzzalupo, I.; Carminati, D.; Giraffa, G. Selection of Lactobacillus plantarum strains to use as starters in fermented table olives: Oleuropeinase activity and phage sensitivity. Food Microbiol. 2013, 34, 81–87. [Google Scholar] [CrossRef]
- Chelliah, R.; Choi, J.G.; Hwang, S.B.; Park, B.J.; Daliri, E.B.M.; Kim, S.H.; Wei, S.; Ramakrishnan, S.R.; Oh, D.H. In vitro and in vivo defensive effect of probiotic LAB against Pseudomonas aeruginosa using Caenorhabditis elegans model. Virulence 2018, 9, 1489–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinheimer, J.A.; Demkow, M.R.; Candioti, M.C. Inhibition of coliform bacteria by lactic cultures. Aust. J. Dairy Technol. 1990, 45, 5–9. [Google Scholar]
- Vijayabaskar, P.; Somasundaram, S. Isolation of Bacteriocin Producing Lactic Acid Bacteria from Fish Gut and Probiotic Activity against Common Fresh Water Fish Pathogen Aeromonas hydrophila. Biotechnology 2008, 7, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Hedberg, M.; Hasslöf, P.; Sjöström, I.; Twetman, S.; Stecksén-Blicks, C. Sugar fermentation in probiotic bacteria–an in vitro study. Oral Microbiol. Immunol. 2008, 23, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Ahmadova, A.; Todorov, S.; Choiset, Y.; Rabesona, H.; Zadi, T.M.; Kuliyev, A.; Franco, B.D.G.D.M.; Chobert, J.-M.; Haertlé, T. Evaluation of antimicrobial activity, probiotic properties and safety of wild strain Enterococcus faecium AQ71 isolated from Azerbaijani Motal cheese. Food Control. 2013, 30, 631–641. [Google Scholar] [CrossRef]
- Kühle, A.V.D.A.; Skovgaard, K.; Jespersen, L. In vitro screening of probiotic properties of Saccharomyces cerevisiae var. boulardii and food-borne Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2005, 101, 29–39. [Google Scholar] [CrossRef]
- Kourelis, A.; Kotzamanidis, C.; Litopoulou-Tzanetaki, E.; Scouras, Z.G.; Tzanetakis, N.; Yiangou, M. Preliminary probiotic selection of dairy and human yeast strains. J. Biol. Res. 2010, 13, 93. [Google Scholar]
- Ogunremi, O.R.; Sanni, A.I.; Agrawal, R. Probiotic potentials of yeasts isolated from some cereal-based N igerian traditional fermented food products. J. Appl. Microbiol. 2015, 119, 797–808. [Google Scholar] [CrossRef]
- Wadstroum, T.; Andersson, K.; Sydow, M.; Axelsson, L.; Lindgren, S.; Gullmar, B. Surface properties of lactobacilli isolated from the small intestine of pigs. J. Appl. Bacteriol. 1987, 62, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Shekrabi, S.P.H.; Soltani, M.; Mehrgan, M.S. Effects of Potential Probiotic Enterococcus casseliflavus (EC-001) on Growth Performance, Immunity, and Resistance to Aeromonas hydrophila Infection in Common Carp (Cyprinus carpio). Probiotics Antimicrob. Proteins 2021, 1–10. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Yu, H.; Gong, J. Lactobacillus Regulates Caenorhabditis elegans Cell Signaling to Combat Salmonella Infection. Front. Immunol. 2021, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Saravanakumar, K.; Daliri, E.B.M.; Kim, J.H.; Lee, J.K.; Jo, H.Y.; Kim, S.H.; Ramakrishnan, S.R.; Madar, I.H.; Wei, S.; et al. Unveiling the potentials of bacteriocin (Pediocin L50) from Pediococcus acidilactici with antagonist spectrum in a Caenorhabditis elegans model. Int. J. Biol. Macromol. 2020, 143, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Botes, M.; Loos, B.; Van Reenen, C.A.; Dicks, L.M.T. Adhesion of the probiotic strains Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 to Caco-2 cells under conditions simulating the intestinal tract, and in the presence of antibiotics and anti-inflammatory medicaments. Arch. Microbiol. 2008, 190, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Saravanakumar, K.; Daliri, E.B.-M.; Kim, J.-H.; Lee, J.-K.; Jo, H.-Y.; Madar, I.H.; Kim, S.-H.; Ramakrishnan, S.R.; Rubab, M.; et al. An effective datasets describing antimicrobial peptide produced from Pediococcus acidilactici—purification and mode of action determined by molecular docking. Data Brief 2020, 31, 105745. [Google Scholar] [CrossRef]
- Fan, K.C.; Lin, J.; Yannuzzi, N.A.; Al-Khersan, H.; Patel, N.A.; Maestre-Mesa, J.; Zaidi, M.; Miller, D.; Flynn, H.W., Jr. In vitro susceptibilities of methicillin-susceptible and resistant staphylococci to traditional antibiotics compared to a novel fluoroquinolone. J. Ophthalmic Inflamm. Infect. 2020, 10, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Zhang, Y.; Zou, K.; Brandman, O.; Luo, C.; Ouyang, Q.; Li, H. Molecular phenotyping of aging in single yeast cells using a novel microfluidic device. Aging Cell 2012, 11, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Sourabh, A.; Kanwar, S.S.; Sharma, O.P. Screening of indigenous yeast isolates obtained from traditional fermented foods of Western Himalayas for probiotic attributes. J. Yeast Fungal Res. 2011, 2, 117–126. [Google Scholar]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Measurement of aggregation properties between probiotics and pathogens: In vitro evaluation of different methods. J. Microbiol. Methods 2007, 71, 71–74. [Google Scholar] [CrossRef]
- Crittenden, R.; Karppinen, S.; Ojanen, S.; Tenkanen, M.; Fagerström, R.; Mättö, J.; Saarela, M.; Mattila-Sandholm, T.; Poutanen, K. In vitro fermentation of cereal dietary fibre carbohydrates by probiotic and intestinal bacteria. J. Sci. Food Agric. 2002, 82, 781–789. [Google Scholar] [CrossRef]
- Sreesankar, E.; Senthilkumar, R.; Bharathi, V.; Mishra, R.K.; Mishra, K. Functional diversification of yeast telomere associated protein, Rif1, in higher eukaryotes. BMC Genomic 2012, 13, 255. [Google Scholar] [CrossRef] [Green Version]
- Nourouzi, J.; Mirzaii, M.; Norouzi, M. Study of Lactobacillus as probiotic bacteria. Iran. J. Public Health 2004, 33, 2. [Google Scholar]

| Isolated Strains | pH | Survival (log CFU mL−1) at Different Time Periods (h) | Survival (%) at Different Time Periods (h) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | 120 | 24 | 48 | 72 | 96 | 120 | ||
| S. boulardii (KT000032) | 1 | 8.34 ± 0.10 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | - | - | - | - | - |
| 2 | 8.36 ± 0.03 | 8.02 ± 0.02 | 7.33 ± 0.12 | 7.27 ± 0.09 | 7.09 ± 0.02 | 6.89 ± 0.12 | 95 b | 87 c | 86 c | 84 d | 82 d | |
| 3 | 8.21 ± 0.32 | 8.16 ± 0.17 | 8.13 ± 0.12 | 7.75 ± 0.03 | 7.13 ± 0.19 | 7.12 ± 0.43 | 98 a | 98 a | 94 b | 87 c | 86 c | |
| 4 | 8.60 ± 0.08 | 8.52 ± 0.19 | 8.50 ± 0.32 | 8.21 ± 0.03 | 8.58 ± 0.31 | 8.00 ± 0.05 | 95 b | 99 a | 96 a | 89 c | 83 d | |
| 5 | 8.86 ± 0.11 | 8.42 ± 0.21 | 8.42 ± 0.05 | 8.29 ± 0.13 | 8.24 ± 0.04 | 6.20 ± 0.07 | 99 a | 99 a | 87 c | 87 c | 86 c | |
| 6 | 8.44 ± 0.22 | 8.23 ± 0.18 | 8.22 ± 0.18 | 8.11 ± 0.19 | 8.12 ± 0.01 | 8.04 ± 0.21 | 97 a | 100 a | 87 c | 87 c | 85 c | |
| 7 | 8.60 ± 0.08 | 8.52 ± 0.19 | 8.50 ± 0.32 | 8.21 ± 0.03 | 8.58 ± 0.31 | 8.00 ± 0.05 | 95 b | 99 a | 96 a | 89 c | 83 d | |
| S. boulardii (KT000033) | 1 | 8.34 ± 0.10 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | ND | ND | ND | ND | ND |
| 2 | 8.13 ± 0.03 | 4.06 ± 0.03 | 4.03 ± 0.02 | 4.45 ± 0.01 | 3.58 ± 0.31 | 2.68 ± 0.07 | 43 g | 38 h | 37 h | 34 i | 24 j | |
| 3 | 8.86 ± 0.11 | 8.42 ± 0.21 | 8.42 ± 0.05 | 8.29 ± 0.13 | 8.24 ± 0.04 | 6.20 ± 0.07 | 99 a | 99 a | 87 c | 87 c | 86 c | |
| 4 | 8.87 ± 0.16 | 8.39 ± 0.22 | 8.39 ± 0.21 | 8.11 ± 0.14 | 8.04 ± 0.31 | 6.86 ± 0.32 | 100 a | 100 a | 85 c | 85 c | 83 d | |
| 5 | 8.73 ± 0.02 | 8.57 ± 0.25 | 8.43 ± 0.06 | 8.45 ± 0.17 | 8.17 ± 0.36 | 7.12 ± 0.01 | 99 a | 98 a | 88 c | 85 c | 84 d | |
| 6 | 8.69 ± 0.02 | 8.41 ± 0.24 | 8.23 ± 0.15 | 8.76 ± 0.06 | 8.41 ± 0.16 | 6.21 ± 0.17 | 99 a | 97 a | 92 b | 88 c | 76 e | |
| 7 | 8.83 ± 0.02 | 8.26 ± 0.26 | 8.19 ± 0.02 | 8.38 ± 0.10 | 8.23 ± 0.08 | 7.15 ± 0.12 | 97 | 97 a | 88 c | 87 c | 86 c | |
| S. boulardii (KT000034) | 1 | 8.34 ± 0.10 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | ND | ND | ND | ND | ND |
| 2 | 8.16 ± 0.14 | 8.18 ± 0.01 | 8.15 ± 0.02 | 8.23 ± 0.43 | 8.12 ± 0.04 | 7.23 ± 0.05 | 97 a | 98 a | 87 c | 86 c | 77 e | |
| 3 | 8.44 ± 0.22 | 8.23 ± 0.18 | 8.22 ± 0.18 | 8.11 ± 0.19 | 8.12 ± 0.01 | 8.04 ± 0.21 | 97 a | 100 a | 87 c | 87 c | 85 c | |
| 4 | 8.92 ± 0.04 | 8.33 ± 0.20 | 8.33 ± 0.01 | 8.12 ± 0.12 | 8.05 ± 0.32 | 7.79 ± 0.41 | 99 a | 99 a | 86 c | 85 c | 83 d | |
| 5 | 8.44 ± 0.14 | 8.27 ± 0.27 | 8.19 ± 0.02 | 7.63 ± 0.19 | 7.12 ± 0.31 | 7.04 ± 0.10 | 99 a | 98 a | 92 b | 87 c | 75 e | |
| 6 | 8.12 ± 0.17 | 8.07 ± 0.28 | 7.32 ± 0.20 | 7.32 ± 0.21 | 7.19 ± 0.40 | 6.20 ± 0.04 | 97 a | 89 | 89 c | 88 c | 77 e | |
| 7 | 8.73 ± 0.02 | 8.57 ± 0.25 | 8.43 ± 0.06 | 8.45 ± 0.17 | 8.17 ± 0.36 | 7.12 ± 0.01 | 99 a | 98 a | 88 c | 85 c | 84 d | |
| S. boulardii (KT000035) | 1 | 8.34 ± 0.10 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | ND | ND | ND | ND | ND |
| 2 | 8.78 ± 0.22 | 8.05 ± 0.23 | 8.05 ± 0.03 | 8.33 ± 0.11 | 8.32 ± 0.05 | 6.32 ± 0.06 | 98 a | 98 | 90 b | 90 b | 79 e | |
| 3 | 8.69 ± 0.02 | 8.41 ± 0.24 | 8.23 ± 0.15 | 8.76 ± 0.06 | 8.41 ± 0.16 | 6.21 ± 0.17 | 99 a | 97 a | 92 b | 88 c | 76 e | |
| 4 | 8.73 ± 0.02 | 8.57 ± 0.25 | 8.43 ± 0.06 | 8.45 ± 0.17 | 8.17 ± 0.36 | 7.12 ± 0.01 | 99 a | 98 a | 88 c | 85 c | 84 d | |
| 5 | 8.83 ± 0.02 | 8.26 ± 0.26 | 8.19 ± 0.02 | 8.38 ± 0.10 | 8.23 ± 0.08 | 7.15 ± 0.12 | 97 a | 97 a | 88 c | 87 c | 86 c | |
| 6 | 8.44 ± 0.14 | 8.27 ± 0.27 | 8.19 ± 0.02 | 7.63 ± 0.19 | 7.12 ± 0.31 | 7.04 ± 0.10 | 99 a | 98 a | 92 b | 87 c | 75 e | |
| 7 | 8.12 ± 0.17 | 8.07 ± 0.28 | 7.32 ± 0.20 | 7.32 ± 0.21 | 7.19 ± 0.40 | 6.20 ± 0.04 | 97 a | 89 c | 89 c | 88 c | 77 e | |
| S. boulardii (KT000036) | 1 | 8.34 ± 0.10 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | ND | ND | ND | ND | ND |
| 2 | 8.26 ± 0.13 | 8.48 ± 0.29 | 7.32 ± 0.21 | 7.12 ± 0.22 | 7.31 ± 0.23 | 6.11 ± 0.13 | 99 a | 87 c | 85 c | 76 e | 74 f | |
| 3 | 8.73 ± 0.02 | 8.57 ± 0.25 | 8.43 ± 0.06 | 8.45 ± 0.17 | 8.17 ± 0.36 | 7.12 ± 0.01 | 99 a | 98 a | 88 c | 85 c | 84 d | |
| 4 | 8.83 ± 0.02 | 8.26 ± 0.26 | 8.19 ± 0.02 | 8.38 ± 0.10 | 8.23 ± 0.08 | 7.15 ± 0.12 | 97 a | 97 a | 88 c | 87 c | 86 c | |
| 5 | 8.44 ± 0.14 | 8.27 ± 0.27 | 8.19 ± 0.02 | 7.63 ± 0.19 | 7.12 ± 0.31 | 7.04 ± 0.10 | 99 a | 98 a | 92 b | 87 c | 75 e | |
| 6 | 8.12 ± 0.17 | 8.07 ± 0.28 | 7.32 ± 0.20 | 7.32 ± 0.21 | 7.19 ± 0.40 | 6.20 ± 0.04 | 97 a | 89 c | 89 c | 88 c | 77 e | |
| 7 | 8.26 ± 0.13 | 8.48 ± 0.29 | 7.32 ± 0.21 | 7.12 ± 0.22 | 7.31 ± 0.23 | 6.11 ± 0.13 | 99 a | 87 c | 85 c | 76 e | 74 e | |
| S. boulardii (KT000037) | 1 | 8.34 ± 0.10 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | ND | ND | ND | ND | ND |
| 2 | 8.69 ± 0.02 | 8.41 ± 0.24 | 8.23 ± 0.15 | 8.76 ± 0.06 | 8.41 ± 0.16 | 6.21 ± 0.17 | 99 a | 97 a | 92 b | 88 c | 76 e | |
| 3 | 8.73 ± 0.02 | 8.57 ± 0.25 | 8.43 ± 0.06 | 8.45 ± 0.17 | 8.17 ± 0.36 | 7.12 ± 0.01 | 99 a | 98 a | 88 c | 85 c | 84 d | |
| 4 | 8.83 ± 0.02 | 8.26 ± 0.26 | 8.19 ± 0.02 | 8.38 ± 0.10 | 8.23 ± 0.08 | 7.15 ± 0.12 | 97 a | 97 a | 88 c | 87 c | 86 c | |
| 5 | 8.69 ± 0.02 | 8.41 ± 0.24 | 8.23 ± 0.15 | 8.76 ± 0.06 | 8.41 ± 0.16 | 6.21 ± 0.17 | 99 a | 97 a | 92 b | 88 c | 76 e | |
| 6 | 8.73 ± 0.02 | 8.57 ± 0.25 | 8.43 ± 0.06 | 8.45 ± 0.17 | 8.17 ± 0.36 | 7.12 ± 0.01 | 99 a | 98 a | 88 c | 85 c | 84 d | |
| 7 | 8.60 ± 0.08 | 8.52 ± 0.19 | 8.50 ± 0.32 | 8.21 ± 0.03 | 8.58 ± 0.31 | 8.00 ± 0.05 | 95 b | 99 a | 96 a | 89 c | 83 d | |
| Isolated Strains | Survival (log CFU mL−1) at 0 h | Survival at Different Bile Salt (oxgall) Concentrations (% w/v) | |||||
|---|---|---|---|---|---|---|---|
| 0.1 (log CFU mL−1) | % | 0.3 (log CFU mL−1) | % | 0.5 (log CFU mL−1) | % | ||
| S. boulardii (KT000032) | 8.12 ± 0.07 | 8.09 ± 0.07 | 99 a | 7.75 ± 0.16 | 95 d | 6.22 ± 0.09 | 77 i |
| S. boulardii (KT000033) | 8.36 ± 0.10 | 8.12 ± 0.17 | 97 c | 7.37 ± 0.31 | 88 g | 5.78 ± 0.21 | 69 k |
| S. boulardii (KT000034) | 8.62 ± 0.03 | 8.36 ± 0.24 | 97 c | 7.56 ± 0.12 | 88 g | 5.66 ± 0.18 | 66 m |
| S. boulardii (KT000035) | 8.35 ± 0.22 | 8.16 ± 0.18 | 98 b | 7.42 ± 0.24 | 89 f | 5.76 ± 0.41 | 69 k |
| S. boulardii (KT000036) | 8.49 ± 0.07 | 8.31 ± 0.07 | 98 b | 7.83 ± 0.13 | 92 e | 6.11 ± 0.04 | 72 j |
| S. boulardii (KT000037) | 8.72 ± 0.07 | 8.44 ± 0.23 | 97 c | 7.45 ± 0.18 | 85 h | 5.89 ± 0.12 | 67 l |
| Isolated Strains | Survival (log CFU mL−1) at 0 h | Survival after 2 h in Pepsin Solution (pH 1.2) (3 mg mL−1) | Survival after 6 h in Pancreatin Solution (pH 8.0) (1 mg mL−1) | ||
|---|---|---|---|---|---|
| log CFU mL−1 | % | log CFU mL−1 | % | ||
| S. boulardii (KT000032) | 8.72 ± 0.07 | 7.41 ± 0.16 | 85 b | 7.63 ± 0.11 | 94 a |
| S. boulardii (KT000033) | 8.36 ± 0.10 | 7.28 ± 0.26 | 87 b | 5.38 ± 0.31 | 64 e |
| S. boulardii (KT000034) | 8.62 ± 0.03 | 7.31 ± 0.45 | 85 b | 5.67 ± 0.52 | 66 b |
| S. boulardii (KT000035) | 8.35 ± 0.22 | 7.44 ± 0.18 | 89 b | 5.58 ± 0.23 | 67 d |
| S. boulardii (KT000036) | 8.49 ± 0.07 | 7.17 ± 0.13 | 84 c | 5.48 ± 0.17 | 65 d |
| S. boulardii (KT000037) | 8.12 ± 0.07 | 5.46 ± 0.22 | 63 e | 5.11 ± 0.27 | 63 b |
| Isolated Strains | Xylene Layer (A600nm) | Aqueous Layer (A600nm) | % | Toluene Layer (A600nm) | Aqueous Layer (A600nm) | % |
|---|---|---|---|---|---|---|
| S. boulardii (KT000032) | 0.85± 0.0.3 | 0.67 ± 0.24 | 79 c | 0.81± 0.33 | 0.73 ± 0.22 | 90 a |
| S. boulardii (KT000033) | 0.87± 0.07 | 0.41 ± 0.18 | 47 f | 0.65 ± 0.23 | 0.37 ± 0.08 | 57 e |
| S. boulardii (KT000034) | 0.69 ± 0.06 | 0.33 ± 0.14 | 48 f | 0.77± 0.13 | 0.55 ± 0.21 | 71 c |
| S. boulardii (KT000035) | 0.58 ± 0.09 | 0.27 ± 0.32 | 47 f | 0.73± 0.12 | 0.45 ± 0.13 | 62 d |
| S. boulardii (KT000036) | 0.83 ± 0.13 | 0.47 ± 0.16 | 57 e | 0.84 ± 0.06 | 0.66 ± 0.12 | 90 a |
| S. boulardii (KT000037) | 0.78 ± 0.14 | 0.38 ± 0.13 | 49 g | 0.81± 0.16 | 0.72 ± 0.17 | 89 b |
| Dysentery-Causing Clinical Pathogens | Isolated Strains | |||||
|---|---|---|---|---|---|---|
| Commercial Probiotics | ||||||
| S. boulardii (KT000032) | S. boulardii (KT000033) | S. boulardii (KT000034) | S. boulardii (KT000035) | S. boulardii (KT000036) | S. boulardii (KT000037) | |
| L. reuteri (Ecoflora) | L. reuteri (KT000042) | S. boulardii (Econorm 250 µg) | L. rhamnosus (GR7) | L. acidophilus (MTCC111) | ||
| E. coli | 22 ± 0.04 b | 15 ± 0.08 c | 15 ± 0.01 c | 16 ± 0.11 c | 14 ± 0.07 d | 26 ± 0.12 a |
| 22 ± 0.13 b | 21 ± 0.04 b | 20 ± 0.05 b | 22 ± 0.13 b | 26 ± 0.04 a | ||
| S. aureus | 25 ± 0.4 a | 15 ± 0.05 c | 18 ± 0.22 c | 11 ± 0.16 b | 18 ± 0.11 c | 19 ± 0.45 c |
| 24 ± 0.2 b | 19 ± 0.14 c | 25 ± 0.19 a | 17 ± 0.07 c | 20 ± 0.17 b | ||
| E. faecalis | 21 ± 0.31 b | 18 ± 0.07 c | 22 ± 0.65 b | 15 ± 0.16 a | 12 ± 0.52 b | 17 ± 0.67 c |
| 17 ± 0.11 c | 17 ± 0.11 c | 17 ± 0.21 c | 14 ± 0.15 d | 18 ± 0.69 c | ||
| M. luteus | 20 ± 0.14 | 15 ± 0.16 a | 16 ± 0.47 c | 17 ± 0.25 c | 11 ± 0.51 b | 14 ± 0.89 d |
| 14 ± 0.6 d | 19 ± 0.18 c | 18 ± 0.07 c | 16 ± 0.21 c | 20 ± 0.27 b | ||
| K. pneumoniae | 14 ± 0.01 c | 17 ± 0.01 c | 11 ± 0.66 b | 15 ± 0.56 c | 22 ± 0.07 b | 16 ± 0.58 c |
| 19 ± 0.22 c | 22 ± 0.03 b | 15 ± 0.81 c | 19 ± 0.78 c | 11 ± 0.16 d | ||
| S. typhi | 20 ± 0.01 b | 15 ± 0.11 b | 15 ± 0.01 a | 17 ± 0.31 c | 24 ± 0.07 b | 22 ± 0.23 b |
| 25 ± 0.17 a | 21 ± 0.13 b | 13 ± 0.1 d | 22 ± 0.25 b | 16 ± 0.12 c | ||
| S. paratyphi A | 13 ± 0.07 d | 17 ± 0.14 a | 12 ± 0.31 b | 15 ± 0.67 c | 17 ± 0.21 c | 25 ± 0.31 a |
| 20 ± 0.15 a | 22 ± 0.03 b | 19 ± 0.25 | 25 ± 0.07 a | 21 ± 0.82 b | ||
| S. paratyphi B | 21 ± 0.44 c | 20 ± 0.05 b | 15 ± 0.27 a | 18 ± 0.80 c | 21 ± 0.51 b | 16 ± 0.38 c |
| 20 ± 0.07 b | 21 ± 0.21 b | 26 ± 0.21 a | 21 ± 0.37 b | 15 ± 0.68 a | ||
| P. mirabilis | 21 ± 0.08 b | 16 ± 0.25 c | 21 ± 0.18 b | 17 ± 0.82 c | 15 ± 0.23 a | 19 ± 0.51 c |
| 16 ± 0.04 c | 22 ± 0.27 b | 22 ± 0.19 | 19 ± 0.55 c | 17 ± 0.36 c | ||
| V. cholerae | 25 ± 0.08 a | 14 ± 0.13 d | 10 ± 0.16 b | 15 ± 0.29 c | 17 ± 0.07 | 22 ± 0.22 b |
| 14 ± 0.03 d | 25 ± 0.51 a | 25 ± 0.68 a | 13 ± 0.27 b | 15 ± 0.41 c | ||
| S. flexneri | 22 ± 0.02 d | 18 ± 0.41 d | 18 ± 0.35 c | 17 ± 0.11 c | 15 ± 0.16 c | 25 ± 0.69 a |
| 18 ± 0.02 d | 26 ± 0.26 a | 21 ± 0.21 b | 17 ± 0.18 c | 18 ± 0.13 c | ||
| S. dysenteriae | 23 ± 0.44 b | 12 ± 0.07 b | 10 ± 0.37 b | 14 ± 0.36 d | 21 ± 0.13 b | 21 ± 0.51 b |
| 22 ± 0.07 b | 19 ± 0.48 c | 17 ± 0.27 c | 18 ± 0.42 c | 27 ± 0.38 a | ||
| P. aeruginosa | 22 ± 0.9 b | 11 ± 0.62 b | 11 ± 0.19 d | 16 ± 0.27 c | 14 ± 0.48 d | 21 ± 0.78 b |
| 21 ± 0.015 b | 27 ± 0.04 a | 19 ± 0.16 c | 15 ± 0.23 c | 22 ± 0.41 b | ||
| Isolated Strains | Autoaggregation (%) | Coaggregation (%) with E. coli | Coaggregation (%) with S. aureus | Coaggregation (%) with S. typhimurium | ||||
|---|---|---|---|---|---|---|---|---|
| 5 h | 24 h | 5 h | 24 h | 5 h | 24 h | 5 h | 24 h | |
| S. boulardii (KT000032) | 78.13 ± 0.11 g | 93.14 ± 0.31 b | 35.04 ± 0.12 | 47.14 ± 0.17 j | 30.14 ± 0.06 j | 41.16 ± 0.06 h | 45.3 ± 0.23 m | 65.07 ± 0.16 k |
| S. boulardii (KT000033) | 47.18 ± 0.12 f | 78.11 ± 0.21 c | 18.17 ± 0.04 l | 24.10 ± 0.14 k | 21.17 ± 0.03 k | 37.41 ± 0.07 h | 15.37 ± 0.31 l | 21.08 ± 0.12 k |
| S. boulardii (KT000034) | 42.14 ± 0.22 g | 84.33 ± 0.23 b | 20.25 ± 0.11 k | 21.07 ± 0.15 k | 27.11 ± 0.07 j | 32.15 ± 0.14 i | 16.28 ± 0.18 l | 24.31 ± 0.03 k |
| S. boulardii (KT000035) | 49.12 ± 0.07 f | 85.18 ± 0.05 a | 16.26 ± 0.15 l | 26.13 ± 0.11 j | 25.09 ± 0.02 j | 35.12 ± 0.08 h | 15.41 ± 0.15 l | 20.17 ± 0.15 k |
| S. boulardii (KT000036) | 68.12 ± 0.18 e | 79.26 ± 0.07 c | 17.46 ± 0.04 l | 28.08 ± 0.11 j | 24.15 ± 0.01 l | 38.33 ± 0.08 h | 16.42 ± 0.32 l | 21.07 ± 0.13 k |
| S. boulardii (KT000037) | 45.12 ± 0.17 d | 85.21 ± 0.28 a | 18.31 ± 0.09 | 22.16 ± 0.26 k | 28.13 ± 0.06 j | 36.33 ± 0.14 h | 17.43 ± 0.26 l | 24.23 ± 0.12 k |
| Antibiotic Susceptibility Based on Disc Diffusion Method (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Antibiotics | Isolated Strains | ||||||
| Commercial Probiotics | |||||||
| Dysentery Causing Clinical Pathogens | |||||||
| L. reuteri (Ecoflora) | L. reuteri (KT000042) | S. boulardii (Econorm 250 µg) | L. rhamnosus (GR7) | L. acidophilus (MTCC111) | S. boulardii (KT000032) | ||
| E. coli | S. aureus | E. faecalis | M. luteus | K. pneumoniae | S. typhi | S. paratyphi A | |
| S. paratyphi B | P. mirabilis | V. cholera | S. flexneri | S. dysentriae | P. aeruginosa | ||
| Amikacin | R | R | R | R | R | R | |
| 17 ± 0.18 | 14 ± 0.25 | 16 ± 0.42 | 19 ± 0.03 | 22 ± 0.02 | 25 ± 0.08 | 21 ± 0.01 | |
| 19 ± 0.35 | 17 ± 0.12 | 15 ± 0.16 | 18 ± 0.08 | 25 ± 0.12 | 17 ± 0.42 | ||
| Amoxycillin | R | R | R | R | R | R | |
| 19 ± 0.02 | 26 ± 0.02 | 22 ± 0.01 | 25 ± 0.03 | 21 ± 0.05 | 17 ± 0.05 | 22 ± 0.12 | |
| 21 ± 0.27 | 22 ± 0.25 | 15 ± 0.05 | 23 ± 0.18 | 21 ± 0.16 | 19 ± 0.06 | ||
| Azithromycin | R | R | R | R | R | R | |
| 13 ± 0.03 | 19 ± 0.01 | 26 ± 0.08 | 22 ± 0.01 | 25 ± 0.05 | 21 ± 0.03 | 20 ± 0.08 | |
| 18 ± 0.02 | 20 ± 0.25 | 11 ± 0.01 | 16 ± 0.25 | 21 ± 0.42 | 25 ± 0.25 | ||
| Benzyl penicillin | R | R | R | R | R | R | |
| R | 9 ± 0.01 | R | 9 ± 0.03 | R | 12 ± 0.01 | R | |
| 07 ± 0.08 | 14 ± 0.42 | R | 8 ± 0.16 | 9 ± 0.12 | 9 ± 0.03 | ||
| Cefalexin | R | R | R | R | R | R | |
| 25 ± 0.02 | 17 ± 0.01 | 15 ± 0.03 | 18 ± 0.08 | 20 ± 0.08 | 11 ± 0.03 | 16 ± 0.12 | |
| 21 ± 0.16 | 19 ± 0.02 | 21 ± 0.12 | 22 ± 0.42 | 25 ± 0.01 | 26 ± 0.25 | ||
| Cefepime | R | R | R | R | R | R | |
| 21 ± 0.02 | 22 ± 0.22 | 21 ± 0.22 | 22 ± 0.02 | 25 ± 0.05 | 26 ± 0.06 | 19 ± 0.16 | |
| 18 ± 0.08 | 20 ± 0.12 | 11 ± 0.03 | 16 ± 0.05 | 21 ± 0.16 | 25 ± 0.05 | ||
| Cefotaxime | R | R | R | R | R | R | |
| 14 ± 0.05 | 16 ± 0.03 | 19 ± 0.05 | 22 ± 0.02 | 25 ± 0.08 | 21 ± 0.05 | 20 ± 0.08 | |
| 11 ± 0.42 | 16 ± 0.08 | 21 ± 0.42 | 25 ± 0.42 | 17 ± 0.05 | 15 ± 0.12 | ||
| Chloramphenicol | R | R | R | R | R | R | |
| 18 ± 0.01 | 20 ± 0.02 | 11 ± 0.03 | 16 ± 0.05 | 21 ± 0.22 | 25 ± 0.02 | 17 ± 0.03 | |
| 15 ± 0.25 | 18 ± 0.42 | 22 ± 0.16 | 25 ± 0.06 | 26 ± 0.01 | 19 ± 0.42 | ||
| Ciprofloxacin | R | ||||||
| R | R | R | R | R | R | ||
| 25 ± 0.03 | 17 ± 0.02 | 15 ± 0.05 | 18 ± 0.06 | 20 ± 0.02 | 11 ± 0.05 | 16 ± 0.42 | |
| 21 ± 0.42 | 19 ± 0.02 | 22 ± 0.25 | 25 ± 0.03 | 21 ± 0.08 | 19 ± 0.16 | ||
| Erythromycin | R | R | R | R | R | R | |
| 14 ± 0.02 | 16 ± 0.22 | 19 ± 0.03 | 22 ± 0.06 | 25 ± 0.04 | 21 ± 0.42 | 18 ± 0.02 | |
| 20 ± 0.42 | 11 ± 0.02 | 16 ± 0.42 | 21 ± 0.23 | 25 ± 0.01 | 17 ± 0.18 | ||
| Gemifloxacin | R | R | R | R | R | R | |
| 20 ± 0.16 | 16 ± 0.04 | 14 ± 0.01 | 18 ± 0.04 | 22 ± 0.69 | 21 ± 0.05 | 22 ± 0.06 | |
| 24 ± 0.14 | 17 ± 0.03 | 14 ± 0.18 | 19 ± 0.16 | 25 ± 0.08 | 27 ± 0.02 | ||
| Gentamicin | R | R | R | R | R | R | |
| 15 ± 0.05 | 23 ± 0.42 | 21 ± 0.05 | 19 ± 0.04 | 17 ± 0.02 | 9 ± 0.06 | 22 ± 0.11 | |
| 19 ± 0.02 | 17 ± 0.08 | 19 ± 0.08 | 22 ± 0.02 | 21 ± 0.06 | 22 ± 0.07 | ||
| Kanamycin | R | R | R | R | R | R | |
| 18 ± 0.05 | 20 ± 0.21 | 11 ± 0.21 | 16 ± 0.01 | 21 ± 0.65 | 25 ± 0.16 | 17 ± 0.05 | |
| 15 ± 0.04 | 18 ± 0.16 | 20 ± 0.06 | 11 ± 0.02 | 16 ± 0.03 | 21 ± 0.38 | ||
| Levofloxacin | R | R | R | R | R | R | |
| 18 ± 0.05 | 20 ± 0.16 | 11 ± 0.02 | 16 ± 0.06 | 21 ± 0.38 | 25 ± 0.14 | 17 ± 0.05 | |
| 15 ± 0.02 | 18 ± 0.05 | 21 ± 0.25 | 25 ± 0.21 | 17 ± 0.02 | 15 ± 0.16 | ||
| Methicillin | R | R | R | R | R | R | |
| 21 ± 0.01 | 19 ± 0.06 | 17 ± 0.42 | 9 ± 0.42 | 22 ± 0.01 | 19 ± 0.01 | 17 ± 0.01 | |
| 17 ± 0.38 | 14 ± 0.16 | 16 ± 0.22 | 19 ± 0.21 | 22 ± 0.23 | 25 ± 0.21 | ||
| Moxifloxacin | R | R | R | R | R | R | |
| 18 ± 0.42 | 20 ± 0.06 | 11 ± 0.05 | 16 ± 0.11 | 21 ± 0.05 | 25 ± 0.21 | 17 ± 0.21 | |
| 15 ± 0.38 | 18 ± 0.05 | 25 ± 0.03 | 17 ± 0.02 | 15 ± 0.16 | 18 ± 0.08 | ||
| Neomycin | R | R | R | R | R | R | |
| 22 ± 0.42 | 17 ± 0.05 | 14 ± 0.05 | 16 ± 0.03 | 19 ± 0.02 | 22 ± 0.01 | 25 ± 0.02 | |
| 18 ± 0.02 | 20 ± 0.01 | 11 ± 0.38 | 16 ± 0.03 | 21 ± 0.04 | 25 ± 0.21 | ||
| Norfloxacin | R | R | R | R | R | R | |
| 18 ± 0.05 | 20 ± 0.38 | 11 ± 0.05 | 16 ± 0.22 | 21 ± 0.12 | 25 ± 0.04 | 17 ± 0.03 | |
| 15 ± 0.06 | 17 ± 0.16 | 15 ± 0.03 | 18 ± 0.01 | 17 ± 0.21 | 15 ± 0.04 | ||
| Ofloxacin | R | R | R | R | R | R | |
| 18 ± 0.01 | 20 ± 0.05 | 11 ± 0.42 | 16 ± 0.05 | 21 ± 0.05 | 25 ± 0.38 | 17 ± 0.05 | |
| 16 ± 0.42 | 19 ± 0.38 | 22 ± 0.04 | 25 ± 0.02 | 21 ± 0.16 | 19 ± 0.42 | ||
| Pefloxacin | R | R | R | R | R | R | |
| 17 ± 0.03 | 14 ± 0.02 | 16 ± 0.42 | 19 ± 0.05 | 22 ± 0.06 | 25 ± 0.04 | 21 ± 0.03 | |
| 19 ± 0.04 | 29 ± 0.06 | 22 ± 0.42 | 25 ± 0.07 | 26 ± 0.16 | 19 ± 0.01 | ||
| Polymyxin-B | R | R | R | R | R | R | |
| 14 ± 0.06 | 16 ± 0.14 | 19 ± 0.03 | 29 ± 0.06 | 25 ± 0.01 | 21 ± 0.22 | 19 ± 0.06 | |
| 16 ± 0.04 | 21 ± 0.25 | 25 ± 0.01 | 17 ± 0.04 | 15 ± 0.25 | 15 ± 0.01 | ||
| Rifampicin | R | R | R | R | R | R | |
| 21 ± 0.8 | 22 ± 0.01 | 15 ± 0.42 | 23 ± 0.04 | 21 ± 0.14 | 17 ± 0.06 | 14 ± 0.22 | |
| 16 ± 0.25 | 19 ± 0.14 | 22 ± 0.18 | 2519 ± 0.06 | 21 ± 0.38 | 19 ± 0.25 | ||
| Roxithromycin | R | R | R | R | R | R | |
| 17 ± 0.14 | 14 ± 0.02 | 16 ± 0.22 | 19 ± 0.03 | 22 ± 0.04 | 25 ± 0.01 | 21 ± 0.03 | |
| 19 ± 0.02 | R | 21 ± 0.42 | 22 ± 0.25 | 19 ± 0.06 | 23 ± 0.04 | ||
| Streptomycin | R | R | R | R | R | R | |
| 21 ± 0.16 | 22 ± 0.16 | 21 ± 0.22 | 22 ± 0.04 | 25 ± 0.01 | 26 ± 0.16 | 19 ± 0.14 | |
| 22 ± 0.12 | 17 ± 0.04 | 14 ± 0.01 | 16 ± 0.38 | 19 ± 0.04 | 22 ± 0.15 | ||
| Sulphadiazine | R | R | R | R | R | R | |
| 15 ± 0.03 | 18 ± 0.22 | 20 ± 0.02 | 19 ± 0.06 | 16 ± 0.11 | 21 ± 0.02 | 19 ± 0.08 | |
| 22 ± 0.02 | 29 ± 0.06 | 17 ± 0.38 | 15 ± 0.25 | 18 ± 0.03 | 23 ± 0.17 | ||
| Sulphamethizole | R | R | R | R | R | R | |
| 25 ± 0.14 | 21 ± 0.22 | 18 ± 0.04 | 20 ± 0.26 | 11 ± 0.04 | 16 ± 0.38 | 29 ± 0.06 | |
| 25 ± 0.25 | 17 ± 0.42 | 14 ± 0.38 | 16 ± 0.43 | 19 ± 0.25 | 22 ± 0.14 | ||
| Teicoplanin | R | R | R | R | R | R | |
| 20 ± 0.22 | 11 ± 0.04 | 16 ± 0.02 | 21 ± 0.12 | 17 ± 0.22 | 17 ± 0.04 | 19 ± 0.01 | |
| 22 ± 0.03 | 19 ± 0.02 | 17 ± 0.16 | 19 ± 0.06 | 22 ± 0.16 | 21 ± 0.02 | ||
| Vancomycin | R | R | R | R | R | R | |
| R | R | R | R | 7 ± 0.01 | 19 ± 0.06 | 8 ± 0.01 | |
| R | R | 9 ± 0.16 | R | R | R | ||
| Tetracycline | R | R | - | R | R | ||
| 25 ± 0.14 | 17 ± 0.22 | 19 ± 0.06 | 18 ± 0.02 | 15 ± 0.03 | 18 ± 0.38 | 20 ± 0.01 | |
| 19 ± 0.06 | 16 ± 0.03 | 21 ± 0.01 | 19 ± 0.04 | 22 ± 0.01 | 19 ± 0.06 | ||
| Meropenem | R | R | R | R | R | R | |
| 17 ± 0.01 | 9 ± 0.38 | 29 ± 0.06 | 19 ± 0.1 | 17 ± 0.16 | 19 ± 0.1 | 22 ± 0.22 | |
| 21 ± 0.16 | 22 ± 0.42 | 22 ± 0.01 | 25 ± 0.11 | 21 ± 0.14 | 18 ± 0.01 | ||
| S. No. | Sugar Fermentation | S. boulardii (KT000032) | |
|---|---|---|---|
| Fermentation of Sugars at 37 °C | Fermentation of Sugars at 30 °C | ||
| 1 | Raffinose (Rf) | −−− | −−− |
| 2 | Rhamnose (Rh) | −−− | −−− |
| 3 | Dulcitol (Du) | −−− | −−− |
| 4 | Sorbitol (Sb) | −−− | −−− |
| 5 | Cellobiose (Ce) | −−− | −−− |
| 6 | Xylose (Xy) | −−− | −−− |
| 7 | Arabinose (Ar) | −−− | −−− |
| 8 | Inulin (In) | −−− | −−− |
| 9 | Fructose (Fc) | −−± | −−− |
| 10 | Mannose (Mo) | −−± | −−− |
| 11 | Sucrose (Su) | −−− | −−− |
| 12 | Lactose (La) | −−± | −−− |
| 13 | Adonitol (Ad) | −−± | −−− |
| 14 | Dextrose (De) | −−± | −−± |
| 15 | Maltose (Ma) | −−± | −−± |
| 16 | Salicin (Sa) | −−− | −−− |
| 17 | Mannitol (Mn) | −−− | −−− |
| 18 | Trehalose (Te) | ±±± | ±±± |
| 19 | Galactose (Ga) | −−± | −−± |
| 20 | Inositol (Is) | −−− | −−− |
| 21 | Melibiose (Mb) | −−− | −−− |
| Isolated Strains | Commercial Probiotics (mm) | ||||
|---|---|---|---|---|---|
| L. reuteri (Ecoflora) | L. reuteri (KT000042) | S. boulardii (Econorm 250 µg) | L. rhamnosus (GR7) | L. acidophilus (MTCC111) | |
| S. boulardii (KT000032) | ND | ND | ND | ND | ND |
| Isolated Strains | Survival (log CFU mL−1) at 0 h | Survival (log CFU mL−1) at 95 °C (mm) | Survival (log CFU mL−1) at 121 °C | |||
|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 120 min | 15 min | ||
| S. boulardii (KT000032) | 8.72 ± 0.07 a | 8.51 ± 0.12 a | 7.16 ± 0.16 b | 6.89 ± 0.06 c | 6.23 ± 0.06 c | 5.86 ± 0.03 d |
| CFS of the Strains | Zone of Inhibition (mm) | Clinical Pathogens | |||||
|---|---|---|---|---|---|---|---|
| 95 °C | 30 °C | 15 °C | |||||
| 15 min | 30 min | 60 min | 120 min | 24 h | 24 h | ||
| S. boulardii (KT000032) | 23 b | 23 b | 21 b | 19 c | 23 b | 23 b | E. coli |
| 20 c | 20 c | 19 c | 19 c | 21 c | 21 c | S. aureus | |
| 21 b | 21 b | 19 c | 18 c | 22 c | 21 c | S. typhi | |
| 18 c | 18 c | 18 c | 15 c | 18 c | 18 c | S. dysenteriae | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelliah, R.; Kim, E.-J.; Daliri, E.B.-M.; Antony, U.; Oh, D.-H. In Vitro Probiotic Evaluation of Saccharomyces boulardii with Antimicrobial Spectrum in a Caenorhabditis elegans Model. Foods 2021, 10, 1428. https://doi.org/10.3390/foods10061428
Chelliah R, Kim E-J, Daliri EB-M, Antony U, Oh D-H. In Vitro Probiotic Evaluation of Saccharomyces boulardii with Antimicrobial Spectrum in a Caenorhabditis elegans Model. Foods. 2021; 10(6):1428. https://doi.org/10.3390/foods10061428
Chicago/Turabian StyleChelliah, Ramachandran, Eun-Ji Kim, Eric Banan-Mwine Daliri, Usha Antony, and Deog-Hwan Oh. 2021. "In Vitro Probiotic Evaluation of Saccharomyces boulardii with Antimicrobial Spectrum in a Caenorhabditis elegans Model" Foods 10, no. 6: 1428. https://doi.org/10.3390/foods10061428
APA StyleChelliah, R., Kim, E.-J., Daliri, E. B.-M., Antony, U., & Oh, D.-H. (2021). In Vitro Probiotic Evaluation of Saccharomyces boulardii with Antimicrobial Spectrum in a Caenorhabditis elegans Model. Foods, 10(6), 1428. https://doi.org/10.3390/foods10061428