Abstract
Hierarchical cluster (HCA) and canonical correlation (CCA) analyses were employed to explore the multivariate relationships among chemical components (proximate, mineral and lipidic components) of lean beef longissimus dorsii lumborum (LDL) and selected carcass traits of cattle fattened on pasture under tropical conditions (bulls, n = 60; steers, n = 60; from 2.5 to 4.0 years of age, estimated by dentition). The variables backfat thickness (BFT), Ca, Mn, Cu, C14:0, C15:0, and C20:0 showed the highest coefficients of variation. Three clusters were defined by the HCA. Out of all carcass traits, only BFT differed significantly (p < 0.001) among clusters. Clusters significantly (p < 0.001) differed for total lipids (TLIPIDS), moisture, dry matter (DM), fatty acid composition, cholesterol content, and mineral composition (except for Fe). The variables that define the canonical variate “CARCASS” were BFT and degree of marbling (MARBLING). TLIPIDS was the main variable for the “PROXIMATE” canonical variate, while C16:0 and C18:1c had the most relevant contribution to the “LIPIDS” canonical variate. BFT and MARBLING were highly cross-correlated with TLIPIDS which, in turn, was significantly affected by the IM lipid content. Carcass traits were poorly correlated with mineral content. These findings allow for the possibility to develop selection criteria based on BFT and/or marbling to sort carcasses, from grass-fed cattle fattened under tropical conditions, with differing nutritional values. Further analyses are needed to study the effects of sex condition on the associations among carcass traits and lipidic components.
1. Introduction
Beef is known as one of the main sources of protein with high biological value, bioavailable minerals (Fe, Zn and P), vitamins of the B-complex (B1, B2, B3 B6 and B12) and other nutritional components (D, E, and β-carotenes) [1,2,3,4,5]. It is also a nutritional source of monounsaturated (MUFA) and essential polyunsaturated (PUFA) fatty acids (omega 3 and omega 6) with dietary and functional properties, and therapeutic effects [6,7,8]. The main benefits of beef consumption for nutrition and health are closely related to its unique chemical composition [2,3,4].
It is well known that intrinsic factors like species, breed, gender, age, and the structure of the type of muscle [3,9,10,11,12,13,14,15], and extrinsic factors such as animal nutrition and pre-slaughter conditions [16,17,18] are largely responsible for the variation found in carcass traits, beef sensory attributes, and nutrient composition. Nutrient composition of grass-fed beef has been a subject of study worldwide [15,19,20,21,22,23] and although there is a general perception that its consumption brings health benefits to consumers, there is no consensus on this matter [24]. Numerous studies on the effect of castration reviewed by Huerta and Ríos [25] have demonstrated that carcasses of castrated males (steers) accumulate more fat than their non-castrated counterparts (bulls); however, the influence of castration on the nutrient composition of lean, grass-fed beef (i.e., fatty acids, cholesterol, and minerals) has been less studied in the tropics, particularly in cattle with Bos indicus influence. A couple of reports in Brazil [26,27] indicate that the intramuscular fat (IMF) of bulls contains more PUFA and exhibit a higher PUFA/SFA ratio than steers. These findings are explained by the larger muscle mass and leaner beef of bulls, and therefore, a more abundant content of membrane phospholipids of muscle cells [28]. The comparison of lean meats from grass-fed bulls vs. steers in cholesterol or mineral content has not indicated significant differences [3].
For decades, the meat industry and scientists have used carcass characteristics to predict palatability-related attributes and/or consumer acceptability. Indeed, most of the carcass quality grading systems rely on the relationships between individual (or combined) carcass traits and sensory attributes of meat [25]. Key characteristics that describe the beef carcass include carcass weight, physiological maturity (often using dentition or ossification as a proxy for age), sex, fat cover and colour, and conformation. Depending upon the country, marbling and lean colour and/or texture have often been added as quality traits to refine the carcass evaluation technique [29,30].
To our knowledge, there is limited information regarding the nutritional quality/value of meat specifically focused on a possible relationship between the anatomical or other physical characteristics of the intact beef carcass and its meat nutrient composition. This information gap needs to be addressed/closed particularly for beef produced under tropical, grass feeding conditions given the alleged health claims linked to its consumption [31]. We propose that, to determine any relationship, all of these traits must be simultaneously considered by using a multivariate analysis approach. Jeong et al. [32] investigated the relationships between the content of IMF, the fatty acid composition, and characteristics of the muscle fibre in the longissimus thoracis of pork. These researchers employed the principal components analysis (PCA) and hierarchical cluster analysis (HCA), an appropriate example of the applicability of this type of statistical approach. Similarly, Patel et al. [33] used multivariate analyses to explore the relationship among animal and carcass characteristics, beef (longissimus thoracis) quality traits, and lean meat mineral composition (20 elements). In this case, the researchers employed a combination of univariate (simple correlation) and multivariate (factorial analysis) techniques that allowed them to compare the relationship between minerals, not only individually but also in a factorial fashion (five factors) with the animal/carcass performance and the beef quality traits. This study [33] only included the carcass weight as one of the three performance characteristics. Both previous investigations [32,33] indicate the need for studying complex relationships employing a multivariate approach, that may include a large number of variables. In this case the Canonical Correlation analysis (CCA), offers a promising multivariate method to complement other techniques. CCA has been widely used in agricultural science [34,35,36,37] to explore the interrelation between multiple variables, relationships that could be symmetric, that is, without a dependency relationship among them, or asymmetric, when one of the sets is dependent and the other is independent.
The underlying principle of CCA is to investigate the relationship between the variables by developing several independent canonical functions that maximize the correlation between the linear composites known as canonical variates [38]. The CCA represents the bivariate correlation between the two canonical variates in a canonical function. The canonical correlation coefficient measures the strength of association between the variable sets under concern. This technique can assist in the analysis of several traits; furthermore, it may indicate the most relevant factors to the set of variables under study [39,40,41].
Knowing the degree of association of the multivariate relationships between the nutrient composition and the quality traits of dressed beef may allow identifying predictors of the meat nutrient composition that can be assessed on the hanging carcass and, eventually, the possibility to develop selection criteria for sorting carcasses with different nutritional values.
This study aimed to explore the multivariate relationships among chemical components (proximate, mineral and lipid components) of lean beef longissimus dorsii lumborum (LDL) and selected carcass traits of cattle fattened on pasture under tropical conditions.
2. Materials and Methods
2.1. Characteristics of the Sample
Carcass traits and nutrient composition data from a randomly selected group of 120 slaughtered cattle (60 bulls and 60 steers; 2.5 to 4.0 years of age, estimated by dentition) were collected for this observational study. This sample was representative of slaughter male cattle derived from the prevailing production systems in the Venezuelan tropics where livestock is mostly fattened on pasture with little or no supplementation [42]. Out of this group, 9 animals were mixed-breed dairy (predominantly Holstein, Brown Swiss, or dual-purpose cattle without a defined breed predominance) x Zebu breeds; and 110 were mixed-breed cattle with a phenotypic predominance of Zebu breeds.
2.2. Harvesting, Carcass Classification and Sample Collection
The animals were harvested at a commercial packing house following the procedures of the Venezuelan Standards of Bioethics and Biosecurity for Research with Living Organism [43], and the Venezuelan Standard for Postmortem Inspection of Cattle [44]. After being weighed, carcasses were chilled at 2–4 °C. After 48 h postmortem, the chilled carcasses were subjected to evaluation. Skeletal and lean maturity (SM and LM, respectively) scores and subcutaneous backfat thickness (BFT) were determined following USDA guidelines [45]. The subcutaneous fat cover (CFINISH) was evaluated using a four-level scale: 1 = Uniform; 2 = Uneven; 3 = In patches; 4 = Devoid [46]. The degree of marbling (MARBLING) was evaluated according to Decreto Presidencial N° 181, using a descriptive scale: 1 = practically devoid, 2 = traces, 3 = slight and 4 = small amount [47].
After evaluation, chilled carcasses were cut out following conventional butchering procedures according to regulation 792-82 of the Venezuelan Commission for Industrial Standard [48], trimmed to 6.4 mm fat cover, and fabricated into commercial cuts. Muscle samples (2.5 cm thick) from the most anterior (cranial) part of the LDL muscle were excised, individually vacuum packaged, identified by animal number, frozen at −30 °C and stored at −20 °C until the final preparation for the proximate analyses. Samples were partially thawed at 4 °C (to avoid fluids losses), trimmed of visible adipose and connective tissue, and homogenized in a Black & Decker™ food processor. Each homogenized sample was subdivided into smaller portions (subsamples) which were packaged in 50 g-zip-lock bags (4–5 bags) and identified by animal number. Bags containing homogenized subsamples were assigned to each type of chemical analysis (proximate, mineral, or lipid profile analysis) and immediately processed accordingly. The remaining bags were preserved at −20 °C as spare samples in the event that a confirmatory analysis was needed. A flowchart (Figure S1, supplementary material) illustrates sample handling for chemical analyses. All the samples were analyzed in duplicate [49].
2.3. Proximate Composition Analysis
Duplicate samples were analyzed for crude protein (CP) content following the Kjeldahl procedure; moisture (WATER) and dry matter (DM) were estimated by weight loss at 105 °C for 24 h, and ash at 550 °C during 6 h [50]. Total lipids (TLIPIDS) content was determined by extracting with a 2:1 chloroform:methanol mixture according to the method of Folch et al. [51] with some modifications as described by Slover & Lanza [52].
2.4. Mineral Analysis
Duplicates of 10.0 g of ground meat were calcined in a furnace at 550 °C for 6 h. Sample handling and mineral analyses were conducted according to the methodology described by Giuffrida-Mendoza et al. [1]. Mineral content was expressed in mg.100 g−1 of fresh tissue.
2.5. Lipid Profile Analysis
Cholesterol content of each steak sample was determined in triplicate, according to the procedure described by Rhee et al. [53].
Fatty acids (FA) were determined by gas chromatography as described by Slover and Lanza [54]. A duplicate of an aliquot of the lipid extract, corresponding to 25 mg of the total lipids of each sample, mixed with the internal standard (Margaric acid, C17:0 methyl ester) was saponified and esterified with BF3/CH3OH [55] to yield fatty acid methyl esters (FAME). FAME were analyzed following the procedure described by Uzcátegui-Bracho et al. [49].
2.6. Data Analysis
The data analysis was performed using the IBM SPSS 23 statistical software [56]. The original, historical data consisted of 120 samples, being reduced to 109 after carrying out preliminary analyses. Univariate analyses were used to evaluate descriptive statistics, kurtosis, skewness, and detection of outliers. Multivariate analyses allowed to detect and treat the possible atypical values and to verify conformity with the basic assumptions of randomness, multivariate normality, and homoscedasticity of the variance. For exploring if any noise was caused for the inclusion of 9 observations (mixed dairy x zebu breed types) the statistical analyses were run again with 100 subjects phenotypically classified as predominantly Zebu crossbreds. The statistical output of this exploratory analysis showed the canonical correlations between the selected carcass traits and the three groups of chemical variables (proximate components, lipid profile, mineral components) were like those found in the previous run with 109 subjects, thus proving that the inclusion of these mixed dairy x Zebu cattle did not cause significant changes in the results. In fact, its inclusion introduced more variability to the sample, which enriched the results.
Two hierarchical cluster analyses (HCA) were performed. The first HCA was applied to explore the presence of any pattern or relationship between the 32 variables under study (except for the categorical variables CFINISH and MARBLING), using the linkage (between groups) method. To measure the degree of association between variables, Pearson’s correlation coefficient was applied with the measurement transformed into absolute values. The second HCA was applied to group all the samples using Ward’s method with the squared Euclidean distance measure and considering the sex condition to describe how the variables are presented within each cluster.
To validate the clusters obtained, an ANOVA with two main factors (sex condition and cluster) was applied on each variable. The results from the two HCA were represented by dendrograms. To analyze the relationship among the subgroups of the variables proximate, mineral, and lipid components with respect to the subgroups of carcass traits, a canonical correlation analysis (CCA) was carried out. Wilk’s Lambda and Bartlett tests were used to determine the significance of canonical correlations.
The acronyms of the variables studied in this research and their definitions are shown in Table 1.

Table 1.
Acronyms of the variables studied and their definitions.
3. Results
3.1. Descriptive Statistics for Carcass Traits, Proximate Composition, Mineral Content, and Fatty Acid Composition of Beef
The descriptive statistics of the experimental data are presented in Table 2 and Table 3. The variables BFT, Ca, Mn, Cu, C14:0, C15:0, and C20:0 showed the highest coefficients of variation. In general, this sample of grass-fed beef carcasses had relative low values of BFT (0.1–1.2 cm) and MARBLING levels. Frequency and percentage distribution of MARBLING levels (i.e., Practically devoid, Traces and Slight amounts) in the filtered data (N = 109) were 48 (44%), 24 (22%) and 37 (33.9%), respectively (values are not presented in tabular form). Among the proximate components, TLIPIDS from separable lean only presented the greatest variation (between 0.93 and 6.67 g.100 g−1). Out of the 30 fatty acids under study, the most abundant were C16:0 (0.028–1.288), C18:0 (0.053–0.705), C18:1c (0.27–1.749) and C18:1t (0.117–0.981). Overall, MUFA constituted 57.65% of the total; PUFA represented less than 5% of the total and the rest (37.35%) corresponded to SFA.

Table 2.
Descriptive statistics for carcass traits proximal, and proximate and mineral contents in beef longissimus lumborum muscle.

Table 3.
Descriptive statistics for cholesterol content and fatty acid profile in beef longissimus lumborum muscle.
3.2. Characterization of the Carcass Traits and Chemical Components of Beef Longissimus Lumborum Muscle by HCA
The first HCA allowed to determine how variables were grouped by degree of similarity as calculated by the squared Euclidean distance with a similarity index ranging from 0 (higher similarity) to 25 (lower similarity); a close distance between variables indicating high correlation. This first HCA also allowed to provide a simple representation of the total data composed of 32 variables and to explore how the variables correlated to each other. Figure 1 shows that most of the variables in this dataset, tended to cluster in the same subgroup (carcass traits, proximate composition, mineral content or lipidic composition). The variable CHOLEST was grouped with LM. The variables Fe and Cu were clustered with ash and CP, and the variables TLIPIDS and C16:1 were proximate with the second smallest distance (Figure 1).
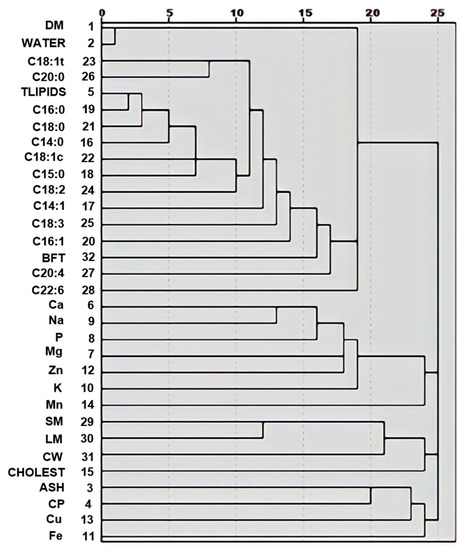
Figure 1.
Dendrogram for variables from the hierarchical cluster analysis. Description of acronyms is presented in Table 1.
The HCA by samples resulted in a dendrogram with three clusters, sufficiently distant to expect relatively different values among the groups (Figure S2, Supplementary Material). ANOVA and multiple range tests (at 5% of significance) were applied to validate and describe the clusters. Results of these analyses are presented in Table 4 and Table 5. Among the carcass traits, only BFT differs significantly (p < 0.001) among clusters (Table 4). In the proximate composition data, TLIPIDS, moisture, and DM resulted different among clusters. With the only exception of Fe, the mineral composition also differed (p < 0.0001). Clusters differed (p < 0.0001) in fatty acid composition and CHOLEST (Table 5; p < 0.05).

Table 4.
Comparison of carcass traits, proximate composition, and mineral content of beef longissimus lumborum muscle between sex conditions and the three clusters.

Table 5.
Comparison of lipid profile of beef longissimus lumborum muscle between sex conditions within the clusters.
Cluster 1 is mainly represented by steers with the highest values in BFT (0.724 cm) and TLIPIDS (4.16 g.100 g−1); therefore, this subgroup also exhibited the highest values in most of the fatty acids evaluated (p < 0.0001). Cluster 2 is mostly composed of bulls with the lowest BFT (0.226 cm) and total lipids (1.81 mg.100 g−1; p < 0.05). On the other hand, cluster 3 was more balanced, comprised of 56.5% of bulls and 53.5% of steers, with similar values in BFT and TLIPIDS; this cluster represented the group of samples with similarities in fat-related traits and differences in mineral content. In general, cluster 3 showed the highest values in Ca, Mg, P, K, Zn, Cu and Mn (p < 0.0001). Figures S3–S6 (Supplementary Materials) illustrated the projection means of variables by each cluster.
3.3. Relationship among Subgroups of Variables by CCA
The first canonical correlation between U (representing the proximate composition traits) and V (representing the subgroup of carcass traits) was significant (p < 0.0001). Canonical redundancy analysis revealed that the first canonical correlation represents 75.96% of the explained variance, which indicates that there is a high degree of association between carcass traits and the proximate composition.
Standardized canonical coefficients or canonical weights of original variables represent their relative contribution to the corresponding canonical variates U (named as “PROXIMATE”) and V (named as “CARCASS”), respectively. Standardized canonical coefficient or canonical weights of TLIPIDS had the greatest contribution to the canonical variate “PROXIMATE” (Table 6). The correlation coefficient (−0.993) also indicated that this variable makes an important contribution to the constitution of this canonical variate.

Table 6.
Standardized canonical coefficient and canonical correlation coefficient between the original variables and its canonical variate “PROXIMATE”.
Table 7 shows the standardized coefficients and correlations coefficients between the original carcass traits and the canonical variate “CARCASS”. The variables MARBLING and BFT were the largest contributors to the formation of this canonical variate (r = −0.836 and r = −0.855, respectively). On the other hand, the variable CFINISH had a moderate contribution to the canonical variate “CARCASS” (r = 0.751). The very low standardized coefficients of SM, LM, and CW indicated an irrelevant contribution of these variables to the canonical variate “CARCASS”.

Table 7.
Standardized canonical coefficient (canonical weights), and canonical correlation coefficient between the original variables and the canonical variate “CARCASS”.
Canonical cross correlation describes the correlation between variables and the opposite canonical variate. The variable TLIPIDS showed the highest canonical cross-loading with the canonical variate “CARCASS” (Table 8). On the other hand, the variables MARBLING, BFT, and CFINISH showed an important canonical cross correlation with the canonical variate “PROXIMATE”. This result indicates a strong and significant linear correlation between these four variables.

Table 8.
Cross correlations (canonical cross loadings) between the variables and the opposite canonical variate.
The variance of the canonical variate “PROXIMATE” associated with the variables of its own group represented 30.70% of the total data variation, which could be attributed to the high loading value of the variable TLIPIDS. The cross variance between “PROXIMATE” and “CARCASS” only accounted for 12.10% of the total variance; a low value that is also associated with the contribution of the TLIPIDS variable. The contribution of the canonical variate “CARCASS” was 34.10% of the total variance. The canonical correlation between PROXIMATE and CARCASS subgroups of variables aligns with the results obtained from the linear correlation between the variables related to fatness (Table 9). The correlation coefficients are all significant and moderate to strong, with values very close to those reached by the components of the canonical variates.

Table 9.
Pearson correlation coefficients among carcass fatness-related variables.
The CCA between the subgroups of variables related to lipid composition traits and the subgroup of carcass traits revealed six canonical correlations. The first canonical correlation was highly significant (p = 0.002), showing a not very strong correlation coefficient (r = 0.629), and represented 59.17% of the explained variability. Table 10 shows the standardized canonical and correlation coefficients between the original variables and their canonical variate “LIPIDS”.

Table 10.
Standardized canonical coefficient (canonical weights), and canonical correlation coefficient between the original variables and their canonical variate “LIPIDS”.
The C16:0 (loading value = −0.796; r = −0.912); and C18:1c (loading value = −0.566; r = −0.878) showed the highest correlation coefficients with the canonical variety “LIPIDS”. This indicates that Palmitic and Oleic acids were the fatty acids that mostly contributed to the conformation of the canonical variate “LIPIDS”. The variable cholesterol content presented a low canonical weight and, therefore, low correlation with its canonical variety.
The main fatty acids (C16:0 and C18:1c) with the highest standardized canonical coefficient also exhibited the highest correlations with the carcass trait variables (Table 11). Other fatty acids, like C14:0, C20:0 and C18:1t presented moderate correlations, but with lesser impact given their low standardized coefficients. On the other hand, carcass traits: CFINISH, BFT, and MARBLING presented a not very strong cross correlation with the canonical variate “LIPIDS” (Table 11).

Table 11.
Cross correlations (canonical cross loadings) between the original variables and “CARCASS” and “LIPIDS” canonical variates.
The variance of the canonical variate “LIPIDS” accounted for 38.8% of the total data variation. The cross variance between “LIPIDS” and “CARCASS” only represented 19.10% of the total variance. This low value is potentially related to a low correlation between these two subgroups of variables, and/or the smaller number of CARCASS variables as compared to the lipidic components.
Six possible canonical correlations were obtained between mineral content and carcass traits; however, none of them were statistically significant. Based on the available data and the statistical technique applied, there is insufficient evidence to demonstrate the existence of any relationship between mineral components and carcass traits.
4. Discussion
The carcasses evaluated in this study are representative of South American tropical cattle fattened on pasture, which presents more variation in degrees of fatness, carcass finish, and conformation traits than their counterparts that are subjected to more standardized, intense feeding protocols. The results obtained in this study also concur with carcass characteristics [18,57,58] and nutrient values [59,60,61,62] reported in previous studies conducted in Venezuela with samples of Longissimus lumborum taken from crossbred cattle varying in age, sex condition, and diet. Also, these values are similar to those reported for the fresh Longissimus muscle derived from Bangladeshi beef (zebu type) finished on pasture [63] and other types of tropical cattle [15]. The mineral content found in this study is within the ranges reported for raw meat from tropical cattle subjected to different management practices [3,15,64,65].
The relatively low Ca content in these beef samples is potentially related to the quality of vegetation consumed/grazed by these animals. Pastures and forages constitute the main food sources for fattening cattle in Venezuela. The nutritional value of pastures depends on the amount of nutrients present in these plant species, which are absorbed from the soil; consequently, it will be the characteristics of the soil that defines the development of the plant with respect to the concentration and availability of the mineral elements present.
According to Araujo [66], tropical grasses can hardly supply all the minerals in amounts adequate to the needs of the animals. The factors that affect the mineral content in forages, in addition to the soil, are: the forage species, the age of the pasture, the yield, the management of the pastures, and climate [66]. Low fertility and high acidity stand out among the most important limitations in quality of most soils in Venezuela. Research carried out in Venezuela to study to the state of mineral nutrition in livestock systems [67,68], showed that calcium levels in pastures were generally poor and this deficiency is reflected in the cattle’s animal tissues. A review of the nutritional value of beef produced under tropical conditions [15], also confirms the low concentrations of this mineral.
The relationship between meat sensory traits and physicochemical characteristics has been studied using multivariate analysis techniques in beef [41,69]; however, no available information was found about the relationships of carcass traits with the chemical composition of the derived meat.
The first HCA allowed a simple representation of the total data with 32 variables and to explore how the variables correlated. The second HCA grouped all the observations by similarities in three clusters with a significant variation in BFT, TLIPIDS, mineral and fatty acid composition. It has been demonstrated that sex greatly influences protein and fat deposition in cattle and defines distinct differences in body composition [27,70,71]; nevertheless, our observations suggest that in grass-fed tropical cattle, the expected differences in body and lipid composition between sexes are not as noticeable. In fact, our results suggest that BFT, TPLIPIDS, and fatty acid composition represented the main variables that defined the clusters. Cluster 3 contained a similar proportion of bulls and steers with the lowest values in BFT and total lipids.
It is noteworthy to highlight that this was not a controlled experiment designed to compare sex conditions. Therefore, genetics, management (slaughter weight and age), nutrition and other confounding factors [72] could affect this type of non-controlled comparison. The inclusion of bulls (intact males) and steers (castrates) in the study was just to provide a balanced random sample of these two sex conditions of slaughter male cattle in the country. Since the fat content is the most variable proximate component in beef, we can hypothesize that the low variability of the sample in levels of marbling (and as a result of IMF), is responsible for this outcome. The low variability in IMF is likely due to two additive factors: genetics and plane of nutrition. Both sex conditions had a common genetic background (Bos indicus) and regardless of sex, it is known that Bos indicus-influenced cattle individuals, exhibits lower levels of marbling when compared to Bos taurus biological types. The second factor could be the low energy content of the grass-based diet which did not facilitate a greater expression of the inherent differences between the sex conditions in terms of lipid content. Finally, it should also be considered that only separable lean was used for chemical analyses. Otherwise, if the meat sample had not been devoid of the surrounding subcutaneous and intermuscular fat depots, the differences between the sex conditions would have been more noticeable because the steers would give loins with a more significant amount of fat per 100 g of fresh tissue than bulls. Needless to say, a relatively greater fat content would bring a concomitant reduction in the concentration of other proximate components.
There is a consensus that the use of a CCA as a multivariate approach is appropriate for evaluating the interrelations among meat quality and carcass traits [41,73]. For this study, CCA is suitable because it measures the magnitude of interrelations between sets of multiple variables [74]. The CCA allows for studying the interrelationship among groups of multiple independent variables and determines the magnitude of the relationships that may exist between subgroups.
We analyzed canonical correlations by paired groups of variables: proximate composition, lipids, and mineral contents with carcass traits. The correlation coefficients between the original variables and the canonical variates allow establishing the weight of each original variable in the conformation of the canonical variate. The main variables that define the “CARCASS” canonical variate were BFT and MARBLING, meaning that these two variables had the highest contribution to the corresponding canonical variate. TLIPIDS was the main contributing variable for the canonical variate “PROXIMATE”, while C16:0 and C18:1c had the largest contribution for the “LIPIDS” canonical variate.
As expected, carcass fatness-related traits (BFT, MARBLING and CFINISH) exhibited the highest cross-correlations with TLIPIDS (Table 7), suggesting that MARBLING is not the only carcass trait that could affect the content of IMF. Canonical weights are important parameters for defining the contribution of original variables to the canonical variates. However, the understanding of canonical loadings and cross-loadings is critical because these values describe the correlation between original and canonical variates. Our findings represent the first evidence of a strong multivariate relationship of quantitative carcass traits with the chemical composition, particularly TLIPIDS, and the fatty acid composition of lean tissue.
In our study, the C16:0 and C18:1c components presented the highest correlations with carcass traits. The palmitic acid (C16:0) represents the main product of the de novo fatty acids synthesized from carbohydrates and volatile fatty acids of the diet, and it can be elongated to stearic acid (C18:0), and then to arachidic (C20:0) [75]. Also, the HCA revealed a high correlation between C16:1 and TLIPIDS (Figure 1).
According to the available data and the CCA applied, there is not enough evidence to assume that there is an association between mineral component variables and carcass traits. These results validate the findings of the HCA which indicate that carcass traits have a weak correlation with the mineral composition of the meat. The results obtained in this work are comparable with those of Duan et al. [11], who reported weak but significant correlations among beef mineral concentrations and carcass traits. According to Duan et al. [11], Mg concentration was positively correlated (p < 0.05) with all carcass traits but negatively correlated with hot carcass weight, while no significant correlation (p > 0.05) was detected between contents of Fe or Zn and carcass traits. Garmyn et al. [76] reported significant correlations between Fe, Zn and marbling levels. Castillo et al. [73] reported that the magnitude of the interrelations among protein, fat, and minerals are different between male (castrated and intact males) and female Saanen goats from 5 to 45 kgs live weight. In our study, individual mineral content did not correlate (p > 0.05) with any carcass trait. Age-related carcass traits (carcass weight, skeletal and lean maturity) were not correlated (p > 0.05) with the proximate, mineral or lipidic compositions.
BFT represents between 10 to 13% of total carcass fat tissue and it is dependent on genetics, nutrition, and finishing systems of ruminants; also, these may be influenced by sex and age, considering that the nutrient dynamics in the ruminant’s body differs between sexes, and these differences become more evident with age [77]. A meta-analysis study by Al-Jammas et al. [78] reported that BFT and USDA yield grade were the variables most highly related to changes in the weight of adipose tissues in the carcass, suggesting that variations in USDA yield grade and BFT may properly explain the differences in meat chemical composition.
The process of IMF deposition depends on many factors such as sex condition, age, and nutrition [72,79,80]. MARBLING, was also significantly and strongly (r > 0.5) related to TLIPIDS, despite exhibiting a very low range of scores [between 1 (Practically devoid) and 3 (Slight); Table 2]. Most likely the reason why marbling was barely second to BFT in the r value (Table 9) was due to the aforementioned narrower range of marbling variation present in these carcasses derived from Bos indicus-influenced, grass-fed cattle. In fact, BFT data (Table 2) show a higher coefficient of variation (%) than that of marbling (69.2% vs. 46.4%). Clearly, the use of a descriptive scale without subdividing each degree of marbling into 100 subunits like the USDA counterpart contributed to diminish its variation in our analysis. Differences in methods of assessment of MARBLING can affect its correlation with IMF%. For instance, Giaretta et al. [81] found that the IMF was more correlated with the percentage of marbling evaluated by the J-image analysis (r = 0.62) than when the USDA scores were used (r = 0.56) while Kruk et al. [82] had reported that the Meats Standards Australia (MSA) marbling ratings were poorly associated with IMF% when compared to other scoring systems. The correlations with IMF% ranged from 0.67–0.79 in this study [82]; however, the authors commented that in other Australian studies, the correlation coefficients with the marbling scores of the Australian Authority for the Uniform Specifications of Meat and Livestock (AUS-MEAT) were lower, ranging from 0.32 to 0.57. Another reason for the not very strong correlation detected between MARBLING and IMF in our study might be related to the very nature of these very lean meats (where “Practically devoid” and “Traces” levels of marbling comprised two thirds of the filtered data). Brackebusch et al. [83] reported a strong linear association between IMF (%) and marbling while Kornasla et al. [84] found that marbling percentage was not very strongly correlated with chemical IMF% (r = 0.60). In fact, Silva et al. [85] pointed out that the association between marbling and IMF is not very strong because part of the IMF is invisible and it also depends on the size and shape of the marbling specks. Furthermore, differences in methodologies of lipid extraction may explain the discrepancies among values reported for correlations between IMF% and marbling levels. According to Siebert et al. [86] when meat is low in fat, significantly more total lipids are extracted with polar solvent mixtures (e.g., chloroform:methanol) due to the phospholipid content of tissue membranes. Therefore, it can be speculated that the total lipid extract of these very lean meats having a much higher component of invisible membrane lipids (e.g., phospholipids and lipoproteins) makes it difficult to achieve the stronger correlations (with the marbling scores) found in highly-marbled carcasses.
Rhee et al. [53] reported the relationships of MARBLING (with eight levels from “Moderately Abundant” to “Practically Devoid”) to CHOLEST of beef longissimus muscle, and showed that only raw steaks with “Practically Devoid” MARBLING level contained significantly less cholesterol (on wet basis) than did raw steaks with any of the other seven MARBLING scores. In the present study, CHOLEST showed a low loading value, indicating a weak association with carcass traits. Catillo et al. [87] reported a close relationship between pork carcass leanness as defined by the EUROP classification system, and the fatty acid composition of backfat. These authors [87] found that as the lean meat content of the carcass decreased from class E to class O, the backfat total content of SFA increased by more than 4 percent, while the total PUFA content decreased about the same percentage; however, we could not find similar reports in beef. Elucidation of the multivariate and quantitative relationship between BFT, MARBLING and fatty acid composition may be useful for a better understanding of the roles of fat deposition on the nutritional composition of beef produced under the conditions described herein.
5. Conclusions
Canonical correlation analysis is an optimized multivariate technique for evaluating the existence or non-existence of relationships between complex groups of variables. In this study it proves to be a powerful tool to study the relationship between the selected set of carcass traits and the proximate, lipid and mineral components, particularly when it is expected that certain degree of interaction exists among these three groups of chemical variables. This work demonstrates an important relationship between backfat thickness, marbling and the content of total lipids and fatty acids in beef LDL muscle from cattle fed on pastures under tropical conditions. Instead, carcass traits are poorly associated with beef mineral content. These findings allow for the possibility to develop selection criteria based on BFT and/or marbling to sort carcasses with differing nutritional values. For the experience gained in beef carcass grading in Venezuela, the evaluation of marbling levels requires more intense training and supervision of graders than the BFT measurement. Moreover, marbling is seldom used to grade beef carcasses in the developing, tropical countries. Therefore, it is more practical to use BFT in future regression analyses to explain the variation in lipid composition of beef longissimus muscle.
Further analyses are needed to determine the potential influence of sex condition on the magnitude of the associations among carcass traits and beef fatty acid composition.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10061364/s1, Figure S1: Graphical description of sample preparation and chemical analyses; Figure S2: Dendrogram for samples from the hierarchical cluster analysis identifying clusters. The samples were numbered randomly from 1 to 109; Figure S3: Projection of variables from the subgroup proximate composition by clusters; Figure S4: Projection of variables from the subgroup mineral content by clusters; Figure S5: Projection of variables from the subgroup lipid profile by clusters; Figure S6: Projection of variables from the subgroup carcass traits by clusters.
Author Contributions
N.H.-L. designed the investigation and field data that supported this research. L.A.d.M. was responsible for the selection of chemical procedures and training of personnel who conducted the chemical analyses and collected lab data that support this research. S.U.-B. conducted the chromatographic analysis of the samples. N.J.-T., E.M.-V. and L.A.d.M. performed statistical analyses, and tabulated results. L.A.d.M., N.J.-T., M.G.-M. and N.H.-L. interpreted, designed, and revised the structure of the manuscript. All authors searched and reviewed the literature, discussed the contents of the manuscript, and approved submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Consejo de Desarrollo Científico y Humanístico de la Universidad del Zulia (CONDES-LUZ), AND Facultad de Agronomía, Universidad del Zulia, Venezuela.
Institutional Review Board Statement
Data collection was carried on in compliance with the criteria for animal care and welfare described in the Bioethics and Biosafety guide of the Fondo Nacional de Ciencia, Innovación y Tecnología (FONACIT) of Venezuela.
Data Availability Statement
Data are not available in public datasets, please contact the authors.
Acknowledgments
The authors are grateful to Matadero Industrial Centro Occidental (MINCO) for their valuable support during the live and carcass data collection, and donation of beef samples. We thank Martin O′Connors for providing further training in carcass evaluation according to the USDA standards. The authors also gratefully acknowledge the cooperation of Daniel Huerta-Sánchez (Department of Economics and Finance, Florida Gulf Coast University) in the review of technical English to improve the statistical discussion, and Javier Aracena in the editing of the supplementary figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giuffrida-Mendoza, M.; Arenas de Moreno, L.; Uzcátegui-Bracho, S.; Rincón-Villalobos, G.; Huerta-Leidenz, N. Mineral content of longissimus dorsi thoracis from water buffalo and Zebu-influenced cattle at four comparative ages. Meat Sci. 2007, 75, 487–493. [Google Scholar] [CrossRef]
- Binnie, M.A.; Barlow, K.; Johnson, V.; Harrison, C. Red meats: Time for a paradigm shift in dietary advice. Meat Sci. 2014, 98, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida-Mendoza, M.; Arenas de Moreno, L.; Huerta-Leidenz, N. Composición nutritiva de la carne de ganado tropical venezolano. An. Venez. Nutr. 2014, 27, 167–176. [Google Scholar]
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Klurfeld, M.D. What is the role of meat in a healthy diet? Anim. Front. 2018, 8, 5–10. [Google Scholar] [CrossRef]
- Balić, A.; Vlaŝi, D.; Žužul, K.; Marinović, B.; Mokos, Z.B. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Macri, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tatsuno, I. Omega-3 polyunsaturated fatty acids focusing on eicosapentaenoic acid and docosahexaenoic acid in the prevention of cardiovascular diseases: A review of the state-of-the-art. Expert. Rev. Clin. Pharmacol. 2021, 14, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Bureš, D.; Bartoň, L. Growth performance, carcass traits and meat quality of bulls and heifers slaughtered at different ages. Czech J. Anim. Sci. 2012, 57, 34–43. [Google Scholar] [CrossRef]
- Cafferky, J.; Hamill, R.M.; Allen, P.; O’Doherty, J.V.; Cromie, A.; Torres, S. Effect of Breed, and gender on meat quality of M. longissimus thoracis et lumborum muscle from Crossbred Beef Bulls and Steers. Foods 2019, 8, 173. [Google Scholar] [CrossRef]
- Duan, Q.; Tait, R.G., Jr.; Schneider, M.J.; Beitz, D.C.; Wheeler, T.L.; Shackelford, S.D.; Cundiff, L.V.; Reecy, J.M. Sire breed effect on beef longissimus mineral concentrations and their relationships with carcass and palatability traits. Meat Sci. 2015, 106, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.-Y.; Hwang, Y.-H.; Joo, S.-T. The relationship between chemical compositions, meat quality, and palatability of the 10 primal cuts from Hanwoo steer. Korean J. Food Sci. An. 2016, 36, 145–151. [Google Scholar] [CrossRef]
- Karakök, S.G.; Ozogul, Y.; Saler, M.; Ozogul, F. Proximate analysis. fatty acid profiles and mineral contents of meats: A comparative study. J. Muscle Foods 2010, 21, 210–223. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 3182746, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Lozano, M.S.R.; Ngapo, T.M.; Huerta-Leidenz, N. Tropical Beef: Is there an axiomatic basis to define the concept? Foods 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Valero, M.V.; Campo, M.M.; Sañudo, C. Some factors that affect ruminant meat quality: From the farm to the fork. Review. Acta Sci. Anim. Sci. 2013, 35, 335–347. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Joo, S.-T. Fatty acid profiles, meat quality, and sensory palatability of grain-fed and grass-fed beef from Hanwoo, American, and Australian crossbred cattle. Korean J. Food Sci. Anim. Resour. 2017, 37, 153–161. [Google Scholar] [CrossRef]
- Jerez-Timaure, N.; Huerta-Leidenz, N. Effects of breed type and supplementation during grazing on carcass traits and meat quality of bulls fattened on improved savannah. Livest. Sci. 2009, 121, 219–226. [Google Scholar] [CrossRef]
- Comparin, M.A.S.; Morais, M.G.; Fernandes, H.J.; Coelho, R.G.; Coutinho, M.A.S.; Ribeiro, C.B.; Menezes, B.B.; Rocha, R.F.A.T. Chemical composition, and fatty acid profile of meat from heifers finished on pasture supplemented with feed additives. Rev. Bras. Saúde Produção Anim. 2015, 16, 606–616. [Google Scholar] [CrossRef]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 1–12. [Google Scholar] [CrossRef]
- Giuffrida-Mendoza, M. Beneficios de la alimentación a pastoreo en la calidad nutritiva de la carne del ganado doble propósito. In Desarrollo Sostenible de la Ganadería de Doble Propósito; González-Stagnaro, C., Soto Belloso, E., Eds.; Fundación Grupo de Investigadores de la Reproducción animal en la Región Zuliana: Maracaibo, Venezuela, 2008; pp. 852–863. [Google Scholar]
- Uzcátegui-Bracho, S.; Rodas-González, A.; Hennig, K.; Arenas de Moreno, L.; Leal, M.; Vergara-López, J.; Jerez-Timaure, N. Composición proximal, mineral y contenido de colesterol del músculo Longissimus dorsi de novillos criollo limonero suplementados a pastoreo. Rev. Cien. 2008, 18, 589–594. [Google Scholar]
- Leheska, J.M.; Thompson, L.D.; Howe, J.C.; Hentges, E.; Boyce, J.; Brooks, J.C.; Shriver, B.; Hoover, L.; Miller, M.F. Effects of conventional and grass feeding systems on the nutrient composition of beef. J. Anim. Sci. 2008, 86, 3575–3585. [Google Scholar] [CrossRef] [PubMed]
- Provenza, F.D.; Scott, L.; Kronberg, S.L.; Gregorini, P. Is grassfed meat and dairy better for human and Environmental Health? Front. Nutr. 2019, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Leidenz, N.; Rios, G. Bovine castration at different stages of growth. II. Carcass characteristics. Rev. Fac. Agron. LUZ 1993, 10, 163–187. [Google Scholar]
- Ruiz, M.R.; Matsushita, M.; Visentainer, J.V.; Hernandez, J.A.; Ribeiro, E.L.d.A.; Shimokomaki, M.; Reeves, J.J.; deSouza, N.E. Proximate chemical composition and fatty acid profiles of Longissimus thoracis from pasture fed LHRH immunocastrated, castrated and intact Bos indicus bulls. S. Afr. J. Anim. Sci. 2005, 35, 13–18. [Google Scholar]
- Aricetti, J.A.; Rotta, P.P.; Prado, R.M.D.; Perotto, D.; Moletta, J.L.; Matsushita, M.; Prado, I.N.D. Carcass characteristics chemical composition and fatty acid profile of longissimus muscle of bulls and steers finished in a pasture system bulls and steers finished in pasture systems. Asian Australas. J. Anim. Sci. 2008, 21, 1441–1448. [Google Scholar] [CrossRef]
- Eichhorn, J.M.; Coleman, L.J.; Wakayama, E.J.; Blomquist, G.J.; Bailey, C.M.; Jenkins, T.G. Effects of breed type and restricted versus ad libitum feeding on fatty acid composition and cholesterol content of muscle and adipose tissue from mature bovine females. J. Anim. Sci. 1986, 63, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorne, R.J.; Thompson, J.M. Meat standards and grading. A world view. Meat Sci. 2010, 86, 227–235. [Google Scholar] [CrossRef]
- Rodas-González, A.; Huerta-Leidenz, N.; Jerez-Timaure, N. Benchmarking Venezuelan quality grades for grass-fed cattle carcasses. Meat Muscle Biol. 2017, 1, 71–80. [Google Scholar] [CrossRef][Green Version]
- Butler, G.; Ali, A.M.; Oladokun, S.; Wang, J.; Davis, D. Forage-fed cattle point the way forward for beef? Future Foods 2021, 3, 100012. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Jeong, T.-C.; Yang, H.-S.; Kim, G.-D. Multivariate analysis of muscle fiber characteristics, intramuscular fat content and fatty acid composition in porcine longissimus thoracis muscle. Livest. Sci. 2017, 202, 13–20. [Google Scholar] [CrossRef]
- Patel, N.; Bergamaschi, M.; Magro, L.; Petrini, A.; Bittante, G. Relationships of a detailed mineral profile of meat with animal performance and beef quality. Animals 2019, 9, 1073. [Google Scholar] [CrossRef]
- Chen, D. Analysis of input and output of China’s agriculture based on canonical correlation. Asian J. Agric. Res. 2011, 3, 9–15. [Google Scholar]
- Kim, T.W.; Kim, C.W.; Noh, C.W.; Kim, S.W.; Kim., I.S. Identification of association between supply of pork and production of meat products in Korea by canonical correlation analysis. Korean J. Food Sci. Anim. Resour. 2018, 38, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Saba, J.; Tavana, S.; Qorbanian, Z.; Shadan, E.; Shekari, F.; Jabbari, F. Canonical Correlation Analysis to Determine the Best Traits for Indirect Improvement of Wheat Grain Yield under Terminal Drought Stress. JAST 2018, 20, 1037–1048. Available online: http://jast.modares.ac.ir/article-23-19920-en.html (accessed on 11 June 2021).
- Tukimat, N.N.A.; Harun, S.; Tadza, M.Y.M. The potential of canonical correlation analysis in multivariable screening of climate model. IOP Conf. Ser. Earth Environ. Sci. 2019, 365, 012025. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis. A Global Perspective, 7th ed.; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2009; ISBN 978-0138132637. [Google Scholar]
- Diel, M.I.; Lúcio, A.D.; Lambrecht, D.M.; Pinheiro, M.V.M.; Sari, B.G.; Olivoto, T.; Valeriano, O.; de Melo, P.J.; de Lima Tartaglia, F.; Luis, A. Canonical correlations in agricultural research: Method of interpretation used leads to greater reliability of results. IJERI 2020, 8, 171–181. [Google Scholar] [CrossRef]
- Wickramasinghe, N.D. Canonical correlation analysis: An introduction to a multivariate statistical analysis. JCCPSL 2019, 25, 37–38. [Google Scholar] [CrossRef]
- Vargas, J.A.C.; Coutinho, J.E.S.; Gomes, D.I.; Alves, K.S.; Maciel, R.P. Multivariate relationship among pH, subcutaneous fat thickness, and color in bovine meat using canonical correlation analysis. Rev. Colomb. Cienc. Pecu. 2021, 34. [Google Scholar] [CrossRef]
- Montero, A.; Huerta-Leidenz, N.; Rodas-González, A.; Arenas de Moreno, L. Deshuese y variación del rendimiento carnicero de canales bovinas en Venezuela: Descripción anatómica el proceso y nomenclatura de cortes equivalentes a los correspondientes norteamericanos. Nacameh 2014, 8, 1–22. Available online: http://nacameh.cbsuami.org (accessed on 22 February 2021). [CrossRef]
- Ministerio del Poder Popular para Ciencia, Tecnología e Industrias Intermedias y Fondo Nacional de Ciencia, Tecnología e Innovación (MCT-FONACIT). Código de Bioética y Bioseguridad, 2nd ed.; Ministerio del Poder Popular para Ciencia, Tecnología e Industrias Intermedias y el Fondo Nacional de Ciencia, Tecnología e Innovación: Caracas, Venezuela, 2002; pp. 1–35. Available online: https://cupdf.com/download/bioetica-fonacit (accessed on 3 April 2021).
- Comisión Venezolana de Normas Industriales. Norma Venezolana 2072-83. Ganado Bovino. Inspección Postmortem; FONDONORMA: Caracas, Venezuela, 1983; pp. 1–10. Available online: http://www.sencamer.gob.ve/sencamer/normas/2072-83.pdf (accessed on 25 February 2021).
- United States Department of Agriculture (USDA). Official United States Standards for Grades of Carcass Beef; Agricultural Marketing Service: Washington, DC, USA, 2017. Available online: https://www.ams.usda.gov/grades-standards/carcass-beef-grades-and-standards (accessed on 11 June 2021).
- Huerta Leidenz, N.; Alvarado, E.; Martínez, L.; Rincón, E. Conformación, acabado y características biométricas de la canal de diferentes clases de bovinos sacrificados en el Estado Zulia. Rev. Fac. Agron. (LUZ) 1979, 5, 522–536. Available online: https://produccioncientificaluz.org/index.php/agronomia/article/view/25841 (accessed on 25 January 2021).
- Decreto Presidencial No. 181: Gaceta Oficial de la República de Venezuela; Nº 35-486; Ministerio de Agricultura y Cria: Caracas, Venezuela, 1994.
- Comisión Venezolana de Normas Industriales (COVENIN). Norma Venezolana 792-82: Carne de Bovino. Definición e Identificación de las Piezas de una Canal; FONDONORMA: Caracas, Venezuela, 1982; pp. 1–10. Available online: http://www.sencamer.gob.ve/sencamer/normas/792-82.pdf (accessed on 22 February 2021).
- Uzcátegui-Bracho, S.; Huerta-Leidenz, N.; Arenas de Moreno, L.; Colina, G.; Jerez-Timaure, N. Contenido de humedad, lípidos totales y ácidos grasos del músculo longissimus crudo de bovinos en Venezuela. Arch. Latinoamer. Nutr. 1999, 49, 171–180. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990; Section 960.39; ISBN 978-093-558-442-4. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipid from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Slover, H.T.; Lanza, E.; Thompson, R.H.; Davis, C.S.; Merola, G.V. Lipids in raw and cooked beef. J. Food Compos. Anal. 1987, 1, 26–37. [Google Scholar] [CrossRef]
- Rhee, K.S.; Thayne, R.; Dutson, T.R.; Smith, G.C.; Hostetler, R.L.; Reiser, R. Cholesterol content of raw and cooked beef Longissimus muscles with different degrees of marbling. J. Food Sci. 1982, 47, 716–719. [Google Scholar] [CrossRef]
- Slover, H.T.; Lanza, E. Quantitative Analysis of Food Fatty Acids by Capillary Gas Chromatography. J. Am. Oil Chem. Soc. 1979, 56, 933–943. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethyl acetals from lipids with boron fluoride- methanol. J. Lipid. Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- IBM SPSS Statistics 23; IBM Corporation: Armonk, NY, USA, 2019.
- Atencio-Valladares, O.; Huerta-Leidenz, N.; Jerez-Timaure, N. Predicción del rendimiento en cortes de carnicería de bovinos venezolanos. Rev. Cien. 2008, 18, 704–714. Available online: https://produccioncientificaluz.org/index.php/cientifica/article/view/15416 (accessed on 22 March 2021).
- Huerta-Leidenz, N.; Rodriguez, R.; Vidal-Ojeda, A.; Vidal-Quintero, A.; Jerez-Timaure, N. Características cárnicas de búfalos de agua vs. vacunos acebuados. Arch. Latinoam. Prod. Anim. 1997, 5 (Suppl. S1), 574–576. [Google Scholar]
- Arenas de Moreno, L.; Vidal, A.; Huerta-Sánchez, D.; Navas, Y.; Uzcátegui-Bracho, S.; Huerta-Leidenz, N. Análisis comparativo proximal y de minerales entre carnes de iguana, res y pollo. Arch. Latinoam. Nutr. 2000, 50, 409–415. [Google Scholar]
- Arenas de Moreno, L.; Ormos-Moreno, R.; Milli-París, S.; Huerta-Leidenz, N.; Uzcátegui-Bracho, S. Efecto de la dieta sobre la composición química de la carne de terneros. Rev. Cien. 2000, 10, 448–452. [Google Scholar]
- Huerta-Montauti, D.; Villa, V.; Arenas de Moreno, L.; Rodas-González, A.; Giuffrida-Mendoza, M.; Huerta-Leidenz, N. Proximate and mineral composition of imported versus domestic beef cuts for restaurant use in Venezuela. J. Muscle Foods 2007, 18, 237–252. [Google Scholar] [CrossRef]
- Uzcátegui-Bracho, S.; Giuffrida-Mendoza, M.; Arenas de Moreno, L.; Jerez-Timaure, N. contenido proximal, lípidos y colesterol de las carnes de res, cerdo y pollo obtenidas de expendios carniceros de la zona sur de Maracaibo. RVTS 2010, 3, 13–29. [Google Scholar]
- Alam, M.K.; Rana, Z.H.; Akhtaruzzaman, M. Comparison of muscle and subcutaneous tissue fatty acid composition of Bangladeshi nondescript deshi bulls finished on pasture diet. J. Chem. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Huerta-Leidenz, N.; Arenas de Moreno, L.; Morón-Fuenmayor, O.; Uzcátegui-Bracho, S. Composición mineral del músculo longissimus crudo derivado de canales bovinas producidas y clasificadas en Venezuela. Arch. Latinoam. Nutr. 2003, 53, 96–101. [Google Scholar]
- Arenas de Moreno, L.; Giuffrida-Mendoza, M.; Bulmes, L.; Uzcátegui-Bracho, S.; Huerta-Leidenz, N.; Jérez-Timaure, N. Efecto de la suplementación estratégica, régimen de implantes y condición sexual sobre la composición proximal y mineral de carne de bovinos cruda y cocida. Rev. Cien. 2008, 18, 65–72. [Google Scholar]
- Araujo, O. La nutrición mineral del ganado vacuno. In Desarrollo Sostenible de la Ganadería de Doble Propósito; González-Stagnaro, C., Soto Belloso, E., Eds.; Fundación Grupo de Investigadores de la Reproducción animal en la Región Zuliana: Maracaibo, Venezuela, 2008; pp. 463–475. [Google Scholar]
- Depablos, L.; Godoy, S.; Claudio, F.; Chicco, C.F.; Ordoñez, J. Nutrición mineral en sistemas ganaderos de las sabanas centrales de Venezuela. Zootec. Trop. 2009, 27, 25–37. [Google Scholar]
- López, M.; Godoy, S.; Alfaro, C.; Chicco, C.F. Evaluación de la nutrición mineral en sabanas bien drenadas al Sur del estado Monagas, Venezuela. Rev. Cien. 2008, 18, 197–206. [Google Scholar]
- Combes, S.; González, I.; Déjean, S.; Baccini, A.; Jehl, N.; Juin, H.; Cauquil, L.; Gabinaud, B.; Lebas, F.; Larzul, C. Relationships between sensory and physicochemical measurements in meat of rabbit from three different breeding systems using canonical correlation analysis. Meat Sci. 2008, 80, 835–841. [Google Scholar] [CrossRef]
- Byers, F.M. Nutritional factors affecting growth of muscle and adipose tissue in ruminants. Fed. Proc. 1982, 41, 2562–2566. [Google Scholar]
- Seideman, S.C.; Cross, H.R.; Oltjen, R.R.; Schanbacher, B.D. Utilisation of the intact male for red meat production: A review. J. Anim. Sci. 1982, 55, 826–840. [Google Scholar] [CrossRef]
- Park, S.J.; Beak, S.H.; Jung, D.J.S.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian-Australas J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef]
- Vargas, J.A.C.; Almeida, A.K.; Härter, C.J.; Souza, A.P.; Fernandes, M.H.M.D.R.; Resende, K.T.D.; Teixeira, I.A.M.D.A. Multivariate relationship among body protein, fat, and macrominerals of male and female Saanen goats using canonical correlation analysis. Rev. Bras. Zootec. 2018, 47, e20170289. [Google Scholar] [CrossRef]
- Ventura, H.T.; Lopes, P.S.; Peloso, J.V.; Guimarães, S.E.F.; Carneiro, A.P.S.; Carneiro, P.L.S. A canonical correlation analysis of the association between carcass and ham traits in pigs used to produce dry-cured ham. Genet. Mol. Biol. 2011, 34, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Marinetti, G.V. Disorders of Fatty Acid Metabolism. In Disorders of Lipids Metabolism, 1st ed.; Plenum Press: New York, NY, USA, 1990; pp. 31–48. [Google Scholar]
- Garmyn, A.J.; Hilton, G.G.; Mateescu, R.G.; Morgan, J.B.; Reecy, J.M.; Tait, R.G., Jr.; Beitz, D.C.; Duan, Q.; Schoonmaker, J.P.; Mayes, M.S.; et al. Nutrient components and beef palatability: Estimation of relationships between mineral concentration and fatty acid composition of Longissimus muscle and beef palatability traits. J. Anim. Sci. 2011, 89, 2849–2858. [Google Scholar] [CrossRef] [PubMed]
- Rotta, P.P.; Prado, R.M.; Prado, I.N.; Valero, M.V.; Visentainer, J.V.; Silva, R.R. The effects of genetic groups, nutrition, finishing systems and gender of Brazilian cattle on carcass characteristics and beef composition and appearance: A review. AJAS 2009, 22, 1718–1734. [Google Scholar] [CrossRef]
- Al-Jammas, M.; Agabriel, J.; Vernet, J.; Ortigues-Marty, I. Quantitative relationships between the tissue composition of bovine carcass and easily obtainable indicators. In Proceedings of the 61st International Congress of Meat Science and Technology (ICoMST), Clermont-Ferrand, France, 23–28 August 2015. [Google Scholar]
- Monteiro, A.C.G.; Santos-Silva, J.; Bessa, R.J.B.; Navas, D.R.; Lemos, J.P.C. Fatty acid composition of intramuscular fat of bulls and steers. Livest. Sci. 2006, 99, 13–19. [Google Scholar] [CrossRef]
- Moloney, A.P.; McGee, M. Factors Influencing the Growth of Meat Animals. In Lawrie´s Meat Science, 8th ed.; Toldrá, F., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 19–47. [Google Scholar]
- Giaretta, E.; Mordenti, A.L.; Canestrari, G.; Brogna, N.; Palmonari, A.; Formigoni, A. Assesment of muscle Longissmus thoracis et lumborum marbling by image analysis and relationships between meat quality parameters. PLoS ONE 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Kruk, Z.A.; Pitchford, W.S.; Siebert, B.D.; Deland, M.B.P.; Bottema, C.D.K. Factors affecting estimation of marbling in cattle and the relationship between marbling scores and intramuscular fat. Anim. Prod. Aust. 2002, 24, 129–132. [Google Scholar]
- Brackebusch, S.; McKeith, F.; Carr, T.; McLaren, D. Relationship between longissimus composition and the composition of other major muscles of the beef carcass. J. Anim. Sci. 1991, 69, 631–640. [Google Scholar] [CrossRef]
- Kornaska, M.; Kuchida, K.; Tarr, G.; Polkinghorne, R.J. Relationship between marbling measures across principal muscles. Meat Sci. 2017, 123, 67–78. [Google Scholar]
- Silva, S.; Teixeira, A.; Font-i-Furnois, M. Intramuscular fat and marbling. In A Handbook of Reference Methods for Meat Quality Assessment, 1st ed.; Font-i-Furnois, M., Candek-Potokar, M., Maltin, C., Prevolnik Povse, M., Eds.; European Cooperation in Science and Technology (COST): Edinburgh, UK, 2015; Chapter 2; pp. 12–21. [Google Scholar]
- Siebert, B.D.; Deland, M.P.; Pitchford, W.S. Breed differences in the fatty acid composition of subcutaneous and intramuscular lipid of early and late maturing, grain-finished cattle. Aust. J. Agric. Res. 1996, 47, 943–952. [Google Scholar] [CrossRef]
- Catillo, G.; Zappaterra, M.; Lo Fiego, D.P.; Steri, R.; Davoli, R. Relationships between EUROP carcass grading and backfat fatty acid composition in Italian Large White heavy pigs. Meat Sci. 2021, 171, 108291. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).