Current Progress in the Utilization of Soy-Based Emulsifiers in Food Applications—A Review
Abstract
1. Introduction
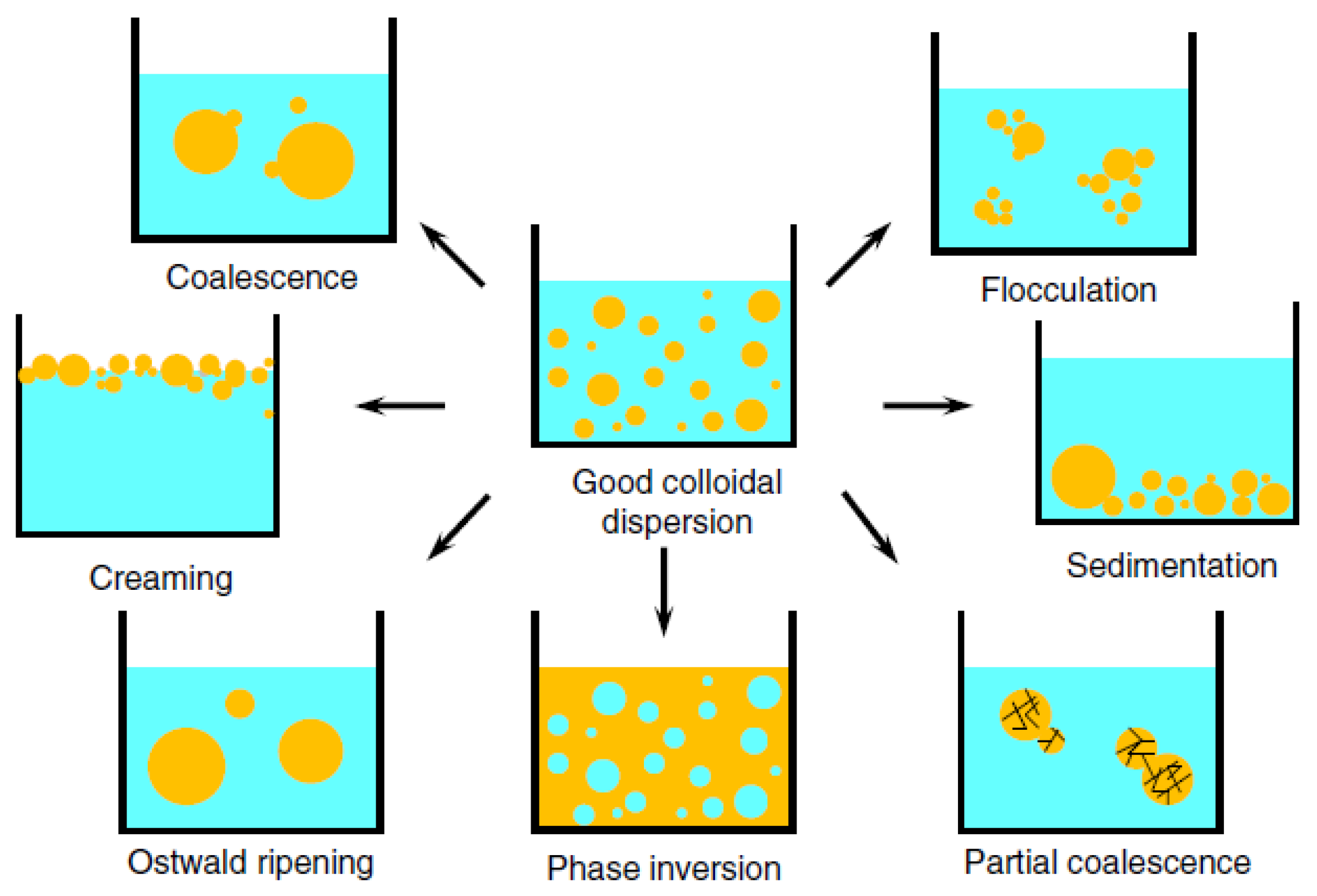
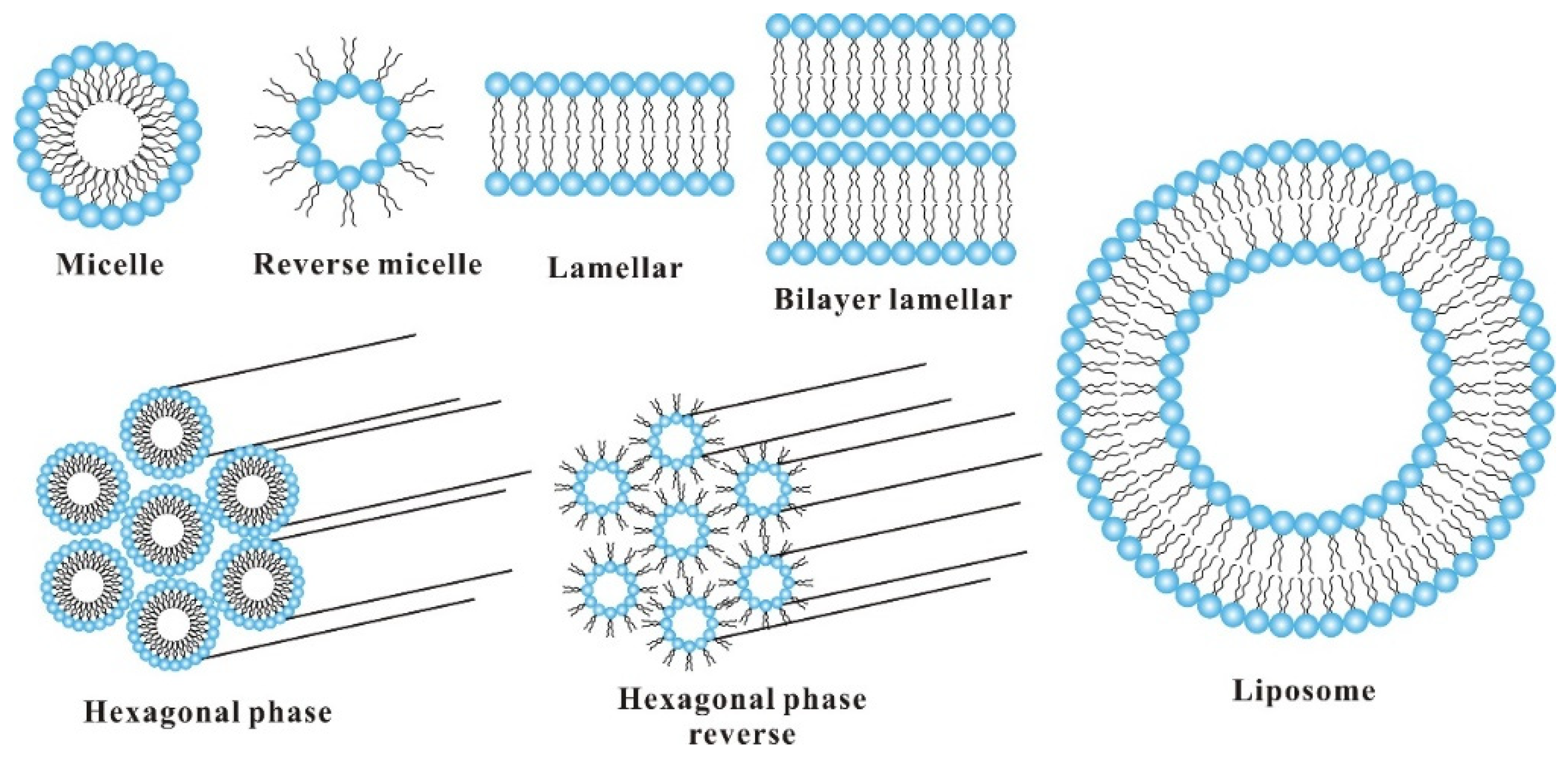
2. Emulsifying Properties
3. Soy Proteins
3.1. Enzyme Treatment
3.2. Thermal Treatment
3.3. Non-Thermal Processing
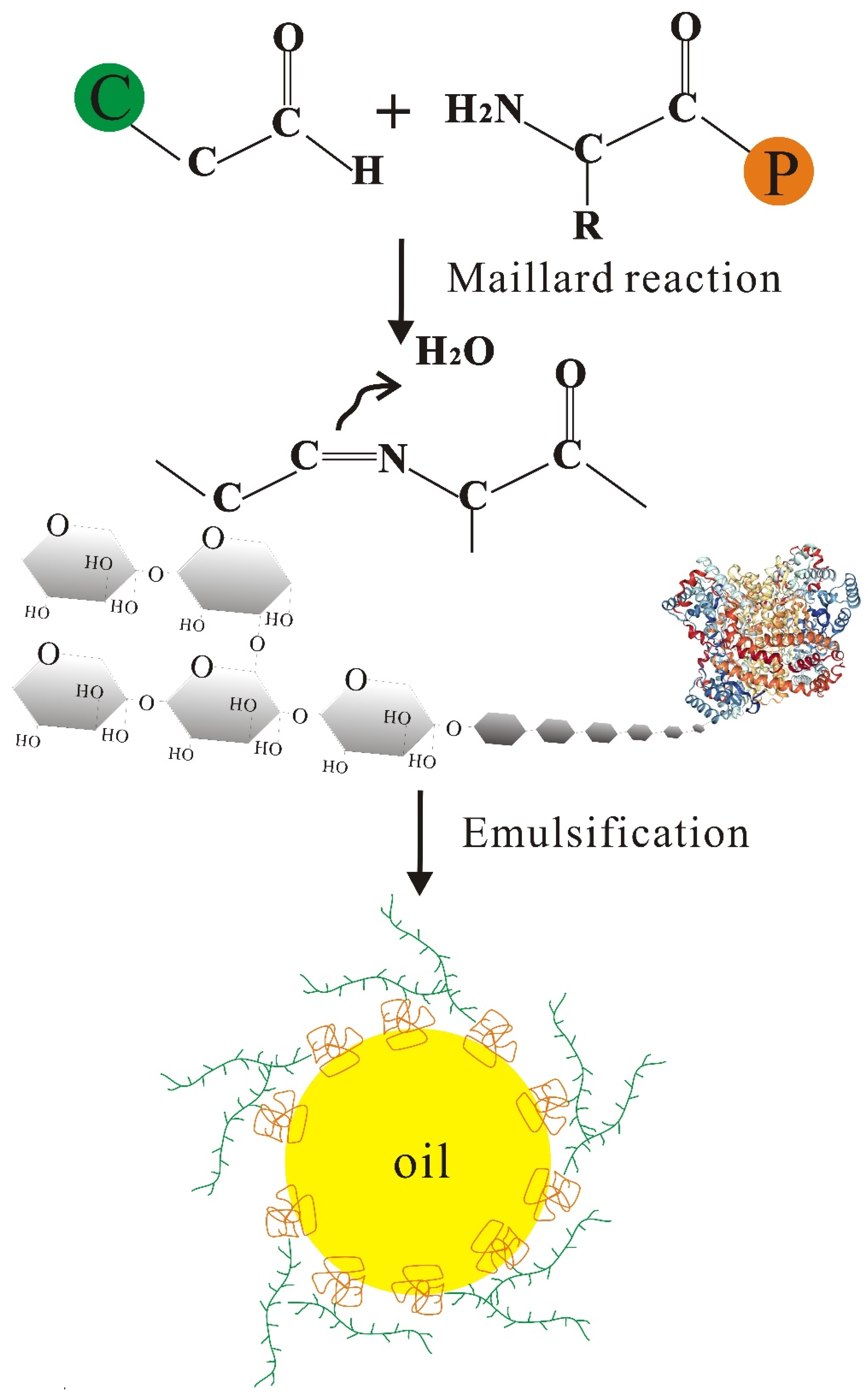
3.4. Glycation Modification
3.5. Fermentation Modification
4. Soy Polysaccharides
5. Soy Lecithin
6. Food Applications
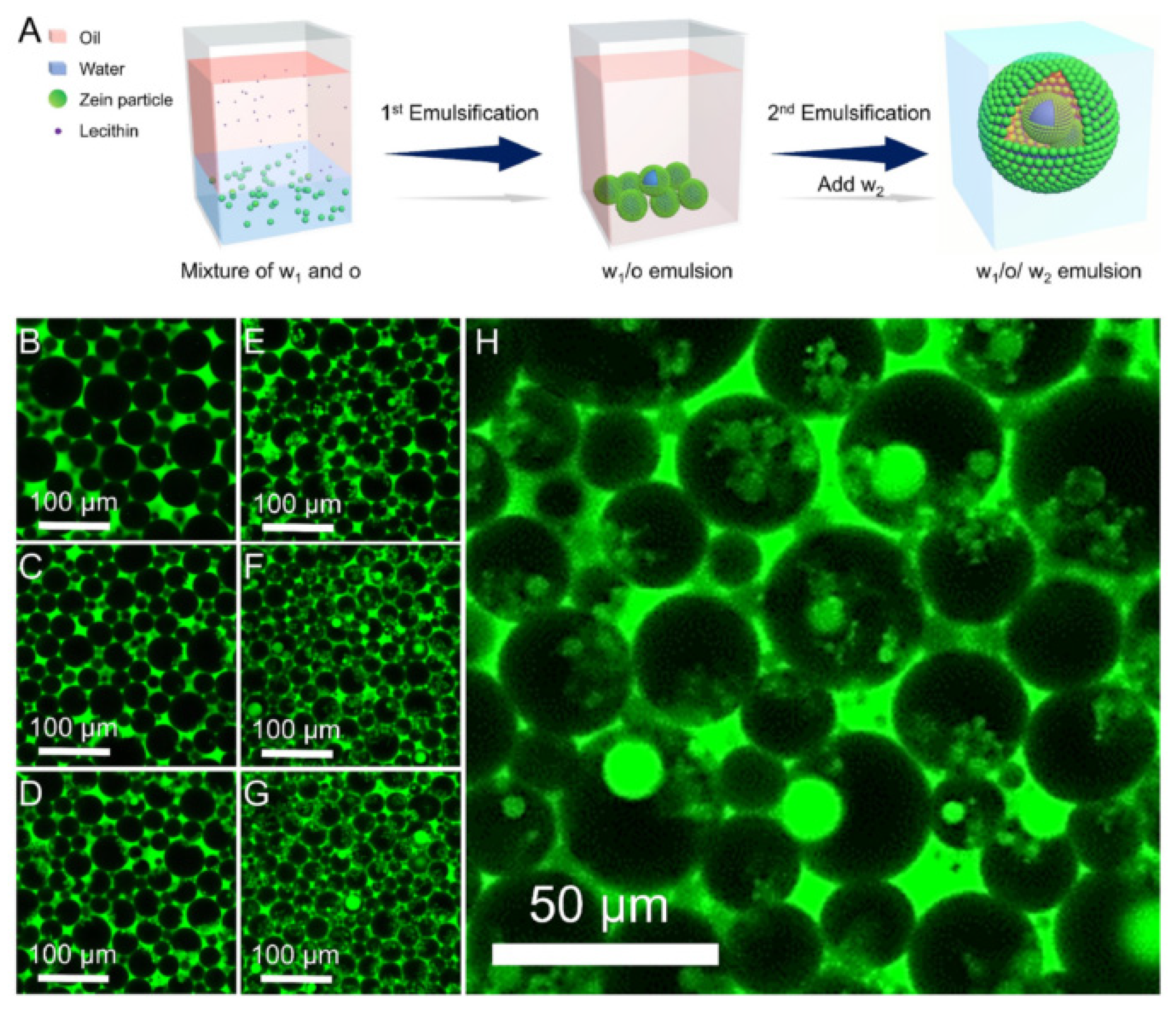
6.1. Bioactive Encapsulation and Delivery
6.2. Fat Replacer
7. Challenges and Future Trends
Funding
Conflicts of Interest
References
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Applications of soy protein hydrolysates in the emerging functional foods: A review. Int. J. Food Sci. Technol. 2020, 55, 421–428. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Kobayashi, I.; Chuah, A.M.; Nakajima, M.; Ichikawa, S. Formulation and stabilization of nano-/microdispersion systems using naturally occurring edible polyelectrolytes by electrostatic deposition and complexation. Adv. Colloid Interface Sci. 2015, 226, 86–100. [Google Scholar] [CrossRef]
- Rivas, H.; Sherman, P. Soy and meat proteins as emulsion stabilizers. 4. The stability and interfacial rheology of O/W emulsions stabilised by soy and meat protein fractions. Colloids Surf. 1984, 11, 155–171. [Google Scholar] [CrossRef]
- Liu, M.; Lee, D.-S.; Damodaran, S. Emulsifying properties of acidic subunits of soy 11S globulin. J. Agric. Food Chem. 1999, 47, 4970–4975. [Google Scholar] [CrossRef]
- Utsumi, S.; Matsumura, Y.; Mori, T. Structure-function relationships of soy proteins. In Food Proteins and Their Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 257–292. [Google Scholar]
- Molina, E.; Papadopoulou, A.; Ledward, D. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Xu, Q.; Nakajima, M.; Liu, Z.; Shiina, T. Soybean-based surfactants and their applications. In Soybean-Applications and Technology; IntechOpen: London, UK, 2011; pp. 341–364. [Google Scholar]
- Tang, C.-H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Shen, P.; Zhou, F.; Zhang, Y.; Yuan, D.; Zhao, Q.; Zhao, M. Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocoll. 2020, 105, 105844. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Zang, X.; Liu, P.; Chen, Y.; Wang, J.; Yu, G.; Xu, H. Improved freeze-thaw stability of o/w emulsions prepared with soybean protein isolate modified by papain and transglutaminase. LWT Food Sci. Technol. 2019, 104, 195–201. [Google Scholar] [CrossRef]
- Corredig, M. Heat-induced changes in oil-in-water emulsions stabilized with soy protein isolate. Food Hydrocoll. 2009, 23, 2141–2148. [Google Scholar]
- Iwabuchi, S.; Watanabe, H.; Yamauchi, F. Observations on the dissociation of. beta.-conglycinin into subunits by heat treatment. J. Agric. Food Chem. 1991, 39, 34–40. [Google Scholar] [CrossRef]
- Iwabuchi, S.; Watanabe, H.; Yamauchi, F. Thermal denaturation of. beta.-conglycinin. Kinetic resolution of reaction mechanism. J. Agric. Food Chem. 1991, 39, 27–33. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jia, S.; Zhang, W.; Ma, L.; Ding, X. Aggregation and emulsifying properties of soybean protein isolate pretreated by combination of dual-frequency ultrasound and ionic liquids. J. Mol. Liq. 2020, 301, 112394. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Lelas, V.; Mason, T.J.; Krešić, G.; Badanjak, M. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009, 93, 386–393. [Google Scholar] [CrossRef]
- Ma, X.; Yan, T.; Hou, F.; Chen, W.; Miao, S.; Liu, D. Formation of soy protein isolate (SPI)-citrus pectin (CP) electrostatic complexes under a high-intensity ultrasonic field: Linking the enhanced emulsifying properties to physicochemical and structural properties. Ultrason Sonochem. 2019, 59, 104748. [Google Scholar] [CrossRef]
- Albano, K.M.; Cavallieri, Â.L.F.; Nicoletti, V.R. Electrostatic Interaction between Soy Proteins and Pectin in O/W Emulsions Stabilization by Ultrasound Application. Food Biophys. 2020, 15, 297–312. [Google Scholar] [CrossRef]
- Ren, X.; Li, C.; Yang, F.; Huang, Y.; Huang, C.; Zhang, K.; Yan, L. Comparison of hydrodynamic and ultrasonic cavitation effects on soy protein isolate functionality. J. Food Eng. 2020, 265, 109697. [Google Scholar] [CrossRef]
- Puppo, M.; Speroni, F.; Chapleau, N.; de Lamballerie, M.; Añón, M.; Anton, M. Effect of high-pressure treatment on emulsifying properties of soybean proteins. Food Hydrocoll. 2005, 19, 289–296. [Google Scholar] [CrossRef]
- Torrezan, R.; Tham, W.P.; Bell, A.E.; Frazier, R.A.; Cristianini, M. Effects of high pressure on functional properties of soy protein. Food Chem. 2007, 104, 140–147. [Google Scholar] [CrossRef]
- Wang, X.-S.; Tang, C.-H.; Li, B.-S.; Yang, X.-Q.; Li, L.; Ma, C.-Y. Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Mo, H. Effects of pulsed electric fields on physicochemical properties of soybean protein isolates. LWT Food Sci. Technol. 2007, 40, 1167–1175. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A.; Milani, E.; Varidi, M. Extruded soy protein as a novel emulsifier: Structure, interfacial activity and emulsifying property. Food Hydrocoll. 2019, 93, 361–373. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, A.; Wang, X.; Xu, N.; Jiang, L. The radiation assisted-Maillard reaction comprehensively improves the freeze-thaw stability of soy protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2020, 103, 105684. [Google Scholar] [CrossRef]
- Kinsella, J.; Whitehead, D. Emulsifying and foaming properties of chemically modified proteins. In Advances in Food Emulsions and Foams; Elsevier Applied Science Publishers Ltd.: London, UK, 1988; pp. 163–188. [Google Scholar]
- Kutzli, I.; Weiss, J.; Gibis, M. Glycation of Plant Proteins Via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application. Foods 2021, 10, 376. [Google Scholar] [CrossRef]
- Liu, J.; Ru, Q.; Ding, Y. Glycation a promising method for food protein modification: Physicochemical properties and structure, a review. Food Res. Int. 2012, 49, 170–183. [Google Scholar] [CrossRef]
- Naik, R.R.; Wang, Y.; Selomulya, C. Improvements of plant protein functionalities by Maillard conjugation and Maillard reaction products. Crit. Rev. Food Sci. Nutr. 2021, 1–26. [Google Scholar] [CrossRef]
- Sivapratha, S.; Sarkar, P. Multiple layers and conjugate materials for food emulsion stabilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 877–892. [Google Scholar] [CrossRef]
- Yang, H.; Su, Z.; Meng, X.; Zhang, X.; Kennedy, J.F.; Liu, B. Fabrication and characterization of Pickering emulsion stabilized by soy protein isolate-chitosan nanoparticles. Carbohydr. Polym. 2020, 247, 116712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Yu, J.; Wang, G.; Wang, X.; Zhang, L. Improving the emulsion freeze-thaw stability of soy protein hydrolysate-dextran conjugates. LWT Food Sci. Technol. 2019, 116, 108506. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Liu, J.; Cui, Q.; Wang, X.; Chen, S.; Jiang, L. Relationship between molecular flexibility and emulsifying properties of soy protein isolate-glucose conjugates. J. Agric. Food Chem. 2019, 67, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, X.H. Structure and property changes of the soy protein isolate glycated with maltose in an ionic liquid through the Maillard reaction. Food Funct. 2019, 10, 1948–1957. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Zhao, G. Fabricating a pickering stabilizer from Okara dietary fibre particulates by conjugating with soy protein isolate via maillard reaction. Foods 2020, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.Z.; Peng, X.Q.; Tang, C.H. Edible pickering high internal phase emulsions stabilized by soy glycinin: Improvement of emulsification performance and pickering stabilization by glycation with soy polysaccharide. Food Hydrocoll. 2020, 103, 105672. [Google Scholar] [CrossRef]
- Li, Q.; He, Q.; Xu, M.; Li, J.; Liu, X.; Wan, Z.; Yang, X. Food-grade emulsions and emulsion gels prepared by soy protein-pectin complex nanoparticles and glycyrrhizic acid nanofibrils. J. Agric. Food Chem. 2020, 68, 1051–1063. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Schweiggert-Weisz, U.; Eisner, P. Soy protein hydrolysates fermentation: Effect of debittering and degradation of major soy allergens. LWT Food Sci. Technol. 2016, 71, 202–212. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Schweiggert-Weisz, U.; Eisner, P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem. 2016, 205, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Qin, W.; Gu, J.; Zhang, H.; Duan, Y.; Ma, H. Structure and functional properties of soy protein isolate-lentinan conjugates obtained in Maillard reaction by slit divergent ultrasonic assisted wet heating and the stability of oil-in-water emulsions. Food Chem. 2020, 331, 127374. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Takahashi, T.; Yoshida, R.; Maeda, H.; Corredig, M. Emulsifying properties of soybean soluble polysaccharide. Food Hydrocoll. 2004, 18, 795–803. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Polysaccharides at fluid interfaces of food systems. Adv. Colloid Interface Sci. 2019, 270, 28–37. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshida, R.; Maeda, H.; Furuta, H.; Corredig, M. Study of the role of the carbohydrate and protein moieties of soy soluble polysaccharides in their emulsifying properties. J. Agric. Food Chem. 2004, 52, 5506–5512. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Maeda, H.; Corredig, M. Influence of heating on oil-in-water emulsions prepared with soybean soluble polysaccharide. J. Agric. Food Chem. 2007, 55, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Maeda, H.; Corredig, M. Emulsifying properties of enzyme-digested soybean soluble polysaccharide. Food Hydrocoll. 2006, 20, 1029–1038. [Google Scholar] [CrossRef]
- Yin, B.; Deng, W.; Xu, K.; Huang, L.; Yao, P. Stable nano-sized emulsions produced from soy protein and soy polysaccharide complexes. J. Colloid Interface Sci. 2012, 380, 51–59. [Google Scholar] [CrossRef]
- Nakamura, A.; Furuta, H.; Kato, M.; Maeda, H.; Nagamatsu, Y. Effect of soybean soluble polysaccharides on the stability of milk protein under acidic conditions. Food Hydrocoll. 2003, 17, 333–343. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshida, R.; Maeda, H.; Corredig, M. The stabilizing behaviour of soybean soluble polysaccharide and pectin in acidified milk beverages. Int. Dairy J. 2006, 16, 361–369. [Google Scholar] [CrossRef]
- Chivero, P.; Gohtani, S.; Yoshii, H.; Nakamura, A. Assessment of soy soluble polysaccharide, gum arabic and OSA-Starch as emulsifiers for mayonnaise-like emulsions. LWT Food Sci. Technol. 2016, 69, 59–66. [Google Scholar] [CrossRef]
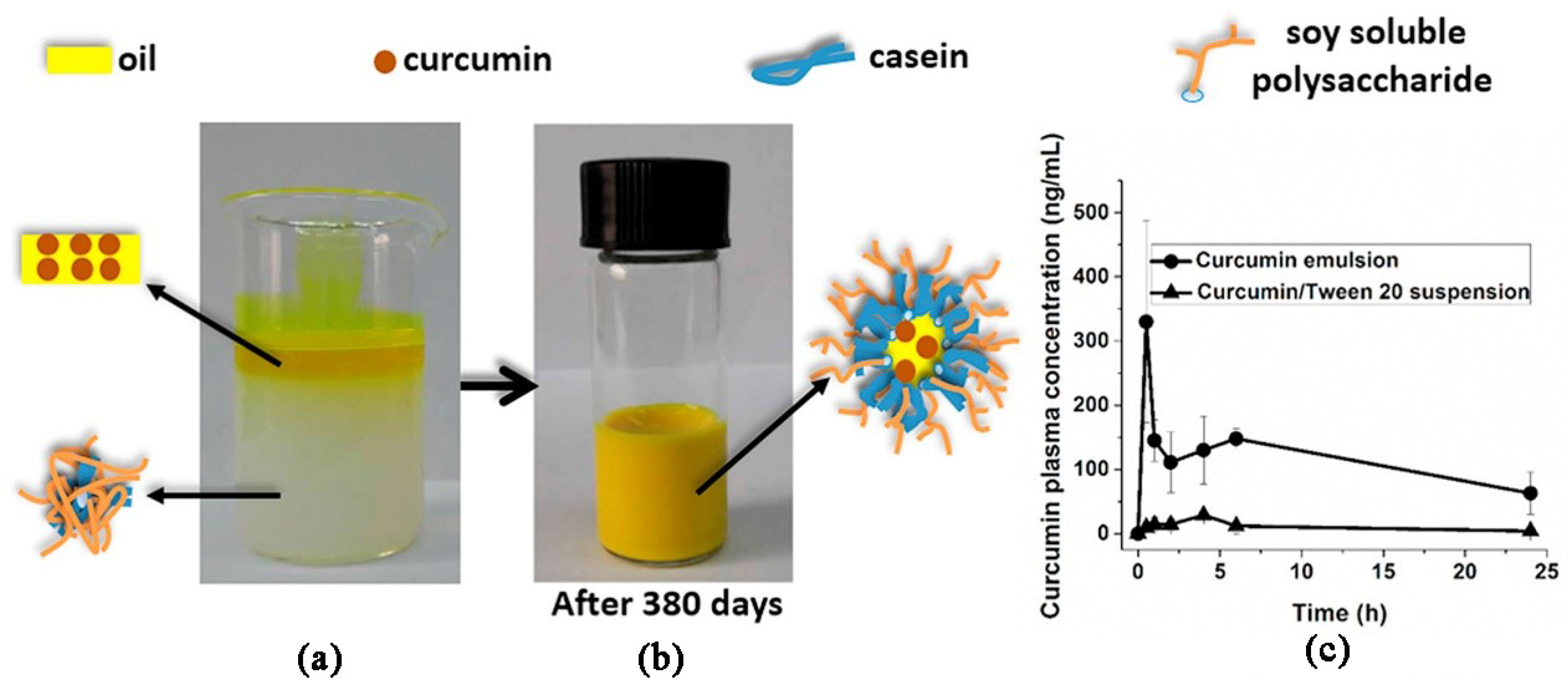
- Xu, G.; Wang, C.; Yao, P. Stable emulsion produced from casein and soy polysaccharide compacted complex for protection and oral delivery of curcumin. Food Hydrocoll. 2017, 71, 108–117. [Google Scholar] [CrossRef]
- Zhan, F.; Shi, M.; Wang, Y.; Li, B.; Chen, Y. Effect of freeze-drying on interaction and functional properties of pea protein isolate/soy soluble polysaccharides complexes. J. Mol. Liq. 2019, 285, 658–667. [Google Scholar] [CrossRef]
- Porfiri, M.C.; Vaccaro, J.; Stortz, C.A.; Navarro, D.A.; Wagner, J.R.; Cabezas, D.M. Insoluble soybean polysaccharides: Obtaining and evaluation of their O/W emulsifying properties. Food Hydrocoll. 2017, 73, 262–273. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade Pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
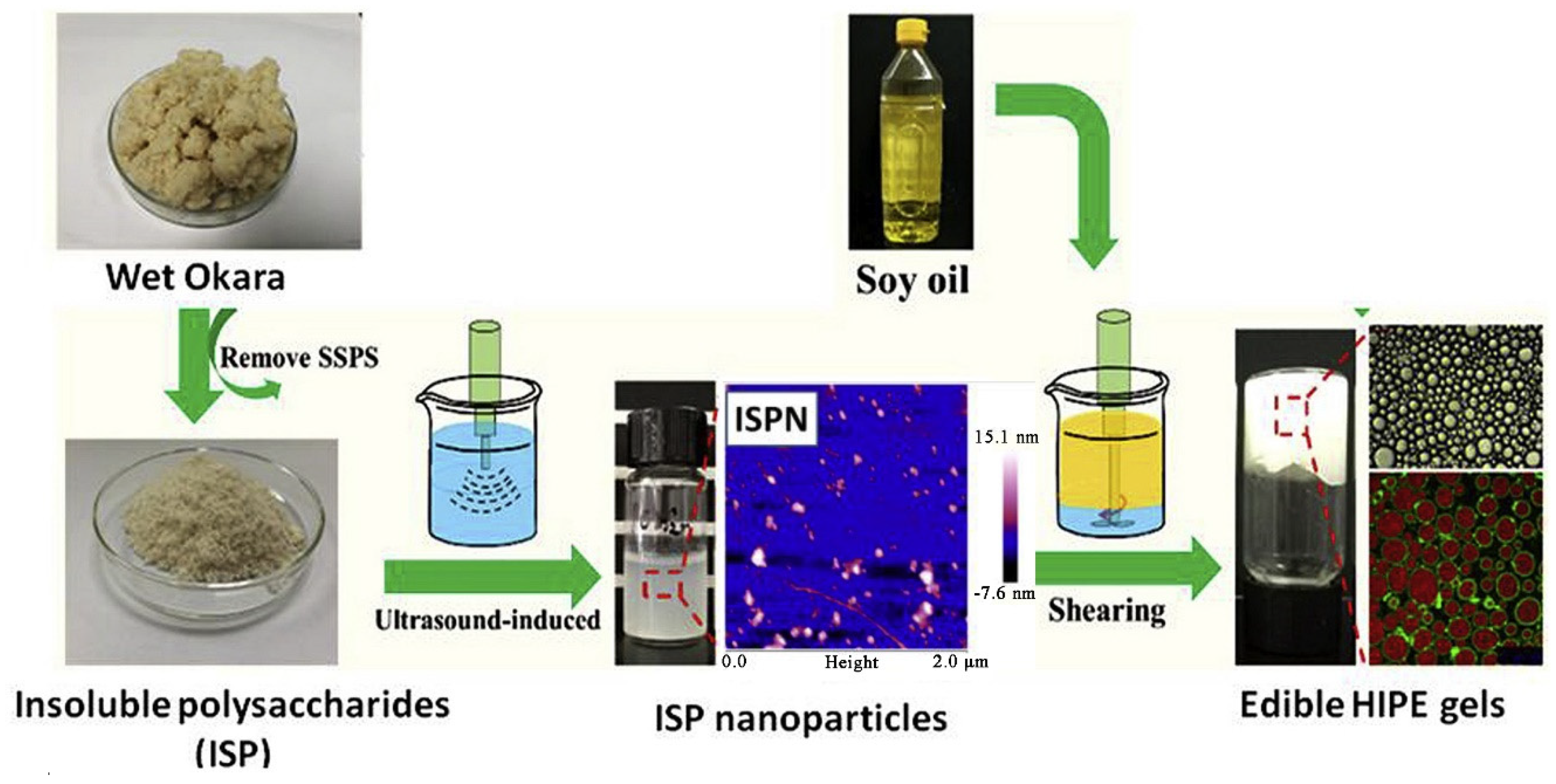
- Yang, T.; Li, X.T.; Tang, C.H. Novel edible pickering high-internal-phase-emulsion gels efficiently stabilized by unique polysaccharide-protein hybrid nanoparticles from Okara. Food Hydrocoll. 2020, 98, 105285. [Google Scholar] [CrossRef]
- Flores-Andrade, E.; Allende-Baltazar, Z.; Sandoval-González, P.E.; Jiménez-Fernández, M.; Beristain, C.I.; Pascual-Pineda, L.A. Carotenoid nanoemulsions stabilized by natural emulsifiers: Whey protein, gum Arabic, and soy lecithin. J. Food Eng. 2021, 290, 110208. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Sui, Z.; Lu, W.; Corke, H. Soybean lecithin-stabilized oil-in-water (O/W) emulsions increase the stability and in vitro bioaccessibility of bioactive nutrients. Food Chem. 2020, 338, 128071. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.K.W.; Chung, C.; Fu, J.T.R.; Sher, A.; Rousset, P.; McClements, D.J. Impact of sodium caseinate, soy lecithin and carrageenan on functionality of oil-in-water emulsions. Food Res. Int. 2019, 123, 779–789. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, P.J.; Horn, A.F.; Jacobsen, C. Influence of casein–phospholipid combinations as emulsifier on the physical and oxidative stability of fish oil-in-water emulsions. J. Agric. Food Chem. 2014, 62, 1142–1152. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Tu, Z.; Zhang, L.; Wang, H.; Tian, M.; Zhang, N. Influence of soy lecithin concentration on the physical properties of whey protein isolate-stabilized emulsion and microcapsule formation. J. Food Eng. 2017, 207, 73–80. [Google Scholar] [CrossRef]
- Shen, Y.; Chang, C.; Shi, M.; Su, Y.; Gu, L.; Li, J.; Yang, Y. Interactions between lecithin and yolk granule and their influence on the emulsifying properties. Food Hydrocoll. 2020, 101, 105510. [Google Scholar] [CrossRef]
- Sandoval-Cuellar, C.E.; de Jesus Perea-Flores, M.; Quintanilla-Carvajal, M.X. In-vitro digestion of whey protein- and soy lecithin-stabilized High Oleic Palm Oil emulsions. J. Food Eng. 2020, 278, 109918. [Google Scholar] [CrossRef]
- Zhuang, X.; Gaudino, N.; Clark, S.; Acevedo, N.C. Novel lecithin-based oleogels and oleogel emulsions delay lipid oxidation and extend probiotic bacteria survival. LWT Food Sci. Technol. 2021, 136, 110353. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, T.; Smits, J.; Huang, X.; Maas, M.; Yin, S.; Ngai, T. Edible high internal phase Pickering emulsion with double-emulsion morphology. Food Hydrocoll. 2021, 111, 106405. [Google Scholar] [CrossRef]
- Tang, C.-H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocoll. 2019, 91, 92–116. [Google Scholar] [CrossRef]
- Wang, S.; Shao, G.; Yang, J.; Zhao, H.; Qu, D.; Zhang, D.; Zhu, D.; He, Y.; Liu, H. Contribution of soybean polysaccharides in digestion of oil-in-water emulsion-based delivery system in an in vitro gastric environment. Food Sci. Technol. Int. 2020, 26, 444–452. [Google Scholar] [CrossRef]
- Paglarini, C.D.S.; Martini, S.; Pollonio, M.A.R. Using emulsion gels made with sonicated soy protein isolate dispersions to replace fat in frankfurters. LWT Food Sci. Technol. 2019, 99, 453–459. [Google Scholar] [CrossRef]
- Balcaen, M.; Steyls, J.; Schoeppe, A.; Nelis, V.; Van der Meeren, P. Phosphatidylcholine-depleted lecithin: A clean-label low-HLB emulsifier to replace PGPR in w/o and w/o/w emulsions. J. Colloid Interface Sci. 2020, 581, 836–846. [Google Scholar] [CrossRef]
- Jalali-Jivan, M.; Abbasi, S. Novel approach for lutein extraction: Food grade microemulsion containing soy lecithin & sunflower oil. Innov. Food Sci. Emerg. Technol. 2020, 66, 102505. [Google Scholar]
- Liu, F.; Tang, C.-H. Soy glycinin as food-grade Pickering stabilizers: Part. III. Fabrication of gel-like emulsions and their potential as sustained-release delivery systems for β-carotene. Food Hydrocoll. 2016, 56, 434–444. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Ruiz-Capillas, C.; Salvador, M.; Herrero, A.M. Emulsion gels as delivery systems for phenolic compounds: Nutritional, technological and structural properties. Food Chem. 2021, 339, 128049. [Google Scholar] [CrossRef] [PubMed]
- Dreher, J.; Blach, C.; Terjung, N.; Gibis, M.; Weiss, J. Influence of protein content on plant-based emulsified and crosslinked fat crystal networks to mimic animal fat tissue. Food Hydrocoll. 2020, 106, 105864. [Google Scholar] [CrossRef]
- Dreher, J.; Blach, C.; Terjung, N.; Gibis, M.; Weiss, J. Formation and characterization of plant-based emulsified and crosslinked fat crystal networks to mimic animal fat tissue. J. Food Sci. 2020, 85, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Utama, D.T.; Jeong, H.S.; Kim, J.; Barido, F.H.; Lee, S.K. Fatty acid composition and quality properties of chicken sausage formulated with pre-emulsified perilla-canola oil as an animal fat replacer. Poult. Sci. 2019, 98, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- de Souza Paglarini, C.; de Figueiredo Furtado, G.; Honório, A.R.; Mokarzel, L.; da Silva Vidal, V.A.; Ribeiro, A.P.B.; Cunha, R.L.; Pollonio, M.A.R. Functional emulsion gels as pork back fat replacers in Bologna sausage. Food Struct. 2019, 20, 100105. [Google Scholar] [CrossRef]
- Pintado, T.; Muñoz-González, I.; Salvador, M.; Ruiz-Capillas, C.; Herrero, A.M. Phenolic compounds in emulsion gel-based delivery systems applied as animal fat replacers in frankfurters: Physico-chemical, structural and microbiological approach. Food Chem. 2021, 340, 128095. [Google Scholar] [CrossRef]
- Chen, Y.B.; Zhu, X.F.; Liu, T.X.; Lin, W.F.; Tang, C.H.; Liu, R. Improving freeze-thaw stability of soy nanoparticle-stabilized emulsions through increasing particle size and surface hydrophobicity. Food Hydrocoll. 2019, 87, 404–412. [Google Scholar] [CrossRef]
- Chen, W.; Liang, G.; Li, X.; He, Z.; Zeng, M.; Gao, D.; Qin, F.; Goff, H.D.; Chen, J. Effects of soy proteins and hydrolysates on fat globule coalescence and meltdown properties of ice cream. Food Hydrocoll. 2019, 94, 279–286. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, physicochemical and sensorial properties of commercial plant-based yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Bhattacharyya, D.K.; Goswami, R.; Bhowal, J. Emulsions stabilized by soy protein nanoparticles as potential functional non-dairy yogurts. J. Sci. Food Agric. 2019, 99, 5808–5818. [Google Scholar] [CrossRef]
- Chung, C.; Sher, A.; Rousset, P.; Decker, E.A.; McClements, D.J. Formulation of food emulsions using natural emulsifiers: Utilization of quillaja saponin and soy lecithin to fabricate liquid coffee whiteners. J. Food Eng. 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Egbert, W.R. Isolated Soy Protein: Technology, Properties, and Applications. Soybeans as Functional Foods and Ingredients; Liu, K., Ed.; AOCS Press: Champaign, IL, USA, 2004; pp. 134–162. [Google Scholar]
- Lee, K.; Ryu, H.; Rhee, K. Protein solubility characteristics of commercial soy protein products. J. Am. Oil Chem. Soc. 2003, 80, 85–90. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.; Kong, Y.; Ma, Z.; Wu, C.; Regenstein, J.M.; Teng, F.; Li, Y. Different commercial soy protein isolates and the characteristics of Chiba tofu. Food Hydrocoll. 2021, 110, 106115. [Google Scholar] [CrossRef]


| Emulsifier | Glycation Conditions | Main Conclusions | References |
|---|---|---|---|
| Soy protein isolate–glucose | Wet heating, 50, 60, 70, 80, and 90 °C, 5 h | The EAI and ESI of the soy protein–glucose isolate were markedly improved under different reaction temperature conditions in comparison to that of untreated SPI. | [37] |
| Soy protein–maltose | Wet heating, 100 °C, 2 h | The 1-butyl-3-methylimidazolium chloride was proved to be a proper medium for protein glycation to increase glycation extent and to improve the emulsifying activity and emulsion stability. | [38] |
| Soy protein isolate–gum acacia | Dry heating, 60 °C, RH 79%, 6 days | The soy protein isolates–gum acacia (SPI-GA) conjugates films containing essential oils showed the highest radical scavenging activity and antibacterial activity. | [39] |
| Soy protein hydrolysate-dextran | Wet heating, 85 °C, 1 h | The soy protein hydrolysate-dextran conjugates produced through the wet method under optimal conditions showed the lowest creaming index and the best freeze–thaw stability. | [36] |
| Soy protein isolate–Okara dietary fibre (ODF) | Dry heating, 60 °C, RH 78%, 6–72 h | The resulting ODF-SPI conjugates were thermally stable and exhibited excellent Pickering emulsion stabilization potentials. | [40] |
| Soy glycinin–soy polysaccharide | Dry heating, 60 °C, RH 78%, 24 h and 72 h | The glycation with soy soluble polysaccharide (SSPS) greatly improved the emulsification performance of soy glycinin, the gel network formation and stability (against heating or freeze–thawing) of the resultant high internal phase emulsions. | [41] |
| Soy protein–pectin | Wet heating, pH 4.5, 95 °C, 30 min | With the addition of glycyrrhizic acid nanofibrils, self-standing soy protein-pectin nanoparticles (SPNPs) stabilized emulsion gels with small droplet size, homogeneous appearance, and microstructure were obtained. | [42] |
| Soy protein isolate–pectin | Dry heating, 60 °C, RH 79%, 1–7 days | The solubility and emulsifying properties were improved after the Maillard reaction and the strong steric-hindrance effect of pectin facilitated the stability of the emulsion. | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L. Current Progress in the Utilization of Soy-Based Emulsifiers in Food Applications—A Review. Foods 2021, 10, 1354. https://doi.org/10.3390/foods10061354
Deng L. Current Progress in the Utilization of Soy-Based Emulsifiers in Food Applications—A Review. Foods. 2021; 10(6):1354. https://doi.org/10.3390/foods10061354
Chicago/Turabian StyleDeng, Lingli. 2021. "Current Progress in the Utilization of Soy-Based Emulsifiers in Food Applications—A Review" Foods 10, no. 6: 1354. https://doi.org/10.3390/foods10061354
APA StyleDeng, L. (2021). Current Progress in the Utilization of Soy-Based Emulsifiers in Food Applications—A Review. Foods, 10(6), 1354. https://doi.org/10.3390/foods10061354






