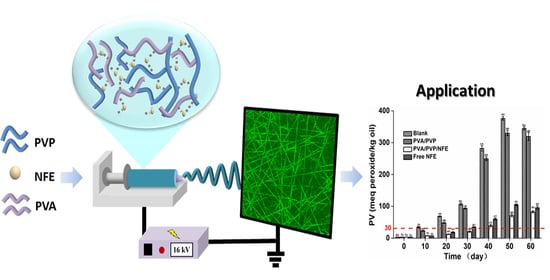
Development of Nervilia fordii Extract-Loaded Electrospun PVA/PVP Nanocomposite for Antioxidant Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Different Solvent Extracts
2.3. Determination of Antioxidant Contents in Nervilia fordii Extracts
- (1)
- Total phenol content (TPC)
- (2)
- Total flavonoid content (TFC)
2.4. Antioxidant Activity Analysis
- (1)
- DPPH assay
- (2)
- Lipid peroxidation (LPO) assay
2.5. Encapsulation of Nervilia fordii Extract by Electrospinning
2.6. Characterization of the Electrospun Fiber Mat
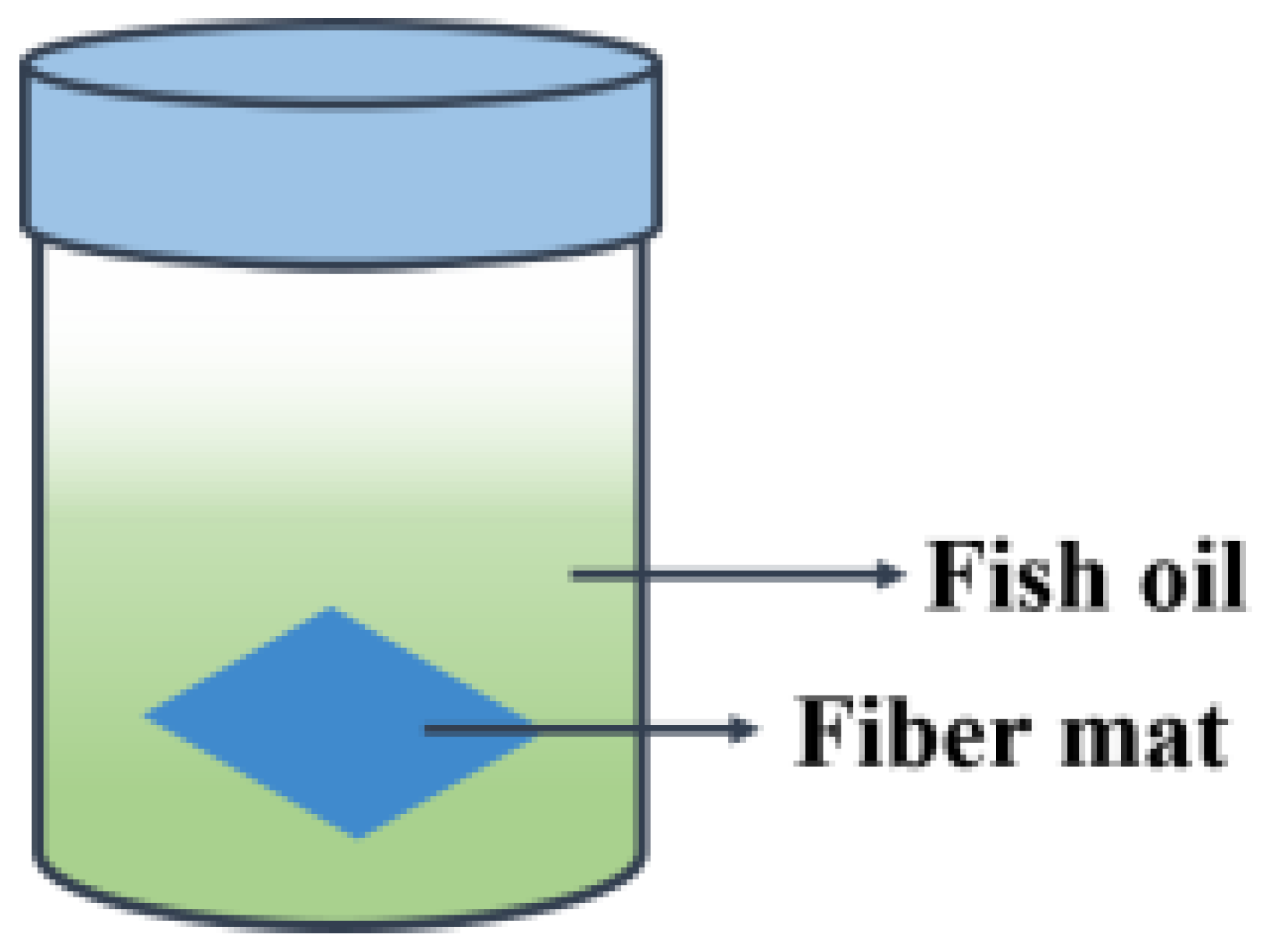
2.7. Oxidative Stability in Accelerated Storage Test
2.8. Statistical Analysis
3. Results
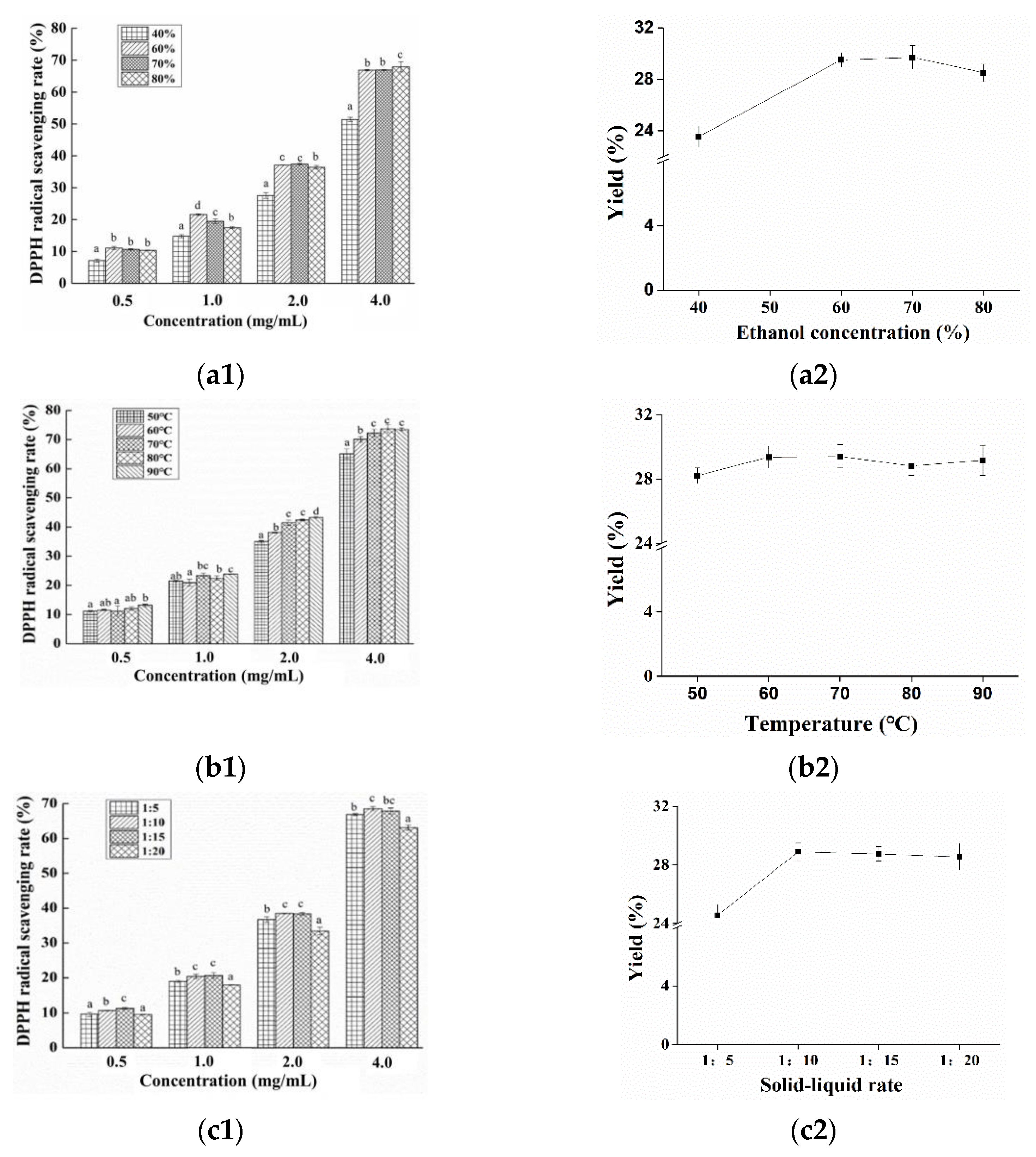
3.1. Preparation of Nervilia fordii Ethanol Extract
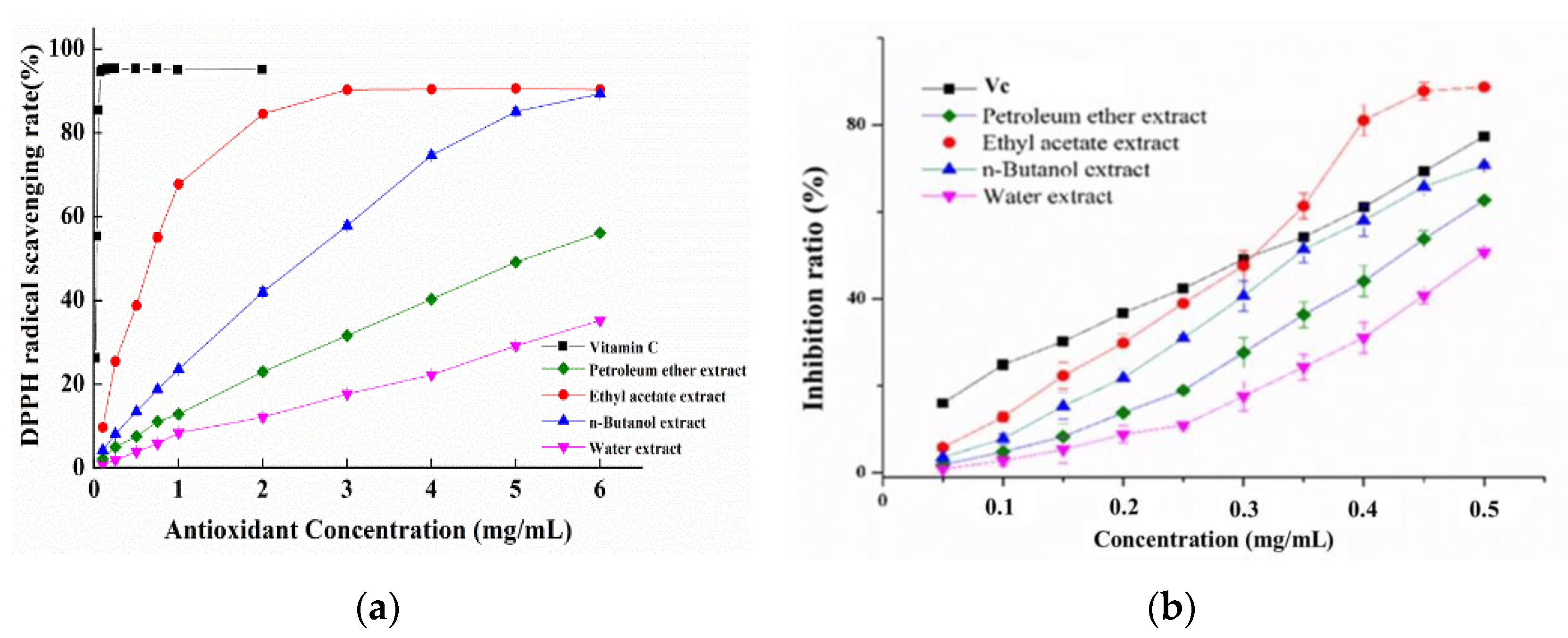
3.2. Antioxidant Capacities of Different Solvent Extracts
3.3. Total Phenol and Flavonoid Contents
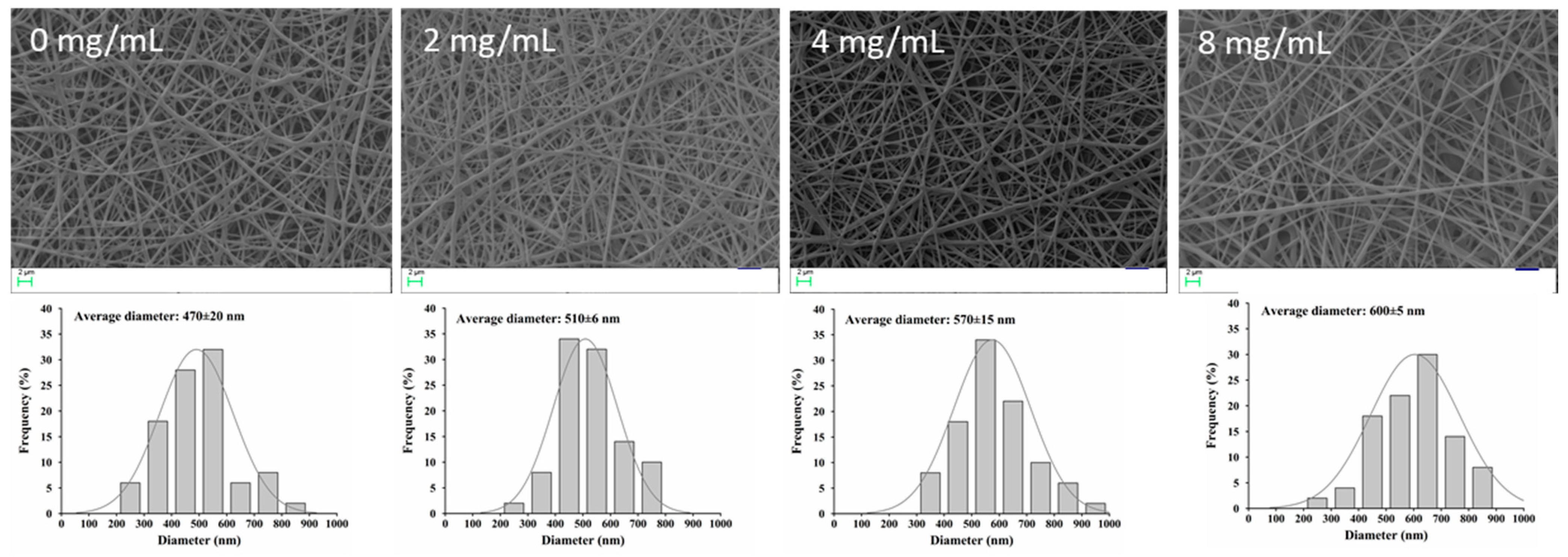
3.4. Encapsulation of NFE into PVA/PVP Electrospun Nanofibers
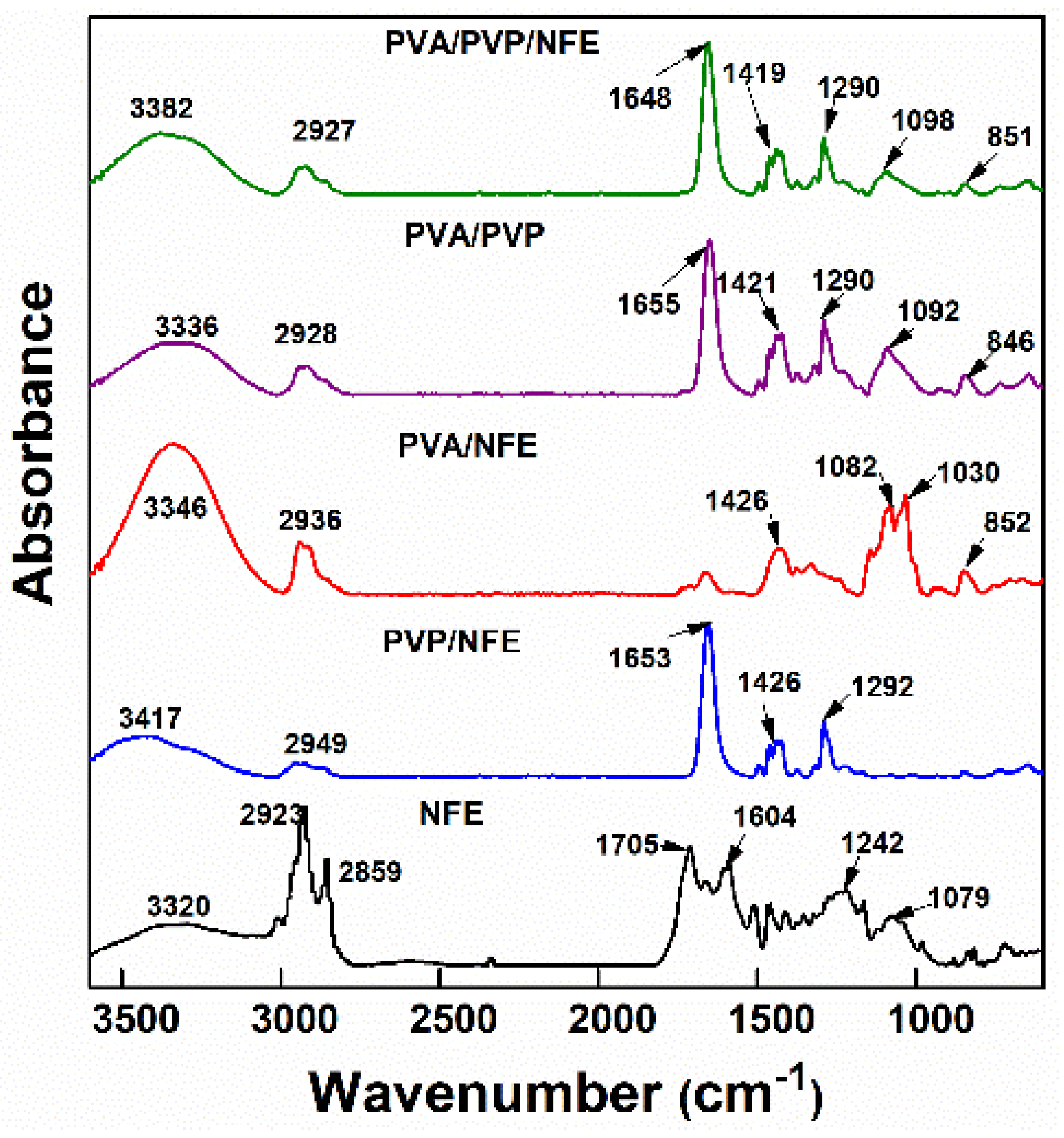
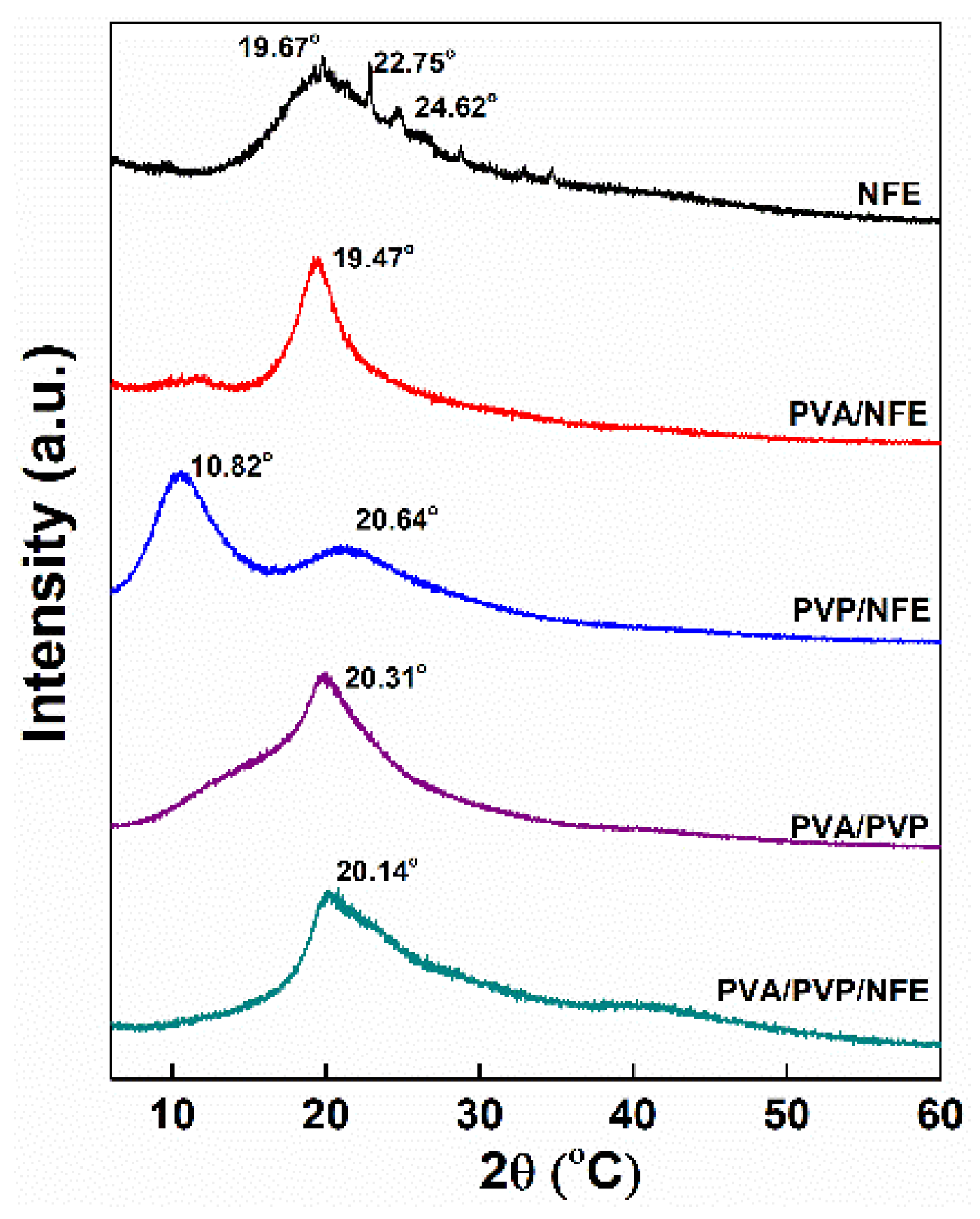
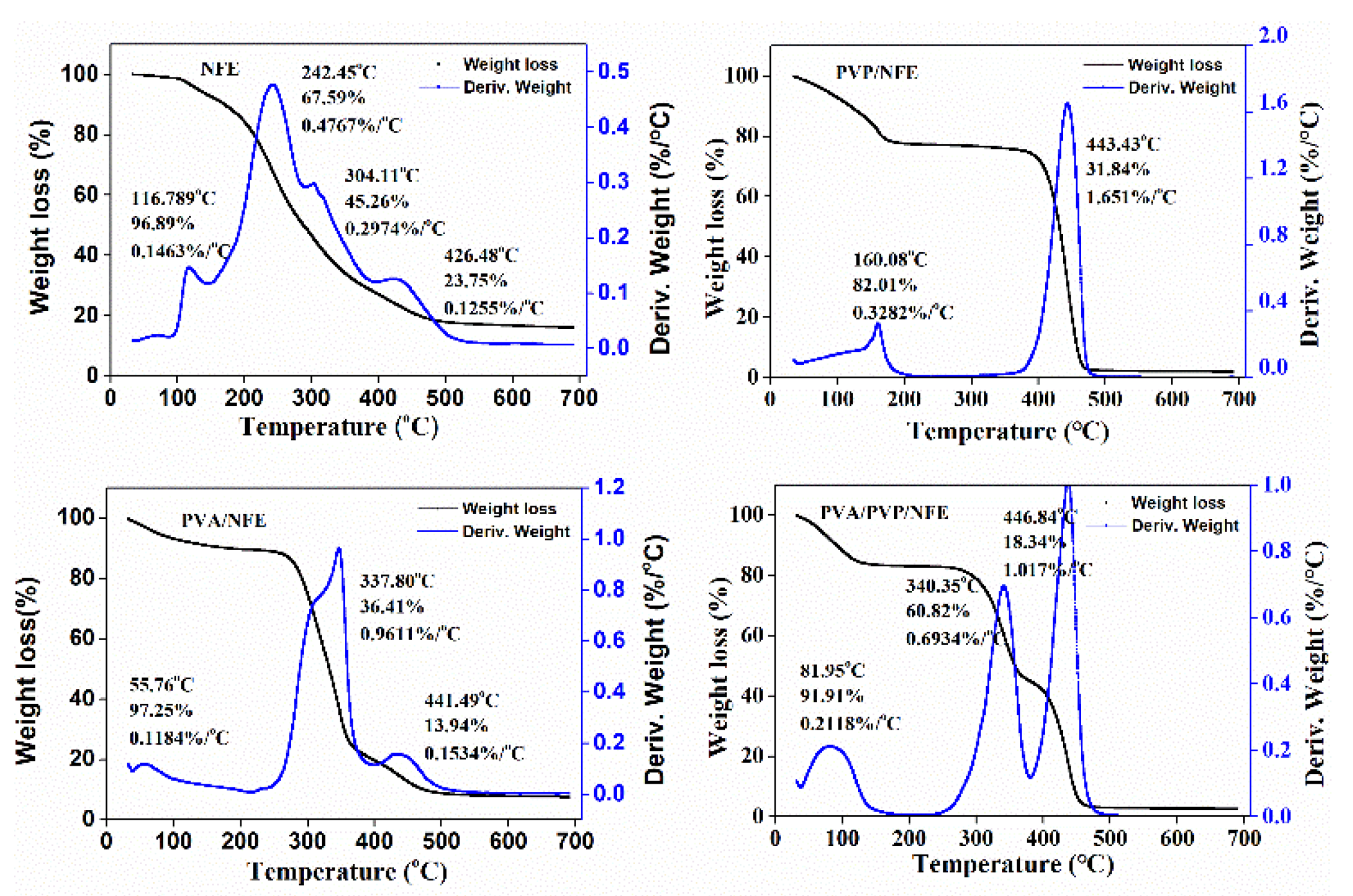
3.5. Characterization of Electrospun Nanofibers
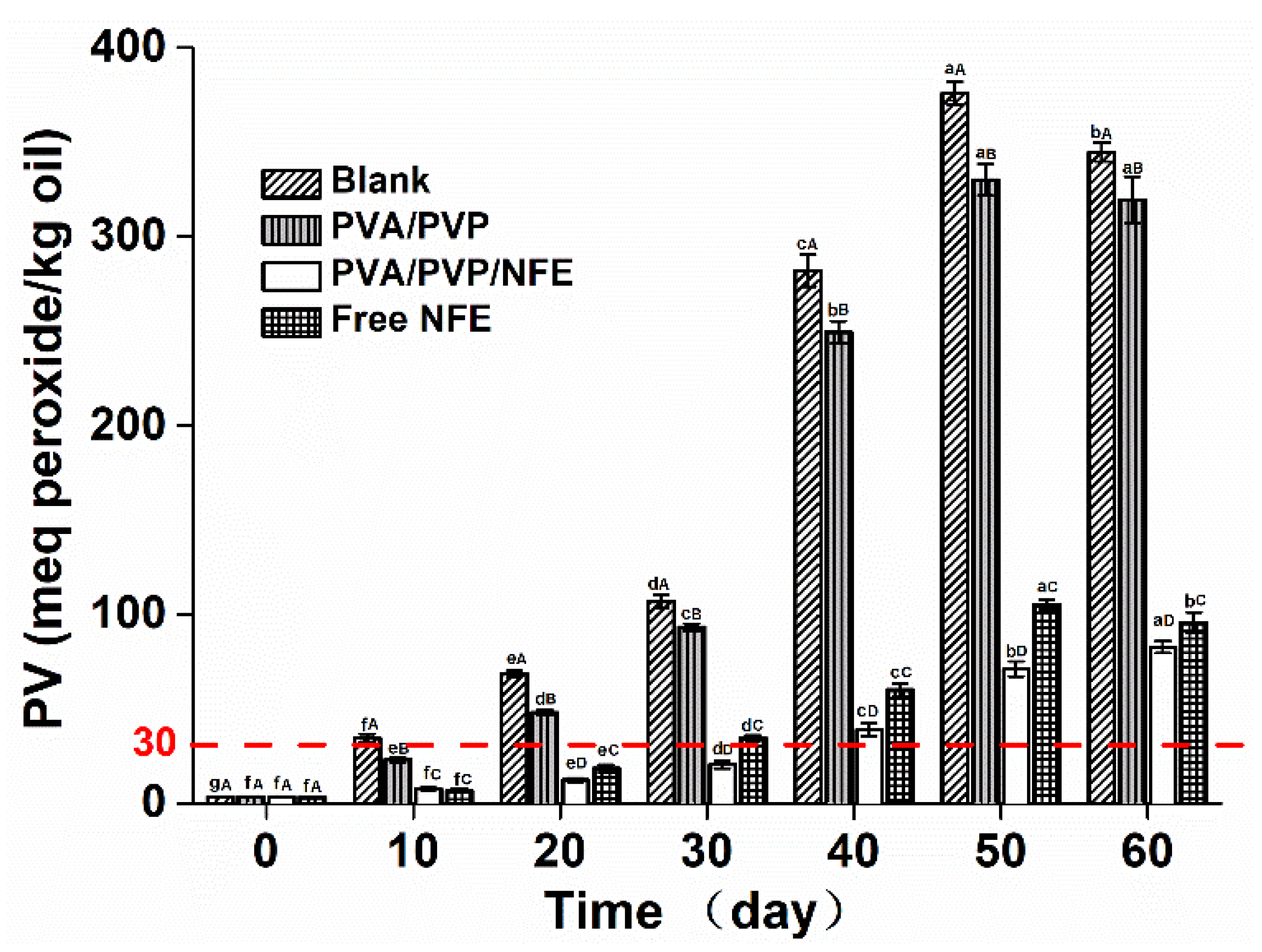
3.6. Effectiveness of the Active Films against Lipid Oxidation of Fish Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 9. [Google Scholar] [CrossRef]
- Yang, S.B.; Kim, E.H.; Kim, S.H.; Kim, Y.H.; Oh, W.; Lee, J.T.; Jang, Y.A.; Sabina, Y.; Ji, B.C.; Yeum, J.H. Electrospinning fabrication of poly(vinyl alcohol)/coptis chinensis extract nanofibers for antimicrobial exploits. Nanomaterials 2018, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.; Zhu, D.H.; Wu, H.; Zong, M.H.; Jing, Y.R.; Han, S.Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.X.; Lin, C.Z.; Zhu, C.C.; Gao, L. Three new flavonol glycosides from Nervilia fordii. Phytochem. Lett. 2012, 5, 104–107. [Google Scholar] [CrossRef]
- Qiu, L.; Jiao, Y.; Xie, J.Z.; Huang, G.K.; Qiu, S.L.; Miao, J.H.; Yao, X.S. Five new flavonoid glycosides from Nervilia fordii. J. Asian Nat. Prod. Res. 2013, 15, 589–599. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Fonseca, L.M.; dos Santos Cruxen, C.E.; Bruni, G.P.; Fiorentini, Â.M.; da Rosa Zavareze, E.; Lim, L.T.; Dias, A.R.G. Development of antimicrobial and antioxidant electrospun soluble potato starch nanofibers loaded with carvacrol. Int. J. Biol. Macromol. 2019, 139, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.M.; Kumar, K.S.; Rajini, N.; Siengchin, S.; Ayrilmis, N.; Rajulu, A.V. A comprehensive review of electrospun nanofibers: Food and packaging perspective. Compos. Part B Eng. 2019, 175, 107074. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Li, J.W.; Weiss, J.; Zhang, H. Core-shell nanofibers electrospun from O/W emulsions stabilized by the mixed monolayer of gelatin-gum Arabic complexes. Food Hydrocoll. 2020, 107, 9. [Google Scholar] [CrossRef]
- Nesic, A.; Ruzic, J.; Gordic, M.; Ostojic, S.; Micic, D.; Onjia, A. Pectin-polyvinylpyrrolidone films: A sustainable approach to the development of biobased packaging materials. Compos. Part B Eng. 2017, 110, 56–61. [Google Scholar] [CrossRef]
- Rahma, A.; Munir, M.M.; Prasetyo, A.; Suendo, V.; Rachmawati, H. Intermolecular interactions and the release pattern of electrospun curcumin-polyvinyl(pyrrolidone) fiber. Biol. Pharm. Bull. 2016, 39, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Haghju, S.; Bari, M.R.; Khaled-Abad, M.A. Affecting parameters on fabrication of beta-D-galactosidase immobilized chitosan/poly (vinyl alcohol) electrospun nanofibers. Carbohyd. Polym. 2018, 200, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.Z.; Liu, Y.R.; Wang, Y.Q.; Tian, F.; Miao, X.M.; Wang, H.; Tang, Y.D. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and e-poly (L-lysine) as antioxidant and antibacterial wound dressing materials. Int. J. Pharm. 2021, 601, 10. [Google Scholar] [CrossRef]
- Bruni, G.P.; de Oliveira, J.P.; Gomez-Mascaraque, L.G.; Fabra, M.J.; Martins, V.G.; Zavareze, E.D.; Lopez-Rubio, A. Electrospun beta-carotene-loaded SPI:PVA fiber mats produced by emulsion-electrospinning as bioactive coatings for food packaging. Food Packag. Shelf Life 2020, 23, 12. [Google Scholar]
- Rybinski, W.; Karamac, M.; Sulewska, K.; Boerner, A.; Amarowicz, R. Antioxidant potential of grass pea seeds from european countries. Foods 2018, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, X.; Jiang, J.; Fan, X.; Du, M.; Shi, X.; Cao, R. Improvement in the oxidative stability of flaxseed oil using an edible guar gum-tannic acid nanofibrous mat. Eur. J. Lipid Sci. Technol. 2019, 121, 1800438. [Google Scholar] [CrossRef]
- Nand, A.V.; Swift, S.; Uy, B.; Kilmartin, P.A. Evaluation of antioxidant and antimicrobial properties of biocompatible low density polyethylene/polyaniline blends. J. Food Eng. 2013, 116, 422–429. [Google Scholar] [CrossRef]
- Altemimi, A.; Watson, D.G.; Kinsel, M.; Lightfoot, D.A. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem. Cent. J. 2015, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.N.; Yang, E.; Yi, C.; Zhao, M.; Jiang, Y. Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. Innov. Food Sci. Emerg. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Aslaner, G.; Sumnu, G.; Sahin, S. Encapsulation of grape seed extract in rye flour and whey protein-based electrospun nanofibers. Food Bioprocess Technol. 2021, 14, 1118–1131. [Google Scholar] [CrossRef]
- İnanç Horuz, T.; Belibağlı, K.B. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food Chem. 2018, 268, 86–93. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.H.; Hu, T.G.; Li, L.; Wu, H. Preparation and characterization of electrospun colon-specific delivery system for quercetin and its antiproliferative effect on cancer cells. J. Agric. Food Chem. 2018, 66, 11550–11559. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tian, Z.; Chen, L. A novel process for microencapsulation of fish oil with barley protein. Food Res. Int. 2011, 44, 2735–2741. [Google Scholar] [CrossRef]
- Gotoh, N.; Wada, S. The importance of peroxide value in assessing food quality and food safety. J. Am. Oil Chem. Soc. 2006, 83, 473–474. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T.; de la Caba, K. Fish gelatin monolayer and bilayer films incorporated with epigallocatechin gallate: Properties and their use as pouches for storage of chicken skin oil. Food Hydrocoll. 2019, 89, 783–791. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.K. Oxygen barrier properties of biaxially oriented polypropylene/polyvinyl alcohol blend films. Polymer 2004, 45, 1599–1607. [Google Scholar] [CrossRef]
| Sample | Content | EC50 c of DPPH | IC50 d of LPO | |
|---|---|---|---|---|
| Total Phenols (mg GAE a/g Extract) | Total Flavonoids (mg RE b/g Extract) | Radical-Quenching Activity (mg Sample/mL) | Suppression Activity (mg Sample/mL) | |
| Ethyl acetate extract (NFE) | 86.67 ± 2.5 | 334.56 ± 4.7 | 0.66 | 0.307 |
| n-Butyl alcohol extract (NFB) | 41.85 ± 1.3 | 125.9 ± 2.6 | 2.43 | 0.347 |
| Water extract | 18.04 ± 1.2 | 46.95 ± 1.5 | 8.36 | 0.492 |
| Petroleum ether extract (NFP) | 15.17 ± 0.8 | 100.36 ± 3.1 | 4.25 | 0.436 |
| Vc | 0.087 | 0.310 | ||
| NFE Concentration (mg/mL) | Viscosity (Pa·S) | Conductivity (μS/cm) | Average Diameter (nm) | DPPH Radical Scavenging Rate (%) |
|---|---|---|---|---|
| 0 | 1535 | 326 | 470 | 0 a |
| 2.0 | 1572 | 315 | 510 | 38.7 ± 1.2 b |
| 4.0 | 1604 | 307 | 570 | 78.4 ± 2.4 c |
| 8.0 | 1693 | 302 | 600 | 80.2 ± 1.9 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, P.; Hu, T.-G.; Wen, Y.; Li, K.-E.; Qiu, W.-P.; He, Z.-L.; Wang, H.; Wu, H. Development of Nervilia fordii Extract-Loaded Electrospun PVA/PVP Nanocomposite for Antioxidant Packaging. Foods 2021, 10, 1728. https://doi.org/10.3390/foods10081728
Wen P, Hu T-G, Wen Y, Li K-E, Qiu W-P, He Z-L, Wang H, Wu H. Development of Nervilia fordii Extract-Loaded Electrospun PVA/PVP Nanocomposite for Antioxidant Packaging. Foods. 2021; 10(8):1728. https://doi.org/10.3390/foods10081728
Chicago/Turabian StyleWen, Peng, Teng-Gen Hu, Yan Wen, Ke-Er Li, Wei-Peng Qiu, Zhi-Lin He, Hong Wang, and Hong Wu. 2021. "Development of Nervilia fordii Extract-Loaded Electrospun PVA/PVP Nanocomposite for Antioxidant Packaging" Foods 10, no. 8: 1728. https://doi.org/10.3390/foods10081728
APA StyleWen, P., Hu, T.-G., Wen, Y., Li, K.-E., Qiu, W.-P., He, Z.-L., Wang, H., & Wu, H. (2021). Development of Nervilia fordii Extract-Loaded Electrospun PVA/PVP Nanocomposite for Antioxidant Packaging. Foods, 10(8), 1728. https://doi.org/10.3390/foods10081728