Lacticaseibacillus rhamnosus Impedes Growth of Listeria spp. in Cottage Cheese through Manganese Limitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Culture Conditions and Inoculum Preparation
2.2. Construction of Red Fluorescent L. innocua
2.3. Agar Well Diffusion Method
2.4. Manufacture of Cottage Cheeses and Inoculation with Listeria spp.
2.5. Proximate Analysis of Cottage Cheeses
2.6. Manufacture of Cottage Cheese Model (CCM) and Curd with B-Milk Model (CBM)
2.7. Monitoring Bacterial Growth and pH in Cottage Cheese
2.8. Monitoring Fluorescence and pH in CBM and Micro-Cottage Cheese Models
2.9. Monitoring Bacterial Growth in Chemically Defined Medium (CDM)
2.10. Monitoring Growth of L. innocua in Whey
3. Results
3.1. Discovery of Lactic Acid Bacteria Strains with Antilisterial Properties in Cottage Cheese
3.2. Lrh-FQ Does Not Produce Antilisterial Bacteriocins under Relevant Conditions
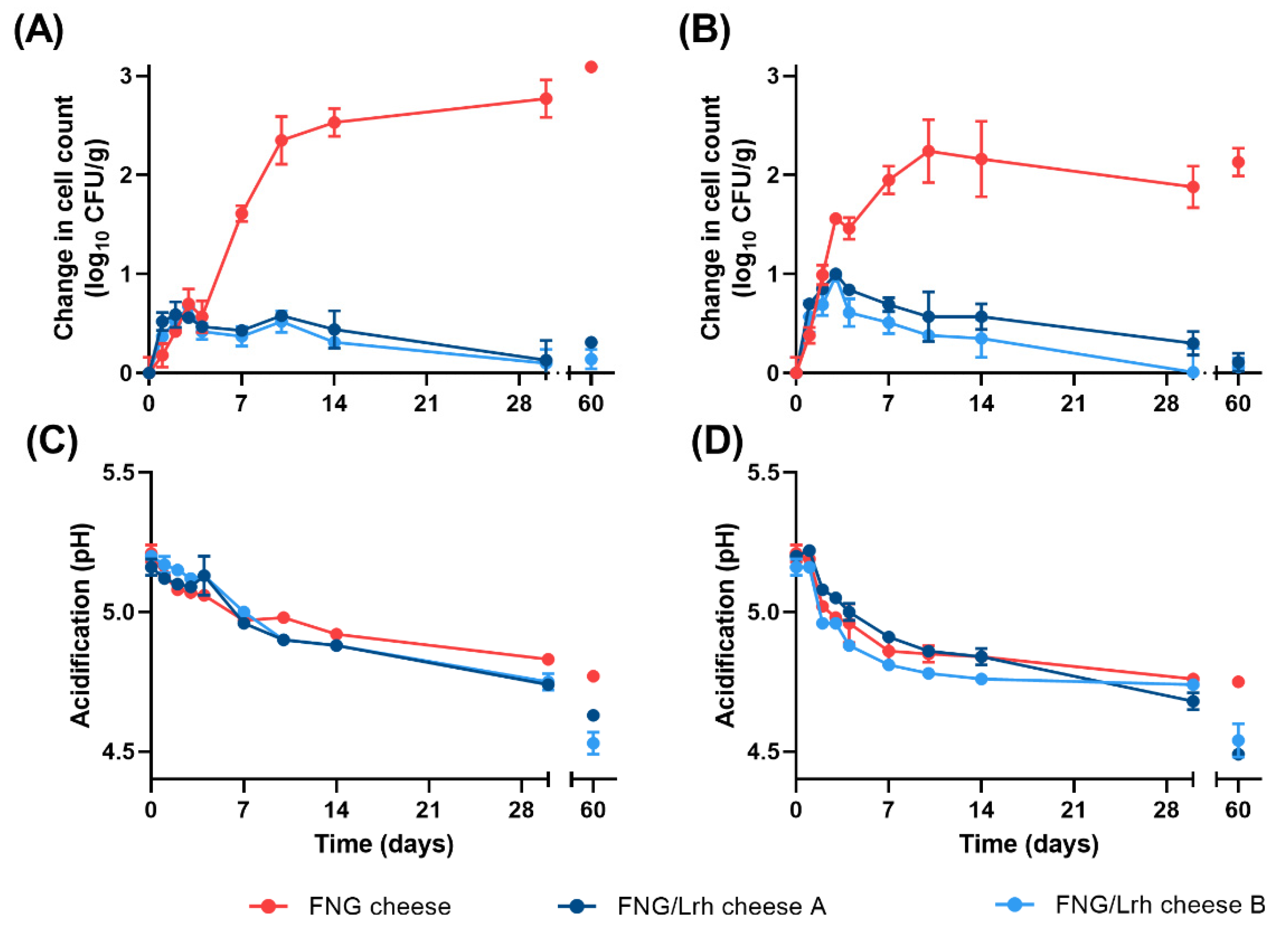
3.3. Growth of L. innocua in Cottage Cheese Is Limited by a Combination of Mn Availability and pH
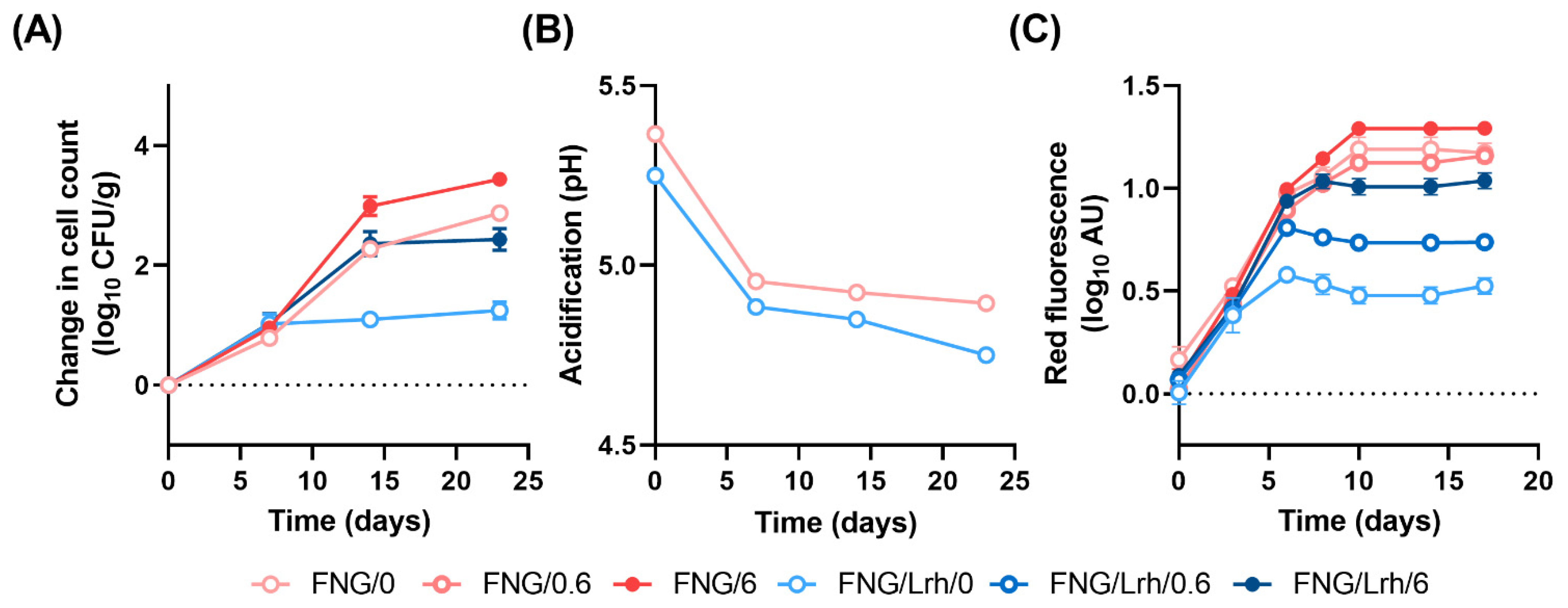
3.4. Listeria spp., Unlike LAB Strains Derived from FNG-10, Require Mn for Growth in Chemically Defined Media
3.5. Addition of Mn Restores Growth of Listeria spp. in Industrial Cottage Cheese
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- CDC. Listeria (Listeriosis). Available online: https://www.cdc.gov/listeria/index.html (accessed on 23 March 2021).
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persistence in food-processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Wiedmann, M.; Teixera, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lawrence, M.L.; Ainsworth, A.J.; Austin, F.W. Comparative assessment of acid, alkali and salt tolerance in Listeria monocytogenes virulent and avirulent strains. FEMS Microbiol. Lett. 2005, 243, 373–378. [Google Scholar] [CrossRef]
- Yousef, A.; Lado, B. Characteristics of Listeria monocytogenes important to food processors. In Listeria, Listeriosis, and Food Safety, 3rd ed.; Ryser, E.T., Marth, E.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 157–213. ISBN 9781420015188. [Google Scholar]
- Vermeulen, A.; Gysemans, K.P.M.; Bernaerts, K.; Geeraerd, A.H.; Van Impe, J.F.; Debevere, J.; Devlieghere, F. Influence of pH, water activity and acetic acid concentration on Listeria monocytogenes at 7 °C: Data collection for the development of a growth/no growth model. Int. J. Food Microbiol. 2007, 114, 332–341. [Google Scholar] [CrossRef] [PubMed]
- McClure, P.J.; Roberts, T.A.; Oguru, P.O. Comparison of the effects of sodium chloride, pH and temperature on the growth of Listeria monocytogenes on gradient plates and in liquid medium. Lett. Appl. Microbiol. 1989, 9, 95–99. [Google Scholar] [CrossRef]
- Phan-Thanh, L.; Montagne, A. Physiological and biochemical aspects of the acid survival of Listeria monocytogenes. J. Gen. Appl. Microbiol. 1998, 44, 183–191. [Google Scholar] [CrossRef]
- Petran, R.L.; Zottola, E.A. A study of factors affecting growth and recovery of Listeria monocytogenes Scott A. J. Food Sci. 1989, 54, 458–460. [Google Scholar] [CrossRef]
- Rosenow, E.M.; Marth, E.H. Growth of Listeria monocytogenes in skim, whole and chocolate milk, and in whipping cream during incubation at 4, 8, 13, 21 and 35 °C. J. Food Prot. 1987, 50, 452–459. [Google Scholar] [CrossRef]
- Junttila, J.R.; Niemelä, S.I.; Hirn, J. Minimum growth temperatures of Listeria monocytogenes and non-haemolytic listeria. J. Appl. Bacteriol. 1988, 65, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.J.; Archer, P.; Banks, J.G. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 1990, 68, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Ryser, E. Incidence and behavior of Listeria monocytogenes in cheese and other fermented dairy products. In Listeria, Listeriosis, and Food Safety, 3rd ed.; Ryser, E.T., Marth, E.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 405–501. ISBN 978-1-4200-1518-8. [Google Scholar]
- Jackson, K.A.; Gould, L.H.; Hunter, J.C.; Kucerova, Z.; Jackson, B. Listeriosis outbreaks associated with soft cheeses, United States, 1998–2014. Emerg. Infect. Dis. 2018, 24, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Lee, H.; Lee, S.; Kim, S.; Yoon, Y. Cheese Microbial Risk Assessments—A Review. Asian Aust. J. Anim. Sci. 2016, 29, 307–314. [Google Scholar] [CrossRef]
- CDC. Listeria Outbreak Linked to Hispanic-Style Fresh and Soft Cheeses. Available online: https://www.cdc.gov/listeria/outbreaks/hispanic-soft-cheese-02-21/details.html (accessed on 23 March 2021).
- Ryser, E.T.; Marth, E.H.; Doyle, M.P. Survival of Listeria monocytogenes during manufacture and storage of cottage cheese. J. Food Prot. 1985, 48, 746–750. [Google Scholar] [CrossRef]
- Chen, J.H.; Hotchkiss, J.H. Growth of Listeria monocytogenes and Clostridium sporogenes in cottage cheese in modified atmosphere packaging. J. Dairy Sci. 1993, 76, 972–977. [Google Scholar] [CrossRef]
- Shank, F.R.; Elliot, E.L.; Wachsmuth, I.K.; Losikoff, M.E. US position on Listeria monocytogenes in foods. Food Control 1996, 7, 229–234. [Google Scholar] [CrossRef]
- Archer, D.L. The evolution of FDA’s policy on Listeria monocytogenes in ready-to-eat foods in the United States. Curr. Opin. Food Sci. 2018, 20, 64–68. [Google Scholar] [CrossRef]
- FDA. Milk Guidance Documents & Regulatory Information System—Grade A Milk Search (GAMS). Available online: https://gams.fda.gov/ (accessed on 4 January 2021).
- De Vuyst, L.; Vandamme, E.J. Antimicrobial potential of lactic acid bacteria. In Bacteriocins of Lactic Acid Bacteria; Springer: Boston, MA, USA, 1994; pp. 91–142. ISBN 978-1-4615-2668-1. [Google Scholar]
- Ross, R.P.; Morgan, S.; Hill, C. Preservation and fermentation: Past, present and future. Int. J. Food Microbiol. 2002, 79, 3–16. [Google Scholar] [CrossRef]
- Daeschel, M.A. Antimicrobial substances from lactic-acid bacteria for use as food preservatives. Food Technol. 1989, 43, 164–167. [Google Scholar]
- Conner, D.E.; Scott, V.N.; Bernard, D.T. Growth, inhibition, and survival of Listeria monocytogenes as affected by acidic conditions. J. Food Prot. 1990, 53, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Ita, P.S.; Hutkins, R.W. Intracellular pH and survival of Listeria monocytogenes Scott A in tryptic soy broth containing acetic, lactic, citric, and hydrochloric acids. J. Food Prot. 1991, 54, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Tremonte, P.; Succi, M.; Iorizzo, M.; Pannella, G.; Lombardi, S.J.; Sturchio, M.; Coppola, R. Detection of antilisterial activity of 3-phenyllactic acid using Listeria innocua as a model. Front. Microbiol. 2018, 9, 1373. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Jeevaratnam, K.; Vidhyasagar, V.; Agaliya, P.J.; Saraniya, A.; Umaiyaparvathy, M. Characterization of an antibacterial compound, 2-hydroxyl indole-3-propanamide, produced by lactic acid bacteria isolated from fermented batter. Appl. Biochem. Biotechnol. 2015, 177, 137–147. [Google Scholar] [CrossRef]
- Dieuleveux, V.; Van Der Pyl, D.; Chataud, J.; Gueguen, M. Purification and characterization of anti-Listeria compounds produced by Geotrichum candidum. Appl. Environ. Microbiol. 1998, 64, 800–803. [Google Scholar] [CrossRef]
- Gummalla, S.; Broadbent, J.R. Tryptophan catabolism by Lactobacillus casei and Lactobacillus helveticus cheese flavor adjuncts. J. Dairy Sci. 1999, 82, 2070–2077. [Google Scholar] [CrossRef]
- Kjos, M.; Borrero, J.; Opsata, M.; Birri, D.J.; Holo, H.; Cintas, L.M.; Snipen, L.; Hernandez, P.E.; Nes, I.F.; Diep, D.B. Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria. Microbiology 2011, 157, 3256–3267. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Siedler, S.; Rau, M.H.; Bidstrup, S.; Vento, J.M.; Aunsbjerg, S.D.; Bosma, E.F.; McNair, L.M.; Beisel, C.L.; Neves, A.R. Competitive exclusion is a major bioprotective mechanism of Lactobacilli against fungal spoilage in fermented milk products. Appl. Environ. Microbiol. 2020, 86, e02312-19. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Edelson, S.G. Effect of pH-dependent, stationary phase acid resistance on the thermal tolerance of Escherichia coli O157:H7. Food Microbiol. 1999, 16, 447–458. [Google Scholar] [CrossRef]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.G.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Puigbò, P.; Guzmán, E.; Romeu, A.; Garcia-Vallvé, S. OPTIMIZER: A web server for optimizing the codon usage of DNA sequences. Nucleic Acids Res. 2007, 35 (Suppl. 2), W126–W131. [Google Scholar] [CrossRef]
- Rud, I.; Jensen, P.R.; Naterstad, K.; Axelsson, L. A synthetic promoter library for constitutive gene expression in Lactobacillus plantarum. Microbiology 2006, 152, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Holo, H.; Nes, I.F. Transformation of Lactococcus by electroporation. Methods Mol. Biol. 1995, 47, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, V.K.; Derkx, P.; Oregaard, G. High-throughput screening for texturing Lactococcus strains. FEMS Microbiol. Lett. 2019, 366, fnz001. [Google Scholar] [CrossRef]
- Dimitrijević, R.; Stojanović, M.; Živković, I.; Petersen, A.; Jankov, R.M.; Dimitrijević, L.; Gavrović-Jankulović, M. The identification of a low molecular mass bacteriocin, rhamnosin A, produced by Lactobacillus rhamnosus strain 68. J. Appl. Microbiol. 2009, 107, 2108–2115. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Moon, G.S. Antilisterial bacteriocin from Lactobacillus rhamnosus CJNU 0519 presenting a narrow antimicrobial spectrum. Korean J. Food Sci. Anim. Resour. 2015, 35, 137–142. [Google Scholar] [CrossRef]
- Oliveira, L.D.C.; Silveira, A.M.M.; Monteiro, A.D.S.; dos Santos, V.L.; Nicoli, J.R.; Azevedo, V.A.d.C.; Soares, S.d.C.; Dias-Souza, M.V.; Nardi, R.M.D. In silico prediction, in vitro antibacterial spectrum, and physicochemical properties of a putative bacteriocin produced by Lactobacillus rhamnosus strain L156.4. Front. Microbiol. 2017, 8, 876. [Google Scholar] [CrossRef]
- Liu, Y.; Ream, A. Gene expression profiling of Listeria monocytogenes strain F2365 during growth in ultrahigh-temperature-processed skim milk. Appl. Environ. Microbiol. 2008, 74, 6859–6866. [Google Scholar] [CrossRef]
- McAuliffe, O.; Hill, C.; Ross, R.P. Inhibition of Listeria monocytogenes in cottage cheese manufactured with a lacticin 3147-producing starter culture. J. Appl. Microbiol. 1999, 86, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, B.; Cocolin, L.; Zeppa, G.; Field, D.; Cotter, P.D.; Hill, C. Technological characterization of bacteriocin producing Lactococcus lactis strains employed to control Listeria monocytogenes in cottage cheese. Int. J. Food Microbiol. 2012, 153, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.A.; Bevis, H.E.; Delves-Broughton, J. The use of the bacteriocin, nisin, as a preservative in ricotta-type cheeses to control the food-borne pathogen Listeria monocytogenes. Lett. Appl. Microbiol. 1997, 24, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.A.S.S.; Lund, B.M. The effect of nisin on Listeria monocytogenes in culture medium and long-life cottage cheese. Lett. Appl. Microbiol. 1996, 22, 433–438. [Google Scholar] [CrossRef]
- Benkerroum, N.; Sandine, W.E. Inhibitory action of nisin against Listeria monocytogenes. J. Dairy Sci. 1988, 71, 3237–3245. [Google Scholar] [CrossRef]
- Mothlagh, A.M.; Holla, S.; Johnson, M.C.; Ray, B.; Field, R.A. Inhibition of Listeria spp. in sterile food systems by pediocin AcH, a bacteriocin produced by Pediococcus acidilactici H. J. Food Prot. 1992, 55, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.M.; Galvin, M.; Ross, R.P.; Hill, C. Evaluation of a spray-dried lacticin 3147 powder for the control of Listeria monocytogenes and Bacillus cereus in a range of food systems. Lett. Appl. Microbiol. 2001, 33, 387–391. [Google Scholar] [CrossRef]
- Pucci, M.J.; Vedamuthu, E.R.; Kunka, B.S.; Vandenbergh, P.A. Inhibition of Listeria monocytogenes by using bacteriocin PA-1 produced by Pediococcus acidilactici PAC 1.0. Appl. Environ. Microbiol. 1988, 54, 2349–2353. [Google Scholar] [CrossRef]
- Huang, E.; Zhang, L.; Chung, Y.K.; Zheng, Z.; Yousef, A.E. Characterization and application of enterocin RM6, a bacteriocin from Enterococcus faecalis. BioMed Res. Int. 2013, 2013, 206917. [Google Scholar] [CrossRef]
- El-Ziney, M.G.; Debevere, J.M. The effect of reuterin on Listeria monocytogenes and Escherichia coli O157:H7 in milk and cottage cheese. J. Food Prot. 1998, 61, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Langa, S.; Martín-Cabrejas, I.; Montiel, R.; Landete, J.M.; Medina, M.; Arqués, J.L. Short communication: Combined antimicrobial activity of reuterin and diacetyl against foodborne pathogens. J. Dairy Sci. 2014, 97, 6116–6121. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.; Cotter, P.D.; Hill, C.; Ross, R.P. The impact of nisin on sensitive and resistant mutants of Listeria monocytogenes in cottage cheese. J. Appl. Microbiol. 2011, 110, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Daeschel, M.A. Nisin resistance of foodborne bacteria and the specific resistance responses of Listeria monocytogenes Scott A. J. Food Prot. 1993, 56, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Rekhif, N.; Atrih, A.; Lefebvre, G. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC 15313 resistant to different bacteriocins produced by lactic acid bacteria strains. Curr. Microbiol. 1994, 28, 237–241. [Google Scholar] [CrossRef]
- Wong, N.P.; LaCroix, D.E.; Alford, J.A. Mineral content of dairy products. II. Cheeses. J. Am. Diet. Assoc. 1978, 72, 608–611. [Google Scholar]
- Nantapo, C.; Muchenje, V. Winter and spring variation in daily milk yield and mineral composition of Jersey, Friesian cows and their crosses under a pasture-based dairy system. S. Afr. J. Anim. Sci. 2014, 43, 17. [Google Scholar] [CrossRef]
- Melo, J.; Andrew, P.W.; Faleiro, M.L. Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: The role of stress responses. Food Res. Int. 2015, 67, 75–90. [Google Scholar] [CrossRef]
- Juttukonda, L.J.; Skaar, E.P. Manganese homeostasis and utilization in pathogenic bacteria. Mol. Microbiol. 2015, 97, 216–228. [Google Scholar] [CrossRef]
- Lopez, C.A.; Skaar, E.P. The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 2018, 23, 737–748. [Google Scholar] [CrossRef]
- Vasconcelos, J.A.; Deneer, H.G. Expression of superoxide dismutase in Listeria monocytogenes. Appl. Environ. Microbiol. 1994, 60, 2360–2366. [Google Scholar] [CrossRef]
- Nakashige, T.G.; Zhang, B.; Krebs, C.; Nolan, E.M. Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol. 2015, 11, 765–771. [Google Scholar] [CrossRef]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef]
- Damo, S.M.; Kehl-Fie, T.E.; Sugitani, N.; Holt, M.E.; Rathi, S.; Murphy, W.J.; Zhang, Y.; Betz, C.; Hench, L.; Fritz, G.; et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 3841–3846. [Google Scholar] [CrossRef]
- Kehl-Fie, T.E.; Skaar, E.P. Nutritional immunity beyond iron: A role for manganese and zinc. Curr. Opin. Chem. Biol. 2010, 14, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, M.; Sonnenburg, J.L.; Cossart, P.; Gordon, J.I. Functional genomic studies of the intestinal response to a foodborne enteropathogen in a humanized gnotobiotic mouse model. J. Biol. Chem. 2007, 282, 15065–15072. [Google Scholar] [CrossRef]
- Zaia, A.A.; Sappington, K.J.; Nisapakultorn, K.; Chazin, W.J.; Dietrich, E.A.; Ross, K.F.; Herzberg, M.C. Subversion of antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm of TR146 epithelial cells after invasion by Listeria monocytogenes. Mucosal Immunol. 2009, 2, 43–53. [Google Scholar] [CrossRef]
- Nisapakultorn, K.; Ross, K.F.; Herzberg, M.C. Calprotectin expression inhibits bacterial binding to mucosal epithelial cells. Infect. Immun. 2001, 69, 3692–3696. [Google Scholar] [CrossRef]
- Jesse, H.E.; Roberts, I.S.; Cavet, J.S. Metal ion homeostasis in Listeria monocytogenes and importance in host–pathogen interactions. Adv. Microb. Physiol. 2014, 65, 83–123. [Google Scholar] [PubMed]
- Colomer-Winter, C.; Flores-Mireles, A.L.; Baker, S.P.; Frank, K.L.; Lynch, A.J.L.; Hultgren, S.J.; Kitten, T.; Lemos, J.A. Manganese acquisition is essential for virulence of Enterococcus faecalis. PLoS Pathog. 2018, 14, e1007102. [Google Scholar] [CrossRef] [PubMed]
- Kajfasz, J.K.; Katrak, C.; Ganguly, T.; Vargas, J.; Wright, L.; Peters, Z.T.; Spatafora, G.A.; Abranches, J.; Lemos, J.A. Manganese uptake, mediated by SloABC and MntH, is essential for the fitness of Streptococcus mutans. mSphere 2020, 5, e00764-19. [Google Scholar] [CrossRef]
- Radin, J.N.; Kelliher, J.L.; Párraga Solórzano, P.K.; Kehl-Fie, T.E. The two-component system ArlRS and alterations in metabolism enable Staphylococcus aureus to resist calprotectin-induced manganese starvation. PLoS Pathog. 2016, 12, e1006040. [Google Scholar] [CrossRef]
- Que, Q.; Helmann, J.D. Manganese homestasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 2000, 35, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Kehres, D.G.; Maguire, M.E. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol. Rev. 2003, 27, 263–290. [Google Scholar] [CrossRef]
- Chander, M.; Setlow, B.; Setlow, P. The enzymatic activity of phosphoglycerate mutase from Gram-positive endospore-forming bacteria requires Mn2+ and is pH sensitive. Can. J. Microbiol. 1998, 44, 759–767. [Google Scholar] [CrossRef] [PubMed]



| Species | Strain | Culture Collection | Serovar # | Origin |
|---|---|---|---|---|
| Listeria monocytogenes | mhl210 | IVH KU | ND | Isolated from pork |
| Listeria monocytogenes | ATCC 13932 | ATCC | 4b | Clinical isolate of spinal fluid of child with meningitis |
| Listeria monocytogenes | DSM 15675 | DSMZ | 4b | Isolated from soft cheese |
| Listeria monocytogenes | FSL R2-501 | ILSI Cornell | 4b | Hispanic-style cheese isolate, associated with NC outbreak |
| Listeria monocytogenes | FSL R2-500 | ILSI Cornell | 4b | Clinical isolate, associated with NC Hispanic-style cheese outbreak |
| Listeria monocytogenes | LM101 | UW-Madison FRI | 4b | Hard salami isolate |
| Listeria monocytogenes | LM310 | UW-Madison FRI | 4b | Goat cheese isolate associated with illness |
| Listeria monocytogenes | LM301 | UW-Madison FRI | 1/2a | Heat-treated Cheddar cheese isolate |
| Listeria monocytogenes | LM108 | UW-Madison FRI | 1/2b | Hard salami isolate |
| Listeria innocua | BL 86/26 | TNO | ||
| Listeria innocua | BL 86/26 + pEFB001 | This study | ||
| Laticaseibacillus rhamnosus | FreshQ® Cheese +LI 1 | Chr. Hansen A/S | ||
| Lactococcus lactis | MG1363 | MoBiTec GmbH | ||
| Lactococcus lactis | MG1363 + pEFB001 | This study | ||
| Lactococcus lactis | FNG-10-1 | Chr. Hansen A/S | ||
| Streptococcus thermophilus | FNG-10-2 | Chr. Hansen A/S |
| Name | Sequence (5′ → 3′) |
|---|---|
| EFB0057 | GAAGAAGGTTTTTATATTACAGCTCCAGATCTAGCGCTATAGTTGTTGACAG |
| EFB0060 | CTTGGTTTTCTAATTTTGGTTCAAAGAAAGCTTTTATTTGTACAGCTCATCC |
| EFB0061 | GGATGAGCTGTACAAATAAAAGCTTTCTTTGAACCAAAATTAGAAAACCAAG |
| EFB0062 | CTGTCAACAACTATAGCGCTAGATCTGGAGCTGTAATATAAAAACCTTCTTC |
| EFB0055 | CAACATCTTCGCTGCAAAGC |
| EFB0056 | CTCTATTCAGGAATTGTCAG |
| Cottage Cheese I (Using FNG-10) | ||||
| Formulation | % Moisture | Water Activity | pH | % NaCl |
| FNG curd | 81.38 ± 0.15 | 0.998 ± 0.002 | 4.49 ± 0.00 | 0.06 ± 0.01 |
| FNG/Lrh-FQ curd | 80.19 ± 0.14 | 0.998 ± 0.002 | 4.52 ± 0.01 | 0.05 ± 0.00 |
| Dressing | 82.84 ± 0.02 | 0.983 ± 0.000 | 6.64 ± 0.01 | 2.16 ± 0.03 |
| FNG cottage cheese composite | 83.19 ± 0.13 | 0.987 ± 0.001 | 5.09 ± 0.01 | 1.08 ± 0.08 |
| FNG/Lrh-FQ cottage cheese composite | 82.61 ± 0.16 | 0.991 ± 0.003 | 5.14 ± 0.02 | 1.10 ± 0.03 |
| Cottage Cheese II (Using FNG-20) | ||||
| Formulation | % Moisture | Water Activity | pH | % NaCl |
| FNG cottage cheese composite | 85.33 ± 0.15 | ND | 5.37 ± 0.05 | 1.04 ± 0.01 |
| FNG/Lrh-FQ cottage cheese composite | 85.23 ± 0.11 | ND | 5.26 ± 0.05 | 1.04 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Gijtenbeek, L.A.; Singer, Q.; Steffensen, L.E.; Neuens, S.; Guldager, H.S.; Bidstrup, S.; Høgholm, T.; Madsen, M.G.; Glass, K.; Siedler, S. Lacticaseibacillus rhamnosus Impedes Growth of Listeria spp. in Cottage Cheese through Manganese Limitation. Foods 2021, 10, 1353. https://doi.org/10.3390/foods10061353
van Gijtenbeek LA, Singer Q, Steffensen LE, Neuens S, Guldager HS, Bidstrup S, Høgholm T, Madsen MG, Glass K, Siedler S. Lacticaseibacillus rhamnosus Impedes Growth of Listeria spp. in Cottage Cheese through Manganese Limitation. Foods. 2021; 10(6):1353. https://doi.org/10.3390/foods10061353
Chicago/Turabian Stylevan Gijtenbeek, Lieke A., Quinn Singer, Louise E. Steffensen, Shannon Neuens, Helle S. Guldager, Susanne Bidstrup, Tina Høgholm, Mikkel G. Madsen, Kathleen Glass, and Solvej Siedler. 2021. "Lacticaseibacillus rhamnosus Impedes Growth of Listeria spp. in Cottage Cheese through Manganese Limitation" Foods 10, no. 6: 1353. https://doi.org/10.3390/foods10061353
APA Stylevan Gijtenbeek, L. A., Singer, Q., Steffensen, L. E., Neuens, S., Guldager, H. S., Bidstrup, S., Høgholm, T., Madsen, M. G., Glass, K., & Siedler, S. (2021). Lacticaseibacillus rhamnosus Impedes Growth of Listeria spp. in Cottage Cheese through Manganese Limitation. Foods, 10(6), 1353. https://doi.org/10.3390/foods10061353







