Influence of Non-Saccharomyces Strains on Chemical Characteristics and Sensory Quality of Fruit Spirit
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials, Yeast Strains, and Chemicals
2.2. Mashing and Fermentation Conditions
2.3. Distillation Process
2.4. Analytical Methods
2.5. Sugars and Organic Acids Analysis (HPLC)
2.6. Volatile Compounds Analysis (GC-FID)
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Investigation of Fermentability of Different Starter Cultures
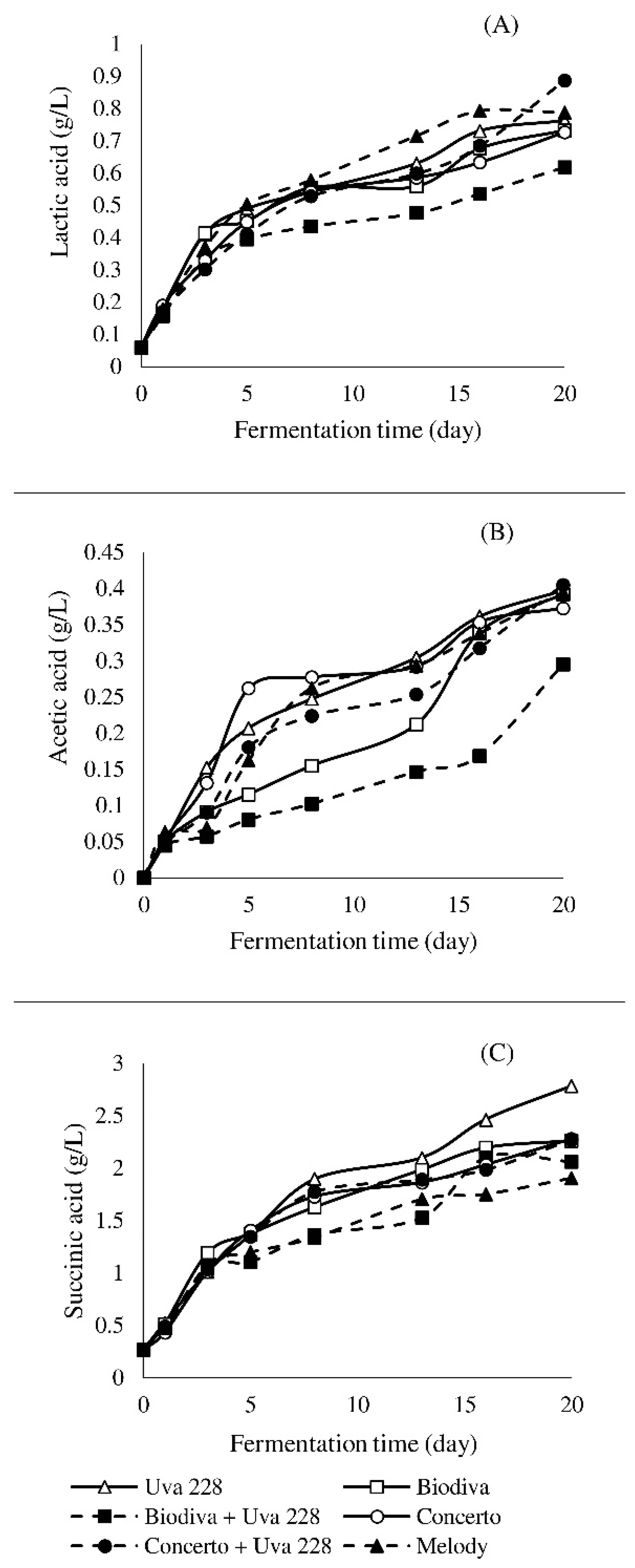
3.2. Analyzed Sugars and Organic Acids Profile during the Fermentation Process
3.3. Analyzed Volatile Compounds in the Apple Distillates
3.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buratti, S.; Benedetti, S. Alcoholic fermentation using electronic nose and electronic tongue. In Electronic Noses and Tongues in Food Science, 1st ed.; Preedy, V., Mendez, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 291–299. ISBN 978-0-12-800243-8. [Google Scholar]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Nissen, P.; Nielsen, D.; Arneborg, N. Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell–cell contact-mediated mechanism. Yeast 2003, 20, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef]
- Lopez-Alvarez, A.; Diaz-Perez, A.L.; Sosa-Aguirre, C.; Macias-Rodriguez, L.; Campos-Garcia, J. Ethanol yield and volatile compound content in fermentation of agave must by Kluyveromyces Marxianus UMPe-1 comparing with Saccharomyces Cerevisiae baker’s yeast used in Tequila production. J. Biosci. Bioeng. 2012, 113, 614–618. [Google Scholar] [CrossRef]
- Amorim, J.C.; Schwan, R.F.; Duarte, W.F. Sugar cane spirit (cachaça): Effects of mixed inoculum of yeasts on the sensory and chemical characteristics. Food Res. Int. 2016, 85, 76–83. [Google Scholar] [CrossRef]
- Duarte, W.F.; Amorim, J.C.; Schwan, R.F. The effects of co-culturing non-Saccharomyces yeasts with S. cerevisiae on the sugar cane spirit (cachaça) fermentation process. Antonie Leeuwenhoek 2013, 103, 175–194. [Google Scholar] [CrossRef]
- Schoorl, N.; Regenbogen, A. Maßanalytische Zuckerbestimmung. Zeitschr. Anal. Chem. 1917, 56, 191–202. [Google Scholar] [CrossRef]
- Chinnici, F.; Spinabelli, U.; Riponi, C.; Amati, A. Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J. Food Compos. Anal. 2005, 18, 121–130. [Google Scholar] [CrossRef]
- MSZ ISO 11132:2013. Sensory Analysis. Methodology. Guidelines for Monitoring the Performance of a Quantitative Sensory Panel; ISO ICS 67.240: Geneva, Switzerland, 2013.
- Satora, P.; Kostrz, M.; Sroka, P.; Tarko, T. Chemical profile of spirits obtained by spontaneous fermentation of different varieties of plum fruits. Eur. Food Res. Technol. 2017, 243, 489–499. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef]
- Herrero, M.; Garcia, L.A.; Diaz, M. Organic acids in cider with simultaneous inoculation of yeast and malolactic bacteria: Effect of fermentation temperature. J. Inst. Brew. 1999, 105, 229–232. [Google Scholar] [CrossRef]
- Tešević, V.; Nikićević, N.; Jovanović, A.; Djoković, D.; Vujisić, L.; Vucković, I.; Bonić, M. Volatile components from old plum brandies. Food Technol. Biotechnol. 2005, 43, 367–372. [Google Scholar]
- Urošević, I.; Nikićević, N.; Stanković, L.; Anđelković, B.; Urošević, T.; Krstić, G.; Tešević, V. Influence of yeast and nutrients on the quality of apricot brandy. J. Serb. Chem. Soc. 2014, 79, 1223–1234. [Google Scholar] [CrossRef]
- Winterová, R.; Mikulíková, R.; Mazáč, J.; Havelec, P. Assessment of the authenticity of fruit spirits by gas chromatography and stable isotope ratio analysis. Czech J. Food Sci. 2008, 26, 368–375. [Google Scholar] [CrossRef]
- Tešević, V.; Nikićević, N.; Milosavljević, S.; Bajić, D.; Vajs, V.; Vučković, I.; Vujisić, L.; Đorđević, I.; Stanković, M.; Veličković, M. Characterization of volatile compounds of “Drenja”, an alcoholic beverage obtained from the fruits of Cornelian cherry. J. Serb. Chem. Soc. 2009, 74, 117–128. [Google Scholar] [CrossRef]
- Díaz-Montano, D.M.; Delia, M.L.; Estarron-Espinosa, M.; Strehaiano, P. Fermentative capability and aroma compound production by yeast strains isolated from Agave tequilana Weber juice. Enzym. Microb. Technol. 2008, 42, 608–616. [Google Scholar] [CrossRef]
- Rusu Coldea, T.E.; Socaciu, C.; Pârv, M.; Vodnar, D. Gas-Chromatographic analysis of major volatile compounds found in traditional fruit brandies from Transylvania, Romania. Not. Bot. Horti Agrobot. 2011, 39, 109–116. [Google Scholar] [CrossRef]
- Spaho, N.; Dürr, P.; Grba, S.; Velagić-Habul, E.; Blesić, M. Effects of distillation cut on the distribution of higher alcohols and esters in brandy produced from three plum varieties. J. Inst. Brew. 2013, 119, 48–56. [Google Scholar] [CrossRef]
- Różański, M.; Pielech-Przybylska, K.; Balcerek, M. Influence of Alcohol Content and Storage Conditions on the Physicochemical Stability of Spirit Drinks. Foods 2020, 9, 1264. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 110/2008 on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Spirit Drinks and Repealing Council Regulation (EEC) No 1576/89; OJEU L39/16: Luxembourg, 2008.

| Refraction (w/w%) | Total Sugars (g/L) | Reducing Sugars (g/L) | Titratable Acidity (g/L) | pH | Volatile Acidity (g/L) | Ethanol (vol%) | Sugars’ Consumption (%) | |
|---|---|---|---|---|---|---|---|---|
| Fresh apple mash | 15.8 (±0.5) | 148.3 (±6.2) | 133 (±5.1) | 5.3 (±0.34) | 3.58 (±0.12) | n.a. | n.a. | n.a. |
| Fermented mash | ||||||||
| Uva228 | 5.2 a (±0.17) | 11.8 a (±2.1) | 11.2 a (±2.3) | 7.6 b (±0.34) | 3.19 a (±0.12) | 0.50 a (±0.08) | 6.00 d (±0.08) | 92 |
| Biodiva | 5.3 ab (±0.18) | 13.3 a (±1.1) | 12.1 a (±1.5) | 7.3 ab (±0.19) | 3.17 a (±0.08) | 0.42 a (±0.11) | 5.20 b (±0.10) | 91 |
| Biodiva + Uva228 | 5.1 a (±0.22) | 10.6 a (±1.3) | 9.5 a (±2.2) | 7.2 ab (±0.23) | 3.10 a (±0.09) | 0.33 a (±0.09) | 5.60 c (±0.15) | 92.9 |
| Concerto | 5.7 b (±0.11) | 19.7 b (±1.7) | 18.2 b (±2.4) | 7.1 ab (±0.17) | 3.16 a (±0.15) | 0.45 a (±0.05) | 4.80 a (±0.10) | 86.7 |
| Concerto + Uva228 | 5.25 ab (±0.2) | 11.3 a (±2.1) | 10.1 a (±1.2) | 6.9 a (±0.13) | 3.14 a (±0.10) | 0.50 a (±0.10) | 5.70 cd (±0.12) | 92.4 |
| Melody | 5.2 a (±0.23) | 13.5 a (±1.9) | 12.5 a (±2.5) | 6.9 a (±0.25) | 3.15 a (±0.13) | 0.36 a (±0.06) | 5.60 c (±0.20) | 90.9 |
| Compound (mg/L Alcohol 100% v/v) | Uva228 | Biodiva | Biodiva + Uva228 | Concerto | Concerto + Uva228 | Melody |
|---|---|---|---|---|---|---|
| Methanol | 1706.07 a (±125.56) | 1710.03 a (±115.62) | 1720.68 a (±134.25) | 1986.88 a (±142.85) | 1933.19 a (±147.21) | 1944.73 a (±131.51) |
| Acetaldehyde | 140.49 a (±17.23) | 133.82 a (±12.56) | 199.32 b (±15.67) | 152.34 a (±14.38) | 125.00 a (±7.45) | 125.49 a (±9.89) |
| Isoamyl alcohol | 329.77 c (±32.27) | 241.17 ab (±27.49) | 297.40 bc (±21.91) | 227.07 ab (±22.63) | 209.87 a (±18.02) | 243.61 ab (±31.71) |
| 1-Propanol | 157.32 a (±11.53) | 172.77 a (±17.78) | 206.10 a (±19.66) | 176.12 a (±10.34) | 167.73 a (±22.24) | 163.78 a (±13.23) |
| 1-Butanol | 3.639 a (±0.56) | 3.380 a (±0.34) | 3.747 a (±0.37) | 4.097 a (±0.16) | 3.828 a (±0.35) | 3.971 a (±0.23) |
| 2-Butanol | 0.293 a (±0.031) | n.d. | 0.604 c (±0.049) | n.d. | 0.428 b (±0.022) | 0.754 d (±0.052) |
| 1-Hexanol | 33.12 ab (±2.46) | 27.35 a (±3.04) | 34.23 b (±2.14) | 43.56 c (±3.56) | 47.62 c (±1.78) | 46.86 c (±4.16) |
| 2-Phenylethanol | 22.44 b (±1.64) | n.d. | 13.24 a (±0.96) | n.d. | 29.84 c (±2.06) | 27.02 c (±1.87) |
| 2-Methyl-1-butanol | 110.56 ab (±9.14) | 88.45 a (±7.52) | 116.89 b (±11.64) | 115.08 b (±12.44) | 149.47 c (±15.02) | 95.50 ab (±8.34) |
| trans-3-Hexen-1-ol | 0.043 ab (±0.005) | 0.018 a (±0.003) | 0.061 bc (±0.010) | 0.080 c (±0.004) | 0.154 d (±0.012) | 0.136 d (±0.011) |
| cis-2-Hexen-1-ol | 0.017 a (±0.002) | 0.017 a (±0.001) | 0.019 a (±0.003) | 0.020 a (±0.003) | 0.018 a (±0.002) | 0.023 a (±0.003) |
| Benzyl alcohol | 0.420 cd (±0.022) | 0.470 d (±0.055) | 0.370 cd (±0.050) | 0.120 a (±0.011) | 0.230 b (±0.033) | 0.335 c (±0.031) |
| Ethyl acetate | 178.50 bc (±10.35) | 147.30 ab (±25.46) | 165.20 abc (±12.26) | 131.60 a (±10.67) | 167.40 abc (±14.24) | 195.50 c (±15.03) |
| Propyl acetate | n.d. | n.d. | n.d. | 0.016 a (±0.003) | 0.012 a (±0.002) | 0.026 b (±0.002) |
| Ethyl hexanoate | 6.026 c (±0.35) | 3.891 a (±0.27) | 4.638 ab (±0.47) | 4.286 ab (±0.56) | 4.024 ab (±0.36) | 4.928 b (±0.28) |
| Ethyl butyrate | 0.022 a (±0.002) | 0.054 b (±0.006) | 0.043 b (±0.003) | 0.049 b (±0.004) | 0.045 b (±0.006) | 0.055 b (±0.010) |
| Isoamyl acetate | 0.033 ab (±0.006) | 0.042 bc (±0.005) | 0.056 c (±0.011) | 0.018 a (±0.002) | 0.027 ab (±0.003) | 0.057 c (±0.010) |
| Phenylethyl acetate | 0.044 a (±0.005) | 0.025 b (±0.010) | 0.055 ab (±0.006) | 0.039 a (±0.006) | 0.053 ab (±0.012) | 0.045 ab (±0.006) |
| Diethyl succinate | 0.245 a (±0.045) | 0.412 c (±0.035) | 0.295 ab (±0.025) | 0.371 bc (±0.023) | 0.387 c (±0.033) | 0.378 c (±0.032) |
| Ethyl octanoate | 3.045 bc (±0.34) | 3.425 c (±0.55) | 2.962 bc (±0.24) | 2.130 a (±0.17) | 2.450 ab (±0.12) | 2.794 abc (±0.31) |
| Ethyl benzoate | 3.805 b (±0.64) | 4.467 c (±0.21) | 4.079 bc (±0.34) | 1.560 a (±0.14) | 2.274 a (±0.27) | 4.773 cd (±0.34) |
| Ethyl formate | n.d. | n.d. | n.d. | 0.563 a (±0.051) | 0.693 b (±0.066) | 0.633 ab (±0.025) |
| Linalool | 0.117 ab (±0.015) | 0.169 bc (±0.021) | 0.129 ab (±0.017) | 0.182 c (±0.031) | 0.150 abc (±0.022) | 0.105 a (±0.011) |
| Technology Purity (Max 5 Points) | Fruit Character (Max 5 Points) | Mouthfeel (Max 5 Points) | Harmony (Max 5 Points) | Total (Max 20 Points) | |
|---|---|---|---|---|---|
| Uva228 | 5 (±0) | 3.93 (±0.46) | 3.73 (±0.59) | 3.46 (±0.63) | 16.1 (±1.24) |
| Biodiva | 5 (±0) | 4.33 (±0.61) | 4.27 (±0.59) | 4.4 (±0.63) | 18 (±1.55) |
| Biodiva + Uva228 | 5 (±0) | 4.26 (±0.59) | 3.93 (±0.46) | 3.73 (±0.46) | 16.9 (±0.79) |
| Concerto | 5 (±0) | 3.8 (±0.56) | 3.8 (±0.67) | 3.4 (±0.73) | 16 (±1.36) |
| Concerto + Uva228 | 5 (±0) | 4.86 (±0.35) | 4.46 (±0.52) | 4.6 (±0.5) | 18.9 (±1.03) |
| Melody | 5 (±0) | 3.53 (±0.64) | 3.46 (±0.74) | 3.2 (±0.77) | 15.2 (±1.69) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fejzullahu, F.; Kiss, Z.; Kun-Farkas, G.; Kun, S. Influence of Non-Saccharomyces Strains on Chemical Characteristics and Sensory Quality of Fruit Spirit. Foods 2021, 10, 1336. https://doi.org/10.3390/foods10061336
Fejzullahu F, Kiss Z, Kun-Farkas G, Kun S. Influence of Non-Saccharomyces Strains on Chemical Characteristics and Sensory Quality of Fruit Spirit. Foods. 2021; 10(6):1336. https://doi.org/10.3390/foods10061336
Chicago/Turabian StyleFejzullahu, Fatjona, Zsuzsanna Kiss, Gabriella Kun-Farkas, and Szilárd Kun. 2021. "Influence of Non-Saccharomyces Strains on Chemical Characteristics and Sensory Quality of Fruit Spirit" Foods 10, no. 6: 1336. https://doi.org/10.3390/foods10061336
APA StyleFejzullahu, F., Kiss, Z., Kun-Farkas, G., & Kun, S. (2021). Influence of Non-Saccharomyces Strains on Chemical Characteristics and Sensory Quality of Fruit Spirit. Foods, 10(6), 1336. https://doi.org/10.3390/foods10061336