Carcass Composition, Meat Quality, Calpain Activity, Fatty Acid Composition and Ribonucleotide Content in Southern Thai Native Goats and Three-Way Crossbred Goats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
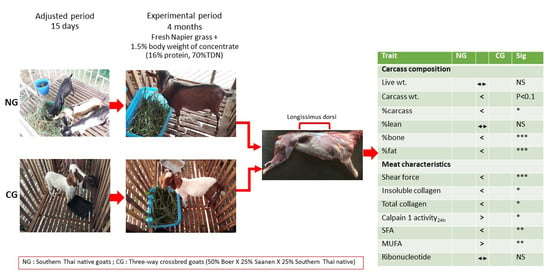
2.2. Experimental Goats and Slaughtering Procedure
2.3. Muscle Sampling
2.4. Meat Characteristics
2.4.1. pH Measurement
2.4.2. Color Measurement
2.4.3. Drip Loss Measurement
2.4.4. Cooking Loss and Shear Force Measurement
2.5. Fatty Acid Analysis
2.6. Cholesterol Analysis
2.7. Ribonucleotide Analysis
2.8. Collagen Analysis
2.9. Casein Zymography
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devendra, C. Dynamics of goat meat production in extensive systems in Asia: Improvement of productivity and transformation of livelihoods. Agrotechnoly 2015, 4, 131. [Google Scholar]
- Food and Agriculture Organization of the United Nation. Agricultural Data. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 December 2019).
- Information Technology Center. National Animal Statistics; Department of Livestock Development: Bangkok, Thailand, 2019.
- Anothaisinthawee, S.; Wattanachant, C.; Nomura, K.; Oishi, T.; Amano, T. Carcass and meat quality of three genotype population in goat breeding for meat purposes in Thailand. J. Agric. Sci. Tokyo Univ. Agric. 2012, 57, 63–70. [Google Scholar]
- Seré, C.; Steinfeld, H.; Groenewold, J. World livestock production systems: Current status, issues and trends. In Proceedings of the a Consultation on Global Agenda for Livestock Research, Nairobi, Kenya, 18–20 January 1995; ILRI: Nairobi, Kenya, 1995. [Google Scholar]
- Anothaisinthawee, S.; Nomura, K.; Oishi, T.; Amano, T. Goat genetic resources and breeding strategies in Thailand. J. Anim. Genet. 2010, 38, 41–48. [Google Scholar] [CrossRef][Green Version]
- USDA. USDA Nutrient Database for Standard Reference, Release 14, Nutrient Data Laboratory. 2002. Available online: http://www.nal.usda.gov/fnic/foodcomp (accessed on 1 December 2019).
- Madruga, M.; Resosemito, F.; Narain, N.; Souza, W.; Cunha, M.; Ramos, J. Effect of raising conditions of goats on physico-chemical quality of its meat. CYTA J. Food 2006, 5, 100–104. [Google Scholar]
- Dunford, E.; Shahidi, F. Flavour of fish meat. In Flavor of Meat, Meat Products and Seafoods, 2nd ed.; Shahidi, F., Ed.; Blackie Academic and Professional: London, UK, 1998; pp. 131–158. [Google Scholar]
- Jones, N.R. Meat and fish flavors; significance of ribomononucleotides and their metabolites. J. Agric. Food Chem. 1969, 17, 712–716. [Google Scholar] [CrossRef]
- Lawrie, R. Meat Science; Pergamon Press: Oxford, UK, 1998. [Google Scholar]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The Calpain System. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Geesink, G.H.; Kuchay, S.; Chishti, A.H.; Koohmaraie, M. μ-Calpain is essential for postmortem proteolysis of muscle proteins1,2. J. Anim. Sci. 2006, 84, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Koohmaraie, M.; Geesink, G. Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci. 2006, 74, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.C.; Casey, N.; Simela, L. Goat meat quality. Small Rumin. Res. 2005, 60, 153–166. [Google Scholar] [CrossRef]
- Babiker, S.; El Khider, I.; Shafie, S. Chemical composition and quality attributes of goat meat and lamb. Meat Sci. 1990, 28, 273–277. [Google Scholar] [CrossRef]
- Chaosap, C.; Sivapirunthep, P.; Takeungwongtrakul, S.; Zulkifli, R.B.M.; Sazili, A.Q.; Chanporn, C.; Panneepa, S.; Sirima, T.; Qurni, S.A. Effects of Zn-L-Selenomethionine on Carcass Composition, Meat Characteristics, Fatty Acid Composition, Glutathione Peroxidase Activity, and Ribonucleotide Content in Broiler Chickens. Food Sci. Anim. Resour. 2020, 40, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Chaosap, C.; Sitthigripong, R.; Sivapirunthep, P.; Pungsuk, A.; Adeyemi, K.; Sazili, A.Q. Myosin heavy chain isoforms expression, calpain system and quality characteristics of different muscles in goats. Food Chem. 2020, 321, 126677. [Google Scholar] [CrossRef]
- Arthur, J.S.C.; Mykles, D.L. Calpain Zymography with Casein or Fluorescein Isothiocyanate Casein. In Calpain Methods and Protocols; Elce, J.S., Ed.; Humana Press: Totowa, NJ, USA, 2000; pp. 109–116. [Google Scholar]
- Khaokhaikaew, S.; Wattanachant, C.; Ngampongsai, W. Effect of Breeds and Rearing Systems of goat on Growth Performance, Carcass Characteristics, Production cost and Economic Return. J. Sci. Technol. Mahasarakham Univ. 2010, 29, 32–43. [Google Scholar]
- Hamoen, J.; Vollebregt, H.; van der Sman, R. Prediction of the time evolution of pH in meat. Food Chem. 2013, 141, 2363–2372. [Google Scholar] [CrossRef]
- Kadim, I.; Mahgoub, O.; Al-Ajmi, D.; Al-Maqbaly, R.; Al-Saqri, N.; Ritchie, A. An evaluation of the growth, carcass and meat quality characteristics of Omani goat breeds. Meat Sci. 2004, 66, 203–210. [Google Scholar] [CrossRef]
- Simela, L.; Webb, E.; Frylinck, L. Effect of sex, age, and pre-slaughter conditioning on pH, temperature, tenderness and colour of indigenous South African goats. S. Afr. J. Anim. Sci. 2004, 34 (Suppl. 1), 208–211. [Google Scholar]
- Pophiwa, P.; Webb, E.; Frylinck, L. Carcass and meat quality of Boer and indigenous goats of South Africa under delayed chilling conditions. S. Afr. J. Anim. Sci. 2017, 47, 794. [Google Scholar] [CrossRef]
- Casey, N.; Norman, H.; Webb, E.C. Managing goat production for meat quality. Small Rumin. Res. 2010, 89, 218–224. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Cornforth, D.P.; Whittier, D. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 2001, 57, 359–363. [Google Scholar] [CrossRef]
- King, D.A.; Voges, K.L.; Hale, D.S.; Waldron, D.F.; Taylor, C.A.; Savell, J.W. High voltage electrical stimulation en-hances muscle tenderness, increases aging response, and improves muscle color from cabrito carcasses. Meat Sci. 2004, 68, 529–535. [Google Scholar] [CrossRef]
- Trach, N.; Phiovankham, B.; Luc, D. Effects of genotype and nutrition on growth performance, carcass characteristics, and meat properties of goats in Laos. J. South. Agric. 2011, 42, 786–790. [Google Scholar]
- Bouton, P.E.; Harris, P.V.; Shorthose, W.R. Effect of ultimate pH upon the water-holding capacity and tenderness of mutton. J. Food Sci. 1971, 36, 435–439. [Google Scholar] [CrossRef]
- Shackelford, S.; Morgan, J.; Cross, H.; Savell, J. Identification of Threshold Levels for Warner-Bratzler Shear Force in Beef Top Loin Steaks. J. Muscle Foods 1991, 2, 289–296. [Google Scholar] [CrossRef]
- Weston, A.; Rogers, R.; Althen, T. Review: The Role of Collagen in Meat Tenderness. Prof. Anim. Sci. 2002, 18, 107–111. [Google Scholar] [CrossRef]
- Astruc, T. Connective tissue: Structure, function, and influence on meat quality. In Encyclopedia of Meat Sciences; Elsevier: Oxford, UK, 2014; pp. 321–328. [Google Scholar]
- Adiwinarti, R.; Kustantinah, B.I.; Rusman, I.E. Improving the performance of local Kacang Goats using ruminally undegradable protein feeds. Asian J. Anim. Sci. 2016, 10, 262–267. [Google Scholar] [CrossRef][Green Version]
- Kannan, G.; Gadiyaram, K.; Galipalli, S.; Carmichael, A.; Kouakou, B.; Pringle, T.; McMillin, K.; Gelaye, S. Meat quality in goats as influenced by dietary protein and energy levels, and postmortem aging. Small Rumin. Res. 2006, 61, 45–52. [Google Scholar] [CrossRef]
- Nagaraj, N.S.; Anilakumar, K.; Santhanam, K.S.V. Changes in the Calpain-Calpastatin and Cathepsin (B, B+L, H AND D) during Postmortem Storage of Goat Muscles. J. Food Biochem. 2007, 26, 75–89. [Google Scholar] [CrossRef]
- Pringle, T.D.; Williams, S.E.; Lamb, B.S.; Johnson, D.D.; West, R.L. Carcass characteristics, the calpain proteinase system, and aged tenderness of Angus and Brahman crossbred steers. J. Anim. Sci. 1997, 75, 2955–2961. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Goll, D.E.; Marchello, J.A.; Duff, G.C.; Thompson, V.F.; Mares, S.W.; Ahmad, H.A. Effect of two dietary concentrate levels on tenderness, calpain and calpastatin activities, and carcass merit in Waguli and Brahman steers1. J. Anim. Sci. 2008, 86, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.-N.; Tsuneishi, E.; Kamiya, M.; Yamada, A. Histological Contribution of Collagen Architecture to Beef Toughness. J. Food Sci. 2010, 75, E73–E77. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.; Bonvillani, A.; Freire, B.; Juárez, M.; Perea, J.; Gómez, G. Effects of genotype and slaughter weight on the meat quality of Criollo Cordobes and Anglonubian kids produced under extensive feeding conditions. Meat Sci. 2009, 83, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, N.W.; Murray, P.; Taylor, D. Total cholesterol concentrations of the muscles in castrated Boer goats. Small Rumin. Res. 2006, 64, 77–81. [Google Scholar] [CrossRef]
- Beserra, F.; Madruga, M.; Leite, A.; da Silva, E.; Maia, E. Effect of age at slaughter on chemical composition of meat from Moxotó goats and their crosses. Small Rumin. Res. 2004, 55, 177–181. [Google Scholar] [CrossRef]
- Pratiwi, N.W.; Murray, P.; Taylor, D.; Zhang, D. Comparison of breed, slaughter weight and castration on fatty acid profiles in the longissimus thoracic muscle from male Boer and Australian feral goats. Small Rumin. Res. 2006, 64, 94–100. [Google Scholar] [CrossRef]
- Abdullah, M.M.H.; Jew, S.; Jones, P.J.H. Health benefits and evaluation of healthcare cost savings if oils rich in monounsaturated fatty acids were substituted for conventional dietary oils in the United States. Nutr. Rev. 2017, 75, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Banskalieva, V.; Sahlu, T.; Goetsch, A. Fatty acid composition of goat muscles and fat depots: A review. Small Rumin. Res. 2000, 37, 255–268. [Google Scholar] [CrossRef]
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Enser, M.; Richardson, R.; Wood, J.; Gill, B.; Sheard, P. Feeding linseed to increase the n-3 PUFA of pork: Fatty acid composition of muscle, adipose tissue, liver and sausages. Meat Sci. 2000, 55, 201–212. [Google Scholar] [CrossRef]
- Rhee, M.-S.; Wheeler, T.; Shackelford, S.; Koohmaraie, M. Variation in palatability and biochemical traits within and among eleven beef muscles. J. Anim. Sci. 2004, 82, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Beriain, M.J.; Bas, P.; Purroy, A.; Treacher, T. Effect of Animal and Nutritional Factors and Nutrition on Lamb Meat Quality; CIHEAM: Zaragoza, Spain, 2000; Volume 52. [Google Scholar]
- Suzuki, A.; Homma, N.; Fukuda, A.; Hirao, K.; Uryu, T.; Ikeuchi, Y. Effects of high pressure treatment on the flavour-related components in meat. Meat Sci. 1994, 37, 369–379. [Google Scholar] [CrossRef]
- Álvarez, C.; Morán, L.; Keenan, D.F.; Mullen, A.-M.; Delgado-Pando, G. Mechanical and Biochemical Methods for Rigor Measurement: Relationship with Eating Quality. J. Food Qual. 2019, 2019, 1894543. [Google Scholar] [CrossRef]
- Tikk, M.; Tikk, K.; Tørngren, M.; Meinert, L.; Aaslyng, M.; Karlsson, A.; Andersen, H. Development of Inosine Monophosphate and Its Degradation Products during Aging of Pork of Different Qualities in Relation to Basic Taste and Retronasal Flavor Perception of the Meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef] [PubMed]
| Traits | NG 1 | CG 1 | p-Value |
|---|---|---|---|
| Initial weight (kg) | 11.80 ± 2.18 | 12.98 ± 1.22 | 0.154 |
| Live weight (kg) | 17.33 ± 2.83 | 18.12 ± 1.52 | 0.446 |
| Carcass (kg) | 7.24 ± 1.43 | 8.22 ± 0.68 | 0.072 |
| Carcass dressing (%) | 41.65 ± 3.00 | 45.52 ± 3.91 | 0.023 |
| Carcass length (cm) | 42.30 ± 2.31 | 44.90 ± 4.68 | 0.132 |
| Lean (kg) | 5.41 ± 0.74 | 5.64 ± 0.51 | 0.262 |
| Bone (kg) | 1.32 ± 0.14 | 1.57 ± 0.08 | 0.0005 |
| Fat (kg) | 0.07 ± 0.09 | 0.18 ± 0.23 | 0.0002 |
| Lean (%) | 63.69 ± 5.68 | 62.15 ± 6.54 | 0.581 |
| Bone (%) | 16.26 ± 0.59 | 18.15 ± 0.48 | <0.0001 |
| Fat (%) | 1.00 ± 0.49 | 2.22 ± 0.79 | 0.0006 |
| Traits | NG 1 | CG 1 | p-Value |
|---|---|---|---|
| pH45 | 6.46 ± 0.36 | 6.55 ± 0.28 | 0.520 |
| pH24 | 5.97 ± 0.45 | 5.74 ± 0.13 | 0.150 |
| L* | 30.01 ± 3.03 | 32.77 ± 5.10 | 0.160 |
| a* | 13.43 ± 1.77 | 13.83 ± 2.99 | 0.725 |
| b* | 13.62 ± 1.69 | 14.78 ± 2.69 | 0.261 |
| Drip loss (%) | 2.07 ± 0.38 | 1.93 ± 0.24 | 0.350 |
| Cooking loss (%) | 29.03 ± 2.64 | 27.29 ± 3.20 | 0.202 |
| Shear force (n) | 32.80 ± 2.80 | 46.30 ± 4.90 | <0.0001 |
| Traits | NG 1 | CG 1 | p-Value |
|---|---|---|---|
| Collagen content 2 | |||
| Soluble collagen | 0.11 ± 0.04 | 0.09 ± 0.03 | 0.211 |
| Insoluble collagen | 3.02 ± 0.51 | 3.67 ± 0.71 | 0.010 |
| Total collagen | 3.50 ± 0.52 | 4.28 ± 0.75 | 0.015 |
| Collagen solubility (%) 3 | 3.18 ± 0.98 | 2.10 ± 0.53 | 0.006 |
| Calpain activity 4 | |||
| Calpain-1 1 h | 1.88 ± 0.14 | 1.74 ± 0.36 | 0.247 |
| Calpain-1 24 h | 0.18 ± 0.02 | 0.14 ± 0.04 | 0.017 |
| Calpain-2 1 h | 1.61 ± 0.18 | 1.58 ± 0.30 | 0.799 |
| Calpain-2 24 h | 0.87 ± 0.09 | 0.88 ± 0.16 | 0.994 |
| Trait | Pearson’s Correlation Coefficient | p-Value |
|---|---|---|
| Collagen content | ||
| Soluble collagen | −0.003 | 0.991 |
| Insoluble collagen | 0.401 | 0.080 |
| Total collagen | 0.461 | 0.047 |
| Collagen solubility (%) | −0.216 | 0.373 |
| Calpain activity | ||
| Calpain-1 1 h | −0.208 | 0.379 |
| Calpain-1 24 h | −0.650 | 0.002 |
| Calpain-2 1 h | −0.082 | 0.731 |
| Calpain-2 24 h | −0.060 | 0.801 |
| Traits | NG 1 | CG 1 | p-Value | |
|---|---|---|---|---|
| Cholesterol 2 | 71.88 ± 15.15 | 79.99 ± 11.38 | 0.178 | |
| Fatty acid composition 3 | ||||
| Myristic acid | C14:0 | 1.63 ± 0.18 | 2.29 ± 0.65 | 0.037 |
| Pentadecanoic acid | C15:0 | 2.32 ± 0.38 | 3.08 ± 1.01 | 0.143 |
| Palmitic acid | C16:0 | 17.89 ± 1.10 | 20.08 ± 1.64 | 0.027 |
| Palmitoleic acid | C16:1 | 1.80 ± 0.43 | 1.80 ± 0.44 | 0.989 |
| Margaric acid | C17:0 | 13.86 ± 2.11 | 12.34 ± 2.17 | 0.253 |
| Heptadecenoic acid | C17:1 | 1.24 ± 0.14 | 1.08 ± 0.18 | 0.114 |
| Stearic acid | C18:0 | 12.63 ± 1.33 | 14.32 ± 1.53 | 0.074 |
| Elaidic acid | C18:1n9t | 0.76 ± 0.07 | 0.81 ± 0.18 | 0.602 |
| Oleic acid | C18:1n9c | 37.00 ± 1.99 | 30.61 ± 3.84 | 0.007 |
| Linoleic acid | C18:2n6c | 3.72 ± 0.91 | 4.67 ± 1.44 | 0.229 |
| α-Linolenic acid | C18:3n3 | 0.51 ± 0.09 | 0.44 ± 0.14 | 0.344 |
| Heneicosylic acid | C21:0 | 0.46 ± 0.06 | 0.36 ± 0.07 | 0.036 |
| Behenic acid | C22:0 | 0.60 ± 0.27 | 1.20 ± 0.36 | 0.011 |
| Tricosylic acid | C23:0 | 4.36 ± 1.17 | 5.04 ± 1.98 | 0.512 |
| Eicosapentaenoic acid | C20:5n3 | 1.22 ± 0.49 | 1.88 ± 0.98 | 0.195 |
| SFA | 53.76 ± 1.93 | 58.73 ± 1.91 | 0.001 | |
| MUFA | 40.80 ± 2.20 | 34.29 ± 3.62 | 0.005 | |
| PUFA | 5.44 ± 1.36 | 6.98 ± 2.39 | 0.226 | |
| DFA | 58.87 ± 1.02 | 55.60 ± 2.42 | 0.114 | |
| PUFA: SFA | 0.10 ± 0.03 | 0.12 ± 0.04 | 0.423 | |
| ω−6 | 3.72 ± 0.91 | 4.66 ± 1.44 | 0.229 | |
| ω−3 | 1.72 ± 0.56 | 2.32 ± 1.07 | 0.283 | |
| ω−6: ω−3 | 2.24 ± 0.49 | 2.32 ± 1.04 | 0.889 |
| Ribonucleotide Content 1 | NG 2 | CG 2 | p-Value |
|---|---|---|---|
| Hypoxanthine | 0.74 ± 0.28 | 0.52 ± 0.12 | 0.091 |
| Inosine | 5.44 ± 1.35 | 5.49 ± 2.21 | 0.959 |
| Inosine monophosphate | 25.61 ± 4.67 | 28.91 ± 6.58 | 0.328 |
| Guanosine monophosphate | 0.82 ± 0.19 | 0.88 ± 0.29 | 0.682 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaosap, C.; Chauychuwong, N.; Chauychuwong, R.; Sriprem, C.; Sivapirunthep, P.; Sazili, A.Q. Carcass Composition, Meat Quality, Calpain Activity, Fatty Acid Composition and Ribonucleotide Content in Southern Thai Native Goats and Three-Way Crossbred Goats. Foods 2021, 10, 1323. https://doi.org/10.3390/foods10061323
Chaosap C, Chauychuwong N, Chauychuwong R, Sriprem C, Sivapirunthep P, Sazili AQ. Carcass Composition, Meat Quality, Calpain Activity, Fatty Acid Composition and Ribonucleotide Content in Southern Thai Native Goats and Three-Way Crossbred Goats. Foods. 2021; 10(6):1323. https://doi.org/10.3390/foods10061323
Chicago/Turabian StyleChaosap, Chanporn, Nantana Chauychuwong, Ratchasak Chauychuwong, Chatchai Sriprem, Panneepa Sivapirunthep, and Awis Qurni Sazili. 2021. "Carcass Composition, Meat Quality, Calpain Activity, Fatty Acid Composition and Ribonucleotide Content in Southern Thai Native Goats and Three-Way Crossbred Goats" Foods 10, no. 6: 1323. https://doi.org/10.3390/foods10061323
APA StyleChaosap, C., Chauychuwong, N., Chauychuwong, R., Sriprem, C., Sivapirunthep, P., & Sazili, A. Q. (2021). Carcass Composition, Meat Quality, Calpain Activity, Fatty Acid Composition and Ribonucleotide Content in Southern Thai Native Goats and Three-Way Crossbred Goats. Foods, 10(6), 1323. https://doi.org/10.3390/foods10061323