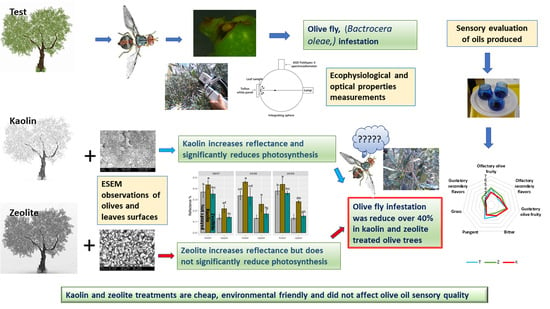
Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Sampling
- (1)
- K: foliar application of kaolin at a dosage of 3.0 kg/100 L of H2O;
- (2)
- Z: foliar application of CHA-zeolitite at a dosage of 0.6 kg/100 L of H2O;
- (3)
- T: control (untreated).
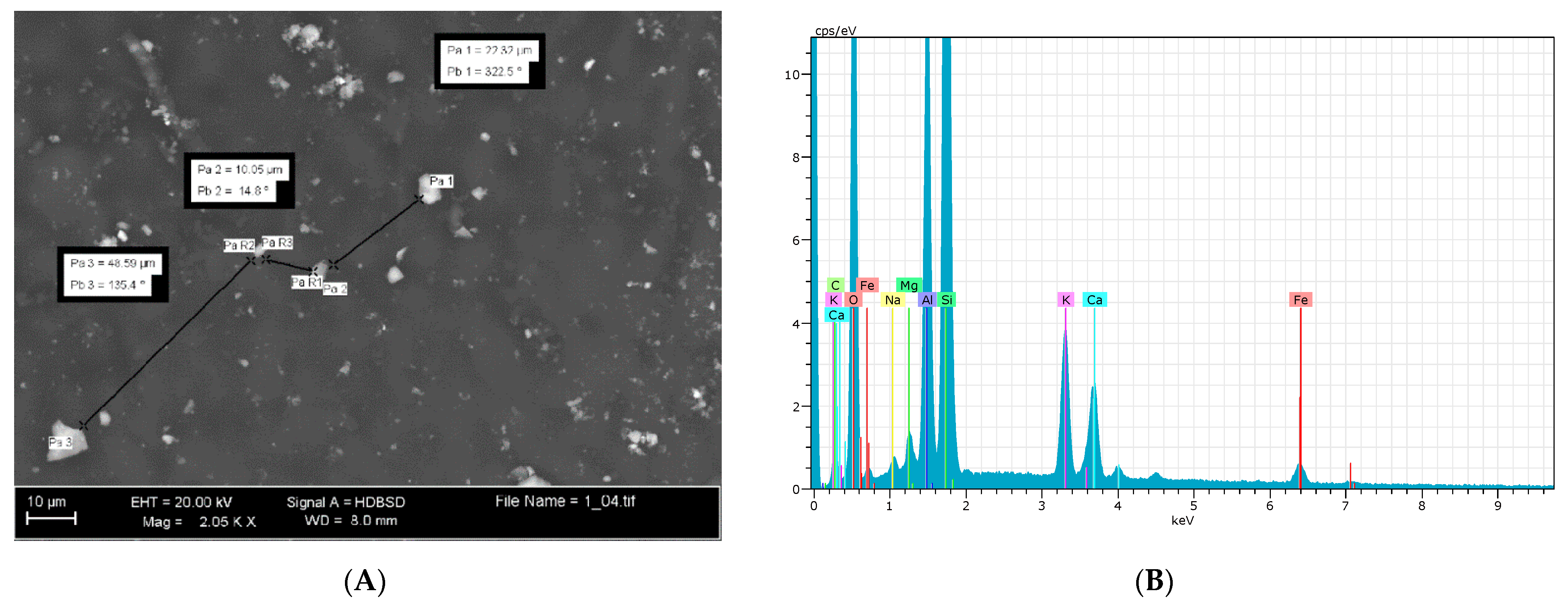
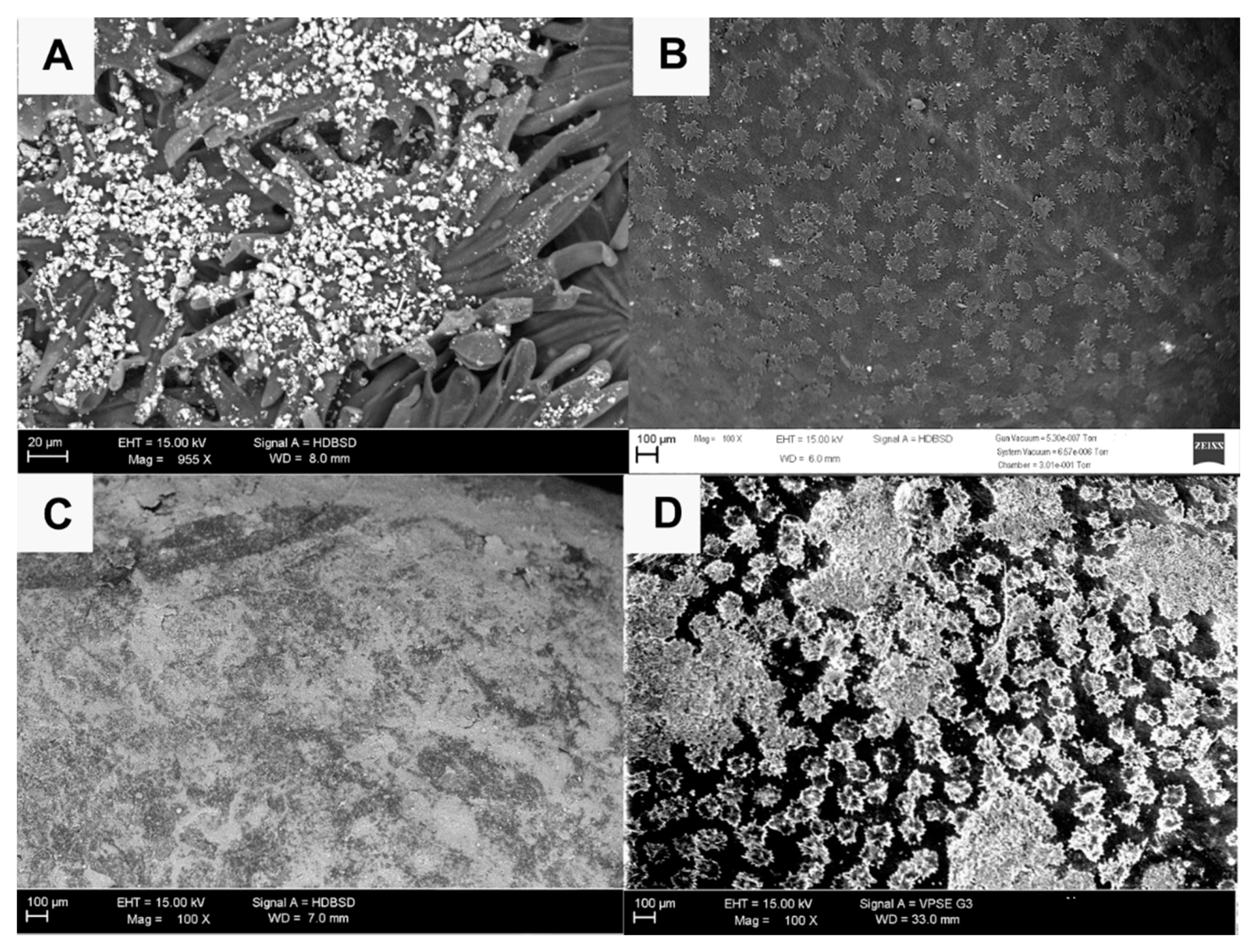
2.2. Environmental Electronic Microscope (ESEM) Observations
2.3. Chemical Analysis on Leaves and Soil Samples
2.4. Ecophyisiological, Optical Properties and Color Leaf Measurements
2.5. Olive Analyses and Olive Oils Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. ESEM Observations
3.2. Chemical Analysis on Leaves and Soil Samples
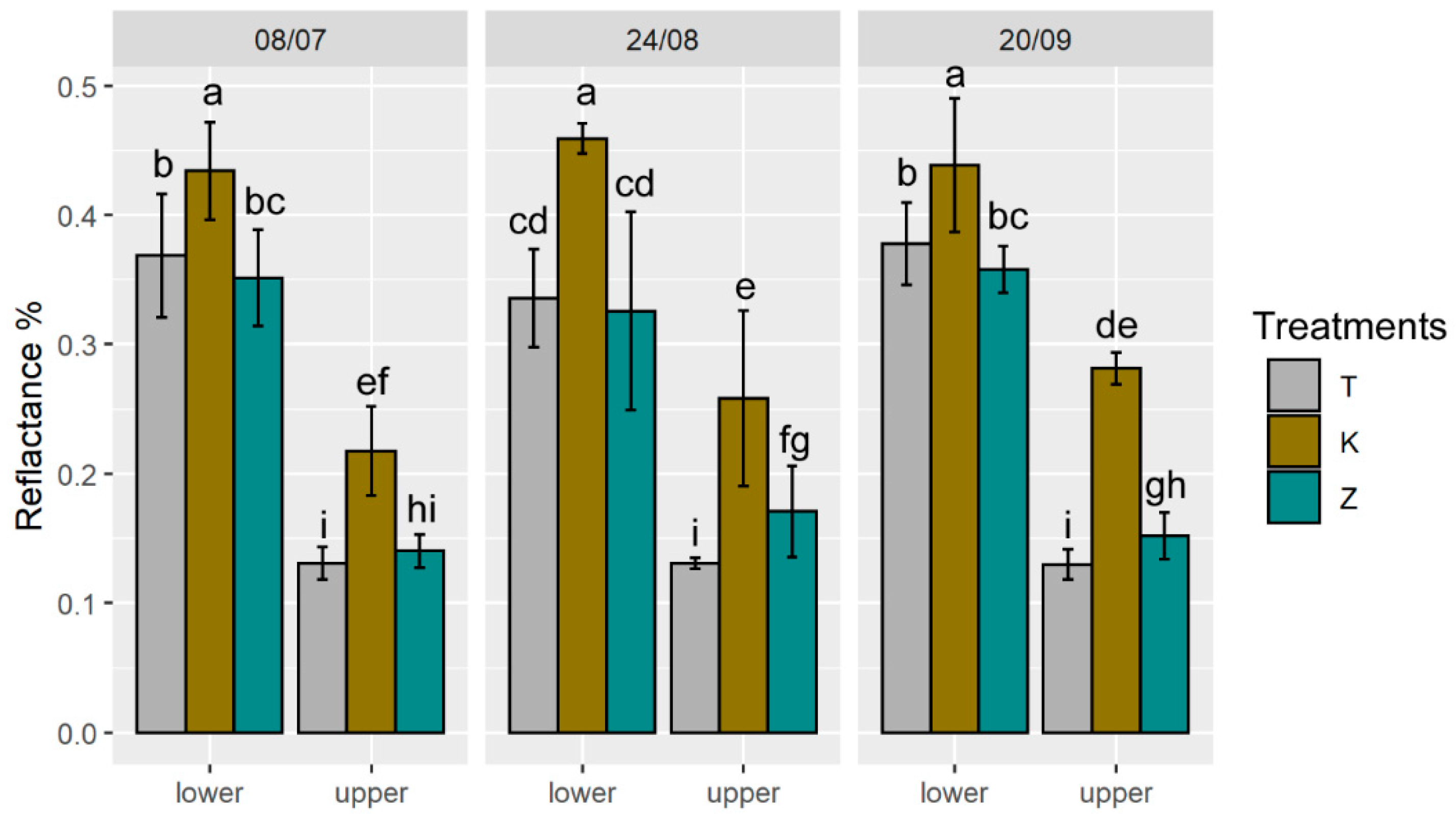
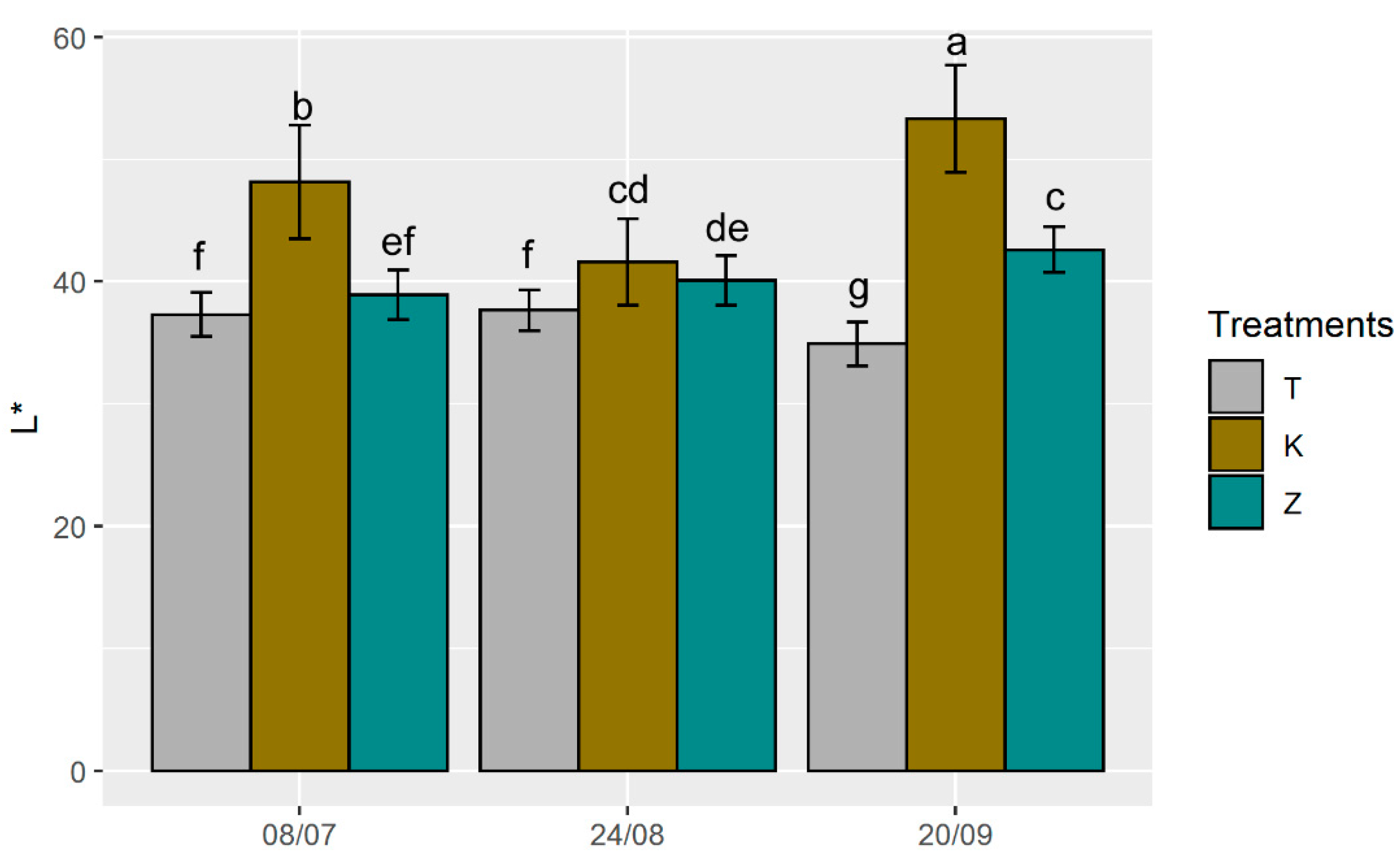
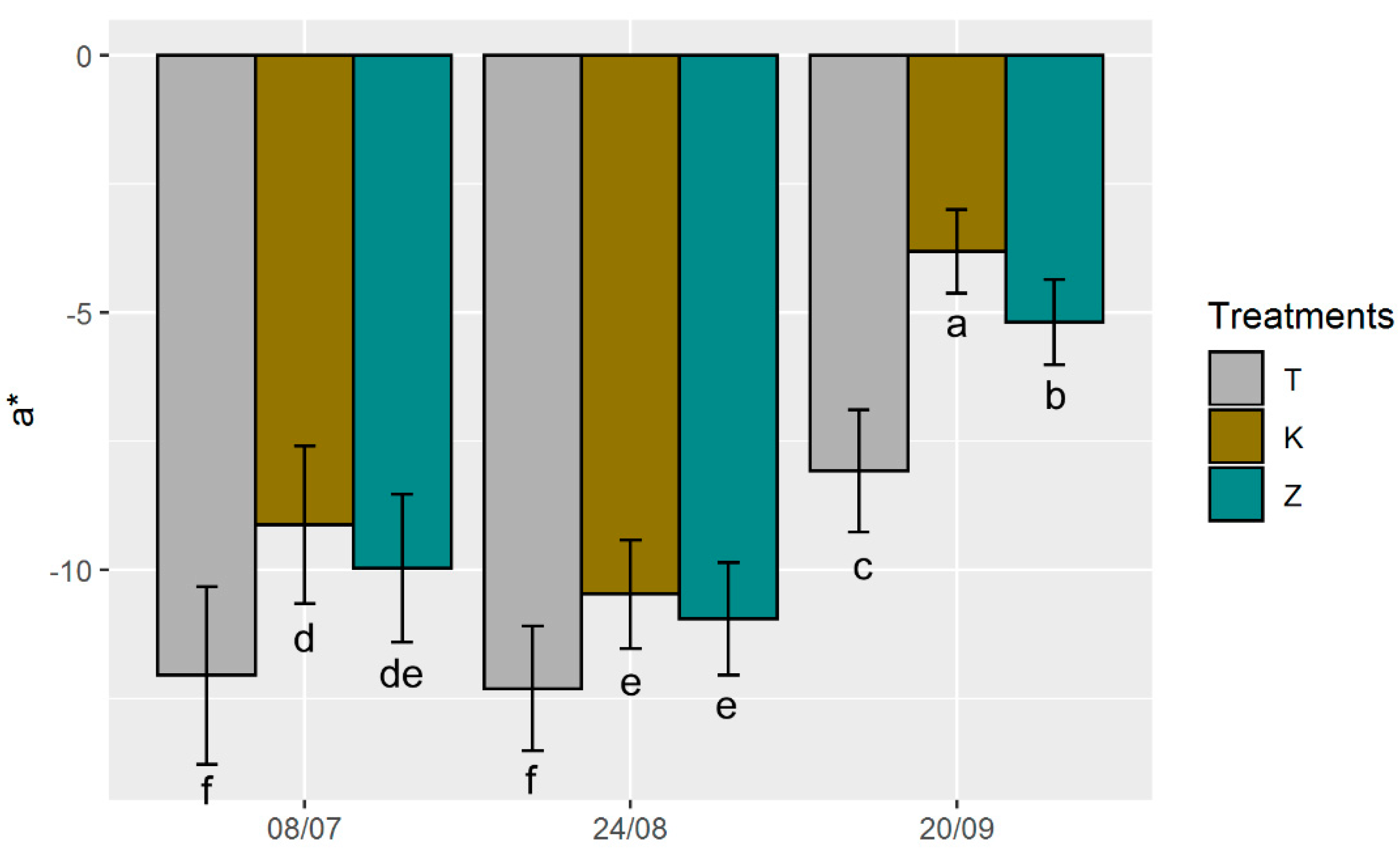
3.3. Ecophysiological Parameters and Optical Properties
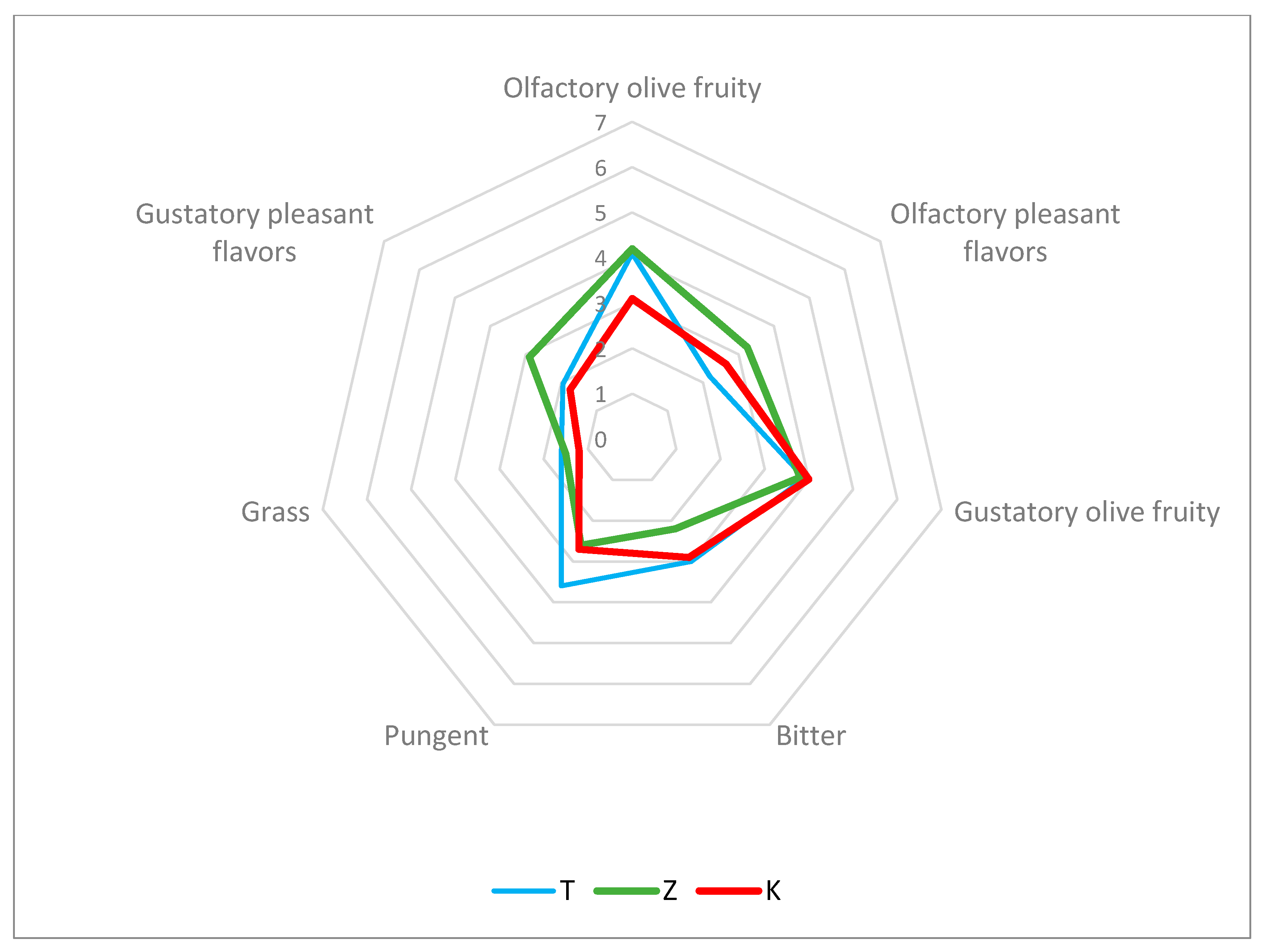
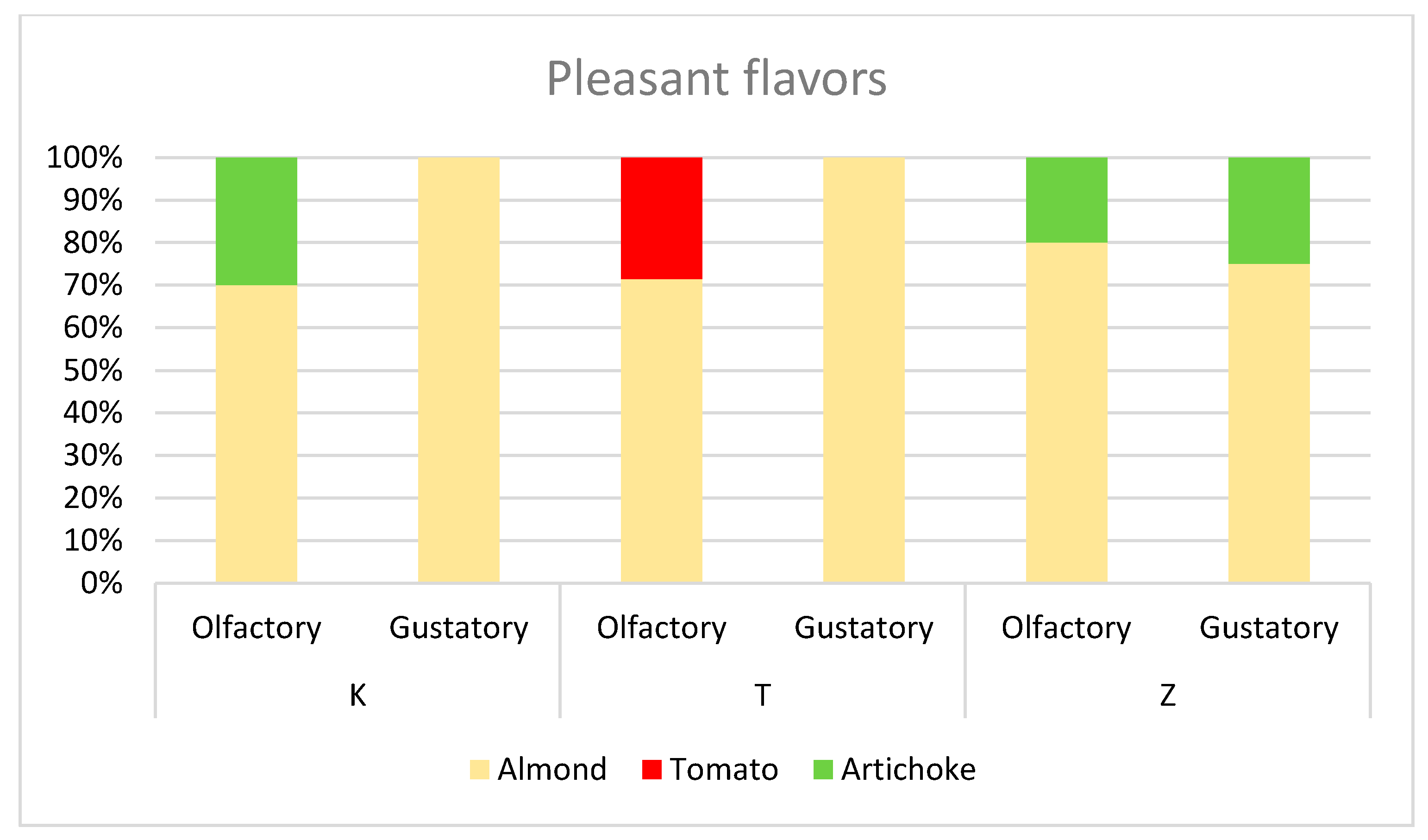
3.4. Olive Analyses and Olive Oil Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haniotakis, G.E. Olive pest control: Present status and prospects. In Proceedings of the IOBC/WPRS Conference on Integrated Protection of Olive Crops, Chania, Greece, 29–31 May 2003. [Google Scholar]
- European Commission. Regulation EC No. 432/2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L136, 1–40. [Google Scholar]
- Volpi, I.; Guidotti, D.; Mammini, M.; Petacchi, R.; Marchi, S. Managing complex datasets to predict Bactrocera oleae infestation at the regional scale. Comput. Electron. Agric. 2020, 179, 105867. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1090/2019 of 26 June 2019. Off. J. Eur. Union 2019, 173, 39–41. [Google Scholar]
- Daane, K.M.; Sime, K.R.; Wang, X.G.; Nadel, H.; Johnson, M.W.; Walton, V.M.; Kirk, A.; Pickett, C.H. Psyttalia lounsburyi (Hymenoptera: Braconidae), potential biological control agent for the olive fruit fly in California. Biol. Control 2008, 44, 79–89. [Google Scholar] [CrossRef]
- Roselli, L.; Clodoveo, M.L.; Corbo, F.; De Gennaro, B. Are health claims a usefull tool to segment the category of extra-virgin olive oil? Threats and opportunities for the Italian olive oil supply chain. Trends Food Sci. Technol. 2017, 68, 176–181. [Google Scholar] [CrossRef]
- Godfrey, L.; Grafton-Cardwell, E.; Kaya, H.; Chaney, W. Microorganisms and their byproducts, nematodes, oils and particle films have important agricultural uses. Calif. Agric. 2005, 59, 35–40. [Google Scholar] [CrossRef][Green Version]
- Brindley, G.W.; Robinson, K. Structure of kaolinite. Nature 1945, 156, 661–662. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Z.; Lei, X.; Yan, C. Defects in structure as the sources of the surface charges of kaolinite. Appl. Clay Sci. 2016, 124, 127–136. [Google Scholar] [CrossRef]
- Dombrowsky, T. The origins of kaolinite—Implication for utlization. In Science of Whiteware; Carty, W., Ed.; Wiley: New York, NY, USA, 2000; pp. 3–12. [Google Scholar]
- Andreola, F.; Castellini, E.; Manfredini, T.; Romagnoli, M. The role of sodium hexametaphosphate in the dissolution process of kaolinite and kaolin. J. Eur. Ceram. Soc. 2004, 24, 2113–2124. [Google Scholar] [CrossRef]
- Kahr, G.; Madsen, F.T. Determination of the cation exchange capacity and the surface area of bentonite, illite and kaolinite by methylene blue adsorption. Appl. Clay Sci. 1995, 9, 327–336. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Q.; Kou, J.; Sun, C.; Li, H. Aggregation mechanism of colloidal kaolinite in aqueous solutions with electrolyte and surfactants. PLoS ONE 2020, 15, e0238350. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, C.; Ferrer, F.; Leus, K.; Spanoghe, P. Removal of pesticides from aqueous solutions by adsorption on zeolites as solid adsorbents. Adsorp. Sci. Technol. 2015, 33, 457–485. [Google Scholar] [CrossRef]
- Kesraoui-Ouki, S.; Cheeseman, C.R.; Perry, R. Natural zeolite utilisation in pollution control: A review of applications to metals’ effluents. J. Chem. Technol. Biotechnol. 1994, 59, 121–126. [Google Scholar] [CrossRef]
- Galli, E.; Passaglia, E. Natural zeolites in environmental engineering. In Zeolites in Chemical Engineering; Holzapfel, H., Ed.; Process Engineering GmbH: Vienna, Austria, 2007; pp. 392–416. [Google Scholar]
- Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Using zeolitic adsorbents to cleanup special wastewater streams: A review. Micropor. Mesopor. Mater. 2015, 214, 224–241. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Passaglia, E. Rietveld structure refinement of NH4-exchanged natural chabazite. Eur. J. Mineral 2006, 18, 351–359. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef]
- Ferretti, G.; Di Giuseppe, D.; Natali, C.; Faccini, B.; Bianchini, G.; Coltorti, M. CN elemental and isotopic investigation in agricultural soils: Insights on the effects of zeolitite amendments. Geochemistry 2017, 77, 45–52. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Khodaei-Joghan, A.; Dolatabadian, A.; Zakikhani, H.; Farmanbar, E. Zeolite-amended cattle manure effects on sunflower yield, seed quality, water use efficiency and nutrient leaching. Soil Tillage Res. 2013, 126, 193–202. [Google Scholar] [CrossRef]
- Colombani, N.; Di Giuseppe, D.; Faccini, B.; Ferretti, G.; Mastrocicco, M.; Coltorti, M. Estimated water savings in an agricultural field amended with natural zeolites. Environ. Process. 2016, 3, 617–628. [Google Scholar] [CrossRef]
- Faccini, B.; Di Giuseppe, D.; Ferretti, G.; Coltorti, M.; Colombani, N.; Mastrocicco, M. Natural and NH4+-enriched zeolitite amendment effects on nitrate leaching from a reclaimed agricultural soil (Ferrara Province, Italy). Nutr. Cycling Agroecosyst. 2018, 110, 327–341. [Google Scholar] [CrossRef]
- Ferretti, G.; Galamini, G.; Deltedesco, E.; Gorfer, M.; Faccini, B.; Zechmeister-Boltenstern, S.; Coltorti, M.; Keiblinger, K.M. Effects of natural and NH4-charged zeolite amendments and their combination with 3, 4-dimethylpyrazole phosphate (DMPP) on soil gross ammonification and nitrification rates. In Geophysical Research Abstracts; Copernicus publication: Munich, Germany, 2019; Volume 21. [Google Scholar]
- Ferretti, G.; Keiblinger, K.M.; Zimmermann, M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Mentlerb, A.; Zechmeister-Boltensternb, S.; Coltorti, M.; Mastrocicco, M. High resolution short-term investigation of soil CO2, N2O, NOx and NH3 emissions after different chabazite zeolite amendments. Appl. Soil Ecol. 2017, 119, 138–144. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. Short-Term Response of Soil Microbial Biomass to Different Chabazite Zeolite Amendments. Pedosphere 2018, 28, 277–287. [Google Scholar] [CrossRef]
- Ferretti, G.; Galamini, G.; Medoro, V.; Coltorti, M.; Di Giuseppe, D.; Faccini, B. Impact of Sequential Treatments with Natural and Na-Exchanged Chabazite Zeolite-Rich Tuff on Pig-Slurry Chemical Composition. Water 2020, 12, 310. [Google Scholar] [CrossRef]
- Ferretti, G.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Mitigation of sodiumrisk in a sandy agricultural soil by the use of natural zeolites. Environ. Monit. Assess. 2018, 190, 646. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Medoro, V.; Tescaro, N.; Faccini, B.; Coltorti, M. Isotherms, Kinetics, and Thermodynamics of NH4+ Adsorption in Raw Liquid Manure by Using Natural Chabazite Zeolite-Rich Tuff. Water 2020, 12, 2944. [Google Scholar] [CrossRef]
- De la Roca, M. Surround® Crop Protectant: La capa protectora natural para cultivos como el olivar. Phytoma España 2003, 148, 82–85. [Google Scholar]
- Saour, G.; Makee, H. A kaolin-based particle film for suppression of the olive fruit fly Bactrocera olCeae Gmelin (Dip., Tephritidae) in olive groves. J. Appl. Entomol. 2003, 128, 28–31. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Sakka, M.; Berillis, P.; Athanassiou, C.G. Insecticidal potential of zeolite formulations against three stored-grain insects, particle size effect, adherence to kernels and influence on test weight of grains. J. Stored Prod. Res. 2016, 68, 93–101. [Google Scholar] [CrossRef]
- McBride, J. Whitewashing agriculture. Agric. Res. 2000, 48, 14–17. [Google Scholar]
- Doraiswamy, P.C.; Rosenberg, N.J. Reflectant Induced Modification of Soybean Canopy Radiation Balance. I. Preliminary Tests with a Kaolinite Reflectant 1. Agron. J. 1974, 66, 224–228. [Google Scholar] [CrossRef]
- Schupp, J.R.; Fallahi, E.; Chun, I.J. Effect of particle film on fruit sunburn, maturity and quality of Fuji’ and Honey crisp apples. HortTechnology 2002, 12, 87–90. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P. Moderate shade can increase net gas exchange and reduce photoinhibition in citrus leaves. Tree Physiol. 2003, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Glenn, D.M. The mechanisms of plant stress mitigation by kaolin-based particle films and applications in horticultural and agricultural crops. HortScience 2012, 47, 710–711. [Google Scholar] [CrossRef]
- Lalancette, N.; Belding, R.D.; Shearer, P.W.; Frecon, J.L.; Tietjen, W.H. Evaluation of hydrophobic and hydrophilic kaolin particle films for peach crop, arthropod and disease management. Pest Manag. Sci. 2005, 61, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, H. Controlled release of preservatives using dealuminated zeolite Y. J. Biochem. Bioph. Meth. 2008, 70, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P. Disguising the leaf surface. In Recent Advances in Crop Protection; Reddy, P., Ed.; Springer: New York, NY, USA, 2012; pp. 91–102. [Google Scholar]
- Carter, G.A. Primary and secondary effect of water content on spectral reflectance of leaves. Am. J. Bot. 1991, 78, 916–924. [Google Scholar] [CrossRef]
- Baldini, E.; Facini, O.; Nerozzi, F.; Rossi, F.; Rotondi, A. Leaf characteristics and optical properties of different woody species. Trees 1997, 12, 73–81. [Google Scholar] [CrossRef]
- Lanza, B.; Di Serio, M.G. SEM characterization of olive (Olea europaea L.) fruit epicuticular waxes and epicarp. Sci. Hortic. 2015, 191, 49–56. [Google Scholar] [CrossRef]
- Spanoghe, P.; De Schampheleire, M.; Van der Meeren, P.; Steurbaut, W. Influence of agricultural adjuvants on droplet spectra. Pest Manag. Sci. 2007, 63, 4–16. [Google Scholar] [CrossRef]
- Gaskin, R.E.; Steele, K.D.; Forster, W.A. Characterising plant surfaces for spray adhesion and retention. N. Zealand Plant Prot. 2005, 58, 179–183. [Google Scholar] [CrossRef]
- Skuterud, R.; Bjugstad, N.; Tyldum, A.; Tørresen, K.S. Effect of herbicides applied at different times of the day. Crop Prot. 1998, 17, 41–46. [Google Scholar] [CrossRef]
- Denaxa, N.K.; Roussos, P.A.; Damvakaris, T.; Stournaras, V. Comparative effects of exogenous glycine betaine, kaolin clay particles and Ambiol on photosynthesis, leaf sclerophylly indexes and heat load of olive cv. Chondrolia Chalkidikis under drought. Sci. Hortic. 2012, 137, 87–94. [Google Scholar] [CrossRef]
- Larbi, A.; Vázquez, S.; El-Jendoubi, H.; Msallem, M.; Abadía, J.; Abadía, A.; Morales, F. Canopy light heterogeneity drives leaf anatomical, eco-physiological, and photosynthetic changes in olive trees grown in a high-density plantation. Photosynth. Res. 2015, 123, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, A.; Magli, M. Ripening of olives var. correggiolo: Modification of oxidative stability of oils during fruit ripening and oil storage. J. Food Agric. Environ. 2004, 2, 193–199. [Google Scholar]
- Uceda, M.; Hermoso, M. La calidad del aceite de oliva. In El Cultivo del Olivo; Barranco, D., Fernàndez-Escobar, R., Rallo, L., Eds.; Junta de Andalucía Ediciones Mundi-Prensa: Madrid, Spain, 2001; pp. 589–614. [Google Scholar]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Gallina Toschi, T. Effect of olive ripening degree on oxidative stability and organoleptic properties of Cv. Nostrana di Brisighella extra virgin olive oil. J. Agric. Food Chem. 2004, 53, 3649–3654. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Kaneda, M.; Terasaki, O.; Zhao, D.Y.; Kim, J.M.; Stucky, G.; Shin, H.J.; Ryoo, R. Direct imaging of the pores and cages of three-dimensional mesoporous materials. Nature 2000, 408, 449–453. [Google Scholar] [CrossRef]
- Hu, P.; Yang, H. Insight into the physicochemical aspects of kaolins with different morphologies. Appl. Clay Sci. 2013, 74, 58–65. [Google Scholar] [CrossRef]
- Curry, E.; Baer, D.; Young, J. X-Ray microanalysis of apples treated with kaolin indicates wax-Embedded Particulate in the Cuticle. In Proceedings of the XXVI International Horticultural Congress: Key Processes in the Growth and Cropping of Deciduous Fruit and Nut Trees, Toronto, ON, Canada, 11–17 August 2002; pp. 497–503. [Google Scholar]
- Tworkoski, T.J.; Michael Glenn, D.; Puterka, G.J. Response of bean to applications of hydrophobic mineral particles. Can. J. Plant Sci. 2002, 82, 217–219. [Google Scholar] [CrossRef]
- Le Grange, M.; Wand, S.J.E.; Theron, K.I. Effect of kaolin applications on apple fruit quality and gas exchange of apple leaves. In Proceedings of the XXVI International Horticultural Congress: Key Processes in the Growth and Cropping of Deciduous Fruit and Nut Trees, Toronto, ON, Canada, 11–17 August 2002; pp. 545–550. [Google Scholar]
- Rosati, A.; Metcalf, S.G.; Buchner, R.P.; Fulton, A.E.; Lampinen, B.D. Effects of kaolin application on light absorption and distribution, radiation use efficiency and photosynthesis of almond and walnut canopies. Ann. Bot. 2007, 99, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wünsche, J.N.; Lombardini, L.; Greer, D.H. ‘Surround’ Particle Film Applications-Effects on Whole Canopy Physiology of Apple. In Proceedings of the XXVI International Horticultural Congress: Key Processes in the Growth and Cropping of Deciduous Fruit and Nut Trees, Toronto, ON, Canada, 11–17 August 2002; pp. 565–571. [Google Scholar]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci. Hortic. 2019, 250, 310–316. [Google Scholar] [CrossRef]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Proietti, P.; Famiani, F. Diurnal and seasonal changes in photosynthetic characteristics in different olive (Olea europaea L.) cultivars. Photosynthetica 2002, 40, 171–176. [Google Scholar] [CrossRef]
- Colavita, G.M.; Blackhall, V.; Valdez, S. Effect of kaolin particle films on the temperature and solar injury of pear fruits. In Proceedings of the XI International Pear Symposium, General Roca, Rio Negro, Argentina, 23–26 November 2010; pp. 609–615. [Google Scholar]

| Treatment | TN (%) | TC (%) | δ15N (‰) | δ13C (‰) |
|---|---|---|---|---|
| T | 1.47 ± 0.02 a | 45.51 ± 1.12 a | −3.71 ± 2.06 a | −27.76 ± 0.28 a |
| K | 1.69 ± 0.17 a | 47.71 ± 2.03 a | −0.53 ± 1.49 a | −28.59 ± 0.42 a |
| Z | 1.44 ± 0.36 a | 47.05 ± 3.39 a | −2.06 ± 1.71 a | −28.43 ± 0.37 a |
| Application Date | Treatment | A 1 μmol CO2 m−2 s−1 | G 2 mmol m⁻² s⁻¹ | Ci 3 μmol CO2 mol air | E 4 mol H2O m−2 s−1 | WUE 5 |
|---|---|---|---|---|---|---|
| 8 July | K | 13.03 ± 0.75 a | 0.39 ± 0.02 a | 321.98 ± 4.05 a | 9.68 ± 0.46 a | 1.38 ± 0.09 a |
| T | 13.14 ± 1.05 a | 0.32 ± 0.02 a | 309.49 ± 4.89 a | 9.82 ± 0.48 a | 1.34 ± 0.09 a | |
| Z | 12.14 ± 0.85 a | 0.33 ± 0.04 a | 309.36 ± 6.20 a | 9.06 ± 0.71 a | 1.42 ± 0.11 a | |
| 24 August | K | 9.98 ± 0.64 a | 0.20 ± 0.02 b | 287.65 ± 5.02 b | 8.01 ± 0.61 b | 1.28 ± 0.07 a |
| T | 11.93 ± 0.78 a | 0.28 ± 0.03 a,b | 295.35 ± 4.57 b | 10.28 ± 0.67 a | 1.18 ± 0.06 a | |
| Z | 12.35 ± 0.59 a | 0.34 ± 0.02 a | 312.77 ± 3.51 a | 9.46 ± 0.58 a,b | 1.37 ± 0.1 a | |
| 20 September | K | 9.5 ± 0.55 b | 0.12 ± 0.01 c | 248.86 ± 6.94 c | 2.91 ± 0.23 b | 3.36 ± 0.15 a |
| T | 13.03 ± 0.49 a | 0.28 ± 0.01 b | 299.98 ± 2.06 b | 5.55 ± 0.21 a | 2.36 ± 0.06 b | |
| Z | 12.19 ± 0.76 a | 0.33 ± 0.01 a | 317.69 ± 2.59 a | 5.99 ± 0.20 a | 2.02 ± 0.08 b |
| Treatment | RI | % Infestation | H2O (%) | Firmness 1 |
|---|---|---|---|---|
| K | 2.6 | 26 | 46.5 ± 1.4 a | 55.0 ± 28.9 |
| T | 2.58 | 70 | 43.2 ± 0.6 b | 52.7 ± 29.9 |
| Z | 2.48 | 34 | 44.7 ± 0.4 a,b | 48.7 ± 28.8 |
| p-value | / | / | 0.038 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotondi, A.; Morrone, L.; Facini, O.; Faccini, B.; Ferretti, G.; Coltorti, M. Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology. Foods 2021, 10, 1291. https://doi.org/10.3390/foods10061291
Rotondi A, Morrone L, Facini O, Faccini B, Ferretti G, Coltorti M. Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology. Foods. 2021; 10(6):1291. https://doi.org/10.3390/foods10061291
Chicago/Turabian StyleRotondi, Annalisa, Lucia Morrone, Osvaldo Facini, Barbara Faccini, Giacomo Ferretti, and Massimo Coltorti. 2021. "Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology" Foods 10, no. 6: 1291. https://doi.org/10.3390/foods10061291
APA StyleRotondi, A., Morrone, L., Facini, O., Faccini, B., Ferretti, G., & Coltorti, M. (2021). Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology. Foods, 10(6), 1291. https://doi.org/10.3390/foods10061291